Abstract
Objectives:
Depression frequently co-occurs with multiple chronic diseases in complex, costly, and dangerous patterns of multimorbidity. The field of health psychology may benefit from evaluating the temporal characteristics of depression’s associations with common diseases, and from determining whether depression is a central connector in multimorbid disease clusters. The present review addresses these issues by focusing on 4 of the most prevalent diseases: hypertension, ischemic heart disease, arthritis, and diabetes.
Method:
Study 1 assessed how prior chronic disease diagnoses were associated with current depression in a large, cross-sectional, population-based study. It assessed depression’s centrality using network analysis accounting for disease prevalence. Study 2 presents a systematic scoping review evaluating the extent to which depression was prospectively associated with the onset of the 4 prevalent chronic diseases.
Results:
In Study 1 depression had the fourth highest betweenness centrality ranking of 26 network nodes and centrally connected many existing diseases and unhealthy behaviors. In Study 2 depression was associated with subsequent incidence of ischemic heart disease and diabetes across multiple meta-analyses. Insufficient information was available about depression’s pro-spective associations with incident hypertension and arthritis.
Conclusions:
Depression is central in patterns of multimorbidity and is associated with incident disease for several of the most common chronic diseases, justifying the focus on screening and treatment of depression in those at risk for developing chronic disease. Future research should investigate the mediating and moderating roles of health behaviors in the association between depression and the staggered emergence over time of clusters of multimorbid chronic diseases.
Keywords: depression, multimorbidity, chronic diseases
Multimorbidity, the occurrence of two or more chronic medical conditions, is a growing public health problem that to date has not been well examined nor effectively addressed in health care settings (Tinetti, Fried, & Boyd, 2012). Nearly 60% of adults meet criteria for multimorbidity, with a significant increase over the last three decades (King, Xiang, & Pilkerton, 2018). Astoundingly, as many as three in five adults aged 65 years or older are afflicted by three or more chronic conditions, with a median of five chronic conditions per person (van den Bussche et al., 2011). Of diseases that co-occur, it appears that depression is the single most commonly comorbid condition in older adults (Sinnige et al., 2013). In fact, people with multimorbid conditions are twice as likely to be depressed as people without multimorbidities (Read, Sharpe, Modini, & Dear, 2017). Depression is a serious problem in its own right that is estimated to affect one in five people in the U.S. in their lifetimes and one in 10 people in a 12-month period (Hasin et al., 2018). Depression carries dire consequences for health, including a 65% greater likelihood of mortality (Cuijpers & Smit, 2002), and the risk of death remains high even among people who consider their physical health to be excellent (Moise et al., 2018).
Multimorbidity and depression covary in a dose-dependent way such that an increasing number of comorbid medical conditions is associated with a greater likelihood of depression and other mental health conditions (Barnett et al., 2012). In addition, it is now established that the relationship between the incidence of physical diseases and depression is likely to be temporally bidirectional. That is, history of disease is often associated with subsequent depression (Hasan, Mamun, Clavarino, & Kairuz, 2015; Huang, Dong, Lu, Yue, & Liu, 2010; Thombs et al., 2006) and depression with future disease incidence (Polenick, Renn, & Birditt, 2018). Depression is common in multimorbidity patterns, but it remains unclear exactly how depression covaries with different diseases in the complex networks that underlie multimorbidity. It is likely that depression is reliably associated with the subsequent onset of certain diseases and also with the existence of or history of other diseases. Given the temporal bidirectionality that may best account for depression’s links with different conditions, research is needed to evaluate the nature of these temporal associations, to gain further insights into the complex interplay between depression and medical multimorbidities.
The present article consists of two studies that examine depression’s multimorbid associations from two temporal perspectives. These assessments are offered as snapshots for ways to consider the complex topic of depression’s role in multimorbid chronic diseases. To start to untangle the inherent complexities of multi-morbidity, Study 1 examines the centrality of depression and multimorbidities from the perspective of how prior diagnoses of the most commonly occurring chronic diseases are associated with current depression. This study draws upon cross-sectionally collected data that include retrospective diagnosis reports and presently reported depressive symptoms. Whereas the development of depression after disease onset has been reliably demonstrated (Huang et al., 2010), the opposite temporal direction between depression and subsequent medical multimorbidity is relatively less well established for many common diseases. Therefore, Study 2 assesses this temporal question using a thorough scoping review(i.e., a systematic review of literature that maps a field of knowledge in a particular area; Pham et al., 2014). Specifically, it examines the extent to which depression is a risk marker for subsequent incidence of the most common chronic diseases. It identifies high-quality systematic reviews—particularly ones including meta-analyses—that examine naturalistic prospective observational studies testing depression’s association with subsequent disease incidence. The rationale for our focus on disease dyads is to bring clarity to the inherently complex subject of multimorbidity. A useful approach to understanding the topic is to reduce the complexity systematically by examining and comparing the discrete parts that make up the whole—that is, the associations of dyads within the larger web of co-occurring conditions. To this end, for the purposes of the Study 2 scoping review and also emphasized in Study 1, we focused on the four diseases that most frequently occur among individuals at greatest risk of multimorbidity (i.e., those 65 years of age and older; Mitchell & Subramaniam, 2005): hypertension (58% prevalence), ischemic heart disease (31%), arthritis (29%), and diabetes (28%; Centers for Medicare and Medicaid Services, 2012).
Study 1: Examination of Current Depression With Preceding Disease Diagnoses
This study builds on the epidemiological literature cited above showing that diseases are associated with depression. This study examines two questions by exploring patterns of multimorbidity that include depression among a variety of common, co-occurring medical conditions and health behaviors in a large, randomly selected, population-based sample (the Nova Scotia Health Survey 1995; NSHS95; MacLean et al., 1996). Primarily, it tests the notion that depression is not merely prevalent among people with multiple medical conditions but that it may also be a central rather than peripheral connector in the network of multimorbidity. Secondarily, the study explores the extent to which history of each of the four most prevalent chronic diseases (hypertension, ischemic heart disease, arthritis diabetes) is associated with current depression in a large, cross-sectional sample. Third, it tests the extent to which a greater number of previously diagnosed multimorbid conditions is associated with likelihood of current depression, when controlling for age, which covaries with medical conditions.
To evaluate the centrality of depression as a connector between chronic conditions, we used methodology originally applied to social networks (Borgatti, 2005; Newman, 2005). Network analyses methods derived from social science have been adapted in behavioral medicine over the past three decades to better understand, map, and measure the relationships and flows of human behaviors (e.g., spread of HIV; Stephenson & Zelen, 1989). More recently, network analysis has been applied to understanding con-stellations of health-related symptoms such as identifying central symptoms underlying poor mental health (Pereira-Morales, Adan, & Forero, 2017) and in networks of bulimia, anxiety, and depression (Levinson et al., 2017). Network analysis has recently influenced conceptualizations of mental health diseases within the field of psychopathology (McNally, 2016; McNally et al., 2015). One of the approach’s primary strengths is its power to reveal patterns of connectivity in complex systems. In this analysis each condition is represented as a “node” in a network. The metric of “betweenness centrality” is a quantification of the concept of connectedness for particular network nodes. In the analyses of symptoms as network nodes, McNally (2016) asserts that activation of a node that has high betweenness centrality will be associated with an increased likelihood of that symptom spreading to multiple clusters of nodes that characterize different disorders (e.g., depression and obsessive–compulsive disorder). In the present study we take a similar approach but apply it at a higher level to mental and physical diseases rather than to individual symptoms. If depression is a relatively central and essential core of the multimorbid network, as hypothesized, then it should have a betweenness ranking in the top 50% of all network nodes; in contrast, if it is a relatively peripheral and unimportant connector, then it should have a betweenness ranking in the bottom 50% of all network nodes.
Method
Across all regions of Nova Scotia in 1995, participants were recruited to complete at-home interviews lasting approximately one hour that were led by one of 29 trained nurses. The interviews assessed a variety of health conditions, mental health disorders, and health behaviors. Eligible participants for the present analysis (N = 2,311; Mage (SD) = 44.11 years (18.52); 49.2% female; 98.7% Caucasian, 0.8% Black, 0.5% Asian; 73.6% completed high school) were those who provided complete data for the following measures: depression, the four prespecified prevalent chronic diseases (hypertension, ischemic heart disease, arthritis, and diabetes), anxiety, and other common medical conditions (stroke, peripheral vascular disease, food-related allergy, non-food-related allergy, asthma, non-arthritis-related back problems, osteoporosis, migraine headaches, chronic bronchitis or emphysema, sinusitis, epilepsy, cancer, stomach intestinal ulcers, urinary incontinence, cataracts, glaucoma, environmental illness), as well as health behaviors (regular smoking, lack of exercise, and alcohol use). The Institutional Review Board of the Dalhousie University in Canada approved the Nova Scotia Health Survey 1995.
The NSHS95 study procedures and population have been previously described (Davidson, Mostofsky, & Whang, 2010; MacLean et al., 1996). Depression was indicated by total scores on the Center for Epidemiologic Studies Depression (CES-D; Radloff, 1977) scale that were greater than or equal to the established, conservative cutpoint of 20 out of 60 points (Himmelfarb & Murrell, 1983). Anxiety was ascertained based on total scores for the trait version of the State–Trait Anxiety Inventory (Spielberger & Gorsuch, 1983). Since no well-established cutpoint exists for the anxiety scale and since anxiety and depression have similar life-time prevalences (Bijl, Ravelli, & Van Zessen, 1998), an anxiety cutpoint was used (49) that resulted in the same proportion of participants with elevated anxiety as with elevated depression (8.2% each). Hypertension, arthritis, diabetes, and all other chronic medical conditions were assessed by asking participants whether they had ever been diagnosed with the conditions. In the case of arthritis, participants were asked if they had ever been diagnosed with arthritis or rheumatism, and therefore the disease category was not restricted to osteoarthritis. Because there was no one measure of ischemic heart disease, this disease was indicated for the purpose of Study 1 by the self-reported occurrence of a heart attack at any prior time. The health behaviors of smoking, lack of exercise, and alcohol use were assessed, respectively, by asking participants whether they presently smoked at least one cigarette per week, whether they regularly exercised at least three times per week (reverse coded such that 1 reflected a relative lack of exercise), and whether they used alcohol at least one time per month.
Betweenness centrality is defined as “the share of times that a node i needs a node k (whose centrality is being measured) in order to reach a node j via the shortest path” (Borgatti, 2005, p. 60). Mathematically, for the present study the betweenness of a node i is represented by xi:
where gi(st) is the number of geodesic paths from node s to node t that pass through i, nst is the total number of geodestic paths from s to t, and n is the total number of nodes in the network (Newman, 2005). In other words, it measures the proportion of all shortest paths among all pairs of nodes that depend on the node in question. For this particular illustration, each node (i.e., disease) was coded dichotomously for each participant as either present or absent. The unit of analysis in this study (i.e., a link between network nodes) was based on the adjusted correlation coefficient (φ/φ max). In order to calculate this adjusted correlation coefficient, a Pearson correlation, φ (phi), was performed for all pairs of conditions (i.e., all potential comorbidities). In order to adjust for differing prevalence levels among paired conditions, each correlation coefficient was adjusted by dividing by the maximum possible correlation coefficient for that association, φmax (phimax; Olivier & Bell, 2013). After calculating the adjusted correlation coefficients of all pairs of nodes, we removed the association coefficients less than | +0.01| that indicated no or negligible relationship to model the network and then calculated network centrality measures using NodeXl Pro Version 1.0.1.406.
Results
Depression had a prevalence of 8.2%. Of the four diseases of interest, arthritis had the highest prevalence (28.4%), followed by hypertension (23.7%), and then ischemic heart disease (specifically, myocardial infarction; 3.7%), and diabetes (3.5%). Multimorbidity was common in this sample with 54.8% and 33.8% of the sample reporting at least two medical conditions and at least three medical conditions, respectively. The number of reported medical conditions per individual ranged from 0 to 10.
Triads of multimorbidity were investigated by examining sets of three connected nodes involving any combination of medical conditions, depression, and/or anxiety (with health behaviors excluded). Of 2,600 hypothetically possible such triads, 1,322 distinct triads of multimorbid conditions actually occurred in this large sample. The single most prevalent triad consisted of hypertension, arthritis, and back problems (3.7%). Depression was present in 21 of the 100 most prevalent triads. The top 10 most common triads that included depression were as follows, in descending order of prevalence: 1) depression, anxiety, back problems (1.6% of the sample); 2) depression, anxiety, arthritis (1.6%); 3) depression, anxiety, nonfood allergies (1.5%); 4) depression, anxiety, hypertension (1.4%); 5) depression, anxiety, migraine(1.4%); 6) depression, hypertension, arthritis (1.4%); 7) depression, arthritis, back problems 1.4%); 8) depression, hypertension, back problems (1.3%); 9) depression, nonfood allergies, arthritis (1.3%); and 10) depression, anxiety, and sinusitis (1.2%).
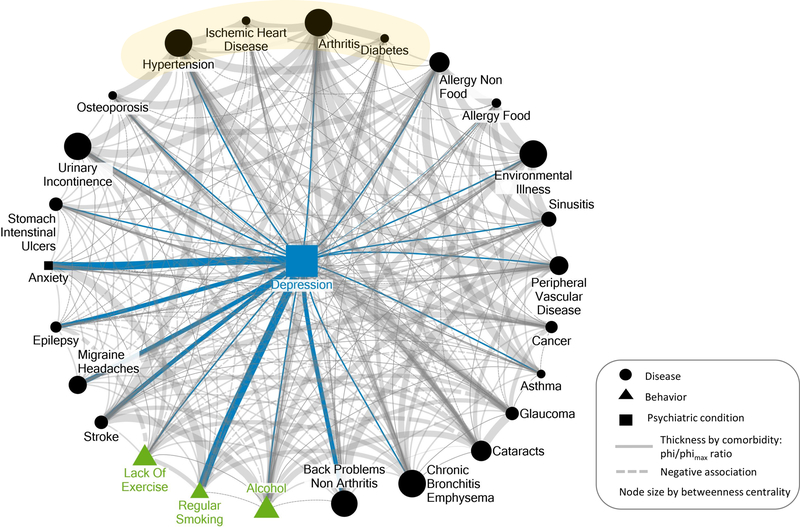
Supporting the first hypothesis, depression ranked as the fourth highest node in betweenness centrality (17.18) among all 26 nodes (range: 0.10–27.11). The only three nodes with higher betweenness centrality were arthritis (27.11), bronchitis/emphysema (23.49), and environmental illness (21.23). The visualization of the multimorbid network in Figure 1 illustrates that depression stands out as a dominant—albeit not the most dominant—link among diseases, unhealthy behavioral factors, and psychological conditions.
Figure 1.
Illustrative visualization of the centrality of depression in a network of multimorbid conditions and behaviors in 2,311 patients with complete data for all represented variables above in the Nova Scotia Health Survey 1995 (MacLean et al., 1996). Gray circular nodes represent medical conditions, blue rectangular nodes represent mood disorders (i.e., depression, anxiety), and green triangles represent unhealthy behaviors. The size of all circular and triangular nodes (i.e., all nodes other than depression) reflects degree of betweenness centrality. The betweenness centrality for depression was the fourth highest (17.18) out of all 26 nodes in the figure. The thickness of the lines connecting nodes represents the ratio of phi/phimax. That ratio was used as the visualized statistic for the association between each pair of nodes to adjust for differences in prevalence among the nodes. Only associations are represented for which the absolute value of the phi/phimax ratio was greater than or equal to .01. The values vary as follows: very thin lines: > = .01 and <.05; thin lines: >= .05 and <.15; medium thickness lines: > = .15 and <.20; thick lines: >= .20 and <.25; very thick lines: > = .25. Positive and negative associations are represented by solid and dotted lines, respectively. Blue lines highlight the associations with depression, and gray lines represent associations among nondepression nodes. See the online article for the color version of this figure.
Regarding the second hypothesis, a self-reported history of 20 of the 21 medical conditions was positively associated with current depression (with the exception of glaucoma, which was —.005). The mean of the association between a history of chronic conditions and current depression [φ/φmax (SD)] was .087 (.058); range:—.005 to .187. Of the four prevalent chronic diseases, only hypertension and arthritis showed associations that were numerically greater than the mean of depression’s associations with all included medical conditions (0.113 for hypertension, 0.091 for arthritis, 0.018 for diabetes, and 0.012 for ischemic heart disease). The four medical conditions with the strongest associations with current depression (all above the M + 1 SD) were back problems not related to arthritis (.187), migraine headaches (.179), epilepsy (.162), and stroke (.156). Even the strongest associations with depression were relatively small in magnitude. Interestingly, unhealthy behaviors showed somewhat stronger associations with depression, although the largest was just small-to-moderate in size: smoking (.251), lack of exercise (.139), and alcohol use (.133).
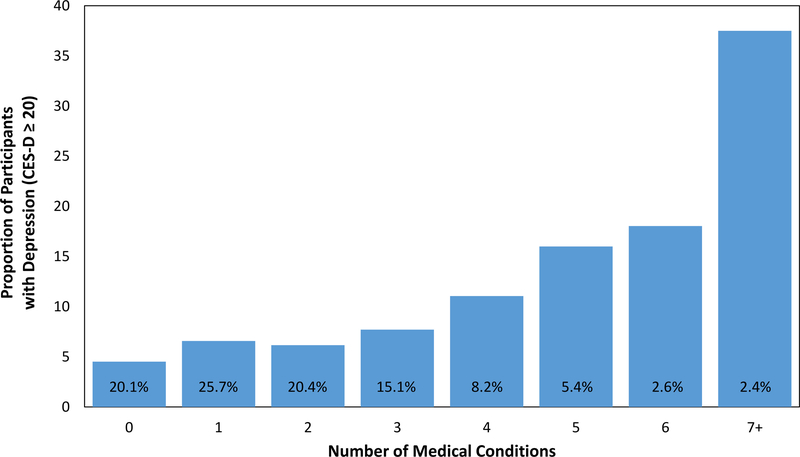
An additional analysis of the 21 medical conditions revealed that, as expected, people with current depression reported a greater number of previously diagnosed multimorbid conditions (M = 3.13 medical conditions, SD = 2.39) than people with depression levels below the cutpoint (M = 1.97, SD = 1.71), t(205.55) = 6.54, p <.001, d = 0.50. In a logistic regression analysis assessing the association between number of previously diagnosed medical conditions and age with current depression status, higher age was associated with lower likelihood of meeting criteria for depression, B = −0.03, adjusted OR 0.97, 95% CI [0.97, 0.98], p <.001, and critically, a higher number of these prior diagnoses was associated with greater likelihood of meeting criteria for depression, B = 0.25, adjusted OR 1.28, 95% CI [1.22, 1.34], p <.001. In a final model in which the interaction of age and number of medical conditions was entered, this interaction was not significant, p = .921, suggesting that age did not moderate the association noted above. See Figure 2 for the association of prior diagnoses and current depression.
Figure 2.
Association between number of previously diagnosed medical conditions and proportion of participants with current depression. The percentage value text appearing over each bar indicates the proportion of participants having the relevant number of medical conditions. CES-D = Center for Epidemiologic Studies Depression. See the online article for the color version of this figure.
Also as expected, people with current depression reported engaging in significantly more unhealthy behaviors (M = 1.42 unhealthy behaviors, SD = 0.91) than people with depression levels below the cutpoint (M = 1.05, SD = 0.86), t(219.15) = 5.45, p <.001, d = 0.41.
Discussion
The visually apparent centrality of depression suggests that depression may be a critical condition to consider in the constellation of multiple morbidities. Our study provides a useful and revealing visual map of the centrality of depression and also lends support to the idea that having prior chronic conditions is associated with current depression diagnosis. Indeed, relative to people without depression, people with depression reported a greater burden of previously diagnosed multimorbid conditions as well as a higher number of unhealthy behaviors.
It remains to be seen whether depression is on the pathway by which multimorbidity occurs. Suls, Green, and Davidson (2016) differentiate the concepts of “simple multimorbidity,” whereby conditions overlap merely by chance, and “associative multimorbidity,” where links are actually causally driven. Critically, regarding that latter category, the visualization cannot verify whether depression is a burdensome comorbidity or a bridge toward new, potentially life-threatening illnesses. Our visualization instead is a hypothesis-generating, thought-provoking finding that prompts further examination of depression as being on the pathway by which multimorbidity occurs.
This study has several limitations. First, despite the relatively large sample, it is important to note that the conditions visualized here represent the multiborbidity constellation of a very specific place and time: a sample of overwhelmingly Caucasian adults living in a maritime, eastern Canadian province in the mid-1990s. Therefore the links among diseases and depression do not necessarily generalize to a wider, contemporary U.S. population with differing demographics. Second, the data were self-reported by people who may have been biased by potentially limited medical knowledge or imperfect memory (“false positives”). Relatedly, although the depression measure is based on a well validated and reliable multiitem scale (Radloff, 1977), other disease diagnoses were assessed with unvalidated, dichotomous, single-item questions. Third, the relative timings of diseases and depression were not entirely certain since the previous medical conditions and health behaviors may have changed substantially by the time that current depression symptoms were assessed (e.g., controlled hypertension, cancer remission) and since depression may have preceded the onset of the chronic illnesses because depression is often chronic and remittent. Fourth, ischemic heart disease was indicated by the incomplete proxy of prior heart attack, and thus a substantial number of cases of true heart disease were likely missed (i.e., false negatives). Fifth, the measures of alcohol use and smoking behavior were necessarily crude and therefore problematic. They could not capture potential U-shaped effects of health behaviors with disease, and they did not differentiate between relatively mild and heavy use. Sixth, there may be some depression-related reporting bias in people with versus without elevated depressive symptoms that may have spuriously influenced the perceived associations between depression and other comorbidities and health behaviors (Rowan, Davidson, Campbell, Dobrez, & MacLean, 2002). Finally, the cross-sectional design did not allow for a determination of the onset of clinical depression subsequent to the diagnoses of the other diseases. Therefore it can only be determined from the present data whether past diagnoses were associated with currently elevated depressive symptoms but not whether the diagnoses were associated with future onset of depressive episodes.
In spite of these limitations, this analysis and visualization serve to illustrate the relative centrality of depression. Of the four chronic diseases examined in this study, this association was most evident for hypertension and arthritis in this sample. Understanding the extent to which depression is associated with the onset of future diseases is an essential next step toward gaining the ability to slow the dangerous, costly growth of multimorbidity’s web. As such, we set forth in Study 2 to understand the ways in which depression predicts the development of chronic illnesses using a scoping review.
Study 2: Scoping Review of Depression as a Prospective Risk Marker for Diseases
The scoping review addresses two questions: (a) Have each of the most prevalent multimorbid medical conditions (hypertension, ischemic heart disease, arthritis, diabetes) been adequately studied to provide a reliable, quantitative evidence about the extent to which depression increases relative risk of subsequent disease development? (b) Does depression increase risk for all, some, or none of these most prevalent chronic conditions?
Method
Selection criteria.
Papers were considered eligible if they met all of the following criteria: (a) The paper involved either a high-quality systematic review and/or meta-analysis, in line with the PRISMA guidelines (Moher, Liberati, Tetzlaff, Altman, & the PRISMA Group, 2009). Relevant to this point, articles published after 2008 were excluded if they did not search at least three databases, and articles published in 2007 or earlier were excluded if they did not search at least two databases. Articles were excluded if they did not specify search criteria or provide specific search terms used or if they did not report at least one of the following: study quality assessment, the functions of the team of reviewers, or the process of data extraction. (b) Papers included depression as a central component. (c) Papers addressed the incidence of four of the most prevalent chronic diseases (hypertension, high cholesterol, ischemic heart disease, arthritis, diabetes; Centers for Medicare and Medicaid Services, 2012). (Although the search criteria also included terms for high cholesterol, only the four true diseases listed above were eligible for inclusion in the final stage of review). Relevant synonymous or highly related terms were included for each of the medical conditions (see the Appendix in online supplemental materials). (d) The types of studies included in the systematic reviews/meta-analyses involved prospective observational cohort designs. Thus, reviews focusing only on interventions, randomized clinical trials, or case-control designs were excluded. (e) Eligible papers were English language publications about human populations.
Search strategy.
The scoping review was conducting by searching the electronic databases OVID Medline, PsycINFO, EMBASE, and the Cochrane Library from the time of database inception until the fourth week of December 2017. All relevant subject headings and free text terms to represent each concept were used for each separate database search. See the Appendix in online supplemental materials for the full search terms used for all databases.
Review phases.
In phase 1 of the review process, titles and abstracts (1,783) were screened to exclude clearly irrelevant articles. Two authors (Jeffrey L. Birk and Louise Falzon) completed this initial phase of the review, discussing questionable cases as needed. In phase 2 of the review process, two reviewers independently assessed full-text articles (238). Conflicting decisions were then resolved through discussion. Three authors (Jeffrey L. Birk, Louise Falzon, and Nathalie Moise) completed this phase of the review. Relevant data, including number of analyzed studies, effect sizes, and confidence intervals, were extracted by Jeffrey L. Birk. Extracted data for a quarter (seven) of eligible references were checked by Louise Falzon and agreement was 100% between the two authors. For a representation of the review process, see the flowchart in Figure S1 in online supplemental material.
Results
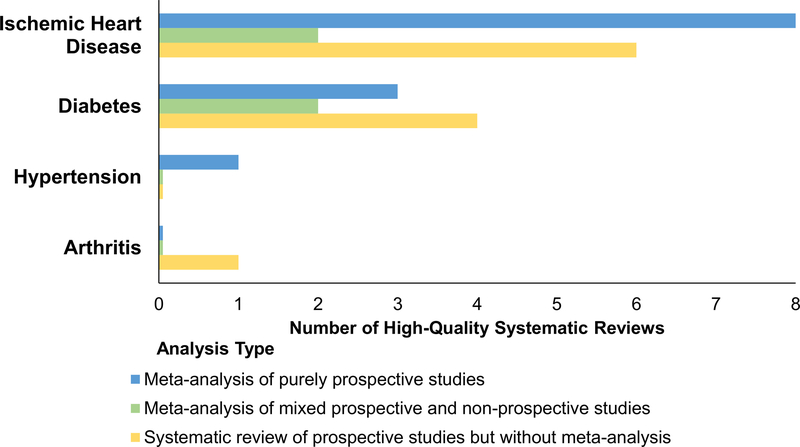
Twenty-five systematic reviews and meta-analyses met inclusion criteria (for extensive details about study characteristics, see Table S1 in online supplemental materials). They were published in the 31-year period spanning 1987 to 2017. They included a total of 16 systematic reviews with meta-analysis (1 hypertension, 10 ischemic heart disease, 0 arthritis, 5 diabetes) as well as nine systematic reviews without meta-analysis that included 11 total studies (0 hypertension, 6 ischemic heart disease, 1 arthritis, 4 diabetes). All papers assessed whether depression conferred a risk for the development of chronic disease (see Figure 3). Below we summarize the finding of the reviews and provide quantitative data from the subset of meta-analyses conducted either on purely prospective studies (ideal) or a mixture of prospective and nonprospective studies. When available, effect sizes and confidence intervals are reported to two decimal places, but a subset of studies only reported these estimates to one decimal place.
Figure 3.
Number of high-quality systematic reviews that investigated depression as a prospective risk marker for the development of medical conditions. Note that 27 total studies were included from the 25 included systematic review papers because one systematic review addressed three of the four diseases (ischemic heart disease, diabetes, and arthritis; Bica et al., 2017). See the online article for the color version of this figure.
Hypertension.
Only one high-quality systematic review assessed risk of incident hypertension (Meng, Chen, Yang, Zheng, & Hui, 2012). This review analyzed 11 studies and determined that the relative risk of developing high blood pressure for people with versus without depression was 1.42 (95% CI [1.09, 1.86]).
Ischemic heart disease.
The earliest included review with meta-analysis indicated that depression was weakly but significantly correlated with subsequent incidence of combined coronary heart disease outcomes (atherosclerosis, angina, myocardial infarction, electrocardiographic abnormalities, cardiac death, or a combination of the above), r = .168, z = 3.77, p <.001, with no relative risk, hazard ratio, or odds ratio reported (Booth-Kewley & Friedman, 1987). The seven meta-analyses of depression as a prospective risk marker for heart disease each estimated significant relative risks, which ranged from 1.30 (95% CI [1.13, 1.76]) to 1.9 (95% CI [1.5, 2.4]; see Table S1 in online supplemental materials for more information; Charlson et al., 2013; Gan et al., 2014; Leung et al., 2012; Nicholson, Kuper, & Hemingway, 2006; Rugulies, 2002; Wu & Kling, 2016; Wulsin & Singal, 2003).
Two meta-analyses that assessed a mixture of longitudinal cohort and case-control studies found a significantly increased risk of coronary heart disease, odds ratio = 1.48 (95% CI [1.29, 1.69]; Van der Kooy et al., 2007) and increased risk of ischemic heart disease-related ventricular tachycardia/ventricular fibrillation: hazard ratio = 1.47 (95% CI [1.23, 1.76]; Shi, Liu, Liang, Hu, & Yang, 2017).
Arthritis.
No eligible meta-analyses assessed depression and subsequent risk of arthritis (osteoarthritis or rheumatoid arthritis). The one eligible systematic review that included arthritis (Bica, Castelló, Toussaint, & Monteso-Curto, 2017) cited a single study showing that depression was prospectively associated with a significant risk for developing arthritis/rheumatism after adjustment for age, sex, and health care utilization, hazard ratio = 1.7 (95% CI[1.3, 2.2]; Patten et al., 2008). No systematic reviews with or without meta-analyses addressed whether depression predicted incidence of osteoarthritis or rheumatoid arthritis as distinct diseases.
Diabetes.
Three meta-analyses of depression as a prospective risk marker for Type 2 diabetes showed significant relative risks (in order of increasing strength) of 1.28 (95% CI [1.14, 1.44]), 1.37 (95% CI [1.14, 1.63]), and 1.41 (95% CI [1.13, 1.76]; Hasan, Clavarino, Mamun, Doi, & Kairuz, 2013; Knol et al., 2006; Yu, Zhang, Lu, & Fang, 2015).
Discussion
Ample, convergent evidence from high-quality meta-analyses reveals that depression is a substantial risk marker for the subsequent development of ischemic heart disease and Type 2 diabetes. Similarly, the current best estimate is that depression also increases the risk of incident hypertension, though only one meta-analysis of 11 studies contributed to this more tentative conclusion. These diseases are among the most prevalent chronic diseases in older adults (Centers for Medicare and Medicaid Services, 2012), and the findings suggest that depression may be a key factor in the development of chronic illness. Given the particularly strong relationship between prior hypertension and arthritis and current depression found in Study 1, further research is needed to elucidate whether depression is associated with a clinically significant prospective elevation in blood pressure and arthritis, the latter of which has had a relative paucity of research relative to the burden of disease.
One limitation of this scoping review was that, although the meta-analyses frequently included relevant behavioral and medical factors as controlled covariates, many biobehavioral pathways linking depression and disease were not assessed. Therefore, low or lack of evidence for a prospective association of depression with subsequent disease onset does not necessarily mean that the disease is not associated with the development of depression at a later time or that depression does not substantially influence health behaviors that indirectly increase risk of the disease.
Critically, this scoping review highlights how research to date has focused on comorbid dyads rather than larger complexes of multimorbidity. That is, this review found no eligible systematic reviews or meta-analyses that examined studies for which depression predicted incidence of triads or larger clusters of multimorbid conditions in the same patients, at least relevant to the highly prevalent chronic conditions that were the focus of this scoping review. Research systematically exploring depression and multimorbid diseases in the same patients is clearly needed. This broader research question was designed to be covered by the literature review search strategy, which included broad terms related to multimorbid webs (e.g., “multimorbidity,” “cluster(s),” “combination(s),” “constellation(s),” “dyad(s),” “triad(s),” “multiple conditions”) in addition to the targeted terms for depression and the diseases of interest (see the Appendix in online supplemental materials). Nonetheless, depression appears to be central and a common pathway for the development of multiple chronic illnesses, which often co-occur. This approach is presented as a model to other researchers to be applied to other highly prevalent conditions that occur in multimorbidity, especially in older people, such as heart failure, chronic kidney disease, chronic obstructive pulmonary disease, Alzheimer’s disease, cancer, asthma, and stroke (Centers for Medicare and Medicaid Services, 2012).
General Discussion
The collective findings of this two-study review suggest several important conclusions. First, depression is a highly central connector in multimorbidity. Second, depression is linked to greater burden in terms of number of chronic diseases. Third, depression is associated with engaging in unhealthy behaviors such as regular smoking, alcohol use, and lack of exercise. Fourth, depression predicts subsequent incidence of some of the most prevalent diseases that commonly occur in multimorbid clusters. The two “snapshot” perspectives in the present paper point to different conclusions about depression-disease associations. Whereas in Study 1, hypertension and arthritis were the common chronic diseases that were most related to depression, in Study 2, prior depression was related to all of the common chronic diseases, with particularly robust evidence for ischemic heart disease, diabetes, and hypertension and a suggestion of a sizable association with arthritis although more study is needed for this last condition. Our study suggests that depression may be central to multiple chronic diseases prevalent in older adults, but the mechanisms through which depression and chronic diseases co-occur remain poorly elucidated. To move beyond these simple temporal studies, employing a biobehavioral framework may be an important next step in addressing depression and multimorbidity (Suls et al., 2016).
Although shared biological factors (e.g., inflammation) may underlie both depression and many chronic diseases (Dantzer, O’Connor, Freund, Johnson, & Kelley, 2008; Goldring & Otero, 2011; Hansson, 2005; Wellen & Hotamisligil, 2005), associated modifiable health behaviors are perhaps just as important to consider if the goal is to reduce the burden of multimorbidity. A consideration of the three health behaviors in the Figure 1 visualization serves to highlight the importance of behavioral factors. Indeed, each of these unhealthy behaviors was associated more strongly with depression in Study 1 than any of the four examined diseases. Prior research has shown that depression has bidirectional relationships with smoking behaviors (Breslau, Peterson, Schultz, Chilcoat, & Andreski, 1998; Chaiton, Cohen, O’Loughlin, & Rehm, 2009), which have also been linked to multiple chronic illnesses (Huxley & Woodward, 2011; Willi, Bodenmann, Ghali, Faris, & Cornuz, 2007; Wolf, D’Agostino, Kannel, Bonita, & Belanger, 1988). Depression and alcohol have similarly bidirectional temporal associations (Boden & Fergusson, 2011). Among people with alcohol disorders, depression predicts even greater future alcohol use, and this correlation is strongest in people with older age (Conner, Pinquart, & Gamble, 2009). Alcohol consumption has adverse effects on many diseases, including several of the most prevalent multimorbid conditions (e.g., hypertension, ischemic heart disease, diabetes; Rehm et al., 2010). Physical inactivity is a third example of an unhealthy behavior that may be prompted by depression given that symptoms of major depression include fatigue and diminished pleasure in activities one usually enjoys (American Psychiatric Association, 2013). Indeed, greater depressive symptoms are associated with lower exercise capacity in patients with heart disease (Papasavvas, Alhashemi, & Micklewright, 2017). As with the other health behaviors above, depression’s links with physical inactivity may be bidirectional and mutually reinforced given that low exercise may be associated with low mood (Biddle, 2000) and given that exercise has been shown to lessen depressive symptoms (Stathopoulou, Powers, Berry, Smits, & Otto, 2006).
With respect to behavioral factors in Study 2, nine of the 12 eligible meta-analyses focusing on prospective studies included papers that controlled for the effects of three behavioral factors highlighted earlier in this paper: alcohol, smoking, and physical activity (Gan et al., 2014; Hasan et al., 2013; Knol et al., 2006; Leung et al., 2012; Meng et al., 2012; Nicholson et al., 2006; Rugulies, 2002; Wulsin & Singal, 2003; Yu et al., 2015). Critically, no meta-analyses controlled for these behaviors in all of the analyzed studies, and thus some of the association may indeed be due, in part, to health behaviors. Related to this point, no meta-analyses quantitatively addressed behavioral factors as potential mediators or moderators of the link between depression and disease, in either temporal order (Kraemer, Stice, Kazdin, Offord, & Kupfer, 2001). In future research health behaviors should be addressed together within networks of depression and multiple diseases, particularly with research investigating depression as a predictor of subsequent disease onset.
Concluding Remarks
Although a causal understanding of the emergence of multimorbidity is not yet possible, the most effective future strategies for managing multimorbid diseases will likely include comprehensive programs that include effective management of depression. A substantial reduction of depressive symptoms in older patients suffering from multimorbid illnesses may be aided greatly not only by traditional interventions such as cognitive–behavioral therapy, but also by proactive prevention of certain diseases. Health psychology would benefit from considering constellations of diseases from the framework of multimorbidity such that no particular disease is necessarily considered the index condition (Suls et al., 2016) and in which all precedent conditions (i.e., diseases, biological factors, health behaviors) are considered in addition to more traditional risk factors (e.g., hypertension) in the temporal accumulation of disease burden. Risk factors and diseases that are often found to precede both the incidence of other chronic diseases and of depression would be especially important targets to identify for prevention efforts (Ridker et al., 2017). To this end, it will be imperative to more precisely ascertain the extent to which depression precedes, co-occurs, and/or follows the diagnosis of the most frequently occurring multimorbid diseases. Prospective observational cohort studies of healthy individuals that focus on the emergence of multiple diseases, including depression, and examine the time course of the development of multimorbid conditions, will be key to this investigation. Future work in longitudinal cohort studies might also consider which unhealthy behavioral factors typically mediate and moderate the relationships between premorbid depression and subsequently emerging patterns of incident diseases, especially in older adults at greatest risk for the development of multimorbidity.
Supplementary Material
Footnotes
Supplemental materials: http://dx.doi.org/10.1037/hea0000737.supp
References
- Ali SS, Khan SA, Khosa F, Aneni EC, Jones A, St Leger AS,…Nasir K. (2017). Noninvasive assessment of subclinical atheroscle-rosis in persons with symptoms of depression. Atherosclerosis, 264, 92–99. 10.1016/j.atherosclerosis.2017.07.010 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: Author. [Google Scholar]
- Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, & Guthrie B (2012). Epidemiology of multimorbidity and implications for health care, research, and medical education: A cross-sectional study. The Lancet, 380, 37–43. 10.1016/S0140-6736(12)60240-2 [DOI] [PubMed] [Google Scholar]
- Bica T, Castelló R, Toussaint LL, & Montesó-Curto P (2017). Depression as a Risk Factor of Organic Diseases:An International Integrative Review. Journal of Nursing Scholarship, 49, 389–399. 10.1111/jnu.12303 [DOI] [PubMed] [Google Scholar]
- Biddle SJ (2000). Emotion, mood and physical activity In Biddle SJH, Fox KR, & Boutcher SH (Eds.), Physical activity and psychological well-being (pp. 63–87). London, England: Routledge. [Google Scholar]
- Bijl RV, Ravelli A, & van Zessen G (1998). Prevalence of psychiatric disorder in the general population: Results of the Netherlands Mental Health Survey and Incidence Study (NEMESIS). Social Psychiatry and Psychiatric Epidemiology, 33, 587–595. 10.1007/s001270050098 [DOI] [PubMed] [Google Scholar]
- Boden JM, & Fergusson DM (2011). Alcohol and depression. Addiction, 106, 906–914. 10.1111/j.1360-0443.2010.03351.x [DOI] [PubMed] [Google Scholar]
- Booth-Kewley S, & Friedman HS (1987). Psychological predictors of heart disease: A quantitative review. Psychological Bulletin, 101, 343–362. 10.1037/0033-2909.101.3.343 [DOI] [PubMed] [Google Scholar]
- Borgatti SP (2005). Centrality and network flow. Social Networks, 27, 55–71. 10.1016/j.socnet.2004.11.008 [DOI] [Google Scholar]
- Breslau N, Peterson EL, Schultz LR, Chilcoat HD, & Andreski P (1998). Major depression and stages of smoking: A longitudinal investigation. Archives of General Psychiatry, 55, 161–166. 10.1001/archpsyc.55.2.161 [DOI] [PubMed] [Google Scholar]
- Brostow DP, Petrik ML, Starosta AJ, & Waldo SW (2017). Depression in patients with peripheral arterial disease: A systematic review. European Journal of Cardiovascular Nursing, 16, 181–193. 10.1177/1474515116687222 [DOI] [PubMed] [Google Scholar]
- Buchberger B, Huppertz H, Krabbe L, Lux B, Mattivi JT, & Siafarikas A (2016). Symptoms of depression and anxiety in youth with type 1 diabetes: A systematic review and meta-analysis. Psychoneuroen-docrinology, 70, 70–84. 10.1016/j.psyneuen.2016.04.019 [DOI] [PubMed] [Google Scholar]
- Camus V, Kraehenbühl H, Preisig M, Büla CJ, & Waeber G (2004). Geriatric depression and vascular diseases: What are the links? Journal of Affective Disorders, 81, 1–16. 10.1016/j.jad.2003.08.003 [DOI] [PubMed] [Google Scholar]
- Centers for Medicare and Medicaid Services. (2012). Chronic conditions among Medicare beneficiaries, chartbook, 2012 edition. Baltimore, MD: Author. [Google Scholar]
- Chaiton MO, Cohen JE, O’Loughlin J, & Rehm J (2009). A systematic review of longitudinal studies on the association between depression and smoking in adolescents. BMC Public Health, 9, 356 10.1186/1471-2458-9-356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlson FJ, Moran AE, Freedman G, Norman RE, Stapelberg NJ, Baxter AJ, …Whiteford HA. (2013). The contribution of major depression to the global burden of ischemic heart disease: A comparative risk assessment. BMC Medicine, 11, 250 10.1186/1741-7015-11-250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner KR, Pinquart M, & Gamble SA (2009). Meta-analysis of depression and substance use among individuals with alcohol use disorders. Journal of Substance Abuse Treatment, 37, 127–137. 10.1016/j.jsat.2008.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuijpers P, & Smit F (2002). Excess mortality in depression: A meta-analysis of community studies. Journal of Affective Disorders, 72, 227–236. 10.1016/S0165-0327(01)00413-X [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, & Kelley KW (2008). From inflammation to sickness and depression: When the immune system subjugates the brain. Nature Reviews Neuroscience, 9, 46–56. 10.1038/nrn2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson KW, Mostofsky E, & Whang W (2010). Don’t worry, be happy: Positive affect and reduced 10-year incident coronary heart disease: The Canadian Nova Scotia Health Survey. European Heart Journal, 31, 1065–1070. 10.1093/eurheartj/ehp603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasure-Smith N, & Lespérance F (2005). Reflections on depression as a cardiac risk factor. Psychosomatic Medicine, 67(Suppl. 1), S19–S25. 10.1097/01.psy.0000162253.07959.db [DOI] [PubMed] [Google Scholar]
- Gan Y, Gong Y, Tong X, Sun H, Cong Y, Dong X, … Lu Z.(2014). Depression and the risk of coronary heart disease: A meta-analysis of prospective cohort studies. BMC Psychiatry, 14, 371 10.2337/dc12-1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring MB, & Otero M (2011). Inflammation in osteoarthritis. Current Opinion in Rheumatology, 23, 471–478. 10.1097/BOR.0b013e328349c2b1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson GK (2005). Inflammation, atherosclerosis, and coronary artery disease. The New England Journal of Medicine, 352, 1685–1695. 10.1056/NEJMra043430 [DOI] [PubMed] [Google Scholar]
- Hasan SS, Clavarino AM, Mamun AA, Doi SA, & Kairuz T (2013). Population impact of depression either as a risk factor or con-sequence of type 2 diabetes in adults: A meta-analysis of longitudinal studies. Asian Journal of Psychiatry, 6, 460–472. 10.1016/j.ajp.2013.09.008 [DOI] [PubMed] [Google Scholar]
- Hasan SS, Mamun AA, Clavarino AM, & Kairuz T (2015). Incidence and risk of depression associated with diabetes in adults: Evidence from longitudinal studies. Community Mental Health Journal, 51, 204–210. 10.1007/s10597-014-9744-5 [DOI] [PubMed] [Google Scholar]
- Hasin DS, Sarvet AL, Meyers JL, Saha TD, Ruan WJ, Stohl M, & Grant BF (2018). Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the united states. Journal of the American Medical Association Psychiatry, 75, 336–346. 10.1001/jamapsychiatry.2017.4602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmelfarb S, & Murrell SA (1983). Reliability and validity of five mental health scales in older persons. Journal of Gerontology, 38, 333–339. 10.1093/geronj/38.3.333 [DOI] [PubMed] [Google Scholar]
- Huang CQ, Dong BR, Lu ZC, Yue JR, & Liu QX (2010). Chronic diseases and risk for depression in old age: A meta-analysis of published literature. Ageing Research Reviews, 9, 131–141. 10.1016/j.arr.2009.05.005 [DOI] [PubMed] [Google Scholar]
- Huxley RR, & Woodward M (2011). Cigarette smoking as a risk factor for coronary heart disease in women compared with men: A systematic review and meta-analysis of prospective cohort studies. The Lancet, 378, 1297–1305. 10.1016/S0140-6736(11)60781-2 [DOI] [PubMed] [Google Scholar]
- Kan C, Silva N, Golden SH, Rajala U, Timonen M, Stahl D, & Ismail K (2013). A systematic review and meta-analysis of the association between depression and insulin resistance. Diabetes Care, 36, 480–489. 10.2337/dc12-1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SJ, & Ismail M (2015). Stress and type 2 diabetes: A review of how stress contributes to the development of type 2 diabetes. Annual Review of Public Health, 36, 441–462. 10.1146/annurev-publhealth-031914-122921 [DOI] [PubMed] [Google Scholar]
- King DE, Xiang J, & Pilkerton CS (2018). Multimorbidity Trends in United States Adults, 1988–2014. Journal of the American Board of Family Medicine, 31, 503–513. 10.3122/jabfm.2018.04.180008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knol MJ, Twisk JW, Beekman AT, Heine RJ, Snoek FJ, & Pouwer F (2006). Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia, 49, 837–845. 10.1007/s00125-006-0159-x [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Stice E, Kazdin A, Offord D, & Kupfer D (2001). How do risk factors work together? Mediators, moderators, and independent, overlapping, and proxy risk factors. The American Journal of Psychiatry, 158, 848–856. 10.1176/appi.ajp.158.6.848 [DOI] [PubMed] [Google Scholar]
- Leung YW, Flora DB, Gravely S, Irvine J, Carney RM, & Grace SL (2012). The impact of premorbid and postmorbid depression onset on mortality and cardiac morbidity among patients with coronary heart disease: Meta-analysis. Psychosomatic Medicine, 74, 786–801. 10.1097/PSY.0b013e31826ddbed [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson CA, Zerwas S, Calebs B, Forbush K, Kordy H, Watson H, … Bulik CM. (2017). The core symptoms of bulimia nervosa, anxiety, and depression: A network analysis. Journal of Abnormal Psychology, 126, 340–354. 10.1037/abn0000254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman JH, Froelicher ES, Blumenthal JA, Carney RM,Doering LV, Frasure-Smith N, … the American Heart Association Statistics Committee of the Council on Epidemiology and Prevention and the Council on Cardiovascular and Stroke Nursing. (2014). Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: Systematic review and recommendations: A scientific statement from the American Heart Association. Circulation, 129 1350–1369. 10.1161/CIR.0000000000000019 [DOI] [PubMed] [Google Scholar]
- MacLean D, Scott J, Beanlands H, Hood R, Cogdon A, LeBlanc B,& Farquharson J (1996). The 1995 Nova Scotia Health Survey. Halifax, Nova Scotia: Department of Health. [Google Scholar]
- McNally RJ (2016). Can network analysis transform psychopathology? Behaviour Research and Therapy, 86, 95–104. 10.1016/j.brat.2016.06.006 [DOI] [PubMed] [Google Scholar]
- McNally RJ, Robinaugh DJ, Wu GW, Wang L, Deserno MK,& Borsboom D (2015). Mental disorders as causal systems: A network approach to posttraumatic stress disorder. Clinical Psychological Science, 3, 836–849. 10.1177/2167702614553230 [DOI] [Google Scholar]
- Meng L, Chen D, Yang Y, Zheng Y, & Hui R (2012). Depression increases the risk of hypertension incidence: A meta-analysis of prospective cohort studies. Journal of Hypertension, 30, 842–851. 10.1097/HJH.0b013e32835080b7 [DOI] [PubMed] [Google Scholar]
- Mitchell AJ, & Subramaniam H (2005). Prognosis of depression in old age compared to middle age: A systematic review of comparative studies. The American Journal of Psychiatry, 162, 1588–1601. 10.1176/appi.ajp.162.9.1588 [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, & the PRISMA Group.(2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine, 6(7), e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moise N, Khodneva Y, Jannat-Khah DP, Richman J, Davidson KW, Kronish IM, … Safford MM. (2018). Observational study of the differential impact of time-varying depressive symptoms on all-cause and cause-specific mortality by health status in community-dwelling adults: The REGARDS study. British Medical Journal Open, 8(1), e017385 10.1136/bmjopen-2017-017385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman ME. (2005). A measure of betweenness centrality based on random walks. Social Networks, 27, 39–54. 10.1016/j.socnet.2004.11.009 [DOI] [Google Scholar]
- Nicholson A, Kuper H, & Hemingway H (2006). Depression as an aetiologic and prognostic factor in coronary heart disease: A meta-analysis of 6362 events among 146 538 participants in 54 observational studies. European Heart Journal, 27, 2763–2774. 10.1093/eurheartj/ehl338 [DOI] [PubMed] [Google Scholar]
- Olivier J, & Bell ML (2013). Effect sizes for 2[H11003]2 contingency tables. [Electronic Resource]. PLoS ONE, 8(3), e58777 10.1371/journal.pone.0058777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papasavvas T, Alhashemi M, & Micklewright D (2017). Association between depressive symptoms and exercise capacity in patients with heart disease. Journal of Cardiopulmonary Rehabilitation and Prevention, 37, 239–249. 10.1097/HCR.0000000000000193 [DOI] [PubMed] [Google Scholar]
- Patten SB, Williams JV, Lavorato DH, Modgill G, Jetté N, & Eliasziw M (2008). Major depression as a risk factor for chronic disease incidence: Longitudinal analyses in a general population cohort. General Hospital Psychiatry, 30, 407–413. 10.1016/j.genhosppsych.2008.05.001 [DOI] [PubMed] [Google Scholar]
- Pereira-Morales AJ, Adan A, & Forero DA (2017). Network analysis of multiple risk factors for mental health in young Colombian adults. Journal of Mental Health. Advance online publication 10.1080/09638237.2017.1417568 [DOI] [PubMed] [Google Scholar]
- Pham MT, Rajić A, Greig JD, Sargeant JM, Papadopoulos A, & McEwen SA (2014). A scoping review of scoping reviews: Advancing the approach and enhancing the consistency. Research Synthesis Methods, 5, 371–385. 10.1002/jrsm.1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polenick CA, Renn BN, & Birditt KS (2018). Dyadic effects of depressive symptoms on medical morbidity in middle-aged and older couples. Health Psychology, 37, 28–36. 10.1037/hea0000573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1, 385–401. 10.1177/014662167700100306 [DOI] [Google Scholar]
- Read JR, Sharpe L, Modini M, & Dear BF (2017). Multimorbidity and depression: A systematic review and meta-analysis. Journal of Affective Disorders, 221, 36–46. 10.1016/j.jad.2017.06.009 [DOI] [PubMed] [Google Scholar]
- Rehm J, Baliunas D, Borges GL, Graham K, Irving H, Kehoe T,… Taylor B. (2010). The relation between different dimensions of alcohol consumption and burden of disease: An overview. Addiction, 105, 817–843. 10.1111/j.1360-0443.2010.02899.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH,Ballantyne C, … the CANTOS Trial Group. (2017). Antiinflammatory therapy with canakinumab for atherosclerotic disease. The New England Journal of Medicine, 377, 1119–1131. 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
- Rowan P, Davidson K, Campbell J, Dobrez D, & MacLean D (2002). Depressive symptoms predict medical care utilization in a population-based sample. Psychological Medicine, 32, 903–908. 10.1017/S0033291702005767 [DOI] [PubMed] [Google Scholar]
- Roy T, & Lloyd CE (2012). Epidemiology of depression and diabetes: A systematic review. Journal of Affective Disorders, 142(Suppl.), S8–S21. 10.1016/S0165-0327(12)70004-6 [DOI] [PubMed] [Google Scholar]
- Rugulies R (2002). Depression as a predictor for coronary heart disease. American Journal of Preventive Medicine, 23, 51–61. 10.1016/S0749-3797(02)00439-7 [DOI] [PubMed] [Google Scholar]
- Shi S, Liu T, Liang J, Hu D, & Yang B (2017). Depression and risk of sudden cardiac death and arrhythmias: A meta-analysis. Psychosomatic Medicine, 79, 153–161. [DOI] [PubMed] [Google Scholar]
- Sinnige J, Braspenning J, Schellevis F, Stirbu-Wagner I, Westert G,& Korevaar J (2013). The prevalence of disease clusters in older adults with multiple chronic diseases—A systematic literature review. PLoS ONE, 8(11), e79641 10.1371/journal.pone.0079641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, & Gorsuch RL (1983). Manual for the state-trait anxiety inventory (form Y) (“self-evaluation questionnaire”). Washington, DC: Consulting Psychologists Press. [Google Scholar]
- Stathopoulou G, Powers MB, Berry AC, Smits JA, & Otto MW (2006). Exercise interventions for mental health: A quantitative and qualitative review. Clinical Psychology: Science and Practice, 13, 179–193. 10.1111/j.1468-2850.2006.00021.x [DOI] [Google Scholar]
- Stephenson K, & Zelen M (1989). Rethinking centrality: Methods and examples. Social Networks, 11, 1–37. 10.1016/0378-8733(89)90016-6 [DOI] [Google Scholar]
- Suls J, Green PA, & Davidson KW (2016). A biobehavioral framework to address the emerging challenge of multimorbidity. Psychosomatic Medicine, 78, 281–289. 10.1097/PSY.0000000000000294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thombs BD, Bass EB, Ford DE, Stewart KJ, Tsilidis KK,Patel U, … Ziegelstein RC. (2006). Prevalence of depression in survivors of acute myocardial infarction. Journal of General Internal Medicine, 21, 30–38. 10.1111/j.1525-1497.2005.00269.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinetti ME, Fried TR, & Boyd CM (2012). Designing health care for the most common chronic condition—Multimorbidity. Journal of the American Medical Association, 307, 2493–2494. 10.1001/jama.2012.5265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vancampfort D, Mitchell AJ, De Hert M, Sienaert P, Probst M, Buys R, & Stubbs B (2015). Type 2 diabetes in patients with major depressive disorder: A meta-analysis of prevalence estimates and predictors. Depression and Anxiety, 32, 763–773. 10.1002/da.22387 [DOI] [PubMed] [Google Scholar]
- van den Bussche H, Koller D, Kolonko T, Hansen H, Wegscheider K, Glaeske G, … Schön G. (2011). Which chronic diseases and disease combinations are specific to multimorbidity in the elderly? Results of a claims data based cross-sectional study in Germany. BMC Public Health, 11, 101 10.1186/1471-2458-11-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kooy K, van Hout H, Marwijk H, Marten H, Stehouwer C, & Beekman A (2007). Depression and the risk for cardiovascular diseases: Systematic review and meta analysis. International Journal of Geriatric Psychiatry, 22, 613–626. 10.1002/gps.1723 [DOI] [PubMed] [Google Scholar]
- Wellen KE, & Hotamisligil GS (2005). Inflammation, stress, and diabetes. The Journal of Clinical Investigation, 115, 1111–1119. 10.1172/JCI25102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willi C, Bodenmann P, Ghali WA, Faris PD, & Cornuz J (2007). Active smoking and the risk of type 2 diabetes: A systematic review and meta-analysis. Journal of the American Medical Association, 298, 2654–2664. 10.1001/jama.298.22.2654 [DOI] [PubMed] [Google Scholar]
- Wolf PA, D’Agostino RB, Kannel WB, Bonita R, & Belanger AJ (1988). Cigarette smoking as a risk factor for stroke. The Framingham Study. Journal of the American Medical Association, 259, 1025–1029. 10.1001/jama.1988.03720070025028 [DOI] [PubMed] [Google Scholar]
- Wu Q, & Kling JM (2016). Depression and the risk of myocardial infarction and coronary death. Medicine, 95(6), e2815 10.1097/MD.0000000000002815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulsin LR, & Singal BM (2003). Do depressive symptoms increase the risk for the onset of coronary disease? A systematic quantitative review. Psychosomatic Medicine, 65, 201–210. 10.1097/01.PSY.0000058371.50240.E3 [DOI] [PubMed] [Google Scholar]
- Yu M, Zhang X, Lu F, & Fang L (2015). Depression and Risk for Diabetes: A Meta-Analysis. Canadian Journal of Diabetes, 39, 266–272. 10.1016/j.jcjd.2014.11.006 [DOI] [PubMed] [Google Scholar]
- Zuidersma M, Thombs BD, & de Jonge P (2011). Onset and recurrence of depression as predictors of cardiovascular prognosis in depressed acute coronary syndrome patients: A systematic review. Psychotherapy and Psychosomatics, 80, 227–237. 10.1159/000322633 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.