Abstract
The objective of this study was to assess breast cancer incidence and mortality rates by molecular subtype for cases diagnosed in New Jersey. Data on all primary, histologically confirmed, invasive breast cancers diagnosed among women between January 1, 2008 and December 31, 2013 were retrieved from the New Jersey State Cancer Registry. Age-adjusted incidence rates were calculated for each subtype, by ageandrace/ethnicity. Logistic regression models, Cox proportional hazards models, and Kaplan Meier curves were used to describe the relative risks for breast cancer incidence, mortality, and survival, respectively. In this population-based sample of 32,770 breast cancer cases, non-Hispanic Blacks (NHBs) had the highest triple-negative breast cancer (TNBC) incidence rate (17.8 per 100,000, 95% CI 16.5–19.2) compared to other races/ethnicities. NHBs had also higher odds of TNBC (OR 2.1, 95% CI 1.95–2.36) and higher hazards of death when diagnosed with TNBC (HR 1.28, 95% CI 1.05–1.56), luminal A (HR 1.64, 95% CI 1.41–1.91), or luminal B (HR 1.54, 95% CI 1.10–2.15) than non-Hispanic Whites (NHWs). Younger women (20–39 years) had higher odds of TNBC (OR 1.77, 95% CI 1.54–2.02) and luminal B (OR 1.56, 95% CI 1.35–1.80) compared to women 50–64 years; minority women had higher odds of non-luminal HER2-expressing and lower odds of luminal A than NHWs. TNBC was associated with the poorest survival rates. These findings highlight a need for enhanced screening to promote earlier diagnosis and improve breast cancer outcomes, particularly in minorities and younger women, which will be essential for achieving health equity.
Keywords: breast cancer, surveillance, incidence, mortality, molecular subtype, diverse population, disparities
INTRODUCTION
In the United States (US), breast cancer is the most commonly diagnosed cancer and the leading cause of cancer-related deaths among women of all age and racial/ethnic groups (ACS, 2017). In 2017, approximately 252,710 new cases of invasive breast cancer were diagnosed and 40,610 breast cancer deaths occurred among US women (ACS, 2017). Breast cancer was the most common cancer diagnosed among New Jersey women from 2010–2014 (NJSCR, 2017). In 2014, New Jersey ranked 9th in the US for breast cancer incidence, with a rate higher than the US average (134.3 per 100,000 vs. 123.9 per 100,000), and elevated age-adjusted rates among Whites (137.8 per 100,000), Blacks (120.8 per 100,000), and Hispanics (105.7 per 100,000), in contrast to US average rates for these groups (124.8, 122.4, and 91.8 per 100,000, respectively) (CDC, 2017). The estimated breast cancer mortality rate was also slightly higher for New Jersey than the US average (21.5 per 100,000 vs. 20.5 per 100,000), with higher rates among Whites and Blacks (20.9 and 30.6 per 100,000, respectively) when compared to US average rates for these groups (20.0 and 28.1 per 100,000, respectively) (CDC, 2017).
Based on global gene expression patterns (Bastien et al., 2012; Network, 2012; Perou et al., 2000; Sorlie et al., 2001; Sweeney et al., 2014) and/or clinical approximation of immunohistochemistry (IHC) expression patterns of the estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) (Bhargava et al., 2009; Morrison et al., 2012; Tamimi et al., 2008), at least four breast cancer subtypes have been identified, including luminal A (ER+/PR+/HER2-), luminal B (ER+/PR+/HER2+), HER2-enriched (luminal, ER+/PR+; non-luminal, ER-/PR-) and triple-negative breast cancer (TNBC, ER-/PR-/HER2-), with differing distributions, risk factors, tumor behaviors and clinical outcomes (Carey et al., 2006; Clarke et al., 2012; Howlader et al., 2014; Kroenke et al., 2014; Sineshaw et al., 2014; Sweeney et al., 2014; Yang et al., 2011). Although gene expression profiles are the gold standard, data show that IHC expression patterns are concordant with gene expression profiles and have substantial clinical utility in subtype classification (Bastien et al., 2012; Carey et al., 2006). As a result, state and regional cancer registries began collecting HER2 data in 2010 (Thornton M).
Distributions of breast cancer subtypes among racially and ethnically diverse populations such as those residing in New Jersey, are important data for understanding cancer disparities and ultimately achieving health equity, particularly in terms of disseminating optimal treatment (Albain et al., 2009; Chlebowski et al., 2005; Dignam, 2001) within the state. We expanded on prior surveillance research by retrospectively collecting and validating two additional years of HER2 data (2008–2009) for invasive breast cancers diagnosed in New Jersey. Our objective was to assess age and racial/ethnic disparities in incidence and mortality by molecular subtype. We calculated age-adjusted incidence and mortality rates for each molecular subtype by age at diagnosis and race/ethnicity; and, compared New Jersey incidence and mortality rates to those of the general US population for diagnosis years 2010 to 2013 (years for which ER, PR, and HER2 data were collected nationally). Finally, we estimated relative risks for breast cancer diagnosis and death by breast cancer subtype.
MATERIALS AND METHODS
Study population and data collection
Data for all primary, histologically confirmed, invasive breast cancers diagnosed among women of all races/ethnicities in New Jersey from January 1, 2008 through December 31, 2013 were retrieved from existing records at New Jersey State Cancer Registry (NJSCR), which is a high-quality, population-based cancer incidence registry established in October 1978 serving the population of New Jersey (currently about 8.9 million residents (McCaig et al., 2002)). Women <20 years of age at breast cancer diagnosis, diagnosed with noninvasive breast cancer, and non-residents of New Jersey diagnosed at an in-state medical facility were excluded from this study as the focus for the current analysis was adult women (age ≥20 years) diagnosed with invasive breast cancer and who reside in the state of New Jersey.
Because cancer registries did not routinely collect HER2 data for incident breast cancer diagnoses until January 1, 2010, HER2 data for diagnosis years 2008 and 2009 were retrospectively collected and coded from pathology records for this study. HER2 data for 2010 through 2013 were reviewed and validated from existing NJSCR records. If there was insufficient information to code HER2 status in existing records, data were obtained by contacting hospital cancer registrars and using in-house pathology reports. Electronic pathology reports were also reviewed to glean additional data. All coding was conducted within the NJSCR database (SEER*DMS).
Classification of ER/PR/HER2 status
An array of standard variables corresponding to Collaborative Stage Site-Specific Factors (SSFs) for breast cancer was used. ER status (SSF 1) and PR status (SSF 2), corresponding to the ER and PR assays, respectively, were coded as positive/elevated, negative/normal, borderline, or unknown (unknown includes test not done, borderline/undetermined, test ordered but results not entered in chart, or unknown for either ER or PR status).
A series of eight (8) additional variables were used to code HER2 status (SSF 8 – SSF 15), and SSF 16 was used to define breast cancer subtype (summary of ER/PR/HER2 status). For HER2 IHC screening, scores 0 and 1+ were coded as negative; score 2+ was coded as borderline; and score 3+ was coded as positive. Fluorescence in situ hybridization (FISH) was performed on IHC borderline cases. Without positive FISH information, tumors scored 2+ by IHC were coded as HER2 negative. FISH results were classified as a range of values: 0–120 was considered negative; 120–180 was considered borderline; and values >180 were considered positive. These results were used to derive the HER2 summary result (SSF 15). The combination of ER, PR and HER2 (SSF 16) was used to classify breast cancer subtype. The final subtype classifications used were luminal A (ER+ and/or PR+/HER2-), luminal B (ER+ and/or PR+/HER2+), non-luminal HER2-expressing (ER-/PR-/HER2+), TNBC (ER-/PR-/HER2-), and unknown. Although included in descriptive analysis (Table 1), cases with unknown breast cancer subtype were excluded from subsequent analyses.
Table 1.
Selected sociodemographic and breast cancer clinicopathologic characteristics among incident breast cancer cases diagnosed in New Jersey, 2008–2013, N = 32,770.
| Characteristics | n | (%) | |
|---|---|---|---|
| Sociodemographic characteristics | |||
| Age at diagnosis (years) | |||
| 20–39 | 1,773 | (5.4) | |
| 40–49 | 6,344 | (19.3) | |
| 50–64 | 12,179 | (37.1) | |
| ≥65 | 12,474 | (38.0) | |
| Race/ethnicity | |||
| White, non-Hispanic | 23,930 | (73.0) | |
| Black, non-Hispanic | 3,829 | (11.7) | |
| Asian/Pacific Islander, non-Hispanic | 1,826 | (5.6) | |
| Hispanic (any race) | 2,831 | (8.6) | |
| Other/unknown | 354 | (1.1) | |
| Breast cancer clinicopathologic characteristics | |||
| Primary site of breast cancer | |||
| Nipple (areolar) | 152 | (0.5) | |
| Central (sub-areolar) | 1,509 | (4.6) | |
| Upper inner quadrant | 3,513 | (10.7) | |
| Lower inner quadrant | 1,767 | (5.4) | |
| Upper outer quadrant | 10,760 | (32.8) | |
| Lower outer quadrant | 2,286 | (7.0) | |
| Axillary tail | 158 | (0.5) | |
| Overlapping lesion | 6,532 | (19.9) | |
| Breast, NOS | 6,093 | (18.6) | |
| Cancer sequence number | |||
| One primary cancer diagnosed | 29,373 | (89.6) | |
| First of two or more primaries diagnosed | 3,397 | (10.3) | |
| Histology | |||
| Adenocarcinoma | 613 | (1.9) | |
| Ductal carcinoma | 23,948 | (73.1) | |
| Lobular carcinoma | 3,074 | (9.4) | |
| Ductal and lobular carcinoma | 1,942 | (5.9) | |
| Mixeda | 1,086 | (3.3) | |
| Other/unknownb | 2,107 | (6.4) | |
| Tumor stagec | |||
| Localized | 20,117 | (61.4) | |
| Regional | 9,810 | (29.9) | |
| Distant | 2,039 | (6.2) | |
| Unknown | 804 | (2.5) | |
| Tumor grade | |||
| Well differentiated | 4,883 | (14.9) | |
| Moderately differentiated | 13,364 | (40.7) | |
| Poorly differentiated | 11,140 | (34.0) | |
| Missing/unknown | 3,383 | (10.3) | |
| ER status (SSF 1) | |||
| Positive | 25,750 | (78.6) | |
| Negative | 5,656 | (17.3) | |
| Borderline | 27 | (0.1) | |
| Other/unknown | 1,337 | (4.1) | |
| PR status (SSF 2) | |||
| Positive | 22,518 | (68.7) | |
| Negative | 8,768 | (26.8) | |
| Borderline | 102 | (0.3) | |
| Other/unknown | 1,382 | (4.2) | |
| HER2 IHC lab value (SSF 8) | |||
| 0 | 4,318 | (13.1) | |
| 1+ | 5,750 | (17.5) | |
| 2+ | 2,760 | (8.4) | |
| 3 | 2,019 | (6.2) | |
| Unknown | 17,923 | (54.4) | |
| HER2 IHC interpretation (SSF 9) | |||
| Positive | 2,484 | (7.7) | |
| Negative | 11,875 | (36.5) | |
| Borderline | 2,414 | (7.4) | |
| Other/unknown | 15,997 | (48.4) | |
| HER2 FISH lab value (SSF 10) | |||
| 1.00–9.79 | 11,889 | (36.3) | |
| 9.80–9.87 | 127 | (0.4) | |
| Other/unknown | 20,754 | (63.3) | |
| HER2 FISH interpretation (SSF 11) | |||
| Positive | 2,185 | (6.7) | |
| Negative | 12,153 | (37.3) | |
| Borderline | 410 | (1.3) | |
| Other/unknown | 18,022 | (54.7) | |
| HER2 CISH lab value (SSF 12) | |||
| 1.00–9.79 | 32 | (0.1) | |
| 9.80–9.87 | 15 | (0.1) | |
| Other/unknown | 32,723 | (99.8) | |
| HER2 CISH interpretation (SSF 13) | |||
| Positive | 30 | (0.1) | |
| Negative | 164 | (0.5) | |
| Borderline | 10 | (0.1) | |
| Other/unknown | 32,566 | (99.4) | |
| HER2 result of other or unknown test (SSF 14) | |||
| Positive | 768 | (2.4) | |
| Negative | 5,351 | (16.5) | |
| Borderline | 166 | (0.5) | |
| Other/unknown | 26,485 | (80.6) | |
| HER2 summary result (SSF 15) | |||
| Positive | 4,373 | (13.5) | |
| Negative | 24,268 | (74.0) | |
| Borderline | 630 | (1.9) | |
| Other/unknown | 3,499 | (10.0) | |
| Breast cancer subtype (SSF 16) | |||
| Luminal A (ER+ and/or PR+/HER2-) | 20,775 | (63.3) | |
| Luminal B (ER+ and/or PR+/HER+) | 2,938 | (8.9) | |
| Non-luminal HER2-expressing (ER-/PR-/HER2+) | 1,396 | (4.2) | |
| Triple-negative (ER-/PR-/HER2-) | 3,346 | (10.2) | |
| Unknown | 4,315 | (13.1) | |
| Vital statusd | |||
| Dead | 5,734 | (17.5) | |
| Alive | 27,036 | (82.5) | |
NOTE: Percentages may not sum to 100 due to rounding. P values were generated using chi-square test. Abbreviations: CS SSF, Collaborative Stage Site-Specific Factor; ER, estrogen receptor; CISH, chromogenic in situ hybridization; FISH, fluorescence in situ hybridization; HER2, human epidermal growth factor 2; IHC, immunohistochemistry; NOS, not otherwise specified; PR, progesterone receptor
Mixed histology includes breast cancers classified as: duct and cribriform carcinoma, duct and mucinous carcinoma, duct and tubular carcinoma, or duct and colloid carcinoma.
Other & unknown histology includes breast cancers classified as: neoplasm, tumor cells, carcinoma NOS, pleomorphic carcinoma, spindle cell carcinoma NOS, pseudosarcomatous carcinoma, small cell carcinoma NOS, papillary carcinoma, squamous cell carcinoma NOS, squamous cell carcinoma keratinizing NOS, basaloid carcinoma, adenocarcinoma, adenocarcinoma NOS, scirrhous adenocarcinoma, adenoid cystic carcinoma, cribriform carcinoma NOS, tubular adenocarcinoma, solid carcinoma NOS, neuroendocrine carcinoma, adenocarcinoma with mixed subtypes, papillary adenocarcinoma NOS, clear cell adenocarcinoma NOS, glycogen-rich carcinoma, mixed cell adenocarcinoma, apocrine adenocarcinoma, papillary serous cystadenocarcinoma, mucinous cystadenocarcinoma NOS, mucin producing adenocarcinoma, signet ring cell carcinoma, comedenocarcinoma NOS, secretory carcinoma of breast, intraductal papillary adenocarcinoma with invasion, intracystic carcinoma NOS, medullary carcinoma NOS, atypical medullary carcinoma, infiltrating ductular carcinoma, Infiltrating lobular mixed with other types of carcinoma, inflammatory carcinoma, Paget’s disease (mammary), Paget’s disease with intraductal carcinoma of breast, adenosquamous carcinoma, adenocarcinoma with spindle cell metaplasia, adenocarcinoma with neuroendocrine differentiation, metaplastic carcinoma NOS, sarcoma NOS, giant cell sarcoma, epithelioid sarcoma, malignant fibrous histiocytoma, liposarcoma NOS, myxoid liposarcoma, leiomyomatosis NOS, spindle cell rhabdomyosarcoma, stromal sarcoma NOS, carcinosarcoma NOS, malignant myoepithelioma, phyllodes tumor, hemangiosarcoma, or osteosarcoma NOS.
SEER summary stage.
Vital status as of December 31, 2014.
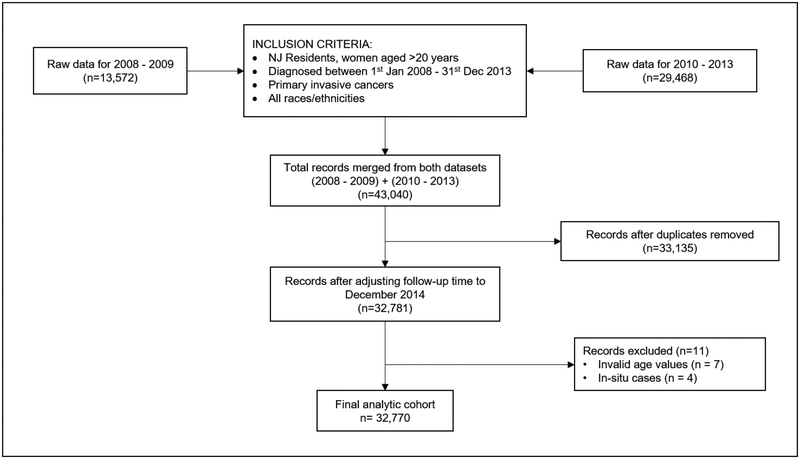
We reviewed SSFs 1, 2, and 8 through 16 and identified 1942 cases that were ineligible (due to unknown/borderline ER, PR, or HER2). We also identified 1442 unresolved cases requiring hospital follow-back (due to unknown or not applicable codes for ER, PR, and/or HER2) and for which we were unable to code SSF 16 (due to insufficient ER, PR, and HER2 information). The final analytical sample included 32,770 women (Figure 1).
Figure 1.
Flow diagram describing the selection of the analytic cohort.
Statistical analysis
Sociodemographic and tumor characteristics were described using frequencies and proportions, and chi-square tests were used to compare the distributions of each variable. Custom incidence files for New Jersey data from 2008 to 2013 were imported into SEER*PREP v2.5.3 (NCI, 2017a) to create a SEER*STAT database for this study. SEER*Stat v8.3.2 (NCI, 2017b) was used to calculate the age-adjusted incidence rates for each breast cancer subtype. The population denominators used to generate rates were based on detailed county population estimates by age, sex and race/ethnicity available in the SEER*STAT database (NCI, 2017b). The 2000 US Standard Population was used for age-specific weights for direct age-adjustment. Subtype-specific incidence rates were generated by age group (20–39, 40–49, 50–64, ≥65), and race/ethnicity (non-Hispanic White [NHW], non-Hispanic Black [NHB], Asian Pacific Islander [API, non-Hispanic], Hispanic, Other/Unknown). Rates were estimated per 100,000 population, and the Tiwari et al. (Tiwari et al., 2006) modification for 95% confidence intervals (CI) was used to quantify the associations between breast cancer subtype and age and race/ethnicity.
We estimated odds ratios (ORs) for breast cancer risk by molecular subtype in New Jersey from 2008 to 2013, overall and by age at diagnosis and race/ethnicity, using multivariable logistic regression models. Cox proportional hazards regression analysis was performed to estimate breast cancer-specific mortality hazard ratios (HRs). Tests for the assumption of proportional hazards were conducted by visual inspection of Schoenfeld residuals and no violations were found. The last date of follow-up for cases was December 31, 2014. Models were adjusted for age at diagnosis, race/ethnicity, and tumor stage. The ORs, HRs and corresponding 95% confidence intervals (Cis) were generated using SAS v9.4 (SAS Institute, Cary, NC). All statistical analyses were two-sided and P <0.05 was considered statistically significant.
RESULTS
Among 32,770 invasive breast cancer cases diagnosed in New Jersey women from 2008 to 2013, 13.1% (n = 4,315) were of unknown subtype. For nearly all cases with unknown subtype (95%), the test result was borderline or uninterpretable; the test was not performed or it was unknown if the test was performed; or, the result was not documented and, therefore, not reported to NJSCR. Chi-square analyses revealed that women with unknown subtype were older (≥65), minority race/ethnicity (NHB, API, Hispanic), diagnosed as distant or unknown stage and deceased at last follow-up. Additionally, 13%, 20% and 3.6% had ER-, PR-, and HER2- breast cancer, respectively.
The distribution of sociodemographic and tumor characteristics is shown in Table 1. Larger proportions of cases were diagnosed among women age 50–64 years (37.1%), ≥65 years (38.0%), and NHWs (73.0%). Almost one-third (32.8%) of breast cancers were diagnosed in the upper outer quadrant, most were histologically classified as ductal carcinoma (73.1%), diagnosed at localized (61.4%) or regional stage (29.9%), and were moderately (40.7%) or poorly differentiated (34.0%). Luminal A was the most common subtype (63.3%), followed by TNBC (10.2%), luminal B (8.9%) and non-luminal HER2-expressing (4.2%).
Age-adjusted breast cancer incidence rates by subtype and age at diagnosis, race/ethnicity, and tumor stage are shown in Table 2. As expected, incidence rates for the luminal A subtype were highest among women ≥65 years (203.3, 95% CI 198.9–207.8), compared to the other subtypes. Among luminal B breast cancer cases, incidence was lowest among women 20–39 years (4.1, 95% CI 3.7–4.7) and highest among those 50–64 years (21.8, 95% CI 20.6–23.1). A similar pattern was observed for the non-luminal HER2-expressing subtype, with the lowest incidence among women 20–39 years (1.9, 95% CI 1.6–2.3) and highest among those 50–64 years (11.3, 95% CI 10.4–12.2). Among TNBC cases, incidence rates increased with age (20–39 years: 4.8, 95% CI 4.3–5.3; 40–49 years: 17.1, 95% CI 15.9–18.5; 50–64 years: 23.0, 95% CI 21.8–24.3; and ≥65 years: 26.0, 95% CI 24.5–27.7).
Table 2.
Age-adjusted breast cancer incidence rates (per 100,000) in New Jersey, by subtype and by age at diagnosis, race/ethnicity, and tumor stage, 2008–2013.
| Breast cancer subtype | Age group | 20–39 years | 40–49 years | 50–64 years | ≥65 years | ||||
| Count | Incidence Rate (95% CI) | Count | Incidence Rate (95% CI) | Count | Incidence Rate (95% CI) | Count | Incidence Rate (95% CI) | ||
| Luminal Aa | 869 | 13.5 (12.7–14.5) | 3,884 | 92.3 (89.4–95.3) | 7,624 | 139.6 (136.4–142.7) | 8,398 | 203.3 (198.9–207.8) | |
| Luminal Bb | 270 | 4.1 (3.7–4.7) | 676 | 16.2 (15.0–17.5) | 1,179 | 21.8 (20.6–23.1) | 813 | 19.8 (18.5–21.3) | |
| Non-luminal HER2- expressingc | 121 | 1.9 (1.6–2.3) | 287 | 6.8 (6.1–7.7) | 611 | 11.3 (10.4–12.2) | 377 | 9.2 (8.2–10.1) | |
| Triple-negatived | 310 | 4.8 (4.3–5.3) | 711 | 17.1 (15.9–18.5) | 1,250 | 23.0 (21.8–24.3) | 1,075 | 26.0 (24.5–27.7) | |
| New Jersey Population | 6,712,391 | 4,104,990 | 5,408,652 | 4,226,407 | |||||
| Breast cancer subtype | Race/ethnicity | 20–39 years | 40–49 years | 50–64 years | ≥65 years | ||||
| Count | Incidence Rate (95% CI) | Count | Incidence Rate (95% CI) | Count | Incidence Rate (95% CI) | Count | Incidence Rate (95% CI) | ||
| Luminal Aa | 15,873 | 72.2 (71.0–73.3) | 1,996 | 51.7 (49.5–54.1) | 1,049 | 44.3 (41.5–47.1) | 1,724 | 45.3 (43.1–47.5) | |
| Luminal Bb | 2,070 | 10.1 (9.7–10.6) | 342 | 8.7 (7.8–9.7) | 203 | 8.0 (6.9–9.2) | 305 | 7.6 (6.8–8.6) | |
| Non-luminal HER2- expressingc | 913 | 4.4 (4.1–4.7) | 213 | 5.4 (4.7–6.2) | 108 | 4.5 (3.6–5.4) | 154 | 3.8 (3.2–4.5) | |
| Triple-negatived | 2,151 | 10.4 (9.9–10.9) | 693 | 17.8 (16.5–19.2) | 162 | 6.6 (5.6–7.7) | 333 | 8.3 (7.4–9.3) | |
| New Jersey Population | 16,228,596 | 3,745,444 | 2,438,511 | 4,714,148 | |||||
NOTE: Risk estimates and 95% confidence intervals (CI) were generated using logistic regression; adjusted for age, race/ethnicity and stage at diagnosis. Counts by age group and race/ethnicity and subtype do not add up to 32,770 (which is the total number of breast cancer cases included in Table 1) due to missing data on race/ethnicity and/or breast cancer subtype among some cases.
Luminal A (ER+ and/or PR+/HER2-);
Luminal B (ER+ and/or PR+/HER2+);
Non-luminal HER2-expressing (ER-/PR-/HER2+);
Triple negative (ER-/PR-/HER2-)
Breast cancer incidence and mortality risks by subtype and by age at diagnosis and race/ethnicity are shown in Table 3. Among all races/ethnicities, luminal A incidence rates were highest, ranging from 51.7 (95% CI 49.5–54.1) among NHBs to 72.2 (95% CI 71.0–73.3) per 100,000 among NHWs. Incidence rates of TNBC among NHBs were nearly twice as high as those among other racial/ethnic groups, (17.8, 95% CI 16.5–19.2). Incidence rates of the non-luminal HER2-expressing subtype were also higher among NHBs (5.4, 95% CI 4.7–6.2).
Table 3.
Multivariable logistic regression and Cox proportional hazards regression models for breast cancer incidence and mortality risk, among incident breast cancer cases diagnosed in New Jersey, by molecular subtype and by age at diagnosis and race/ethnicity, 2008–2013.
| Luminal Aa | Luminal Bb | Non-luminal HER2- expressingc | Triple-negatived | |
|---|---|---|---|---|
| Incidence | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| Age at diagnosis (years) | ||||
| 20–39 | 0.62 (0.56–0.68) | 1.56 (1.35–1.80) | 1.22 (0.10–1.50) | 1.77 (1.54–2.02) |
| 40–49 | 0.95 (0.89–1.02) | 1.10 (1.00–1.21) | 0.87 (0.76–1.01) | 1.10 (1.00–1.20) |
| 50–64 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| ≥65 | 1.22 (1.16–1.29) | 0.67 (0.61–0.73) | 0.61 (0.54–0.69) | 0.84 (0.77–0.92) |
| Race/ethnicity | ||||
| NHW | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| NHB | 0.59 (0.55–0.63) | 0.93 (0.82–1.05) | 1.31 (1.12–1.53) | 2.15 (1.95–2.36) |
| API | 0.78 (0.71–0.86) | 1.24 (1.06–1.44) | 1.55 (1.27–1.90) | 0.94 (0.80–1.11) |
| Hispanic | 0.76 (0.70–0.82) | 1.05 (0.92–1.19) | 1.20 (1.00–1.44) | 1.19 (1.05–1.35) |
| Mortality | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) |
| Age at diagnosis (years) | ||||
| 20–39 | 0.96 (0.74–1.25) | 0.81 (0.50–1.30) | 1.10 (0.66–1.84) | 0.99 (0.74–1.34) |
| 40–49 | 0.80 (0.68–0.95) | 0.65 (0.43–0.97) | 0.75 (0.49–1.17) | 1.03 (0.82–1.30) |
| 50–64 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| ≥65 | 1.51 (1.33–1.71) | 1.69 (1.28–2.24) | 2.21 (1.62–3.01) | 1.25 (1.03–1.53) |
| Race/ethnicity | ||||
| NHW | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| NHB | 1.64 (1.41–1.91) | 1.54 (1.10–2.15) | 1.34 (0.95–1.91) | 1.28 (1.05–1.56) |
| API | 1.00 (0.76–1.31) | 0.59 (0.28–1.25) | 0.99 (0.56–1.74) | 1.00 (0.69–1.46) |
| Hispanic | 0.94 (0.75–1.17) | 1.06 (0.68–1.64) | 0.81 (0.47–1.39) | 1.14 (0.86–1.52) |
NOTE: Risk estimates and 95% confidence intervals (CI) were generated using logistic regression for incidence and Cox proportional hazards regression for breast cancer specific mortality; adjusted for age, race/ethnicity and stage at diagnosis. Bolded values represent statistical significance.
Luminal A (ER+ and/or PR+/HER2-);
Luminal B (ER+ and/or PR+/HER2+);
Non-luminal HER2-expressing (ER-/PR-/HER2+);
Triple negative (ER-/PR-/HER2-)
NHBs (OR 2.15, 95% CI 1.95–2.36), Hispanics (OR 1.19, 95% CI 1.05–1.35), and younger women (20–39 years: OR 1.77, 95% CI 1.54–2.02 and 40–49 years: OR 1.10, 95% CI 1.00–1.20) had higher odds of TNBC compared to NHWs and women aged 50–64 years, respectively. Younger women also had higher odds of luminal B breast cancer (OR 1.56, 95% CI 1.35–1.80). Women ≥65 years, however, had higher odds of the luminal A subtype (OR 1.22, 95% CI 1.16–1.29) and lower odds of all other subtypes. All minority women had higher odds of developing non-luminal HER2-expressing breast cancer compared to NHW women (NHB: OR 1.31, 95% CI 1.12–1.53; API: OR 1.55, 95% CI 1.27–1.90; Hispanic: OR 1.20, 95% CI 1.00–1.44). APIs had an increased risk of the luminal B subtype (OR 1.24, 95% CI 1.06–1.44) compared to NHWs.
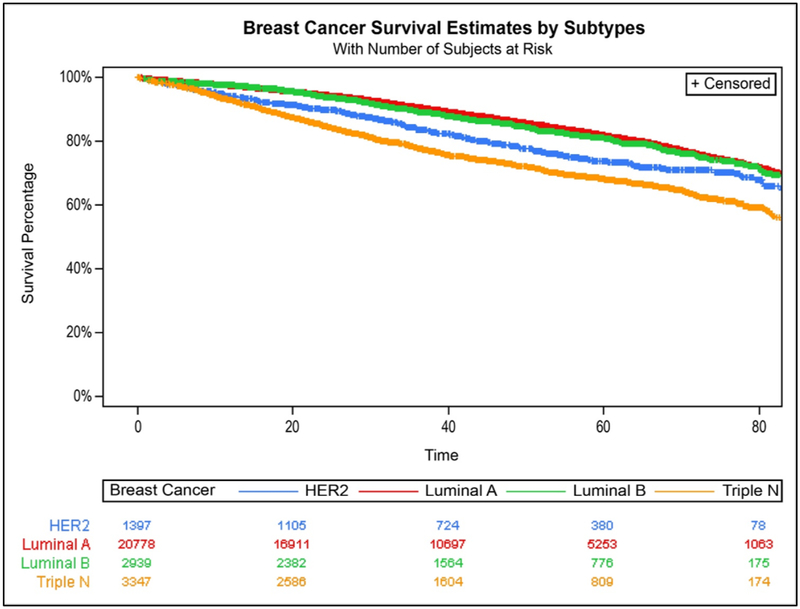
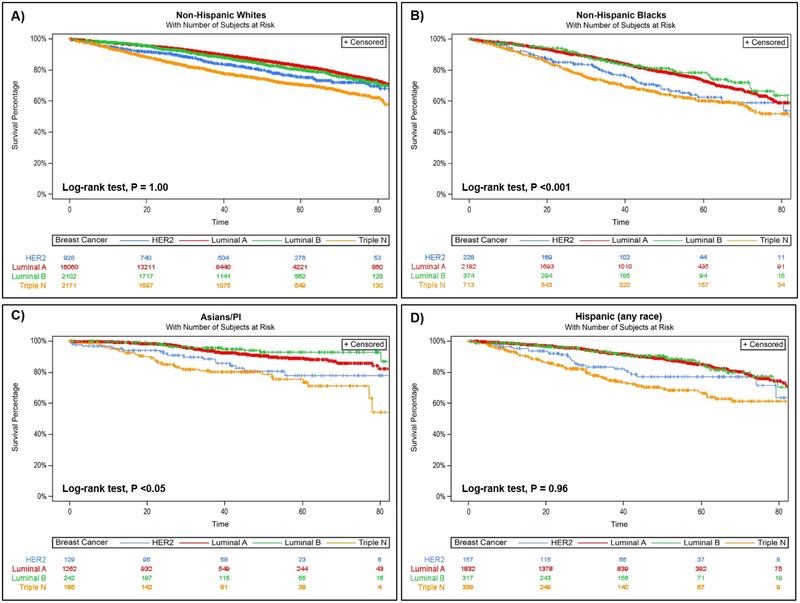
When compared to women 50–64 years, those ≥65 years had higher risk of breast cancer death regardless of subtype, but the risk of death was more than double among those with the nonluminal HER2-expressing subtype (HR 2.21; 95% CI 1.62–3.01). Women 40–49 years had lower risk of breast cancer death when diagnosed with luminal A and luminal B subtypes. NHBs had increased risk of breast cancer death for all subtypes except nonluminal HER2-expressing subtype compared to NHW, with HRs ranging from 1.28 (95% 1.05–1.56) for TNBC to 1.64 (95% 1.41–1.91) for luminal A. As shown in the Kaplan-Meier survival curves (adjusted for age, race/ethnicity, and tumor stage), women diagnosed with TNBCs had the poorest breast cancer-specific survival, followed by those diagnosed with the non-luminal HER2-expressing subtype (P <0.0001; Figure 2). Analysis stratified by race/ethnicity (Figure 3) suggested that this was likely driven by TNBC diagnosed among NHBs (P <0.001) and APIs (P <0.05).
Figure 2.
Breast cancer survival by subtype, New Jersey, 2008–2013
Figure 3.
Breast cancer survival by breast cancer subtype, stratified by race/ethnicity, A) Non-Hispanic Whites; B) Non-Hispanic Blacks; C) Asians/Pacific Islanders; and D) Hispanics (any race), in New Jersey, 2008–2013. Note: models were adjusted for age at diagnosis and stage at diagnosis.
DISCUSSION
In this study, which is the largest population-based sample of breast cancer in New Jersey to date (N = 32,770), we demonstrated the feasibility of retrospectively coding HER2 data not previously recorded in NJSCR files for cases diagnosed in 2008 and 2009. Analyses of these data showed that NHB women had the highest age-adjusted incidence rates of TNBCs (17.8 per 100,000) compared to all other racial/ethnic groups, which ranged from 6.6 (API) to 10.4 (NHW). NHBs also had a 28% higher risk of breast cancer death than their NHW counterparts, which is consistent with the literature (Clarke et al., 2012; DeSantis et al., 2016; Howlader et al., 2014; Noone et al., 2016; Parise et al., 2009). In terms of incidence, young women (20–39 years) had higher risks of TNBC and luminal B breast cancers compared to women 50–64 years, and Hispanic women had higher risks of non-luminal HER2-expressing and TNBC subtypes than NHWs. Our findings also showed TNBCs were associated with the poorest survival.
Incidence of the non-luminal HER2-expressing subtype was highest among NHBs compared to other racial/ethnic groups, while rates were lowest among Hispanics and similar between NHWs and APIs. The latter finding is in contrast to several studies, which have shown the highest rates for this subtype to be among APIs compared to NHWs (Clarke et al., 2012; Howlader et al., 2014; Parise et al., 2009; Sineshaw et al., 2014). We suspect that our findings are suggestive of differences in tumor biology and/or etiologic mechanisms of TNBCs and non-luminal HER2-expressing breast cancers associated with racial/ethnic exposures, which are also related to poorer outcomes, as reported herein and elsewhere (Akinyemiju et al., 2015; Carey et al., 2006; Leone et al., 2015; Li et al., 2017; Llanos et al., 2015; Sorlie et al., 2001; Warner et al., 2015). Additionally, it is quite possible that our finding of similar rates of the non-luminal HER2-expressing subtype among APIs and NHWs could be reflective of differences in subgroups of APIs that reside in New Jersey (as compared to populations in other states), that may be underrepresented in prior breast cancer epidemiology studies. This warrants further analysis. Many studies have focused on the TNBC subtype due to its aggressive nature and limited treatment options, but it should be noted that non-luminal HER2-expressing tumors are also associated with relatively poor survival, have similar penetrance among minority women, and exhibit features that are indicative of a more aggressive phenotype than the luminal A subtype. Poorer survival among TNBC and non-luminal HER2-expressing breast cancer cases may also relate to lack of timely and optimal/guideline-concordant treatment, particularly among racial/ethnic minorities and underserved populations (Bustami et al., 2014; Chen and Li, 2015; Daly and Olopade, 2015; Freedman et al., 2013; George et al., 2015; Hassett et al., 2016; Reeder-Hayes et al., 2014; Sheppard et al., 2015). A recent study suggested differences in response to treatment by race/ethnicity even when subtype was the same (Rauscher et al., 2017), warranting further analysis. Population-based studies with the ability to explore etiologic, risk factor, and prognostic differences by subtype are critically needed to better understand disparities and achieve health equity for breast cancer outcomes.
As we consider the burden of breast cancer in New Jersey, another important finding was that approximately 30% and 6% of breast cancers were diagnosed at regional stage and distant stage, respectively. Given the high risk of mortality associated with later stage diagnosis reported here and elsewhere (Markossian and Hines, 2012; Tian et al., 2012; Tian et al., 2011), it is important to address this issue at the population level. In fact, data suggests a high-degree of spatial variation in late-stage breast cancer incidence in New Jersey (Roche et al., 2016). Future research to evaluate the geographic distribution of molecular subtypes is needed given the observed disparities in breast cancer incidence and mortality in New Jersey.
There were some limitations of this study that should be considered. First, our use of hormone receptor expression by IHC rather than gene expression for classifying breast cancer subtypes was a limitation, although one could argue that gene expression has its limitations as well. Studies have shown good concordance between IHC and gene expression for classifying the major subtypes (Bastien et al., 2012; Carey et al., 2006), supporting utility of IHC, and its use in the SEER program. Another limitation was that >10% of the cases had unknown breast cancer subtype, due to incomplete reporting of hormone receptor data or inconsistencies in reporting, particularly for HER2, as a result of variations in reporting sources (e.g., physician’s private offices vs. larger hospitals/medical facilities). Additionally, we were unable to account for known (and suspected) breast cancer risk factors (e.g. age at menarche, age at menopause, menopausal status, family history of breast cancer, parity, BMI) (Althuis et al., 2004; Ambrosone et al., 2015; Bethea et al., 2016; Hwang et al., 2005; Krieger, 2008; Rosenberg et al., 2016), which could have strengthened our analysis. Nonetheless, the distribution of breast cancer subtypes reported herein were consistent with other studies (Bastien et al., 2012; Howlader et al., 2014; Morrison et al., 2012; Network, 2012; Perou et al., 2000; Sorlie et al., 2001; Sweeney et al., 2014). Bias that may have resulted from missing data is also a concern, particularly for missing or misclassified race/ethnicity. However, this is minimal due to the stringent data quality standards promulgated by the North American Association of Central Cancer Registries (NAACCR). Prior studies have assessed the use of standard registry data and have demonstrated sufficient reliability of race and ethnicity variables (Clegg et al., 2007; Knowlton et al., 2014; Patel et al., 2005). The exclusion of 13% of New Jersey breast cancer cases due to unknown ER/PR/HER2 status is another source of bias. As our analysis revealed, women with unknown subtype were older (≥65), minority race/ethnicity, diagnosed at distant stage or have unknown stage, and deceased at the time of last follow-up. The exclusion of these women would have likely biased our results toward the null, thereby underestimating incidence rates, risks of nonluminal HER2-expression and TNBC subtypes, and risks of breast cancer death. There were also important strengths of this study, including a large, population-based sample of racially and ethnically diverse women with data on ER, PR and HER2 status. In fact, this is the largest dataset currently available with breast cancer subtypes in New Jersey.
Findings reported herein highlight a need for enhanced screening among some subgroups of women to promote earlier diagnosis, and improve breast cancer outcomes. Understanding the mechanisms leading to the development of each breast cancer subtype is essential and will play a major role in improving prognosis and addressing breast cancer outcomes disparities; and, may contribute to improved treatment options that will hopefully reduce the observed breast cancer mortality and survival differences by molecular subtype.
Acknowledgements
We sincerely thank Jie Li, MPH, Gerald Harris, PhD, Annette Werts, Rudmilla Chowdhury, CTR, and Adrian Botchway, CTR at the New Jersey State Cancer Registry for their contributions to this work. This study was supported by the National Cancer Institute (Cancer Center Support Grant Number P30 CA072720) through a New Investigator Award and K01 CA193527 awarded to A.A.M. Llanos. Cancer Epidemiology Services, including the New Jersey State Cancer Registry, receives financial support from the: Surveillance, Epidemiology, and End Results Program of the National Cancer Institute, National Institutes of Health, under contract HHSN 261201300021I and control No. N01-PC-2013–00021; National Program of Cancer Registries, Centers for Disease Control and Prevention, under cooperative agreement 5U58/DP003931; the State of New Jersey and Rutgers Cancer Institute of New Jersey.
Footnotes
Conflict of interest
The authors have no conflicts of interest to declare.
REFERENCES
- ACS (2017). American Cancer Society Breast Cancer Facts & Figures 2017–2018 (Atlanta, GA: American Cancer Society, Inc.). [Google Scholar]
- Akinyemiju T, Moore JX, and Altekruse SF (2015). Breast cancer survival in African-American women by hormone receptor subtypes. Breast cancer research and treatment 153, 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albain KS, Unger JM, Crowley JJ, Coltman CA Jr., and Hershman DL (2009). Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. Journal of the National Cancer Institute 101, 984–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althuis MD, Fergenbaum JH, Garcia-Closas M, Brinton LA, Madigan MP, and Sherman ME (2004). Etiology of hormone receptor-defined breast cancer: a systematic review of the literature. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 13, 1558–1568. [PubMed] [Google Scholar]
- Ambrosone CB, Zirpoli G, Hong CC, Yao S, Troester MA, Bandera EV, Schedin P, Bethea TN, Borges V, Park SY, et al. (2015). Important Role of Menarche in Development of Estrogen Receptor-Negative Breast Cancer in African American Women. Journal of the National Cancer Institute 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastien RR, Rodriguez-Lescure A, Ebbert MT, Prat A, Munarriz B, Rowe L, Miller P, Ruiz-Borrego M, Anderson D, Lyons B, et al. (2012). PAM50 breast cancer subtyping by RT-qPCR and concordance with standard clinical molecular markers. BMC medical genomics 5, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea TN, Rosenberg L, Castro-Webb N, Lunetta KL, Sucheston-Campbell LE, Ruiz-Narvaez EA, Charlot M, Park SY, Bandera EV, Troester MA, et al. (2016). Family History of Cancer in Relation to Breast Cancer Subtypes in African American Women. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 25, 366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava R, Striebel J, Beriwal S, Flickinger JC, Onisko A, Ahrendt G, and Dabbs DJ (2009). Prevalence, morphologic features and proliferation indices of breast carcinoma molecular classes using immunohistochemical surrogate markers. International journal of clinical and experimental pathology 2, 444–455. [PMC free article] [PubMed] [Google Scholar]
- Bustami RT, Shulkin DB, O’Donnell N, and Whitman ED (2014). Variations in time to receiving first surgical treatment for breast cancer as a function of racial/ethnic background: a cohort study. JRSM Open 5, 2042533313515863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Newtork (2012). Comprehensive molecular portraits of human breast tumours. Nature 490, 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, et al. (2006). Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. Jama 295, 2492–2502. [DOI] [PubMed] [Google Scholar]
- CDC (2017). U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2014 Incidence and Mortality Web-based Report (Atlanta: Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute; ). [Google Scholar]
- Chen L, and Li CI (2015). Racial disparities in breast cancer diagnosis and treatment by hormone receptor and HER2 status. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 24, 1666–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebowski RT, Chen Z, Anderson GL, Rohan T, Aragaki A, Lane D, Dolan NC, Paskett ED, McTiernan A, Hubbell FA, et al. (2005). Ethnicity and breast cancer: factors influencing differences in incidence and outcome. Journal of the National Cancer Institute 97, 439–448. [DOI] [PubMed] [Google Scholar]
- Clarke CA, Keegan TH, Yang J, Press DJ, Kurian AW, Patel AH, and Lacey JV Jr. (2012). Age-specific incidence of breast cancer subtypes: understanding the black-white crossover. Journal of the National Cancer Institute 104, 1094–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg LX, Reichman ME, Hankey BF, Miller BA, Lin YD, Johnson NJ, Schwartz SM, Bernstein L, Chen VW, Goodman MT, et al. (2007). Quality of race, Hispanic ethnicity, and immigrant status in population-based cancer registry data: implications for health disparity studies. Cancer Causes Control 18, 177–187. [DOI] [PubMed] [Google Scholar]
- Daly B, and Olopade OI (2015). A perfect storm: How tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin 65, 221–238. [DOI] [PubMed] [Google Scholar]
- DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, and Jemal A (2016). Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA Cancer J Clin 66, 31–42. [DOI] [PubMed] [Google Scholar]
- Dignam JJ (2001). Efficacy of systemic adjuvant therapy for breast cancer in African-American and Caucasian women. Journal of the National Cancer Institute Monographs, 36–43. [DOI] [PubMed] [Google Scholar]
- Freedman RA, He Y, Winer EP, and Keating NL (2013). Racial/Ethnic differences in receipt of timely adjuvant therapy for older women with breast cancer: are delays influenced by the hospitals where patients obtain surgical care? Health Serv Res 48, 1669–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George P, Chandwani S, Gabel M, Ambrosone CB, Rhoads G, Bandera EV, and Demissie K (2015). Diagnosis and surgical delays in African American and white women with early-stage breast cancer. J Womens Health (Larchmt) 24, 209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassett MJ, Schymura MJ, Chen K, Boscoe FP, Gesten FC, and Schrag D (2016). Variation in breast cancer care quality in New York and California based on race/ethnicity and Medicaid enrollment. Cancer 122, 420–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlader N, Altekruse SF, Li CI, Chen VW, Clarke CA, Ries LA, and Cronin KA (2014). US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. Journal of the National Cancer Institute 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang ES, Chew T, Shiboski S, Farren G, Benz CC, and Wrensch M (2005). Risk factors for estrogen receptor-positive breast cancer. Archives of surgery 140, 58–62. [DOI] [PubMed] [Google Scholar]
- Knowlton R, Gershman S, Solis A, and Das B (2014). An assessment of the reliability of race, Hispanic ethnicity, birthplace, and tobacco history data in the Massachusetts cancer registry, 2005–2009. J Registry Manag 41, 5. [PubMed] [Google Scholar]
- Krieger N (2008). Hormone therapy and the rise and perhaps fall of US breast cancer incidence rates: critical reflections. International journal of epidemiology 37, 627–637. [DOI] [PubMed] [Google Scholar]
- Kroenke CH, Sweeney C, Kwan ML, Quesenberry CP, Weltzien EK, Habel LA, Castillo A, Bernard PS, Factor RE, Kushi LH, et al. (2014). Race and breast cancer survival by intrinsic subtype based on PAM50 gene expression. Breast cancer research and treatment 144, 689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone JP, Leone J, Zwenger AO, Iturbe J, Vallejo CT, and Leone BA (2015). Prognostic significance of tumor subtypes in male breast cancer: a population-based study. Breast cancer research and treatment 152, 601–609. [DOI] [PubMed] [Google Scholar]
- Li X, Yang J, Peng L, Sahin AA, Huo L, Ward KC, O’Regan R, Torres MA, and Meisel JL (2017). Triple-negative breast cancer has worse overall survival and cause-specific survival than non-triple-negative breast cancer. Breast cancer research and treatment 161, 279–287. [DOI] [PubMed] [Google Scholar]
- Llanos AA, Chandwani S, Bandera EV, Hirshfield KM, Lin Y, Ambrosone CB, and Demissie K (2015). Associations between sociodemographic and clinicopathological factors and breast cancer subtypes in a population-based study. Cancer Causes Control 26, 1737–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markossian TW, and Hines RB (2012). Disparities in late stage diagnosis, treatment, and breast cancer-related death by race, age, and rural residence among women in Georgia. Women Health 52, 317–335. [DOI] [PubMed] [Google Scholar]
- McCaig C, Perks CM, and Holly JM (2002). Intrinsic actions of IGFBP-3 and IGFBP-5 on Hs578T breast cancer epithelial cells: inhibition or accentuation of attachment and survival is dependent upon the presence of fibronectin. Journal of cell science 115, 4293–4303. [DOI] [PubMed] [Google Scholar]
- Morrison DH, Rahardja D, King E, Peng Y, and Sarode VR (2012). Tumour biomarker expression relative to age and molecular subtypes of invasive breast cancer. British journal of cancer 107, 382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCI (2017a). Surveillance Research Program, National Cancer Institute. Surveillance, Epidemiology, and End Results Program. SEER*Prep Software version 2.5.3.
- NCI (2017b). Surveillance Research Program, National Cancer Institute. Surveillance, Epidemiology, and End Results Program. SEER*Stat Software version 8.3.2.
- NJSCR (2017). New Jersey State Cancer Registry Annual Report Cancer Incidence and Mortality in New Jersey, 2010–2014. (Trenton, New Jersey: Cancer Epidemiology Services, New Jersey Department of Health; ). [Google Scholar]
- Noone AM, Cronin KA, Altekruse SF, Howlader N, Lewis DR, Petkov VI, and Penberthy L (2016). Cancer incidence and survival trends by subtype using data from the Surveillance Epidemiology and End Results Program, 1992–2013. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parise CA, Bauer KR, Brown MM, and Caggiano V (2009). Breast cancer subtypes as defined by the estrogen receptor (ER), progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) among women with invasive breast cancer in California, 1999–2004. Breast J 15, 593–602. [DOI] [PubMed] [Google Scholar]
- Patel DA, Knowles A, Schwartz AG, and Schwartz K (2005). Evaluation of African-American and white racial classification in a surveillance, epidemiology, and end results cancer registry. Ethn Dis 15, 713–719. [PubMed] [Google Scholar]
- Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, et al. (2000). Molecular portraits of human breast tumours. Nature 406, 747–752. [DOI] [PubMed] [Google Scholar]
- Rauscher GH, Silva A, Pauls H, Frasor J, Bonini MG, and Hoskins K (2017). Racial disparity in survival from estrogen and progesterone receptor-positive breast cancer: implications for reducing breast cancer mortality disparities. Breast cancer research and treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeder-Hayes KE, Meyer AM, Dusetzina SB, Liu H, and Wheeler SB (2014). Racial disparities in initiation of adjuvant endocrine therapy of early breast cancer. Breast cancer research and treatment 145, 743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche LM, Niu X, Stroup AM, and Henry KA (2016). Disparities in Female Breast Cancer Stage at Diagnosis in New Jersey: A Spatial-Temporal Analysis. J Public Health Manag Pract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg L, Bethea TN, Viscidi E, Hong CC, Troester MA, Bandera EV, Haiman CA, Kolonel LN, Olshan AF, Ambrosone CB, et al. (2016). Postmenopausal Female Hormone Use and Estrogen Receptor-Positive and -Negative Breast Cancer in African American Women. Journal of the National Cancer Institute 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard VB, Oppong BA, Hampton R, Snead F, Horton S, Hirpa F, Brathwaite EJ, Makambi K, Onyewu S, Boisvert M, et al. (2015). Disparities in breast cancer surgery delay: the lingering effect of race. Ann Surg Oncol 22, 2902–2911. [DOI] [PubMed] [Google Scholar]
- Sineshaw HM, Gaudet M, Ward EM, Flanders WD, Desantis C, Lin CC, and Jemal A (2014). Association of race/ethnicity, socioeconomic status, and breast cancer subtypes in the National Cancer Data Base (2010–2011). Breast cancer research and treatment 145, 753–763. [DOI] [PubMed] [Google Scholar]
- Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, et al. (2001). Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proceedings of the National Academy of Sciences of the United States of America 98, 10869–10874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney C, Bernard PS, Factor RE, Kwan ML, Habel LA, Quesenberry CP Jr., Shakespear K, Weltzien EK, Stijleman IJ, Davis CA, et al. (2014). Intrinsic subtypes from PAM50 gene expression assay in a population-based breast cancer cohort: differences by age, race, and tumor characteristics. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 23, 714–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamimi RM, Baer HJ, Marotti J, Galan M, Galaburda L, Fu Y, Deitz AC, Connolly JL, Schnitt SJ, Colditz GA, et al. (2008). Comparison of molecular phenotypes of ductal carcinoma in situ and invasive breast cancer. Breast cancer research: BCR 10, R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton M, e. Standards for Cancer Registries Volume II: Data Standards and Data Dictionary, Record Layout Version 12.2 (Springfield, IL: North American Association of Central Cancer Registries; ). [Google Scholar]
- Tian N, Goovaerts P, Zhan FB, Chow TE, and Wilson JG (2012). Identifying risk factors for disparities in breast cancer mortality among African-American and Hispanic women. Womens Health Issues 22, e267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian N, Wilson JG, and Zhan FB (2011). Spatial association of racial/ethnic disparities between late-stage diagnosis and mortality for female breast cancer: where to intervene? Int J Health Geogr 10, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari RC, Clegg LX, and Zou Z (2006). Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res 15, 547–569. [DOI] [PubMed] [Google Scholar]
- Warner ET, Tamimi RM, Hughes ME, Ottesen RA, Wong YN, Edge SB, Theriault RL, Blayney DW, Niland JC, Winer EP, et al. (2015). Racial and Ethnic Differences in Breast Cancer Survival: Mediating Effect of Tumor Characteristics and Sociodemographic and Treatment Factors. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 33, 2254–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XR, Chang-Claude J, Goode EL, Couch FJ, Nevanlinna H, Milne RL, Gaudet M, Schmidt MK, Broeks A, Cox A, et al. (2011). Associations of breast cancer risk factors with tumor subtypes: a pooled analysis from the Breast Cancer Association Consortium studies. Journal of the National Cancer Institute 103, 250–263. [DOI] [PMC free article] [PubMed] [Google Scholar]