Abstract
Objective:
To describe the frequency, intensity, and duration of urologic chronic pelvic pain syndrome symptom exacerbations (“flares”), as well as risk factors for these features, in the Multidisciplinary Approach to the Study of Chronic Pelvic Pain Epidemiology and Phenotyping longitudinal study.
Participants and methods:
Current flare status (“urologic or pelvic pain symptoms that are much worse than usual”) was ascertained at each bi-weekly assessment. Flare characteristics, including start date, and current intensity of pelvic pain, urgency, and frequency (scales of 0–10), were assessed for participants’ first three flares and at three randomly selected times when they did not report a flare. Generalized linear and mixed effects models were used to investigate flare risk factors.
Results:
Of the 385 eligible participants, 24.2% reported no flares, 22.9% reported 1 flare, 28.3% 2–3 flares, and 24.6% ≥4 flares, up to a maximum of 18 during the 11-month follow-up (median incidence rate=0.13/bi-weekly assessment, range=0.00–1.00). Pelvic pain (mean=2.63 point increase) and urologic symptoms (mean=1.72) were both significantly worse during most flares (60.6%), with considerable within-participant variability (26.2–37.8%). Flare duration varied from 1–150 days (94.3% within-participant variability). In adjusted analyses, flares were more common, symptomatic, and/or longer-lasting in female participants and those with worse non-flare symptoms, bladder hypersensitivity, and chronic overlapping pain conditions.
Conclusion:
In this foundational flare study, we found that pelvic pain and urologic symptom flares were common, but variable in frequency and manifestation. We also identified sub-groups of participants with more frequent, symptomatic, and/or longer-lasting flares for targeted flare management/prevention and further study.
Keywords: Epidemiology, interstitial cystitis, prostatitis, symptom flare-up
INTRODUCTION
Interstitial cystitis/bladder pain syndrome (IC/BPS) and chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) are characterized by persistent pelvic or bladder pain and urologic symptoms, such as urgency and frequency. The etiology of these conditions, collectively referred to as urologic chronic pelvic pain syndrome (UCPPS), is unknown and both are difficult to diagnose and treat.1,2 They occur in approximately 1–7% of the population,3–6 and contribute to significant reductions in physical and mental health, sexual health, and work productivity, as well as considerable personal and societal healthcare expenditures.4,7–11
Similar to many other chronic pain conditions, UCPPS symptoms are not static, but instead fluctuate over time. Symptom exacerbations (“flares”) vary widely in presentation, with pelvic pain intensity ranging from mild to severe, flare duration from minutes to months, and flare frequency from less than once per year to multiple times per day, based on patient surveys and focus groups.12,13 A similarly wide range of flare frequency has been observed in the few longitudinal studies conducted to date. In a previous IC/BPS cohort study, 67.9% of participants reported no flares over the two-year follow-up, whereas 5.7% reported up to 3–4 flares (defined as urinary tract infection (UTI) symptoms).14 Similarly, in our small, previous UCPPS longitudinal study, 51.8% of participants reported no flares over the one-year follow-up, whereas 12.5% reported 3–4 flares (defined as urologic or pelvic pain symptoms that are much worse than usual lasting at least one day) over the 20 days they completed symptom diaries.15 Both of these studies were limited, however, to specific manifestations of flares (UTI symptoms or day(s)-long duration), and neither examined risk factors for greater flare burden. Therefore, we used data from the Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Epidemiology and Phenotyping study to describe the frequency and characteristics of the full range of flares, and risk factors for these features over the one-year follow-up.
PARTICIPANTS AND METHODS
Study design
The MAPP Epidemiology and Phenotyping Study was a one-year, multi-site longitudinal study designed to study the “usual-care” natural history of UCPPS and to identify sub-groups of patients with possible differing etiology and clinical course.16,17 Participants completed an extensive battery of questionnaires at bi-annual in-person visits and a shorter set at online bi-weekly assessments. The MAPP Epidemiology and Phenotyping Study was approved by the institutional review boards at each site and the data coordinating center. All participants provided written informed consent.
Flare assessment
Flares were assessed and characterized as part of a case-crossover study of flare triggers embedded into the main longitudinal study.18 Briefly, at each bi-annual in-person and bi-weekly assessment, participants were asked to report their current flare status using the following question: “Are you currently experiencing a flare of your urologic or pelvic pain symptoms? By this we mean, are you currently experiencing symptoms that are much worse than usual” (Appendix Table 1). If participants responded affirmatively, they were directed to a second questionnaire – the Brief Flare Risk Factor Questionnaire17 – that included additional questions about their: 1) flare start date to identify individual flares and calculate a crude (truncated) estimate of flare duration; and 2) current levels of pelvic pain, urgency, and frequency to characterize the intensity of participants’ pelvic pain and urologic symptoms during flares (using the maximum of urgency and frequency to describe urologic symptoms because of their high degree of correlation). This additional questionnaire was administered for the first three reported flares and at three randomly selected times when participants did not report a flare (once per 4-month period). Recalled symptom data averaged over the past week (which could possibly include some flare and non-flare days) was also used to describe symptoms during flares.
Risk factor assessment
Possible risk factors included study quarter; sex; age; duration of symptoms and symptoms of IC/BPS or CP/CPPS at baseline (males only); average non-flare pelvic pain and urologic symptom intensity from visit 3 onwards; and baseline self-reported bladder hypersensitivity,19 whole body pain widespread-ness,20 and presence and number of chronic overlapping pain conditions (COPCs).21 We also examined pelvic pain and urologic symptom trajectories22 from visit 3 onwards as possible correlates of flare burden.
Statistical analysis
For the flare frequency analyses, we limited the sample to participants who reported their current flare status at least three times. Crude incidence rates were calculated by dividing the number of assessments at which participants reported a flare (not taking into consideration flare start date, as this was assessed for three flares only) by the number of completed assessments per participant. Relative rates were calculated by generalized linear models, using the number of completed assessments as the offset; generalized estimating equations for robust variance estimation; and Poisson, negative binomial, or generalized Poisson distributions depending on the dispersion of the data.23–25 Models were initially adjusted for study site, sex, age, and average non-flare pelvic pain and urologic symptom intensity as markers of participants’ overall clinical status. Adjustment for baseline flare status, which might influence participants’ baseline phenotypic characteristics and correlate with greater flare frequency, was also considered, but not retained, because of its minimal influence on risk factor estimates once non-flare symptom intensity was included in the models. Final models were additionally adjusted for variables that remained significantly associated with flare frequency: bladder hypersensitivity and number of COPCs.
For the flare characteristic analyses, we limited the sample to participants who completed the Brief Flare Risk Factor Questionnaire at least twice, once each when they were and were not experiencing a flare. Flares were required to have started in the past two weeks to avoid double-counting. Changes in symptom intensity during flares and risk factors for more painful flares, those with worse urologic symptoms, and longer flares were estimated by linear and generalized linear mixed effects models, clustering flares by participant.26,27 Cut-off points for more symptomatic flares were determined by classification and regression tree analyses,28 using data from our previous site-specific survey.13 This survey asked participants to report their average pelvic pain, urgency, frequency (scales of 0–10), and bother (none, only a little, some, and a lot) for flares lasting minutes, hours, and days. We used these data to perform two analyses each for pelvic pain and urologic symptoms, one to identify cut-off points for flare symptom intensity associated with “a lot” of bother (≥7 out of 10 for pelvic pain and urologic symptoms), and a second to identify cut-off points for change in intensity during flares associated with “a lot” of bother among participants with non-flare symptom values <7 (≥4 for pain and urologic symptoms). These two cut-off points were then combined into one outcome, similar to previous depression analyses.29 We used the median flare duration (≥5 days) in the full MAPP study to define longer flares rather than site-specific data because the distribution assessed on the site-specific survey was considerably shorter than in the full study. Within-participant variability in flare intensity and duration was calculated as 1 minus the intra-class correlation coefficient.
Two-sided p-values<0.05 were considered statistically significant. All analyses were performed using SAS version 9.4.
RESULTS
Of the 424 enrolled participants, we excluded two who became pregnant and 19 who did not provide flare status information at least three times during follow-up, leaving 403 participants in the analysis. Forty-five percent of participants were male and most identified as White (88.3%, Appendix Table 2). The average age was 43.6 years and the average condition duration was 8.60 years. At baseline, participants reported a moderate level of UCPPS symptoms (mean=5.08–5.56 out of 10) and many had bladder hypersensitivity (82.9%), extra-pelvic pain (73.7%) and/or a COPC (38.0%).
Flare frequency
Participants provided information on their flare status an average of 17.6 times (range: 3 (2.0%) to 25 (11.8%)), with wide variation in the number of flares reported (range: 0 (19.5%) to 20 (0.3%); median incidence rate=0.13/assessment). Consistent with our previous observation of early symptom regression in this cohort, flare frequency was considerably higher at baseline (0.28/assessment) than later during follow-up (0.11/assessment post-visit 12). As this higher baseline flare frequency may not have been representative of participants’ typical frequency, but possibly part of their motivation to join MAPP, we limited all further analyses to visit 3 onwards (n=385 participants). In this more restricted sample, we observed similarly wide variability in flare frequency, with 24.2% of participants reporting no flares, 22.9% 1 flare, 28.3% 2–3 flares, and 24.6 ≥4, up to a maximum of 18 flares over the 11-month follow-up (0.3% of participants; median incidence rate=0.13/assessment, range=0.00–1.00/assessment; Appendix Figure 1).
Risk factors for greater flare frequency
In unadjusted analyses, female sex; younger age; IC/BPS (versus CP/CPPS) symptoms (males only); greater average non-flare pelvic pain and urologic symptom intensity; bladder hypersensitivity; extra-pelvic pain; several individual COPCs, increasing number of COPCs, and any COPC; and worsening pelvic pain and urologic symptom trajectories were each associated with greater flare frequency (Table 1). After adjustment, findings for IC/BPS symptoms (males only), bladder hypersensitivity, ≥2 COPCs, and worsening pain and urologic symptom trajectories remained statistically significant.
Table 1.
Relative rates (RRs) and 95% confidence intervals (CIs) of flares in urologic chronic pelvic pain syndrome participants (n=3821) in the Multidisciplinary Approach to the Study of Chronic Pelvic Pain Epidemiology and Phenotyping Study, 2009–2013.
| Crude RR (95% CI) |
Adjusted RR2
(95% CI) |
Adjusted RR3
(95% CI) |
|
|---|---|---|---|
| Increasing study quarter (continuous) | 1.01 (0.92–1.11) | 1.05 (0.96–1.15) | 1.04 (0.96–1.14) |
| Female sex | 1.48 (1.20–1.82) | 1.29 (1.03–1.61) | 1.16 (0.92–1.46) |
| Increasing age (continuous in years) | 0.84 (0.75–0.94) | 0.88 (0.79–0.99) | 0.92 (0.82–1.03) |
| Increasing symptom duration (continuous in years) | 0.97 (0.87–1.08) | 0.95 (0.84–1.08) | 0.94 (0.83–1.06) |
| Baseline IC/BPS versus CP/CPPS symptoms (males only) | 1.42 (1.01–2.01) | 1.71 (1.03–2.85) | 1.62 (1.00–2.64) |
| Average non-flare pelvic pain intensity (continuous from 0–10) | 1.13 (1.08–1.18) | 1.02 (0.95–1.09) | 1.03 (0.96–1.11) |
| Average non-flare urologic symptom intensity (continuous from 0–10) |
1.14 (1.09–1.19) | 1.11 (1.04–1.18) | 1.06 (0.98–1.14) |
| Bladder hypersensitivity: | |||
| Neither painful filling nor urgency | 1.00 | 1.00 | 1.00 |
| Either painful filling or urgency | 1.63 (1.18–2.26) | 1.55 (1.12–2.15) | 1.58 (1.15–2.20) |
| Both painful filling and urgency | 2.19 (1.61–2.96) | 1.75 (1.29–2.37) | 1.73 (1.28–2.35) |
| Number of extra-pelvic regions with pain:4 | |||
| 0 | 1.00 | 1.00 | 1.00 |
| 1–2 | 1.34 (1.03–1.74) | 1.21 (0.94–1.56) | 1.16 (0.90–1.50) |
| 3–7 | 1.34 (1.03–1.75) | 1.08 (0.83–1.41) | 1.00 (0.75–1.33) |
| Chronic overlapping pain conditions: | |||
| Chronic fatigue syndrome | 2.04 (1.53–2.73) | 1.43 (1.08–1.90) | 1.16 (0.82–1.63) |
| Fibromyalgia | 1.41 (0.96–2.07) | 1.01 (0.74–1.38) | 0.71 (0.50–1.03) |
| Irritable bowel syndrome | 1.22 (0.98–1.53) | 1.05 (0.85–1.29) | 0.96 (0.77–1.19) |
| Migraines | 1.44 (1.13–1.83) | 1.26 (0.99–1.60) | 1.12 (0.83–1.51) |
| Temporomandibular joint disorder | 1.31 (1.02–1.68) | 1.08 (0.86–1.37) | 0.82 (0.59–1.14) |
| Vulvodynia | 1.41 (1.02–1.95) | 1.22 (0.90–1.66) | 1.15 (0.84–1.55) |
| Number of conditions: | |||
| 0 | 1.00 | 1.00 | 1.00 |
| 1 | 1.25 (0.98–1.59) | 1.14 (0.88–1.48) | 1.13 (0.87–1.45) |
| 2 or more | 1.82 (1.40–2.36) | 1.34 (1.03–1.75) | 1.30 (1.01–1.68) |
| Any condition | 1.33 (1.07–1.64) | 1.04 (0.85–1.27) | 0.89 (0.70–1.14) |
| Pelvic pain trajectory over follow-up | |||
| Improving | 1.00 | 1.00 | 1.00 |
| Stable | 0.95 (0.73–1.24) | 0.83 (0.65–1.06) | 0.85 (0.67–1.08) |
| Worsening | 1.47 (1.15–1.87) | 1.39 (1.10–1.75) | 1.42 (1.13–1.78) |
| Urologic symptom trajectory over follow-up | |||
| Improving | 1.00 | 1.00 | 1.00 |
| Stable | 0.95 (0.73–1.23) | 0.88 (0.69–1.12) | 0.90 (0.71–1.14) |
| Worsening | 1.41 (1.11–1.79) | 1.34 (1.05–1.70) | 1.33 (1.05–1.68) |
CP/CPPS=chronic prostatitis/chronic pelvic pain syndrome; IC/BPS=interstitial cystitis/bladder pain syndrome.
Excludes 3 participants who reported flares at all biweekly assessments.
Adjusted for site, sex, age, and average non-flare symptom intensity, as appropriate.
Adjusted for site, sex, age, average non-flare symptom intensity, bladder hypersensitivity, and number of chronic overlapping pain conditions, as appropriate.
Seven extra-pelvic regions (back, head, right leg, left leg, right arm, left arm, and trunk) were created similar to previous analyses, using data from the Body Pain Inventory.
Flare characteristics
Among participants who completed the Brief Flare Risk Factor Questionnaire at least twice (n=807 non-flare and 716 flare assessments from 297 participants), non-flare values ranged from 0–10 for both pelvic pain (mean=3.15) and urologic symptoms (mean=3.35), with four participants reporting values of 10 for pelvic pain and/or urologic symptoms (Table 2, Figure 1). These values were significantly worse during flares (mean=5.78 for pelvic pain and 5.07 for urologic symptoms, ranges: 0–10). Almost all participants reported values >0 for pelvic pain and urologic symptoms during flares, except for two with all values of 0. On average, symptoms increased by 2.63 (range: −4 to 9) for pelvic pain and 1.72 (range: −6 to 8) for urologic symptoms during flares. Similar increases were observed for other UCPPS symptoms and overall pain in the week before participants’ flare assessments. With respect to flare duration (reported for 579 flares), 20.0% of flares had lasted 1–2 days by the time of participants’ flare assessment, 40.2% 3–6 days, 24.2% 7–10 days, and 15.5% >10 days (range=1–150 days). In general, current pelvic pain decreased slightly, but significantly, with increasing flare duration, whereas current urologic symptoms increased slightly with increasing duration.
Table 2.
Flare and non-flare symptoms in urologic chronic pelvic pain syndrome participants in the Multidisciplinary Approach to the Study of Chronic Pelvic Pain Epidemiology and Phenotyping Study, 2009–2013.
| N | Non-flare (Mean (SD)/%)1 |
Flare (Mean (SD)/%)2 |
Mean change during flares |
P-value | |
|---|---|---|---|---|---|
| Urologic chronic pelvic pain syndrome symptoms: | |||||
| Current symptoms | |||||
| Pelvic pain (range: 0–10) | 287 | 3.15 (2.31) | 5.78 (2.15)3 | 2.63 | <0.0001 |
| Urologic symptoms (maximum of urgency and frequency, range: 0–10) | 289 | 3.35 (2.47) | 5.07 (2.69)4 | 1.72 | <0.0001 |
| Symptoms in the past week | |||||
| Any pain or discomfort in the (%): | |||||
| Female: | |||||
| Entrance to the vagina | 173 | 38.5 | 52.9 | 14.4 | 0.0014 |
| Vagina | 174 | 39.2 | 52.2 | 13.0 | 0.004 |
| Urethra | 175 | 44.9 | 63.6 | 18.7 | <0.0001 |
| Male: | |||||
| Tip of the penis (not related to urination) | 120 | 32.7 | 45.8 | 13.1 | 0.02 |
| Testicles | 120 | 29.2 | 38.2 | 9.0 | 0.11 |
| Perineum | 120 | 43.5 | 49.8 | 6.3 | 0.28 |
| Any genital site | 295 | 63.0 | 79.2 | 16.2 | <0.0001 |
| Number of genital sites with pain (range: 0–3) |
295 | 1.15 (1.09) | 1.53 (1.08) | 0.38 | <0.0001 |
| Any pain or burning during urination | 295 | 41.6 | 58.7 | 17.1 | <0.0001 |
| Any pain or discomfort during or after sexual intercourse | 272 | 32.2 | 43.3 | 11.1 | 0.002 |
| Any pain or discomfort with bladder filling | 295 | 48.5 | 61.4 | 12.9 | 0.0006 |
| Any pain or discomfort relieved by voiding | 295 | 54.2 | 63.7 | 9.4 | 0.011 |
| Frequency of a sensation of incomplete emptying (0=not at all to 5=almost always) | 295 | 1.81 (1.65) | 2.16 (1.69) | 0.35 | 0.007 |
| Overall symptoms: | |||||
| Current symptoms | |||||
| Overall whole body pain (range: 0–10)5 | 122 | 3.41 (2.56) | 4.36 (2.59) | 0.95 | 0.004 |
| Symptoms in the past week | |||||
| Worst pain (range: 0–10)5 | 122 | 5.42 (2.88) | 6.51 (2.49) | 1.10 | 0.001 |
| Average pain (range: 0–10)5 | 120 | 3.88 (2.27) | 4.63 (2.16) | 0.74 | 0.009 |
Includes a maximum of 807 non-flare values.
Includes a maximum of 716 flare values.
Taking flare duration into account (n=579 flares), these values were 6.09 for 1–3 day-long flares, 5.70 for 4–5 day-long flares, 5.75 for 6–8 day-long flares, and 5.62 for >8 day-long flares (p<0.0001).
These values were 5.10 for 1–3 day-long flares, 5.10 for 4–5 day-long flares, 5.00 for 6–8 day-long flares, and 5.14 for >8 day-long flares (p<0.0001).
Assessed by the Brief Pain Inventory, which was administered every two months. Responses collected within two weeks of a flare/non-flare assessment were used.
Figure 1. Flare and non-flare symptoms in urologic chronic pelvic pain syndrome participants in the Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Epidemiology and Phenotyping Study, 2009–2013.
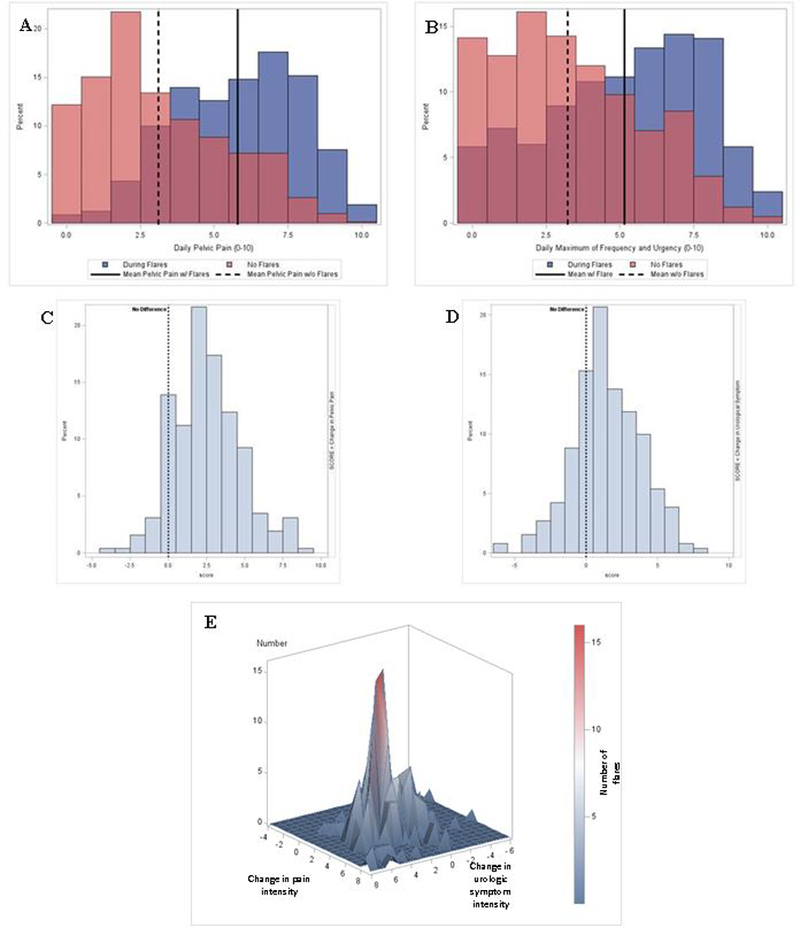
On each bi-weekly assessment of the one-year MAPP study follow-up, participants were asked to report whether they were currently experience a flare of their urologic or pelvic pain symptoms (i.e., “symptoms that are much worse than usual”). If participants responded affirmatively, they were directed to a second questionnaire on which they were asked about their current intensity of pelvic pain, urinary urgency, and frequency on scales of 0-10. This questionnaire was completed for the first three reported flares. Participants were also asked to complete the second questionnaire at three randomly-selected times when they did not report a flare. Figure A describes the distribution of pelvic pain intensity reported at non-flare (light pink) and flare (blue) assessments. Overlapping values are indicated in dark pink. Figure B describes the distribution of urologic symptom intensity (i.e., the maximum of urgency and frequency intensity) at non-flare (light pink) and flare (blue) assessments. Changes in pelvic pain and urologic symptom intensity during flares are described in Figures C and D, using the closest non-flare and flare assessments for comparison. Figure E presents the joint distribution of changes in pelvic pain and urologic symptom intensity during flares.
Considering patterns of symptoms, most flares involved an increase (≥1 point above non-flare symptoms) in both pelvic pain and urologic symptoms (60.6%), with smaller percentages involving increases in pelvic pain (20.1%) or urologic symptoms only (5.8%). Four percent (4.3%) of flares had stable symptoms and 9.2% had a decrease (Figure 1). When flares from the same participant were examined, a high degree of within-participant variability was observed (pelvic pain=37.8% of the total variance, urologic symptoms=26.2%, and duration=94.3%), indicating that participants did not always experience the same intensity or duration of symptoms during flares, but that these characteristics could vary.
Risk factors for flare characteristics
In unadjusted analyses, female sex, worse non-flare pelvic pain and urologic symptom intensity, and fibromyalgia were associated with more painful flares (Table 3). With the exception of fibromyalgia, these factors were also associated with flares with worse urologic symptoms, as was earlier study quarter, longer condition duration, IC/BPS symptoms (males only), and bladder hypersensitivity. Having a stable urologic symptom trajectory was protective for flares with worse urologic symptoms. Finally, worse non-flare pain intensity, irritable bowel syndrome, and any COPC were associated with longer flares.
Table 3.
Odds ratios (ORs) and 95% confidence intervals (CIs) of greater flare pelvic pain intensity, urologic symptom intensity, and duration in urologic chronic pelvic pain syndrome participants (n=286) in the Multidisciplinary Approach to the Study of Chronic Pelvic Pain Epidemiology and Phenotyping Study, 2009–2013.
| Pelvic pain intensity1 | Urologic symptom intensity2 | Duration3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Crude OR | Adjusted OR4 | Adjusted OR5 | Crude OR | Adjusted OR4 | Adjusted OR5 | Crude OR | Adjusted OR4 | Adjusted OR5 | |
| Increasing study quarter (continuous) | 0.88 | 0.95 | 0.96 | 0.79* | 0.86 | 0.87 | 0.87 | 0.87 | 0.87 |
| Female sex | 2.16*** | 2.00** | 2.12** | 2.29*** | 2.33** | 2.54*** | 1.09 | 0.99 | 1.01 |
| Increasing age (continuous in years) | 0.86 | 0.98 | 0.96 | 1.12 | 1.35* | 1.30* | 0.97 | 1.01 | 1.01 |
| Increasing symptom duration (continuous in years) | 1.11 | 1.04 | 1.05 | 1.23* | 1.13 | 1.14 | 1.07 | 1.02 | 1.03 |
| Baseline IC/BPS versus CP/CPPS symptoms (males only) |
1.38 | 1.47 | 1.51 | 4.04* | 2.58 | 1.91 | 0.63 | 0.70 | 0.72 |
| Average non-flare pelvic pain intensity (continuous from 0–10) |
1.39*** | 1.28** | 1.29** | 1.30*** | 0.95 | 0.96 | 1.09* | 1.17* | 1.17* |
| Average non-flare urologic symptom intensity (continuous from 0–10) | 1.32*** | 1.10 | 1.11 | 1.46*** | 1.51*** | 1.50*** | 1.03 | 0.93 | 0.94 |
| Increasing bladder hypersensitivity: | |||||||||
| Neither painful filling or urgency | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Either painful filling or urgency | 1.08 | 0.85 | 0.82 | 5.44*** | 5.16** | 4.77** | 0.87 | 1.02 | 1.03 |
| Both painful filling and urgency | 1.67# | 0.96 | 0.96 | 7.08*** | 4.29** | 4.19** | 0.77 | 0.83 | 0.83 |
| Number of non-pelvic regions with pain:6 | |||||||||
| 0 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 1–2 | 0.76 | 0.56# | 0.57# | 0.70 | 0.55# | 0.58# | 1.58# | 1.42 | 1.42 |
| 3–7 | 1.21 | 0.75 | 0.81 | 1.03 | 0.57# | 0.69 | 1.63# | 1.58 | 1.59 |
| Chronic overlapping pain conditions (COPCs): | |||||||||
| Chronic fatigue syndrome | 1.62# | 0.62 | 0.64 | 1.64 | 0.64 | 0.89 | 0.84 | 0.89 | 0.80 |
| Fibromyalgia | 2.34* | 1.46 | 2.49# | 1.27 | 0.70 | 1.27 | 1.11 | 0.97 | 0.90 |
| Irritable bowel syndrome | 1.18 | 0.90 | 0.95 | 1.06 | 0.72 | 0.77 | 1.55* | 1.68* | 1.77* |
| Migraines | 1.39 | 1.16 | 1.61 | 0.96 | 0.75 | 1.13 | 1.16 | 1.23 | 1.32 |
| Temporomandibular joint disorder | 0.97 | 0.65 | 0.62 | 0.97 | 0.64 | 0.90 | 0.85 | 0.88 | 0.73 |
| Vulvodynia | 1.03 | 0.80 | 0.85 | 1.30 | 0.82 | 0.95 | 1.14 | 1.22 | 1.22 |
| Number of conditions: | |||||||||
| 0 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 1 | 1.03 | 0.85 | 0.85 | 0.76 | 0.56# | 0.54# | 1.18 | 1.21 | 1.22 |
| 2 or more | 1.63# | 0.78 | 0.78 | 1.33 | 0.54# | 0.52# | 1.03 | 1.15 | 1.16 |
| Any condition | 1.37 | 0.88 | 0.96 | 1.04 | 0.63# | 0.69 | 1.63* | 1.74** | 2.02** |
| Pelvic pain trajectory over follow-up | |||||||||
| Improving | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Stable | 0.90 | 0.69 | 0.70 | 0.74 | 0.60# | 0.66 | 0.73 | 0.56* | 0.53* |
| Worsening | 0.71 | 0.65 | 0.65 | 0.70 | 0.61# | 0.67 | 0.82 | 0.76 | 0.73 |
| Urologic symptom trajectory over follow-up | |||||||||
| Improving | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Stable | 0.86 | 0.75 | 0.75 | 0.57* | 0.54* | 0.57# | 0.81 | 0.73 | 0.71 |
| Worsening | 1.22 | 1.07 | 1.08 | 1.32 | 1.14 | 1.11 | 0.69 | 0.65# | 0.66# |
0.05≤p<0.10
0.01≤p<0.05
0.001≤p<0.01
p<0.001
CP/CPPS=chronic prostatitis/chronic pelvic pain syndrome; IC/BPS=interstitial cystitis/bladder pain syndrome.
Defined as a change in pelvic pain of ≥4 or a level of pelvic pain ≥7 on a scale of 0–10 during a flare.
Defined as a change in urologic symptoms (maximum of urgency and frequency) of ≥4 or a level of urologic symptoms ≥7 on a scale of 0–10 during a flare.
Defined as a duration ≥5 days, the median flare duration in this population.
Adjusted for site, sex, age, and average non-flare symptom intensity, as appropriate.
Adjusted for site, sex, age, average non-flare symptom intensity, bladder hypersensitivity, and number of chronic overlapping pain conditions, as appropriate.
Seven extra-pelvic regions (back, head, right leg, left leg, right arm, left arm, and trunk) were created similar to previous analyses, using data from the Body Pain Inventory.
In adjusted analyses, female sex and greater non-flare pelvic pain intensity remained significantly associated with more painful flares; and female sex, greater non-flare urologic symptom intensity, and bladder hypersensitivity remained significantly associated with flares with worse urologic symptoms. Older age increased the odds of flares with worse urologic symptoms. Finally, worse non-flare pain intensity, irritable bowel syndrome, and any COPC remained significantly associated with longer flares. Having a stable pelvic pain trajectory was protective.
DISCUSSION
In this foundational flare study - the largest and most comprehensive to date - we found that pelvic pain and urologic symptom flares were common, but variable in frequency, intensity, and duration. This variability was observed both across and within participants. We also found that female sex, worse non-flare symptoms, and bladder hypersensitivity or COPCs were independently associated with greater flare burden, including greater flare frequency, symptom intensity, and/or duration.
To our knowledge, only two previous studies have documented flare frequency over time in participants to avoid recall inaccuracy.14,15 Similar to our analysis, these studies (one of which included a small subset of our participants) observed a wide range in flare frequency, although one was considerably lower (67.9% 0 and 5.7% 3–4 flares) than our distribution (24.4% 0 and 20.5% 3–4 flares), most likely because of its more restrictive flare definition (i.e., UTI symptoms). It is also possible we may have double-counted flares lasting >2 weeks, as these could have been captured on more than one bi-weekly assessment. Nevertheless, our estimates are still likely lower than the true frequency of flares because we did not capture flares that occurred between bi-weekly assessments. Our estimates also did not distinguish flares by intensity or duration, both of which have been found to contribute to their degree of impact and bother.12,13 Therefore, future studies should consider using additional methods, such as diaries or ecological momentary assessment, to obtain more accurate estimates of flare frequency, both overall and by flare type.
Similar to flare frequency, our descriptive findings for flare characteristics are consistent with those from the small number of studies conducted to date.12,13,15 These studies, many of which included a small subset of our participants, found that, although most flares involved significant increases in both pelvic pain and urologic symptoms, they could also vary in type (pelvic pain only, urologic symptoms only, or both) and intensity (mild to severe) of symptoms, both across and within participants. These findings are consistent with our observations of: 1) increases in both pelvic pain and urologic symptoms in a large proportion of flares (60.7%); 2) wide ranges in flare symptom intensity (0–10); 3) wide ranges in changes in symptom intensity during flares (−6 to 9); and 4) considerable within-participant variability in symptom intensity (26.2–37.8% of the total variance). Of note, our large sample size also allowed us to observe a small number of flares not previously described by other studies with seemingly stable or decreasing symptom levels. These unexpected flares may potentially be explained by: 1) inclusion of non-flare symptom values of 10, precluding notable symptom increases during flares; 2) changes in symptoms during flares besides pelvic pain, urgency, and frequency, such as genital pain or pain with urination only; 3) long time lags between non-flare and flare assessments, making these values less comparable; and 4) participant error. Similar to previous studies, we also observed wide variability in flare duration, ranging from 1–150 days. However, our distribution was likely shifted more towards longer flares than previous surveys and focus groups because of length-biased sampling30 (i.e., longer flares would be more likely to be captured on bi-weekly assessments than shorter flares). Alternatively, our estimates may be slightly under-estimated because flare duration was truncated at bi-weekly assessments. More accurate estimates will require following participants for the full duration of their flare.
Finally, our findings for flare risk factors are consistent with those from the limited literature on this topic to date.13–15 In their cohort study of IC/BPS patients, Stanford and McMurphy14 observed that participants with higher average baseline pain/urgency/frequency scores were borderline significantly more likely to report a UTI-symptom flare than those with lower scores, similar to our observation of crude and lesser-adjusted positive associations for greater non-flare symptom intensity with flare frequency. We also found that participants with worse non-flare symptoms were more likely to report flares with greater symptom intensity and duration (pelvic pain only), and that those with worsening pelvic pain and urologic symptom trajectories were more likely to report greater flare frequency, consistent with focus group participant reports that non-flare and flare symptoms worsen and improve together – i.e., that when their condition is “bad”, they experience worse non-flare symptoms, as well as more frequent, symptomatic, and longer flares.12 An additional novel finding from our study was that associations between non-flare symptoms and flare frequency attenuated after adjustment for bladder hypersensitivity and number of COPCs, suggesting they may have been explained by these two characteristics rather than by a link between worse non-flare symptoms and greater flare frequency – for instance, as a marker of overall worse clinical status.
With respect to sex, we previously observed that female participants were more likely to report days-long flares13,15 and less likely to report minutes-long flares than male participants in minimally-adjusted, site-specific analyses.13 As our current study design may have been more likely to capture day(s)- rather than minutes-long flares, we believe these previous findings are consistent with our crude and lesser-adjusted observations of greater flare frequency among female than male participants and, at least, not inconsistent with our observation of no difference in flare duration by sex among the sample of longer flares likely captured by our study design. Further novel findings from our study were: 1) that associations between female sex and greater flare frequency attenuated after adjustment for bladder hypersensitivity and COPCs, suggesting they may have been explained by these two factors; and 2) that female participants were independently more likely to report greater flare symptom intensity than male participants.
In addition to non-flare symptoms and sex, we found that participants with bladder hypersensitivity or COPCs (various individual, any, or multiple COPCs), but not extra-pelvic pain, had greater flare frequency, worse urologic symptoms during flares (bladder sensitivity only), and longer flares (COPCs only) in adjusted analyses. These novel, but slightly contradictory, findings suggest that both peripheral and central pain mechanisms may potentially contribute to flare burden. Finally, although some associations did not persist in adjusted analyses, this does not mean that certain sub-groups did not experience greater flare burden – only that their burden was more related to their bladder hypersensitivity and COPCs than to other characteristics.
Although our study is the largest and most detailed cohort study of flares to date, it is still limited by use of data collected for a different research purpose (i.e., to identify flare triggers with purposefully minimal ascertainment/characterization of flares). As such, we did not have information on flares that occurred between bi-weekly assessments, the full duration of flares, or characteristics of all flares (only a maximum of three flares/participant). These limitations reduced our ability to: 1) describe the frequency of flares overall and by symptom intensity and duration; 2) describe the full distribution of flare intensity and duration; and 3) detect associations, especially for flare intensity and duration, as the 1–3 flares included per participant may not have been representative of their typical flare experience. Finally, a further major limitation is our minimal collection of information on UCPPS therapies, precluding analysis of their influence on flare burden.
In summary, we found that the majority of participants experienced at least one flare of their pelvic pain and urologic symptoms during the 11-month follow-up, with wide variability in flare frequency, intensity, and duration between and within participants. In general, flares were independently more common, symptomatic, and/or longer-lasting in female participants, and those with worse non-flare symptoms, bladder hypersensitivity, or COPCs. Together, these findings provide foundational, descriptive information on flares and identify patient sub-groups to target and study further for flare management and prevention.
ACKNOWLEDGMENTS
We thank the research staff at the MAPP discovery sites and the data coordinating center for implementing the MAPP Study, and the participants for their generous participation.
This work was supported by the US National Institutes of Health/National Institute of Diabetes and Digestive and Kidney disease (U01 DK082315, U01 DK82316, U01 DK82325, U01 DK82333, U01 DK82342, U01 DK82344, U01 DK82345, and U01 DK82370).
Appendix Figure 1. Frequency of urologic chronic pelvic pain syndrome flares in the Multidisciplinary Approach to the Study of Chronic Pelvic Pain Epidemiology and Phenotyping Study, 2009–2013.

On each bi-weekly assessment of the MAPP study, participants were asked to report whether they were currently experiencing a flare of their urologic or pelvic pain symptoms (i.e., “symptoms that are much worse than usual”). The percentage of participants who reported each number of flares over the last 11 months of the one-year study is plotted.
Appendix Table 1.
Summary of information used to characterize flares and determine risk factors for and correlates of flare burden
| Timing of assessment | Items/questionnaires used for assessment | |
|---|---|---|
| Flare status and characteristics | ||
| Flare status | Each bi-annual and bi-weekly assessment | • “Are you currently experiencing a flare of your urologic or pelvic pain symptoms? By this we mean, are you currently experiencing symptoms that are much worse than usual?” |
| Pelvic pain intensity | First three flares and three randomly selected non-flare assessments | • “Think about the pain, pressure, and discomfort associated with your bladder/prostate and/or pelvic region. On average, how would you rate your current symptoms?” (scale: 0–10) • Painful flares defined as a change in pelvic pain ≥4 or a level of pelvic pain ≥7 during a flare; cut-off points determined by classification and regression tree analysis, using data from our previous site-specific flare survey31 |
| Urologic symptom intensity | First three flares and three randomly selected non-flare assessments | • “Urgency is defined as the urge or pressure to urinate. On average, how would you rate your current urgency?” (scale: 0–10) • “Think about your frequency of urination. On average, how would you rate your current frequency of urination?” (scale: 0–10) • Urologic symptom intensity defined as the maximum of urgency and frequency. • Flares with worse urologic symptoms defined as a change in urologic symptoms ≥4 or a level of urologic symptoms ≥7 during a flare; cut-off points determined by classification and regression tree analysis, using data from our previous site-specific flare survey1 |
| Intensity of additional UCPPS symptoms | Each bi-annual and bi-weekly assessment | • Items from the Genitourinary Pain Index2 (genital pain; pain/burning/discomfort during urination, during/after sexual intercourse, with bladder filling, or relieved by voiding; and a sensation of incomplete emptying) |
| Intensity of overall pain | Each bi-annual and bi-monthly assessment |
• Items from the Body Pain Inventory3 (current overall whole body pain, worst and average whole body pain in the past week) |
| Flare duration | First three flares and three randomly selected non-flare assessments | • “Did your flare start in the past two weeks? If yes, how many days have you been experiencing your current flare?” • Flare duration calculated as the difference in days between the dates of flare onset and questionnaire completion • Long flares defined as those greater or equal to the median flare duration (≥5 days) |
| Risk factors for greater flare frequency, symptom intensity, and duration | ||
| Study quarter | N/A | • Derived from participants’ dates of initiation and completion of the one-year MAPP follow-up |
| Sex | Baseline | • “What is your gender?” |
| Age | Baseline | • “What is your date of birth?” |
| Duration of symptoms | Baseline | • “Do you know when your chronic pelvic pain symptoms first began? If yes, at what age did they first begin?” |
| Symptoms of IC/BPS or CP/CPPS (males only) | Baseline | • Symptoms of IC/BPS defined as a report of an unpleasant sensation of pain, pressure or discomfort, perceived to be related to the bladder and/or pelvic region, associated with lower urinary tract symptoms. Symptoms must have been present for the majority of the time during any 3 months in the previous 6 months and for the majority of the time during the most recent 3 months. • Symptoms of CP/CPPS defined as a report of pain or discomfort in any of the 8 domains of the Male Genitourinary Pain Index (area between the rectum and testicles, testicles, tip of the penis (not related to urination), below your waist in your pubic or bladder area, during urination, during or after sexual climax, as your bladder fills, or relieved by voiding). Symptoms must have been present for the majority of the time during any 3 months in the previous 6 months. |
| Average non-flare pelvic pain intensity | Each bi-annual and bi-weekly assessment | • “Think about the pain, pressure, and discomfort associated with your bladder/prostate and/or pelvic region. On average, how would you rate these symptoms during the past 2 weeks?” (scale of 0–10) • Calculated as the average of all bi-annual and bi-weekly values from visit 3 onwards |
| Average non-flare urologic symptom intensity | Each bi-annual and bi-weekly assessment | • Urgency is defined as the urge or pressure to urinate. On average, how would you rate the urgency that you have felt during the past 2 weeks?” (scale of 0–10) • Think about your frequency of urination. On average, how would you rate your frequency of urination during the past 2 weeks? (scale of 0–10) • Urologic symptom intensity defined as the maximum of urgency and frequency. • Calculated as the average of all bi-annual and bi-weekly values from visit 3 onwards |
| Bladder hypersensitivity | Baseline | • RAND RICE questionnaire4
• Used to categorize participants into those with neither painful filling nor painful urgency, either painful filling or urgency, or both in the past three months5 |
| Pain widespread-ness | Baseline | • Items from the Body Pain Inventory3
• Used to create seven extra-pelvic regions (back, head, right leg, left leg, right arm, left arm, and trunk).6 |
| Presence and number of COPCs | Baseline | • Items from the Complex Medical Symptom Inventory7
• Used to define symptoms of chronic fatigue syndrome, fibromyalgia, irritable bowel syndrome, migraines, temporomandibular joint disorder, and vulvodynia |
| Correlates of greater flare frequency, symptom intensity, and duration | ||
| Pelvic pain trajectory | Each bi-annual and bi-weekly assessment | • Pelvic pain defined as the sum of the Genitourinary Pain Index pain sub-score and question 4 of the Interstitial Cystitis Symptom Index8 • Trajectories were estimated by functional clustering using participants’ bi-annual and bi-weekly pain values from visit 3 onwards.9 |
| Urologic symptom trajectory | Each bi-annual and bi-weekly assessment | • Urologic symptoms defined as the sum of the Genitourinary Pain Index urinary sub-score and questions 1–3 from the Interstitial Cystitis Symptom Index.8 • Trajectories were estimated by functional clustering using participants’ bi-annual and bi-weekly pain values from visit 3 onwards.9 |
UCPPS=urologic chronic pelvic pain syndrome
Appendix Table 2.
Baseline demographic and clinical characteristics of all urologic chronic pelvic pain syndrome participants combined and separately for those in the flare frequency and flare characteristics analyses; Multidisciplinary Approach to the Study of Chronic Pelvic Pain Epidemiology and Phenotyping Study, 2009–2013.
| All MAPP participants |
Participants in the analysis of: |
|||
|---|---|---|---|---|
| Flare frequency | Flare characteristics | |||
| N=424 | N=403 | N=3851 | N=297 | |
| Demographic characteristics: | ||||
| Male sex (%) | 45.1 | 44.7 | 44.2 | 40.4 |
| White race (%) | 88.2 | 88.3 | 88.8 | 89.6 |
| Hispanic ethnicity (%) | 6.6 | 5.7 | 5.5 | 5.4 |
| Age (years, mean, SD) | 43.4 (15.1) | 43.6 (15.2) | 43.9 (15.3) | 43.3 (15.0) |
| Duration of symptoms (years, mean, SD) |
8.50 (10.6) | 8.60 (10.5) | 8.79 (10.7) | 8.52 (10.2) |
| Baseline urologic chronic pelvic pain syndrome symptoms (mean, SD): | ||||
| Pelvic pain (range: 0–10) | 5.07 (2.20) | 5.08 (2.20) | 5.08 (2.19) | 5.18 (2.16) |
| Urologic symptoms (maximum of urgency and frequency, range: 0–10) | 5.53 (2.48) | 5.56 (2.46) | 5.53 (2.45) | 5.62 (2.37) |
| Pain severity score (0–28)2 | 14.95 (5.62) | 14.93 (5.59) | 14.98 (5.57) | 15.29 (5.58) |
| Urinary severity score (0–25)3 | 12.61 (6.22) | 12.68 (6.20) | 12.68 (6.17) | 12.89 (6.24) |
| Bladder hypersensitivity (%): | ||||
| Neither painful filling nor urgency | 17.5 | 17.1 | 17.1 | 13.8 |
| Either painful filling or urgency | 31.6 | 30.8 | 30.7 | 30.6 |
| Both painful filling and urgency | 50.9 | 52.1 | 52.2 | 55.6 |
| Baseline extra-pelvic pain symptoms: | ||||
| Number of extra-pelvic regions with pain (%): | ||||
| 0 | 25.5 | 26.3 | 26.5 | 24.2 |
| 1–2 | 37.7 | 37.5 | 36.9 | 38.7 |
| 3–7 | 36.8 | 36.2 | 36.6 | 37.0 |
| Chronic overlapping pain conditions (%): | ||||
| Chronic fatigue syndrome | 10.6 | 10.4 | 10.4 | 12.1 |
| Fibromyalgia | 9.2 | 8.4 | 8.1 | 9.4 |
| Irritable bowel syndrome | 30.4 | 30.3 | 29.9 | 31.0 |
| Migraines | 23.4 | 23.6 | 22.3 | 24.2 |
| Temporomandibular joint disorder | 23.8 | 23.3 | 23.1 | 24.6 |
| Vulvodynia | 19.3 | 18.8 | 18.6 | 18.1 |
| Number of conditions | ||||
| 0 | 53.8 | 53.6 | 54.6 | 51.2 |
| 1 | 26.7 | 27.5 | 27.3 | 28.6 |
| 2 or more | 19.3 | 18.9 | 18.2 | 20.2 |
| Any condition | 38.4 | 38.0 | 37.4 | 39.7 |
N=382 in the flare frequency risk factor analyses, after excluding 3 participants who reported flares at all bi-weekly assessments.
Defined as the sum of the Genitourinary Pain Index pain sub-score and question 4 of the Interstitial Cystitis Symptom Index.
Defined as the sum of the Genitourinary Pain Index urinary sub-score and questions 1 to 3 of the Interstitial Cystitis Symptom Index.
REFERENCES
- 1.Sutcliffe S, Colditz GA, Goodman MS, Pakpahan R, Vetter J, Ness TJ, et al. Urological chronic pelvic pain syndrome symptom flares: characterisation of the full range of flares at two sites in the Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network. BJU international. 2014; 114(6):916–25. doi: 10.1111/bju.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clemens JQ, Calhoun EA, Litwin MS, McNaughton-Collins M, Kusek JW, Crowley EM, et al. Validation of a modified National Institutes of Health chronic prostatitis symptom index to assess genitourinary pain in both men and women. Urology. 2009; 74(5):983–7 quiz 7 e1-3. doi: 10.1016/j.urology.2009.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994; 23(2):129–38. Epub 1994/03/01. [PubMed] [Google Scholar]
- 4.Berry SH, Elliott MN, Suttorp M, Bogart LM, Stoto MA, Eggers P, et al. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol. 2011; 186(2):540–4. doi: 10.1016/j.juro.2011.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai HH, Krieger JN, Pontari MA, Buchwald D, Hou X, Landis JR, et al. Painful Bladder Filling and Painful Urgency are Distinct Characteristics in Men and Women with Urological Chronic Pelvic Pain Syndromes: A MAPP Research Network Study. J Urol. 2015. 194(6):1634–41. doi: 10.1016/j.juro.2015.05.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai HH, Jemielita T, Sutcliffe S, Bradley CS, Naliboff B, Williams DA, et al. Characterization of Whole Body Pain in Urological Chronic Pelvic Pain Syndrome at Baseline: A MAPP Research Network Study. J Urol. 2017; 198(3):622–31. doi: 10.1016/j.juro.2017.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams DA, Schilling S. Advances in the assessment of fibromyalgia. Rheum Dis Clin North Am. 2009; 35(2):339–57. doi: 10.1016/j.rdc.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffith JW, Stephens-Shields AJ, Hou X, Naliboff BD, Pontari M, Edwards TC, et al. Pain and Urinary Symptoms Should Not be Combined into a Single Score: Psychometric Findings from the MAPP Research Network. J Urol. 2016; 195 (4 Pt 1):949–54. doi: 10.1016/j.juro.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naliboff BD, Stephens AJ, Lai HH, Griffith JW, Clemens JQ, Lutgendorf S, et al. Clinical and Psychosocial Predictors of Urological Chronic Pelvic Pain Symptom Change in 1 Year: A Prospective Study from the MAPP Research Network. J Urol. 2017; 1984):848–57. doi: 10.1016/j.juro.2017.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Footnotes
DISCLOSURES:
Siobhan Sutcliffe: Nothing to disclose
Robert Gallop: Nothing to disclose
H. Henry Lai: Clinical trial of an investigational drug for Allergan; Scientific Advisory Board for Aquinox and Teva; Product registry for Medtronic
Gerald L. Andriole: Nothing to disclose
Catherine S. Bradley: Nothing to disclose
Gisela Chelimsky: Co-owner of PAinStakers LLC
Thomas Chelimsky: Nothing to disclose
J. Quentin Clemens: Nothing to disclose
Graham A. Colditz: Nothing to disclose
Bradley Erickson: Nothing to disclose
James W. Griffith: Nothing to disclose
Jayoung Kim: Nothing to disclose
John N. Krieger: Nothing to disclose
Jennifer Labus: Grants from University of California – Los Angeles
Bruce D. Naliboff: Nothing to disclose
Larissa V. Rodriguez: Nothing to disclose
Suzette E. Sutherland: Unrestricted educational grant/Advisory Board/Consultant for Boston Scientific; Clinical research for Fempulse, Pelvital, and Allergan; Advisory Board/Consultant for Axonics.
Bayley J. Taple: Nothing to disclose
J. R. Landis: Nothing to disclose
REFERENCES
- 1.Polackwich AS, Shoskes DA. Chronic prostatitis/chronic pelvic pain syndrome: a review of evaluation and therapy. Prostate Cancer Prostatic Dis. 2016;19(2):132–8. doi: 10.1038/pcan.2016.8.. [DOI] [PubMed] [Google Scholar]
- 2.Hanno PM, Erickson D, Moldwin R, Faraday MM, American Urological A. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J Urol. 2015;193(5):1545–53. doi: 10.1016/j.juro.2015.01.086. [DOI] [PubMed] [Google Scholar]
- 3.Barry MJ, Link CL, McNaughton-Collins MF, McKinlay JB, Boston Area Community Health I. Overlap of different urological symptom complexes in a racially and ethnically diverse, community-based population of men and women. BJU international. 2008;101(1):45–51. doi: 10.1111/j.1464-410X.2007.07191.x. [DOI] [PubMed] [Google Scholar]
- 4.Berry SH, Elliott MN, Suttorp M, Bogart LM, Stoto MA, Eggers P, et al. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol. 2011;186(2):540–4. doi: 10.1016/j.juro.2011.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clemens JQ, Link CL, Eggers PW, Kusek JW, Nyberg LM, Jr., McKinlay JB, et al. Prevalence of painful bladder symptoms and effect on quality of life in black, Hispanic and white men and women. J Urol. 2007;177(4):1390–4. doi: 10.1016/j.juro.2006.11.084. [DOI] [PubMed] [Google Scholar]
- 6.Suskind AM, Berry SH, Ewing BA, Elliott MN, Suttorp MJ, Clemens JQ. The prevalence and overlap of interstitial cystitis/bladder pain syndrome and chronic prostatitis/chronic pelvic pain syndrome in men: results of the RAND Interstitial Cystitis Epidemiology male study. J Urol. 2013;189(1):141–5. doi: 10.1016/j.juro.2012.08.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McNaughton Collins M, Pontari MA, O’Leary MP, Calhoun EA, Santanna J, Landis JR, et al. Quality of life is impaired in men with chronic prostatitis: the Chronic Prostatitis Collaborative Research Network. Journal of general internal medicine. 2001;16(10):656–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bogart LM, Suttorp MJ, Elliott MN, Clemens JQ, Berry SH. Prevalence and correlates of sexual dysfunction among women with bladder pain syndrome/interstitial cystitis. Urology. 2011;77(3):576–80. doi: 10.1016/j.urology.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith KB, Pukall CF, Tripp DA, Nickel JC. Sexual and relationship functioning in men with chronic prostatitis/chronic pelvic pain syndrome and their partners. Archives of sexual behavior. 2007;36(2):301–11. doi: 10.1007/s10508-006-9086-7. [DOI] [PubMed] [Google Scholar]
- 10.Beckett MK, Elliott MN, Clemens JQ, Ewing B, Berry SH. Consequences of interstitial cystitis/bladder pain symptoms on women’s work participation and income: results from a national household sample. J Urol. 2014;191(1):83–8. doi: 10.1016/j.juro.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clemens JQ, Markossian T, Calhoun EA. Comparison of economic impact of chronic prostatitis/chronic pelvic pain syndrome and interstitial cystitis/painful bladder syndrome. Urology. 2009;73(4):743–6. doi: 10.1016/j.urology.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sutcliffe S, Bradley CS, Clemens JQ, James AS, Konkle KS, Kreder KJ, et al. Urological chronic pelvic pain syndrome flares and their impact: qualitative analysis in the MAPP network. International urogynecology journal. 2015;26(7):1047–60. doi: 10.1007/s00192-015-2652-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sutcliffe S, Colditz GA, Goodman MS, Pakpahan R, Vetter J, Ness TJ, et al. Urological chronic pelvic pain syndrome symptom flares: characterisation of the full range of flares at two sites in the Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network. BJU international. 2014;114(6):916–25. doi: 10.1111/bju.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanford E, McMurphy C. There is a low incidence of recurrent bacteriuria in painful bladder syndrome/interstitial cystitis patients followed longitudinally. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(5):551–4. doi: 10.1007/s00192-006-0184-9. [DOI] [PubMed] [Google Scholar]
- 15.Sutcliffe S, Colditz GA, Pakpahan R, Bradley CS, Goodman MS, Andriole GL, et al. Changes in symptoms during urologic chronic pelvic pain syndrome symptom flares: findings from one site of the MAPP Research Network. Neurourology and urodynamics. 2015;34(2):188–95. doi: 10.1002/nau.22534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clemens JQ, Mullins C, Kusek JW, Kirkali Z, Mayer EA, Rodriguez LV, et al. The MAPP research network: a novel study of urologic chronic pelvic pain syndromes. BMC urology. 2014;14:57. doi: 10.1186/1471-2490-14-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landis JR, Williams DA, Lucia MS, Clauw DJ, Naliboff BD, Robinson NA, et al. The MAPP research network: design, patient characterization and operations. BMC urology. 2014;14:58. doi: 10.1186/1471-2490-14-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sutcliffe S, Jemielita T, Lai HH, Andriole GL, Bradley CS, Clemens JQ, et al. A Case-Crossover Study of Urological Chronic Pelvic Pain Syndrome Flare Triggers in the MAPP Research Network. J Urol. 2018;199(5):1245–51. doi: 10.1016/j.juro.2017.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai HH, Krieger JN, Pontari MA, Buchwald D, Hou X, Landis JR, et al. Painful Bladder Filling and Painful Urgency are Distinct Characteristics in Men and Women with Urological Chronic Pelvic Pain Syndromes: A MAPP Research Network Study. J Urol. 2015;194(6):1634–41. doi: 10.1016/j.juro.2015.05.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai HH, Jemielita T, Sutcliffe S, Bradley CS, Naliboff B, Williams DA, et al. Characterization of Whole Body Pain in Urological Chronic Pelvic Pain Syndrome at Baseline: A MAPP Research Network Study. J Urol. 2017;198(3):622–31. doi: 10.1016/j.juro.2017.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams DA, Schilling S. Advances in the assessment of fibromyalgia. Rheum Dis Clin North Am. 2009;35(2):339–57. doi: 10.1016/j.rdc.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naliboff BD, Stephens AJ, Lai HH, Griffith JW, Clemens JQ, Lutgendorf S, et al. Clinical and Psychosocial Predictors of Urological Chronic Pelvic Pain Symptom Change in 1 Year: A Prospective Study from the MAPP Research Network. J Urol. 2017;198(4):848–57. doi: 10.1016/j.juro.2017.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stokes ME, Davis CS, Koch GG. Categorical Data Analysis Using SAS. 3rd ed Cary, NC: SAS Institute Inc; 2012. [Google Scholar]
- 24.Hilbe JM. Negative Binomial Regression. 2nd ed Cambridge: Cambridge University Press; 2012. [Google Scholar]
- 25.Cameron AC, Trivedi PK. Regression analysis of count data. 2nd ed New York: Cambridge University Press; 2013. [Google Scholar]
- 26.Verbeke G, Molenberghs G. Linear mixed models for longitudinal data. New York: Springer Verlag; 2009. [Google Scholar]
- 27.Molenberghs G, Verbeke G. Models for discrete longitudinal data. New York: Springer Verlag; 2005. [Google Scholar]
- 28.Breiman L, Friedman JH, Olshen RA, Stone CJ. Classification and Regression Trees. New York: Wadsworth, Inc.; 1984. [Google Scholar]
- 29.Tang TZ, DeRubeis RJ, Beberman R, Pham T. Cognitive changes, critical sessions, and sudden gains in cognitive-behavioral therapy for depression. J Consult Clin Psychol. 2005;73(1):168–72. doi: 10.1037/0022-006X.73.1.168. [DOI] [PubMed] [Google Scholar]
- 30.A Dictionary of Epidemiology. 6th ed Oxford: Oxford University Press; 2014. [Google Scholar]


