Abstract
Cancer led to the deaths of more than 9 million people worldwide in 2018,1 and most of these deaths are due to metastatic tumor burden. While in most cases we still do not know why cancer is lethal, we know that a total tumor burden of one kilogram – equivalent to one trillion cells – is not compatible with life. While localized disease is curable through surgical removal or radiation, once cancer has spread, it is largely incurable. The inability to cure metastatic cancer lies, at least in part, to the fact that cancer is resistant to all known compounds and anti-cancer drugs. The source of this resistance remains undefined.2 In fact, the vast majority of metastatic cancers are resistant to all currently available anti-cancer therapies, including chemotherapy, hormone therapy, immunotherapy, and systemic radiation. Thus, despite decades – even centuries – of research, metastatic cancer remains lethal and incurable.3 We present historical and contemporary evidence that the key actuators of this process – of tumorigenesis, metastasis, and therapy resistance – are polyploid giant cancer cells.
Keywords: Polyploid giant cancer cells, keystone species, therapeutic resistance, stemness, metastasis, cancer ecology
The term cancer is derived from the Greek word for crab, used by Hippocrates to describe solid malignant tumors, circa 400 BC. The word metastasis, from the Greek for “displacement,” was formally described by French physician Joseph Récamier in 1829 in his treatise Recherches sur le traitement du cancer (translated Research on Treatment of Cancer).4 By this time, the idea that cancer spreads from its primary site was well appreciated and cell theory was established and accepted, though the routes or origins of metastases were not clear. In 1889, Stephen Paget, a surgeon at the West London Hospital and the Metropolitan Hospital in London, UK, performed an autopsy series of 735 women who died of fatal breast cancer.5 In this Lancet publication, he addressed the common theories of metastasis held by his colleagues and presented his data that demonstrated a clear pattern of metastatic spread. His now-famous “seed and soil hypothesis” remains the framework for all modern cancer metastasis research: “… every single cancer cell must be regarded as an organism, alive and capable of development. When a plant goes to seed, its seeds are carried in all directions; but they can only live and grow if they fall on congenial soil.” In concluding this landmark paper, Paget states, “The best work in the pathology of cancer is now being done by those who…are studying the nature of the seed.”5
In modern cancer biology, it is well accepted that metastatic spread is a stochastically rare event; the overwhelming majority of cells that leave the primary tumor will not establish a secondary tumor.6 Likewise, we also know that patients with metastatic disease, even with an initial response to systemic therapy, will eventually fail and their disease will recur. Tumor burden falling below levels of radiographic and biochemical detection indicates that the resistant tumor is derived from a single or a few cancer cells that are intrinsically resistant or develop resistance in response to therapeutic selective pressure.3 This phenomenon of tumor growth from a single or a few cells is supported by phylogenetic analysis.7–9 Importantly, it appears that both metastasis and therapeutic resistance is mediated by only one or a few cells.
To better understand cancer, tumors may be described and modeled as ecosystems, with cancer cell species co-existing in a complex habitat with host cell species.6,9–18 The cancer cells, the body’s normal cells, and the tumor microenvironment in which they reside and influence make up the cancer ecosystem. In many ecosystems, the community structure and ecosystem integrity are dependent on a single and often low-abundant species termed the keystone species.13,19 Keystone species are named after the architectural keystone of an arch. If the keystone is removed, the arch – or the ecosystem – collapses. Keystone species exert a disproportionally large effect on the ecosystem relative to their abundance.19 While there are relatively few individuals within the keystone species in any given community, they occupy a unique and nonredundant niche within the ecosystem – they are not replaceable. Examples of keystone species include the wolves of Yellowstone and the elephants of the Serengeti. Loss of these keystone species had a cascading negative effect on all the other species of the ecosystem, causing fundamental changes and even collapse of the ecosystem structure.
We propose here that the cancer ecosystem is dependent upon a keystone species: a rare population of cells that has the capacity to survive the harsh conditions of the of the tumor microenvironment (e.g., hypoxia, low nutrients, low pH), to metastasize, and to mediate therapeutic resistance by surviving treatment and then repopulating tumors with resistant cancer cells. While these keystone cancer cells survive, metastatic cancer will remain incurable. If we can identify and eliminate the keystone cancer cells, the tumor ecosystem will collapse, making the bulk tumor cells vulnerable to traditional therapies, opening the door for the opportunity for cancer cure. These rare keystone cancer cells, however, remain undefined.
For centuries, physicians and scientists could only observe the natural history of cancer within a patient, without understanding the basic units of the disease: cancer cells. With the invention and wide adoption of the microscope, cancer biologists were finally able to see what cancer cells looked like – to truly study the nature of the seed. With the discovery of cells in 1665 by Robert Hooke, the field of cell biology was born. Johannes Müller, a pioneer in tissue microscopy and histology, published some of the first descriptions and illustrations of cancer cells in his 1838 book Ueber den feinern Bau und die Formen der krankhaften Geschwülste (translated: On the Finer Structure and Form of Morbid Tumors) (Figure 1A).20 By 1839 cell theory was formally codified, attributed to plant biologist Matthais Schleiden and Müller’s students Theodor Schwann and Rudolph Virchow, and has served as the basis of all modern cell and molecular biology.21 Virchow, the “father of pathology” and cancer and metastasis biologist, recorded further descriptions of cancer cells in his book Die Cellularpathologie in ihrer Begründung auf physiologische und pathologische Gewebenlehre (translated: Cellular pathology as based upon Physiological and Pathological Histology), based on his lectures given to the Pathological Institute of Berlin in 1858 (Figure 1B).22,23
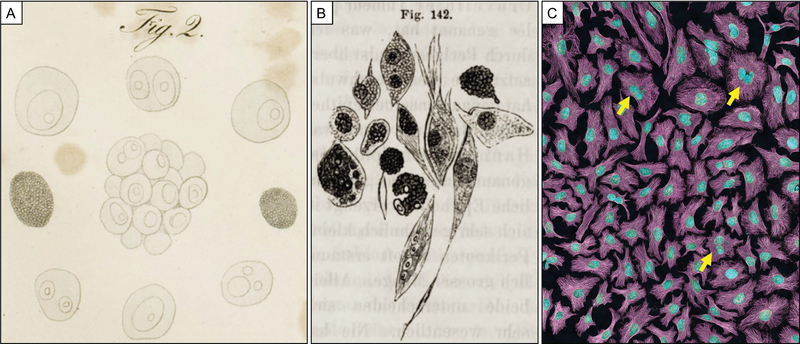
Figure 1. Historical evidence of polyploid giant cancer cells.
(A) Illustration (Plate II, Fig 2) from Ueber den feinern Bau und die Formen der krankhaften Geschwülste (translated: On the Finer Structure and Form of Morbid Tumors) by Johannes Müller, 1838; Caption translates “Cell spheres with germ cells and the nuclei of the germ cells…of Carcinoma reticulare.” (Public domain, CC BY-SA 4.0) (B) Illustration (Fig 142) from Die Cellularpathologie in ihrer Begründung auf physiologische und pathologische Gewebenlehre (translated: Cellular pathology as based upon Physiological and Pathological Histology) by Rudolph Virchow, 1858 (translated by Frank Chance); Caption: “Various, polymorphous cancer-cells…two with multiplication of nuclei. 300 diameters.” (Public domain, CC BY-NC 4.0) (C) PGCCs in HeLA cell culture indicated by arrows. Multiphoton fluorescence image: cytoskeletal microtubules, magenta; DNA, cyan. (Image by NIH, public domain, CC-PD-Mark)
These discoveries made possible through advances in technology gave the cancer research community the ability to observe and record the phenotypes of cancer cells within primary tumors and metastatic lesions, but early studies were limited to autopsy studies and static tissue sections. The advent of cell culture enabled researchers to observe, perturbate, and phenotype cancer cells over time. George Gey and Margaret Gey successfully isolated cervical cancer cells from Henrietta Lacks in 1951 to generate the first cell line, HeLa, still in use today.24 Since that time, hundreds of cancer cell lines have been isolated or generated through genetic transformation, enabling new discoveries in cancer cell and molecular biology, as well as advancements in anti-cancer therapies.
Despite all of these discoveries - from the description of the progression of the disease and necessity of the “seed and soil” in 1889,5 to the visualization and description of cancer cells from patient tumors in 1838–1858, 20,22 to the widespread use of cancer cell lines in vitro starting in 195124 – we still do not understand, nor can we cure, metastatic cancer. What have we missed?
Prostate cancer biologist Dr. John Isaacs often reminds us that “The most powerful tool we have is our eyes.” Have we stopped seeing what is under the microscope? We are blinded by the assumptions of what we expect to see when we look at cancer cells: monolayers or spheroids of more-or-less differentiated epithelial-like cells. Looking through the microscope, at histological sections or cell cultures, we pick out nuclei and cell borders, search for regular patterns of cell size and morphology. We have been trained for generations to dismiss aberrant cells as artifact of the technique or protocol. ResearchGate, the social media forum that allows scientists to seek advice from other researchers worldwide, is peppered with questions about unusual cells in culture. Posters typically respond that the cells are artifact of some external pressure (e.g., old media, over confluence, loss of CO2 conditions, viral manipulation) or are irreversibly senescent cells that will not survive passaging. Even in our cartoons describing cancer progression and the metastatic cascade, the cells follow a uniform prototype – cuboid for proliferative cells, spindle-shaped for invasive cells. This is what we teach and this is what we learn. While the stochastic data we have indicates that the critical mediators of lethal and incurable disease appear to be a rare population, we only base our observations on the majority population.
In looking at the hand-drawn illustrations of Müller and Virchow, it is immediately apparent that they observed the inherent cellular heterogeneity of a tumor. While Müller’s hand-drawn illustrations are dominated by the typical more-or-less differentiated epithelial-type cells, there are examples of giant multinucleated or large nucleated cells that he specifically highlights (Figure 1A).20 Virchow described the heterogeneity of cancer cells in his volume as “… curious bodies, provided with large nuclei and nucleoli, which are described as the specific, polymorphous cells of cancer.” His illustration of the cancer cells shows great phenotypic heterogeneity, including very large cells with multiple nuclei (Figure 1B).22,23
These cells, few in number but persistent within cancer cell populations, may be a cancer keystone species. Close examination of any cell culture flask of any solid tumor type will reveal similar non-typical cells that are morphologically distinct (i.e., non-cuboid and non-spindle shaped) cells with large cytoplasmic region and high DNA content as a single large nucleus or within multiple nuclei. Indeed, a polyploid giant cancer cell is evident in the first published photographs of HeLa cells, the first cancer cell line developed (Plate 39),25 and this rare population of cells persists today (Figure 1C).
Polyploid giant cancer cells (PGCCs) were observed and recorded at least 180 years ago, and have been visualized in cell culture, the workhorse of cancer cell biologists, for 65 years. The formation of PGCCs following therapeutic intervention, including chemotherapy and radiation, and upon conditioning in hypoxia, simulating the tumor microenvironment, has been described in the literature. Most measures of cell response to therapy, including dose response curves generated through viability or proliferation assays, do not account for the presence or phenotype of the very rare population of cells that survives treatment below the limit of detection of the assay. It has been assumed by most researchers that observed PGCCs do not survive and die due to mitotic catastrophe subsequent to multipolar cell division. Indeed, the only way to appreciate the presence of PGCCs in tissue culture at all is to directly observe them through microscopy.
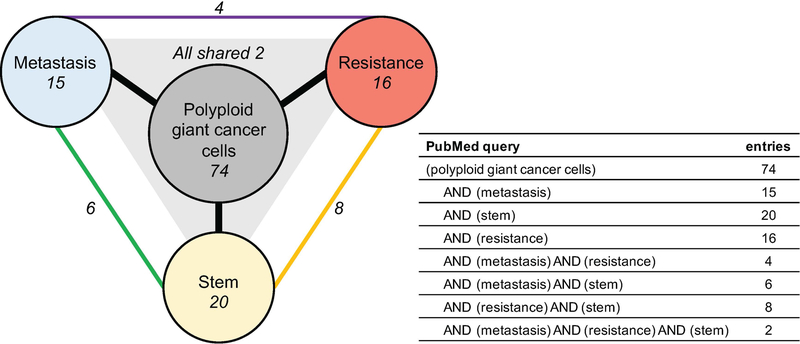
There is a small body of literature specifically related to PGCCs (74 PubMed listed entries with query: [polyploid giant cancer cells]26–99 versus 3,857,567 with query [cancer]; accessed 05/10/2019; Figure 2), but this literature, taken holistically, makes a compelling case for defining them as the essential keystone cancer species and actuators of tumorigenesis, therapeutic resistance, and recurrence in metastatic disease. Keystone species are relatively few in number, but have a significant impact on the health and composition of the ecosystem. In this case, the survival of keystone cancer species mediates 1) metastatic spread, 2) survival of cancer cells during and after therapeutic insult and 3) clonal expansion to generate a clinically significant tumor mass. In cancer biology terminology, this translates to PGCCs playing critical roles in all 3 capacities: in metastasis, therapeutic resistance, and having stem-like capacity to asymmetrically divide to give rise to a clonal population of cancer cells. Of the 74 publications, 15 address metastasis, 16 discuss therapeutic resistance, and 20 explore stem-like characteristics (Figure 2). Notably, only 2 publications combine all three essential characteristics under the investigation of PGCCs.
Figure 2. PubMed queries of the polyploid giant cancer cell literature.
PubMed-listed entries of indicated queries accessed on 05/10/2019. Entries that are listed with multiple search terms are indicated by connecting edges.
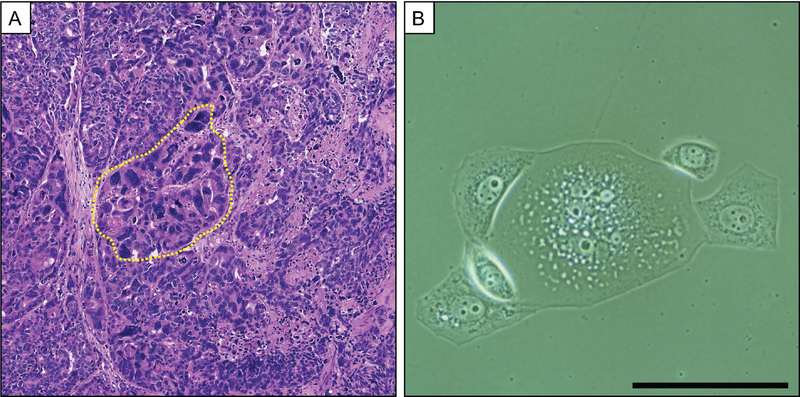
The presence of PGCCs has been described in a multiple cancer types (breast, ovarian, colon, melanoma, lung, pancreas, urinary bladder, renal, thyroid, prostate), but systematic analyses to assess PGCC status with clinical prognostic have not been performed.43,54,60,92,100–110 For example, PGCCs have been documented in a PCa patient with pT3bN1Mx, Gleason 5+4=9 (Grade group 5) PCa (Figure 3A). At time of radical prostatectomy, the primary tumor and 7/12 lymph nodes were positive for focal regions of pleomorphic giant cells. Alharbi, et al. analyzed a series of 30 cases of PCa patients with PGCC present in the diagnostic specimen collected from 2005 to 2018.111 Presence of PGCCs in PCa diagnostic specimens indicates aggressive disease and is associated with a rapid disease course and death. Of the men with a new PCa diagnosis with >1 year follow up, 7/19 (37%) were dead at a median of 8 months. 4/7 (57%) men who had a previous history of PCa were dead at a median of 7 months after diagnosis of recurrent PCa. This is in grim contrast to reported PCa-specific mortality of <5% at 2 years and 10% at 4 years for men with similar nonmetastatic PCa diagnoses (Gleason score 9 and 10).112 Notably, despite their apparent role in mediating aggressive disease, PGCCs typically make up a small minority of the assessed tumor region (5–20%). These striking data highlight the likely role for these keystone PGCCs as actuators of rapid lethal disease progression.
Figure 3. Polyploid giant cancer cells in prostate cancer in vivo and in vitro.
(A) H&E image of a lymph node prostate cancer metastasis with PGCCs (one region indicated by yellow border). (B) Phase image of a PC3 PGCC undergoing asymmetric division to form mononuclear and typical-sized daughter cells. PC3 cells were cultured with 10nM Docetaxel for 3 days followed by 4 days in Docetaxel-free media. (scale = 200 um)
Studies conducted in yeast, drosophila, cancer models, and clinical data suggest that the polyploidy state mediates therapy-resistant phenotypes.113 PGCCs have been observed emerging in response to a variety of genotoxic stresses, including anti-cancer therapy such as radiation and chemotherapy, as well as tumor microenvironment-simulating hypoxia36,56,65,73,78,97,114–122. In addition to simply emerging in response to stressors, there is evidence that PGCCs contribute to overall therapeutic resistance. Cells derived from PGCCs that form upon Cisplatin treatment have increased resistance to cytotoxic drugs.78 There is also evidence in castration-resistant prostate cancer (CRPCa) that PGCCs drive resistance to taxane-based chemotherapy.65,73,117–119,122 The mechanism of this multi-therapy resistance phenotype remains unknown. One hypothesis is that that PGCCs enter a protective and reversible state of therapeutic-induced-senescence, allowing them to survive therapy and later reenter cell cycle to form to daughter cells.123 Studies have shown that PGCCs express a stem-like phenotype (e.g., expression of self-renewal markers).124,125 Moreover, there is strong evidence that PGCCs can asymmetrically divide to give rise to daughter cells of typical size and ploidy (Figure 3B).65,97,122 PGCCs have been shown to re-enter the cell cycle and either undergo error prone aberrant mitoses or an error prone process of cell division independent of a mitotic spindle that uses budding or bursting called amitosis or neosis.80,85,93,126,127 This stem-like phenotype of asymmetric division gives PGCCs the capacity to generate a clinically evident metastasis of majority non-PGCC cells. In addition, there is recent data that PGCCs that form in response to hypoxia, such as would be found in the primary tumor microenvironment, and in response to therapy have increased metastatic potential, including increased mesenchymal phenotype as well as enhanced migration and invasion.42,97,128
As the field of PGCC research grows, it is important to set a definition of the cell type of interest. Pathologists have borrowed language from Virchow, describing regions of “polymorphous giant cells,” and there are other reports of “osteoclast-like cells” in tumor sections, describing multinucleated cancer cells. PGCCs have two phenotypic defining characteristics: 1) polyploidy (though not necessarily multinucleation) and 2) relatively large size. Most solid tumors and cancer cell lines are aneuploid (i.e., have an abnormal number of chromosomes or segments of chromosomes). Polyploidy describes a multiple of the baseline set of chromosomes that does not have an upper limit (e.g., 4n, 6n, or 16n). In the case of polyploidy observed in an aneuploid cancer cell line, therefore, it would be a multiple of that “aneuploid n.” Importantly, polyploidy does not require multinucleation and can simply present as a single large nucleus, though cells with multiple nuclei are likely polyploid. The other defining phenotypic characteristic of PGCCs is their “giant” morphology (Figure 3A–B). PGCCs are physically and visually larger than their surrounding sister cells, not just with elevated genomic content, but also cytoplasmic area. Further research is needed to assess if size or deformability of PGCCs is biologically significant.
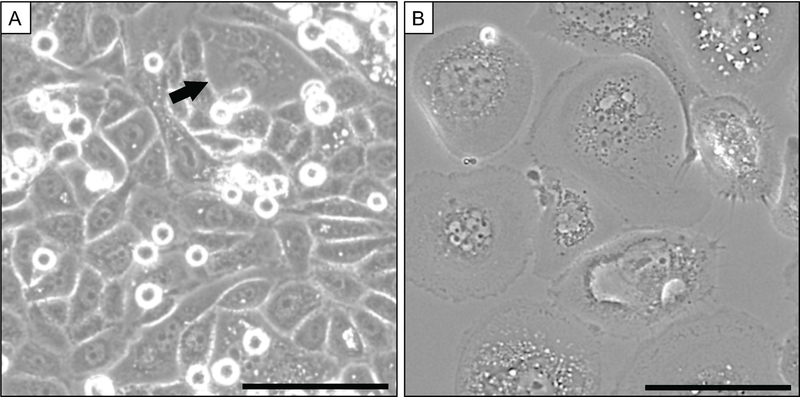
In order to understand and eventually target PGCCs, it will be important to both study PGCCs in isolation as well as in their native ecosystem, especially to appreciate the initial emergence of PGCCs in the primary tumor ecosystem. While there are not currently any biomarkers for PGCCs, either for monitoring in vivo or for isolation, this should be an area for future research. The most commonly used assays to study PGCCs rely on microscopy of in vitro cultures in order to capture essential PGCC events, such as formation and cell division. Currently, there are no commonly adopted high-throughput methods to isolate a pure population of PGCCs. One of the most common methods to quantify (and in rare cases to isolate) PGCCs in a population is flow-cytometry using standard cell cycle analysis to isolate relative >4n cells (e.g., with 7AAD or other DNA stain. Addition of Cyclin-B1 staining can be used discriminate the G2/M diploid cells that have undergone S phase and so have 4n genomic content. Overall, however, such flow-cytometry methods drastically reduce viability and are impractical due to the long assay time. Theoretically, researchers may be able to take advantage of the relative size difference of PGCCs compared to the other cancer cells in the population using size-exclusion techniques such as have been developed for circulating tumor cell research, but such a method has not been widely adopted. Importantly, conventional laboratory assays designed to assess efficacy of anti-cancer therapy (e.g., viability or proliferation assays taken days after treatment of cells in vitro or tumor recurrence measured weeks after treatment of tumor-bearing animals in vivo) do not account for the rare population of surviving PGCCs that exist below the limit of detection until they reenter cell cycle.62 The majority of modern methods to count and assess cell viability do not require the investigator to actually look at the remaining population. Observing a population of PC-3 cells 72 hours after treatment with a LD90 dose of docetaxel reveals that the majority of cells are PGCCs (Figure 4) (personal communication, Amend and Pienta). Assessing PGCCs in vivo, either in histological sections or in liquid biopsies, has its own challenges. It is difficult to assess cell membranes from a typical H&E stain, making polyploidy difficult to ascertain, though focal regions of majority PGCC phenotype (called “polymorphous giant cells” or “osteoclast-like cells”) have been reported. Addition of a membrane stain followed by careful evaluation by a skilled pathologist would provide an opportunity for assessing single or rare PGCC status in patient samples. The presence of PGCCs in the circulation has not been systematically assessed. In liquid biopsy research, it is important to carefully review the algorithm requirements. Many such automated counting systems define a cell as one with a single and/or small nucleus, and so would automatically eliminate any multinucleated or large-nucleated cell from analysis, including possible PGCCs.
Figure 4. Polyploid giant cancer cells are the majority population following docetaxel treatment in vitro.
(A) PC3 cell culture at baseline contains rare PGCCs (arrow). (B) After treatment for 72 hours with LD90 docetaxel, PGCCs are the dominant population and virtually no non-PGCC cells remain. (Phase contrast; scale = 100 um)
While we and others have highlighted the likely role for PGCCs in mediating disease resistance, there are no currently available therapies to specifically target these cells. Indeed, PGCCs emerge in response to all tested standard-of-care therapeutics. As they have a unique phenotype, however, PGCCs may have unique vulnerabilities. For instance, it is clear that in order to divide, it is likely that PGCCs may have to use different cell division machinery than non-polyploid cells (e.g., microtubule organizing center [MTOC] assembly). With such elevated DNA content, it is likely that cell cycle checkpoints may also represent a viable therapeutic target. As discussed above, there is evidence that PGCCs exit the cell cycle and enter a quiescent state. Restraining the cells in that G0 state may represent a way, not to eliminate the PGCCs, but to prevent the lethal tumor burden that arises when the PGCCs reenter cell cycle. Clearly, this is a critical area of further research – to define therapeutic targets and determine optimal delivery to eliminate the keystone PGCCs from a tumor. Importantly, the PGCCs represent a minority population of the tumor burden, any anti-PGCC therapy can be used in combination with current standard-of-care that will eliminate the bulk of the tumor population.
Metastatic cancer remains incurable because a subset of cells within a tumor has intrinsic or develops resistance to anti-cancer therapy. While standard-of-care hormone therapy, chemotherapy, or radiation may reduce overall tumor size, only a single or a few cancer cell “seeds” are required to mediate metastasis and therapy resistance. These keystone cancer cells, while few in number, exert a large effect on the tumor ecosystem. PGCCs have been observed for more than a century since the first descriptions of cancer cells by Müller and Virchow. It is clear from the limited available clinical data that presence of PGCCs in localized or recurrent prostate cancer indicates a dismal prognosis. The current PGCC literature, though limited, suggests that this distinctive phenotype of cancer cell can 1) initiate the metastatic cascade, 2) survive “lethal” doses of therapeutic, and 3) asymmetrically divide to generate typical cancer cells with increased resistance to different classes of anti-cancer therapy. To cure metastasis, the PGCCs actuating metastasis, therapy resistance, and tumor outgrowth must be eliminated.
Acknowledgments:
This work was supported by The Patrick C. Walsh Prostate Cancer Research Fund (to S.R.A. and R.H.A.), the Prostate Cancer Foundation (to S.R.A. and K.J.P), NSF PHY-1659940 (to R.H.A.), and NCI U54CA143803, CA163124, CA093900, and CA143055 (to K.J.P.). The authors thank Robert Axelrod and Robert Gatenby and the members of the Brady Urological Institute, especially Dr. John Isaacs, for thoughtful discussion.
Funding acknowledgement:
S.R.A.: The Patrick C. Walsh Prostate Cancer Research Fund and the Prostate Cancer Foundation
R.H.A.: NSF PHY-1659940 and The Patrick C. Walsh Prostate Cancer Research Fund
K.J.P: NCI grants U54CA143803, CA163124, CA093900, and CA143055 and the Prostate Cancer Foundation
Footnotes
Disclosure / Conflict of Interest Statement:
The authors have no disclosures.
References:
- 1.Cancer IAfRo. Latest Global Cancer Data: Cancer Burden Rises to 18.1 Million New Cases and 9.6 Million Cancer Deaths in 2018 2018. In: September.
- 2.Pienta KJ, Partin AW, Coffey DS. Cancer as a disease of DNA organization and dynamic cell structure. Cancer research 1989;49(10):2525–2532. [PubMed] [Google Scholar]
- 3.Amend SR, de Groot AE, Torga G, et al. Ten unanswered questions in cancer: “If this is true, what does it imply”? American journal of clinical and experimental urology 2018;6(2):26–31. [PMC free article] [PubMed] [Google Scholar]
- 4.Récamier JCA. Recherches sur le traitement du cancer: par la compression méthodique simple ou combinée, et sur l’histoire générale de la même maladie. Vol 1: Gabon; 1829. [Google Scholar]
- 5.Paget S The distribution of secondary growths in cancer of the breast. The Lancet 1889;133(3421):571–573. [PubMed] [Google Scholar]
- 6.de Groot AE, Roy S, Brown JS, Pienta KJ, Amend SR. Revisiting Seed and Soil: Examining the Primary Tumor and Cancer Cell Foraging in Metastasis. Molecular cancer research : MCR 2017;15(4):361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haffner MC, Mosbruger T, Esopi DM, et al. Tracking the clonal origin of lethal prostate cancer. The Journal of clinical investigation 2013;123(11):4918–4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greaves M, Maley CC. Clonal evolution in cancer. Nature 2012;481(7381):306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maley CC, Aktipis A, Graham TA, et al. Classifying the evolutionary and ecological features of neoplasms. Nature reviews Cancer 2017;17(10):605–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amend SR, Pienta KJ. Ecology meets cancer biology: the cancer swamp promotes the lethal cancer phenotype. Oncotarget 2015;6(12):9669–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amend SR, Roy S, Brown JS, Pienta KJ. Ecological paradigms to understand the dynamics of metastasis. Cancer letters 2016;380(1):237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pienta KJ, Robertson BA, Coffey DS, Taichman RS. The cancer diaspora: Metastasis beyond the seed and soil hypothesis. Clinical cancer research : an official journal of the American Association for Cancer Research 2013;19(21):5849–5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kareva I Cancer ecology: Niche construction, keystone species, ecological succession, and ergodic theory. Biological Theory 2015;10(4):283–288. [Google Scholar]
- 14.Ibrahim-Hashim A, Gillies RJ, Brown JS, Gatenby RA. Coevolution of tumor cells and their microenvironment:“niche construction in cancer”. In: Ecology and Evolution of Cancer Elsevier; 2017:111–117. [Google Scholar]
- 15.Gillies RJ, Brown JS, Anderson AR, Gatenby RA. Eco-evolutionary causes and consequences of temporal changes in intratumoural blood flow. Nature Reviews Cancer 2018:1. [DOI] [PMC free article] [PubMed]
- 16.Lloyd MC, Cunningham JJ, Bui MM, Gillies RJ, Brown JS, Gatenby RA. Darwinian dynamics of intratumoral heterogeneity: not solely random mutations but also variable environmental selection forces. Cancer research 2016;76(11):3136–3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alfarouk KO, Ibrahim ME, Gatenby RA, Brown JS. Riparian ecosystems in human cancers. Evolutionary applications 2013;6(1):46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang KR, Mooney SM, Zarif JC, Coffey DS, Taichman RS, Pienta KJ. Niche inheritance: a cooperative pathway to enhance cancer cell fitness through ecosystem engineering. Journal of cellular biochemistry 2014;115(9):1478–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paine RT. A note on trophic complexity and community stability. The American Naturalist 1969;103(929):91–93. [Google Scholar]
- 20.Müller J Über den feinern Bau und die Formen der krankhaften Geschwülste. G. Reimer; 1838.
- 21.Mazzarello P A unifying concept: the history of cell theory. Nature cell biology 1999;1(1):E13. [DOI] [PubMed] [Google Scholar]
- 22.Virchow R Die krankhaften Geschwülste: dreissig Vorlesungen gehalten während des Wintersemesters 1862–1863 an der Universität zu Berlin. Vol 4: A. Hirschwald; 1867. [Google Scholar]
- 23.Virchow R Cellular Pathology as Based Upon Physiological and Pathological Histology JB Lippincott; 1863. [DOI] [PubMed] [Google Scholar]
- 24.Gey G Tissue culture studies of the proliferative capacity of cervical carcinoma and normal epithelium. Cancer Res 1952;12:264–265. [Google Scholar]
- 25.Scherer WF, Syverton JT, Gey GO. Studies on the propagation in vitro of poliomyelitis viruses: IV. Viral multiplication in a stable strain of human malignant epithelial cells (strain HeLa) derived from an epidermoid carcinoma of the cervix. Journal of Experimental Medicine 1953;97(5):695–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The 150 most important questions in cancer research and clinical oncology series: questions 94–101 : Edited by Cancer Communications. Cancer communications (London, England) 2018;38(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abe R, Raza A, Preisler HD, Tebbi CK, Sandberg AA. Chromosomes and causation of human cancer and leukemia. LIV. Near-tetraploidy in acute leukemia. Cancer genetics and cytogenetics 1985;14(1–2):45–59. [DOI] [PubMed] [Google Scholar]
- 28.Alameddine RS, Hamieh L, Shamseddine A. From sprouting angiogenesis to erythrocytes generation by cancer stem cells: evolving concepts in tumor microcirculation. Biomed Res Int 2014;2014:986768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bracey TS, Williams AC, Paraskeva C. Inhibition of radiation-induced G2 delay potentiates cell death by apoptosis and/or the induction of giant cells in colorectal tumor cells with disrupted p53 function. Clinical cancer research : an official journal of the American Association for Cancer Research 1997;3(8):1371–1381. [PubMed] [Google Scholar]
- 30.Braune EB, Tsoi YL, Phoon YP, et al. Loss of CSL Unlocks a Hypoxic Response and Enhanced Tumor Growth Potential in Breast Cancer Cells. Stem cell reports 2016;6(5):643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Butler MG, Dahir GA, Schwartz HS. Molecular analysis of transforming growth factor beta in giant cell tumor of bone. Cancer genetics and cytogenetics 1993;66(2):108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen HZ, Ouseph MM, Li J, et al. Canonical and atypical E2Fs regulate the mammalian endocycle. Nat Cell Biol 2012;14(11):1192–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen J, Niu N, Zhang J, et al. Polyploid Giant Cancer Cells (PGCCs): The Evil Roots of Cancer. Current cancer drug targets 2019;19(5):360–367. [DOI] [PubMed] [Google Scholar]
- 34.Chitikova ZV, Gordeev SA, Bykova TV, Zubova SG, Pospelov VA, Pospelova TV. Sustained activation of DNA damage response in irradiated apoptosis-resistant cells induces reversible senescence associated with mTOR downregulation and expression of stem cell markers. Cell Cycle 2014;13(9):1424–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cong B, Ohsawa S, Igaki T. JNK and Yorkie drive tumor progression by generating polyploid giant cells in Drosophila. Oncogene 2018;37(23):3088–3097. [DOI] [PubMed] [Google Scholar]
- 36.Erenpreisa J, Ivanov A, Wheatley SP, et al. Endopolyploidy in irradiated p53-deficient tumour cell lines: persistence of cell division activity in giant cells expressing Aurora-B kinase. Cell biology international 2008;32(9):1044–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erenpreisa J, Roach HI. Aberrations of cell cycle and cell death in normal development of the chick embryo growth plate. Mechanisms of ageing and development 1999;108(3):227–238. [DOI] [PubMed] [Google Scholar]
- 38.Erenpreisa JA, Cragg MS, Fringes B, Sharakhov I, Illidge TM. Release of mitotic descendants by giant cells from irradiated Burkitt’s lymphoma cell line. Cell biology international 2000;24(9):635–648. [DOI] [PubMed] [Google Scholar]
- 39.Eriksson D, Blomberg J, Lindgren T, et al. Iodine-131 induces mitotic catastrophes and activates apoptotic pathways in HeLa Hep2 cells. Cancer biotherapy & radiopharmaceuticals 2008;23(5):541–549. [DOI] [PubMed] [Google Scholar]
- 40.Faivre J, Frank-Vaillant M, Poulhe R, et al. Centrosome overduplication, increased ploidy and transformation in cells expressing endoplasmic reticulum-associated cyclin A2. Oncogene 2002;21(10):1493–1500. [DOI] [PubMed] [Google Scholar]
- 41.Fei F, Li C, Wang X, et al. Syncytin 1, CD9, and CD47 regulating cell fusion to form PGCCs associated with cAMP/PKA and JNK signaling pathway. Cancer medicine 2019. [DOI] [PMC free article] [PubMed]
- 42.Fei F, Qu J, Liu K, et al. The subcellular location of cyclin B1 and CDC25 associated with the formation of polyploid giant cancer cells and their clinicopathological significance. Lab Invest 2019;99(4):483–498. [DOI] [PubMed] [Google Scholar]
- 43.Fei F, Zhang D, Yang Z, et al. The number of polyploid giant cancer cells and epithelial-mesenchymal transition-related proteins are associated with invasion and metastasis in human breast cancer. J Exp Clin Cancer Res 2015;34:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gardner RL, Davies TJ. Lack of coupling between onset of giant transformation and genome endoreduplication in the mural trophectoderm of the mouse blastocyst. The Journal of experimental zoology 1993;265(1):54–60. [DOI] [PubMed] [Google Scholar]
- 45.Ghanizadeh-Vesali S, Zekri A, Zaker F, et al. Significance of AZD1152 as a potential treatment against Aurora B overexpression in acute promyelocytic leukemia. Annals of hematology 2016;95(7):1031–1042. [DOI] [PubMed] [Google Scholar]
- 46.Glassmann A, Carrillo Garcia C, Janzen V, et al. Staurosporine Induces the Generation of Polyploid Giant Cancer Cells in Non-Small-Cell Lung Carcinoma A549 Cells. Analytical cellular pathology (Amsterdam) 2018;2018:1754085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gong Y, DeFrias DV, Nayar R. Pitfalls in fine needle aspiration cytology of extraadrenal paraganglioma. A report of 2 cases. Acta Cytol 2003;47(6):1082–1086. [DOI] [PubMed] [Google Scholar]
- 48.Hiraoka H Effects of thermotherapy on the morphology and cell cycle of a transplanted ascites hepatoma in rats. The Japanese journal of experimental medicine 1990;60(1):31–37. [PubMed] [Google Scholar]
- 49.Huang G, Meng L, Tsai RY. p53 Configures the G2/M Arrest Response of Nucleostemin-Deficient Cells. Cell death discovery 2015;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalejs M, Ivanov A, Plakhins G, et al. Upregulation of meiosis-specific genes in lymphoma cell lines following genotoxic insult and induction of mitotic catastrophe. BMC cancer 2006;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klisch K, Schraner EM, Boos A. Centrosome Clustering in the Development of Bovine Binucleate Trophoblast Giant Cells. Cells, tissues, organs 2017;203(5):287–294. [DOI] [PubMed] [Google Scholar]
- 52.Kuhn EM, Therman E. The behavior of heterochromatin in mouse and human nuclei. Cancer genetics and cytogenetics 1988;34(1):143–151. [DOI] [PubMed] [Google Scholar]
- 53.Lin KC, Torga G, Sun Y, et al. The role of heterogeneous environment and docetaxel gradient in the emergence of polyploid, mesenchymal and resistant prostate cancer cells. Clin Exp Metastasis 2019;36(2):97–108. [DOI] [PubMed] [Google Scholar]
- 54.Liu G, Wang Y, Fei F, et al. Clinical characteristics and preliminary morphological observation of 47 cases of primary anorectal malignant melanomas. Melanoma Res 2018;28(6):592–599. [DOI] [PubMed] [Google Scholar]
- 55.Liu J The dualistic origin of human tumors. Seminars in cancer biology 2018;53:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lopez-Sanchez LM, Jimenez C, Valverde A, et al. CoCl2, a mimic of hypoxia, induces formation of polyploid giant cells with stem characteristics in colon cancer. PloS one 2014;9(6):e99143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Losick VP, Fox DT, Spradling AC. Polyploidization and cell fusion contribute to wound healing in the adult Drosophila epithelium. Curr Biol 2013;23(22):2224–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu YC, Lee YR, Liao JD, et al. Reversine Induced Multinucleated Cells, Cell Apoptosis and Autophagy in Human Non-Small Cell Lung Cancer Cells. PloS one 2016;11(7):e0158587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lukacs G, Balazs G, Zs-Nagy I, Juhasz F. [Prognostic significance of nuclear DNA content in highly malignant thyroid tumors]. Wiener klinische Wochenschrift 1990;102(9):253–256. [PubMed] [Google Scholar]
- 60.Lv H, Shi Y, Zhang L, et al. Polyploid giant cancer cells with budding and the expression of cyclin E, S-phase kinase-associated protein 2, stathmin associated with the grading and metastasis in serous ovarian tumor. BMC cancer 2014;14:576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Manti L, Davies HE, Venables S, Bowen ID, Court JB. Correlation between the clonogenic initial slope and the response of polykaryon-forming units: the behavior of strains defective in XRCC5 and ATM and the heritability of small variations in radioresponse. Radiation research 2000;154(6):650–658. [DOI] [PubMed] [Google Scholar]
- 62.Mirzayans R, Andrais B, Murray D. Roles of Polyploid/Multinucleated Giant Cancer Cells in Metastasis and Disease Relapse Following Anticancer Treatment. Cancers 2018;10(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mirzayans R, Andrais B, Scott A, Paterson MC, Murray D. Single-cell analysis of p16(INK4a) and p21(WAF1) expression suggests distinct mechanisms of senescence in normal human and Li-Fraumeni Syndrome fibroblasts. Journal of cellular physiology 2010;223(1):57–67. [DOI] [PubMed] [Google Scholar]
- 64.Mirzayans R, Andrais B, Scott A, Wang YW, Murray D. Ionizing radiation-induced responses in human cells with differing TP53 status. International journal of molecular sciences 2013;14(11):22409–22435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mittal K, Donthamsetty S, Kaur R, et al. Multinucleated polyploidy drives resistance to Docetaxel chemotherapy in prostate cancer. British journal of cancer 2017;116(9):1186–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morita Y, Takahashi A, Yamamoto K, Miki T, Murakami N, Miura O. Secondary near-tetraploidy with double der(15)t(15;17) in acute promyelocytic leukemia in relapse. Cancer genetics and cytogenetics 2004;149(2):131–136. [DOI] [PubMed] [Google Scholar]
- 67.Murata H, Kusuzaki K, Takeshita H, et al. Cytofluorometric DNA ploidy analysis in giant cell tumor of bone: histologic and prognostic value. Cancer letters 1999;136(2):223–229. [DOI] [PubMed] [Google Scholar]
- 68.Nair JS, de Stanchina E, Schwartz GK. The topoisomerase I poison CPT-11 enhances the effect of the aurora B kinase inhibitor AZD1152 both in vitro and in vivo. Clinical cancer research : an official journal of the American Association for Cancer Research 2009;15(6):2022–2030. [DOI] [PubMed] [Google Scholar]
- 69.Nelson DA, Tan TT, Rabson AB, Anderson D, Degenhardt K, White E. Hypoxia and defective apoptosis drive genomic instability and tumorigenesis. Genes & development 2004;18(17):2095–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Niculescu VF. The reproductive life cycle of cancer: Hypotheses of cell of origin, TP53 drivers and stem cell conversions in the light of the atavistic cancer cell theory. Medical hypotheses 2019;123:19–23. [DOI] [PubMed] [Google Scholar]
- 71.Niu N, Mercado-Uribe I, Liu J. Dedifferentiation into blastomere-like cancer stem cells via formation of polyploid giant cancer cells. Oncogene 2017;36(34):4887–4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Niu N, Zhang J, Zhang N, et al. Linking genomic reorganization to tumor initiation via the giant cell cycle. Oncogenesis 2016;5(12):e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ogden A, Rida PC, Knudsen BS, Kucuk O, Aneja R. Docetaxel-induced polyploidization may underlie chemoresistance and disease relapse. Cancer letters 2015;367(2):89–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Olive PL, Banath JP, Durand RE. Development of apoptosis and polyploidy in human lymphoblast cells as a function of position in the cell cycle at the time of irradiation. Radiation research 1996;146(6):595–602. [PubMed] [Google Scholar]
- 75.Parris GE. Clinically significant cancer evolves from transient mutated and/or aneuploid neoplasia by cell fusion to form unstable syncytia that give rise to ecologically viable parasite species. Medical hypotheses 2005;65(5):846–850. [DOI] [PubMed] [Google Scholar]
- 76.Pasitka L, van Noort D, Lim W, Park S, Mandenius CF. A Microbore Tubing Based Spiral for Multistep Cell Fractionation. Analytical chemistry 2018;90(21):12909–12916. [DOI] [PubMed] [Google Scholar]
- 77.Pere-Vedrenne C, Prochazkova-Carlotti M, Rousseau B, et al. The Cytolethal Distending Toxin Subunit CdtB of Helicobacter hepaticus Promotes Senescence and Endoreplication in Xenograft Mouse Models of Hepatic and Intestinal Cell Lines. Frontiers in cellular and infection microbiology 2017;7:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Puig PE, Guilly MN, Bouchot A, et al. Tumor cells can escape DNA-damaging cisplatin through DNA endoreduplication and reversible polyploidy. Cell biology international 2008;32(9):1031–1043. [DOI] [PubMed] [Google Scholar]
- 79.Qu Y, Zhang L, Rong Z, He T, Zhang S. Number of glioma polyploid giant cancer cells (PGCCs) associated with vasculogenic mimicry formation and tumor grade in human glioma. J Exp Clin Cancer Res 2013;32:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rajaraman R, Guernsey DL, Rajaraman MM, Rajaraman SR. Stem cells, senescence, neosis and self-renewal in cancer. Cancer Cell Int 2006;6:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rohnalter V, Roth K, Finkernagel F, et al. A multi-stage process including transient polyploidization and EMT precedes the emergence of chemoresistent ovarian carcinoma cells with a dedifferentiated and pro-inflammatory secretory phenotype. Oncotarget 2015;6(37):40005–40025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saltel F, Giese A, Azzi L, et al. Unr defines a novel class of nucleoplasmic reticulum involved in mRNA translation. Journal of cell science 2017;130(10):1796–1808. [DOI] [PubMed] [Google Scholar]
- 83.Shu Z, Row S, Deng WM. Endoreplication: The Good, the Bad, and the Ugly. Trends in cell biology 2018;28(6):465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sliwinska MA, Mosieniak G, Wolanin K, et al. Induction of senescence with doxorubicin leads to increased genomic instability of HCT116 cells. Mechanisms of ageing and development 2009;130(1–2):24–32. [DOI] [PubMed] [Google Scholar]
- 85.Sundaram M, Guernsey DL, Rajaraman MM, Rajaraman R. Neosis: a novel type of cell division in cancer. Cancer Biol Ther 2004;3(2):207–218. [DOI] [PubMed] [Google Scholar]
- 86.Tatake RJ, Rajaram N, Damle RN, Balsara B, Bhisey AN, Gangal SG. Establishment and characterization of four new squamous cell carcinoma cell lines derived from oral tumors. Journal of cancer research and clinical oncology 1990;116(2):179–186. [DOI] [PubMed] [Google Scholar]
- 87.Walen KH. The origin of transformed cells. studies of spontaneous and induced cell transformation in cell cultures from marsupials, a snail, and human amniocytes. Cancer genetics and cytogenetics 2002;133(1):45–54. [DOI] [PubMed] [Google Scholar]
- 88.Wright M, Lacorre-Arescaldino I, Macquet JP, Daffe M. Induction of polyploid nuclei in the plasmodium of Physarum polycephalum by platinum antitumor compounds. Cancer research 1984;44(2):777–783. [PubMed] [Google Scholar]
- 89.Yang Z, Yao H, Fei F, et al. Generation of erythroid cells from polyploid giant cancer cells: re-thinking about tumor blood supply. Journal of cancer research and clinical oncology 2018;144(4):617–627. [DOI] [PubMed] [Google Scholar]
- 90.Zhang D, Wang Y, Zhang S. Asymmetric cell division in polyploid giant cancer cells and low eukaryotic cells. Biomed Res Int 2014;2014:432652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang D, Yang X, Yang Z, et al. Daughter Cells and Erythroid Cells Budding from PGCCs and Their Clinicopathological Significances in Colorectal Cancer. J Cancer 2017;8(3):469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang L, Ding P, Lv H, et al. Number of polyploid giant cancer cells and expression of EZH2 are associated with VM formation and tumor grade in human ovarian tumor. Biomed Res Int 2014;2014:903542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang S, Mercado-Uribe I, Hanash S, Liu J. iTRAQ-based proteomic analysis of polyploid giant cancer cells and budding progeny cells reveals several distinct pathways for ovarian cancer development. PloS one 2013;8(11):e80120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang S, Mercado-Uribe I, Liu J. Generation of erythroid cells from fibroblasts and cancer cells in vitro and in vivo. Cancer letters 2013;333(2):205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang S, Mercado-Uribe I, Liu J. Tumor stroma and differentiated cancer cells can be originated directly from polyploid giant cancer cells induced by paclitaxel. Int J Cancer 2014;134(3):508–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang S, Mercado-Uribe I, Sood A, Bast RC, Liu J. Coevolution of neoplastic epithelial cells and multilineage stroma via polyploid giant cells during immortalization and transformation of mullerian epithelial cells. Genes & cancer 2016;7(3–4):60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang S, Mercado-Uribe I, Xing Z, Sun B, Kuang J, Liu J. Generation of cancer stem-like cells through the formation of polyploid giant cancer cells. Oncogene 2014;33(1):116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang S, Zhang D, Yang Z, Zhang X. Tumor Budding, Micropapillary Pattern, and Polyploidy Giant Cancer Cells in Colorectal Cancer: Current Status and Future Prospects. Stem cells international 2016;2016:4810734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zou LJ, Li GQ, Gong LL, et al. [Expression of aurora-A kinase in human lung cancer cell lines PG, A549, and NCI-H460]. Ai zheng = Aizheng = Chinese journal of cancer 2005;24(7):792–795. [PubMed] [Google Scholar]
- 100.Wolberg WH, Street WN, Mangasarian OL. Importance of nuclear morphology in breast cancer prognosis. Clinical cancer research : an official journal of the American Association for Cancer Research 1999;5(11):3542–3548. [PubMed] [Google Scholar]
- 101.Imai Y, Morishita S, Ikeda Y, et al. Immunohistochemical and molecular analysis of giant cell carcinoma of the pancreas: a report of three cases. Pancreas 1999;18(3):308–315. [DOI] [PubMed] [Google Scholar]
- 102.Lorentzen M Giant cell tumor of the ovary. Virchows Arch A Pathol Anat Histol 1980;388(1):113–122. [DOI] [PubMed] [Google Scholar]
- 103.Molberg KH, Heffess C, Delgado R, Albores-Saavedra J. Undifferentiated carcinoma with osteoclast-like giant cells of the pancreas and periampullary region. Cancer 1998;82(7):1279–1287. [DOI] [PubMed] [Google Scholar]
- 104.Mosnier JF, Balique JG. Pleomorphic giant cell carcinoma of the esophagus with coexpression of cytokeratin and vimentin and neuroendocrine differentiation. Arch Pathol Lab Med 2000;124(1):135–138. [DOI] [PubMed] [Google Scholar]
- 105.Nai GA, Amico E, Gimenez VR, Guilmar M. Osteoclast-like giant cell tumor of the pancreas associated with mucus-secreting adenocarcinoma. Case report and discussion of the histogenesis. Pancreatology 2005;5(2–3):279–284. [DOI] [PubMed] [Google Scholar]
- 106.O’Connor RC, Hollowell CM, Laven BA, Yang XJ, Steinberg GD, Zagaja GP. Recurrent giant cell carcinoma of the bladder. J Urol 2002;167(4):1784. [PubMed] [Google Scholar]
- 107.Pokieser W, Ulrich W, Neuhold N, Hoebling W, Hurtl I. Giant cells in poorly differentiated (insular) carcinoma of the thyroid. Acta Cytol 2003;47(1):108–110. [PubMed] [Google Scholar]
- 108.Sakai Y, Kupelioglu AA, Yanagisawa A, et al. Origin of giant cells in osteoclast-like giant cell tumors of the pancreas. Hum Pathol 2000;31(10):1223–1229. [DOI] [PubMed] [Google Scholar]
- 109.Lopez-Beltran A, Eble JN, Bostwick DG. Pleomorphic giant cell carcinoma of the prostate. Arch Pathol Lab Med 2005;129(5):683–685. [DOI] [PubMed] [Google Scholar]
- 110.Shen R, Wen P. Clear cell renal cell carcinoma with syncytial giant cells: a case report and review of the literature. Arch Pathol Lab Med 2004;128(12):1435–1438. [DOI] [PubMed] [Google Scholar]
- 111.Alharbi AM, De Marzo AM, Hicks JL, Lotan TL, Epstein JI. Prostatic Adenocarcinoma With Focal Pleomorphic Giant Cell Features: A Series of 30 Cases. Am J Surg Pathol 2018;42(10):1286–1296. [DOI] [PubMed] [Google Scholar]
- 112.Leapman MS, Cowan JE, Simko J, et al. Application of a Prognostic Gleason Grade Grouping System to Assess Distant Prostate Cancer Outcomes. Eur Urol 2017;71(5):750–759. [DOI] [PubMed] [Google Scholar]
- 113.Coward J, Harding A. Size Does Matter: Why Polyploid Tumor Cells are Critical Drug Targets in the War on Cancer. Front Oncol 2014;4:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sagona AP, Stenmark H. Cytokinesis and cancer. FEBS letters 2010;584(12):2652–2661. [DOI] [PubMed] [Google Scholar]
- 115.Nakayama Y, Igarashi A, Kikuchi I, Obata Y, Fukumoto Y, Yamaguchi N. Bleomycin-induced over-replication involves sustained inhibition of mitotic entry through the ATM/ATR pathway. Experimental cell research 2009;315(15):2515–2528. [DOI] [PubMed] [Google Scholar]
- 116.Illidge TM, Cragg MS, Fringes B, Olive P, Erenpreisa JA. Polyploid giant cells provide a survival mechanism for p53 mutant cells after DNA damage. Cell biology international 2000;24(9):621–633. [DOI] [PubMed] [Google Scholar]
- 117.Makarovskiy AN, Siryaporn E, Hixson DC, Akerley W. Survival of docetaxel-resistant prostate cancer cells in vitro depends on phenotype alterations and continuity of drug exposure. Cellular and molecular life sciences : CMLS 2002;59(7):1198–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Martin SK, Kyprianou N. Exploitation of the Androgen Receptor to Overcome Taxane Resistance in Advanced Prostate Cancer. Advances in cancer research 2015;127:123–158. [DOI] [PubMed] [Google Scholar]
- 119.Martin SK, Pu H, Penticuff JC, Cao Z, Horbinski C, Kyprianou N. Multinucleation and Mesenchymal-to-Epithelial Transition Alleviate Resistance to Combined Cabazitaxel and Antiandrogen Therapy in Advanced Prostate Cancer. Cancer research 2016;76(4):912–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lucanus AJ, Yip GW. Kinesin superfamily: roles in breast cancer, patient prognosis and therapeutics. Oncogene 2018;37(7):833–838. [DOI] [PubMed] [Google Scholar]
- 121.Peled A, Schwartz D, Elkind NB, Wolkowicz R, Li R, Rotter V. The role of p53 in the induction of polyploidity of myelomonocytic leukemic M½ cells. Oncogene 1996;13(8):1677–1685. [PubMed] [Google Scholar]
- 122.Lin KC, Torga G, Sun Y, et al. The role of heterogeneous environment and docetaxel gradient in the emergence of polyploid, mesenchymal and resistant prostate cancer cells. Clin Exp Metastasis 2019. [DOI] [PubMed]
- 123.Elmore LW, Di X, Dumur C, Holt SE, Gewirtz DA. Evasion of a single-step, chemotherapy-induced senescence in breast cancer cells: implications for treatment response. Clinical cancer research : an official journal of the American Association for Cancer Research 2005;11(7):2637–2643. [DOI] [PubMed] [Google Scholar]
- 124.Lagadec C, Vlashi E, Della Donna L, Dekmezian C, Pajonk F. Radiation-induced reprogramming of breast cancer cells. Stem Cells 2012;30(5):833–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Salmina K, Jankevics E, Huna A, et al. Up-regulation of the embryonic self-renewal network through reversible polyploidy in irradiated p53-mutant tumour cells. Experimental cell research 2010;316(13):2099–2112. [DOI] [PubMed] [Google Scholar]
- 126.Jiang Q, Zhang Q, Wang S, et al. A Fraction of CD133+ CNE2 Cells Is Made of Giant Cancer Cells with Morphological Evidence of Asymmetric Mitosis. J Cancer 2015;6(12):1236–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wheatley D Growing evidence of the repopulation of regressed tumours by the division of giant cells. Cell biology international 2008;32(9):1029–1030. [DOI] [PubMed] [Google Scholar]
- 128.Lin KC, Torga G, Wu A, et al. Epithelial and mesenchymal prostate cancer cell population dynamics on a complex drug landscape. Convergent science physical oncology 2017;3(4). [DOI] [PMC free article] [PubMed] [Google Scholar]