Abstract
Objectives:
DMT-DALDA (H-Dmt-D-Arg-Phe-Lys-NH2; Dmt=2’,6’-dimethyltyrosine) is a selective mu opioid agonist. We sought to characterize efficacy, tolerance, dependence and side effect profile when given by continuous intrathecal infusion.
Methods:
Adult male Sprague Dawley rats were prepared with chronic intrathecal catheters and osmotic mini-pumps to deliver vehicle (saline), DMT-DALDA or morphine. Hind paw thermal escape latencies were assessed. In addition, effects upon intraplantar formalin-evoked flinching and withdrawal after 14 days of infusion were examined. The flare response after intradermal delivery was examined in the canine model.
Results:
i) Intrathecal infusion of 0.3–30 pmol/μl/h of DMT-DALDA or 37.5 nmol/μL/h of morphine over 7 or 14 days resulted in a dose-dependent increase in thermal escape latency. The maximum antinociceptive effect was observed between 1 and 4 days after start of infusion with preserved cornea, blink, placing and stepping. By days 12–14, response latencies were below baseline. ii) On days 2–4 of DMT DALDA infusion, the pan opioid receptor antagonist naloxone, but not the delta-preferring antagonist naltrindole, antagonized the analgesic effects. iii) Assessment of formalin flinching on day 1 following IT DMT-DALDA Infusion showed significant analgesia in phases 1 and 2. On day 6 of infusion there was minimal effect, while on day 13, there was an increase in flinching). iv) On days 7 and 14 of infusion naloxone resulted in prominent withdrawal signs indicating dependence and withdrawal. v) Intradermal morphine and DMT DALDA both yield a naltrexone-insensitive, cromolyn-sensitive flare in the canine model at similar concentrations.
Conclusions:
These data suggest that DMT-DALDA is a potent, spinally active agonist with a propensity to produce tolerance dependence and mast cell degranulation. While it was equiactive to morphine in producing mast cell degranulation, it was >1000 fold more potent in producing analgesia, suggesting a possible lower risk in producing a spinal mass at equianalgesic doses.
Keywords: Intrathecal infusion, DMT1-DALDA, mu opioid receptor, tolerance, dependence, withdrawal, formalin flinching
INTRODUCTION
The intrathecal delivery of mu opioid agonists can produce a potent and selective analgesia in animal models and human patients. This spinal effect reflects the presynaptic location of mu opioid receptors on small nociceptive primary afferents, which by blocking voltage gated calcium channels prevents transmitter release from those afferents [1–3]. In companion work we systematically examined the antinociceptive effects of the bolus delivery of a family of peptides built on the endogenous mu opioid peptide: dermorphin. In this allied work we observed that one of the analogues, DMT-DALDA (H-Dmt-D-Arg-Phe-Lys-NH2), which displays a high affinity at the mu receptor (Kiμ = 0.143 65 nM) and high receptor selectivity for mu vs. delta and kappa opioid receptors (Kiδ/Kiμ> 10,000 and Kiκ/Kiμ> 100) [4], and an extraordinary potency in blocking a spinal reflex (the tail-flick) [5], hind paw withdrawal to a thermal stimulus and a reduction in formalin-evoked flinching [6] after bolus IT administration.
Of interest, several of these peptides carry a net positive charge at physiological pH and are thus very hydrophilic, which suggests a long residency time in the intrathecal space, a property supporting an extended duration of action after intrathecal delivery. Indeed, following bolus delivery of equiactive doses, durations of action comparable to morphine were observed [6, 7]. To further characterize, the spinal actions of DMT-DALDA and, for comparison, morphine, we undertook the following studies: i) systematic time and concentration-effect curves for chronically infused intrathecal DMT-DALDA/morphine; ii) receptor pharmacology of spinally infused DMT-DALDA/morphine using naloxone (a pan-opiate antagonist) and naltrindole (a delta receptor preferring antagonist) and iii) examining the ability of DMT DALDa and morphine to produce mast cell degranulation, a mechanism proposed to mediate the opiate receptor independent generation of an intrathecal mass using the naltrexone insensitive, cutaneous flare observed after intradermal delivery [8].
MATERIALS AND METHODS
Animal care was in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication 85–23, Bethesda, MD) and as approved by the institutional Animal Care and Use Committee of the University of California, San Diego, CA.
Rat Studies.
Animals.
Male, Sprague-Dawley rats (225–300 g; Harlan Indianapolis, IN) were individually housed in standard cages and maintained on a 12-hour light/dark cycle (lights on at 7 AM). Testing occurred during the light cycle. Food and water were available ad libitum.
Surgical Preparation.
The rats were implanted with intrathecal catheters connected to subcutaneous osmotic mini-pumps (Alzet®) for continuous drug delivery, as described previously [9]. In the current work, rats were anesthetized by induction with 4% isoflurane in a room air/oxygen mixture (1:1), the anesthesia was maintained with 2% isoflurane delivery by mask, and the animal placed in a head holder. The cisternal membrane was exposed through a midline incision and a catheter passed into the intrathecal space to the level of the L2–L3 spinal segments (8.5 cm). The catheter was externalized on the top of the head. Rats were given carprofen (5 mg/kg, in lactated Ringer’s solution) subcutaneously and allowed to recover under a heat lamp. Preloaded mini-pumps were inserted subcutaneously on the lateral neck and connected to the spinal catheters preloaded with saline. Catheters were constructed from two pieces of medical-grade polyethylene tubing fitted with an additional length of polyethylene to connect to the pumps. The implanted portion (0.20 mm ID x 0.36 mm OD; Scientific Commodities, Inc., USA, #BB31695-polyethylene/08) was heat-fused to the externalized portion (0.28 mm ID x 0.64 mm OD; Scientific Commodities, Inc., #BB31695-polyethylene/1). For connection to pumps, the externalized portion of the catheter was then heat-fused to an additional length of polyethylene tubing (0.76 mm ID x 1.22 mm OD; Scientific Commodities, Inc., #BB31695-polyethylene/4). Catheters were packaged and sterilized by ethylene oxide before use.
Drugs and Chemicals.
DMT-DALDA (MW=981) was synthesized as previously described [4, 10]: Morphine2SO4 (MW=759) was obtained from Merck Pharmaceuticals (Rahway, NJ). Naloxone hydrochloride and naltrindole were obtained from Sigma Chemical (St. Louis, MO). Isoflurane was purchased from VETone (Meridian, ID).
Behavioral Testing
On day 0, animals prepared with catheters were randomly selected for study. Before initiation of drug delivery, baseline behavioral and testing data were taken. At selected times after infusion of the test or control article, these data were again collected. All assessments were made with observer blinded as to the drug being delivered. Animals were euthanized upon completion of the final test period.
General Functional Evaluation.
These studies were not targeted at defining the side effect profile of the intrathecally delivered drugs except in so far as it would impact upon the ability to display the appropriate behavioral response in nociceptive assessment. Accordingly, end points included: i) absence of spontaneous activity in the test environment (otherwise evoked by a hand clap) [11], ii) changes in motor function as evidenced by presence of a Straub (stiff) tail, and iii) presence of severe hind limb dysfunction as defined by loss of hind paw placing and stepping reflex, loss of weight bearing and/or failure of symmetrical ambulatory patterns.
Acute Thermal Escape Thresholds.
A Hargreaves type hind paw thermal stimulator system was employed [12], constructed in the engineering lab in the Department of Anesthesiology at the University of California, San Diego (now constructed by Torrey Pines Instruments, San Diego, CA). This system allowed the direction of a focused light beam on the plantar surface of the paw, through a glass plate upon which the rat stood. Surface temperature was maintained at 30°C. Rats were placed in individual Plexiglas chambers on the thermal escape surface and allowed to acclimate for 30 min before testing. A brisk withdrawal of the paw after the initiation of the thermal stimulus was taken as the response. Lack of a response within 20 sec was cause to terminate the test and assign the score of “20 sec”. Latency measurements were taken for the right and left hind paws and averaged. Measurements were then made at various time points after drug administration. Each time point signifies the time at which the hind paws were tested. Unless otherwise stated, animals were assessed for thermal escape latencies at baseline and again periodically after initiation of infusion.
Formalin-Induced Flinching.
Animals were allowed to acclimate in individual Plexiglas chambers for 30 minutes before experimental manipulation and a soft metal band was placed around the hind paw being injected. Flinching was evoked by a unilateral subcutaneous injection of formalin (2.5%, 50 μl) into the dorsal paw. Flinching of the injected paw was quantified by a device which detected the flinching movement of the paw with the metal band (constructed in the engineering lab in the Department of Anesthesiology at the University of California, San Diego; now constructed by Torrey Pines Instruments, San Diego, CA) [13].
Flinches were counted in 1-minute intervals for 60 minutes and analyzed as cumulative flinch count during phase 1 (0–10 min) and phase 2 (11–60 min).
Assessment of Withdrawal Profile.
To quantify the magnitude of the withdrawal syndrome, each animal was scored for the presence or absence of 6 withdrawal signs during the 15-min interval after the injection of the antagonist: diarrhea (passage of boli); agitation (increased spontaneous activity in cage); wet dog shakes/shivering/piloerection; salivation (wetness around muzzle); vocalization (spontaneous squeaking); chromodachyorrhea (porphyrin around orbits). For analysis each observation was scored as 1 for a maximum score of 6 [14].
Rat Study Paradigm.
Dose response curve: Groups of animals were prepared with intrathecal catheters and pumps (Alzet® 2001) preloaded to deliver saline (Vehicle); or DMT-DALDA 0.3, 1, 3 or 10 pmol/μL/hour. Each animal was periodically examined over the next 7 days for their thermal escape latency.
Formalin evoked flinching study: Groups of animals were prepared with intrathecal catheters and pumps (Alzet 2002) preloaded to deliver saline (Vehicle); or DMT-DALDA (10 pmol/μL/hour). Flinching was then examined, respectively, after 1, 6 or 13 days of infusion.
Antagonists and withdrawal syndrome study: To quantify the magnitude of dependence and withdrawal syndrome, groups of rats were infused for 14 days with intrathecal infusion of DMT-DALDA (10 pmol/hour), MS (37.5 nmol/hour), or saline. On day 2, baseline thermal escape and withdrawal measures were assessed, and half of the animals were randomly assigned to receive either naloxone (Nx) (1 mg/kg), intraperitoneal (IP) or naltrindole (Ntd) (3 mg/kg, IP). On day 3, baseline measures were again taken and the animals receiving Nx on day 2 received Ntd and those receiving Ntd received Nx. On days 12 and 14 this sequence was repeated. Thermal escape latency and the presence or absence of Straub tail 15 min after injection of Nx, and Ntd were compared between each group using t-test on early phase (days 2,4) and late phase (days 12,14).
Canine Mast Cell Studies
These studies were carried out as previously described in detail [8].
Animals:
Male beagle dogs (N = 3; Ridglan Farms Inc., Mt. Horeb, WI, or equivalent), 12–16 months of age and weighing approximately 9–16 kg, were housed in runs with wood shavings and given ad libitum access to food and water. In brief, for the study of cutaneous flare, animals were anesthetized with propofol (5 μg kg− 1 min− 1), intubated, maintained under spontaneous ventilation with 1.0–2.0% isoflurane and 60% N20/40% O2 (approximate values) and continuously monitored for end-tidal gases and saturation. Body temperature was maintained with an underbody heating pad.
Drug delivery protocol.
For drug study, two rows of 9 or 10 injections each (50 μL) were made lateral to the midline at time 0. Resulting skin flares were measured at 30 min post-injection without knowledge of injectate. Flare areas (A) were calculated in square millimeters as an oval (A = 3.14 a ∗ b, where a = half-length of long axis and b = half-length of short axis).
Drug delivery sequences
Drugs studied were compound 48–80 (a mast cell degranulating polymer; MW = 167 Da, estimated), morphine sulfate (379 Da) and DMT-DALDA (MW = 981 Da). Each study drug was examined at three concentrations randomly assigned to be delivered along with vehicle injections (saline, 0.9%) and the positive control (compound 48/80). Sodium chloride (0.9%) was the vehicle for all drugs. In separate experiments, the effects of the MC stabilizer cromolyn (10 mg/kg, IM) were examined to assess the role of MC degranulation in the observed flare. To determine the role of the opioid receptor in the observed flare effects, animals were pretreated with the opioid antagonist naltrexone (1 mg/kg, IM) given 30 min prior to intradermal injection of the highest concentration of each test article employed.
Statistical Analysis
Data were compiled in Excel (v.14.4.9, Microsoft Corporation, USA), and statistical analyses were performed using Prism (v.6.0, GraphPad Software, Inc., USA). Parametric data (escape latencies, area under the curve (AUC), flare areas) were presented as means ± SD. Nonparametric data (Indices of withdrawal) were presented as medians and quartiles. Thermal escape latencies were normalized by converting them to percentage of the maximum possible effect (Post–Pre)/20 sec – Pre) × 100 and the area under the percentage max possible effect curve calculated over a defined time interval in days (AUC). For cutaneous flare, log dose effect curves were prepared plotting flare area vs. drug concentration, and best fit regression lines calculated to determine slope and the concentrations required to produce a fixed flare size (50 mm2) with 95% confidence intervals.
Calculation of group sizes based on rats receiving intrathecal saline using AUC (0–4 hours: 448 ± 246, n = 10) or peak thermal escape latency at 30 min (10.6 ± 1.1 sec, n = 10) yielded estimated group sizes of 4. For formalin groups, sizes were estimated based on defining a 30% reduction in the AUC in chronically implanted rats receiving intrathecal saline assuming a ß = 0.8 and p = 0.05. For formalin flinching Phase 2, mean ± SD (n = 12) = 1458 ± 220 flinches indicates a group size of 3–4. (https://www.stat.ubc.ca/~rollin/stats/ssize/n2.html)
RESULTS
Concentration dependency of IT DMT-DALDA infusion (1.0 μl/hr): thermal escape
The intrathecal infusion of 0.3, 1, 3, and 10 pmol/μL/hr of DMT-DALDA (Fig. 1A, B) over 7 days resulted in a concentration-dependent increase in thermal escape latency with the maximum effect observed between 1–4 days after initiation of infusion. Over the 7-day period there was an evident reduction in the antinociceptive action of the IT DMT-DALDA. In this concentration range, no motor dysfunction was observed, e.g. preserved cornea, blink and placing/stepping reflexes normal and symmetrical ambulation). The most sensitive measure of a motor effect was the appearance of Straub tail which was observed at doses of 1 pmol/hr. As indicated, this phenomenon disappeared after 4 days (Fig. 1C). At 100 pmol/μL/hr or more, a strong, reversible, truncal rigidity which precluded behavioral testing was observed that lasted approximately 2 days (Fig. 1D).
Figure 1.
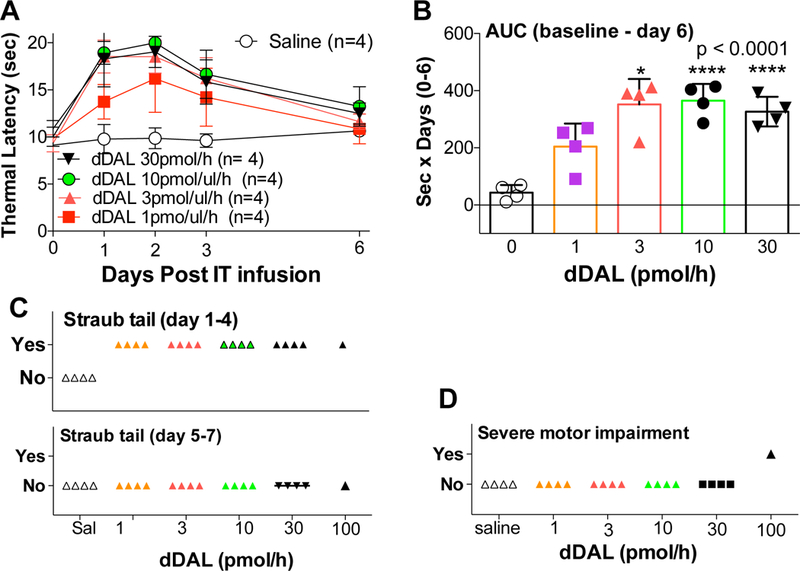
(A): Dose response curve of thermal latencies (mean ± SD: seconds) vs. time post IT infusion of DMT-DALDA (dDAL) or saline vehicle. (B) Scatter graph of area under the time course of the thermal escape curve (AUC: 0–6 d) of IT doses of DMT-DALDA and saline vehicle. One-way ANOVA analysis was performed on AUC, with a Dunnett’s post hoc comparison test comparing drug effects to vehicle showed * p<0.05, **** p<0.0001. (C) Scatter graph of the observance of incidence of animals displaying Straub tail during the days 1–4 or 5–7 period. (D) Scatter graph of the incidence of animals displaying severe motor dysfunction at any time during the time course.
Continuous IT infusion of DMT-DALDA (0.5 μL/hr): thermal escape.
To permit longer periods of infusion we repeated the DMT-DALDA studies performed with 1 μL/hr above with an infusion rate of 0.5 μL/hr. at a concentration of 20 pmoL/μL (e.g. equivalent to 10 pmol/hr), with the 7-day AUC for the 10 pmoL/hr dose given at 1 μL/hr and 0.5 μL/ hr dosing being: 365 ± 22 and 359 ± 43 sec x days, respectively, with transient expression of Straub tail. As shown in Figure 2A comparable results were obtained, with peak effects observed at 3 days and return to baseline displayed recovery to baseline by day 7 and below baseline by days 12–14 (Fig. 2D).
Figure 2:
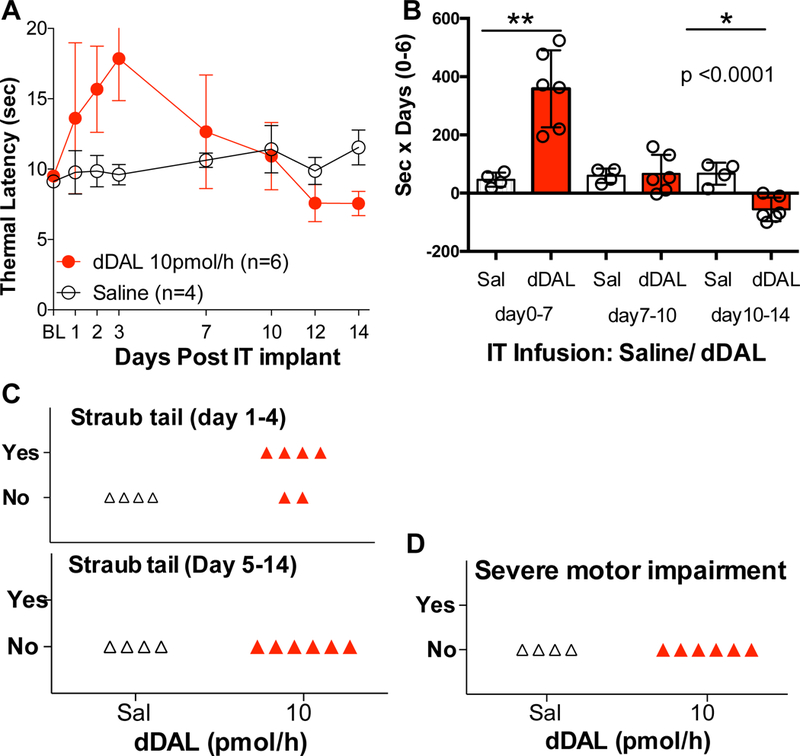
(A) Thermal latencies (Mean± SD: seconds) vs. time post-initiation of IT infusion of DMT-DALDA (dDAL: 10 pmol/hour (0.5 μl/hour of 20 pmol/μl, 14 days) or saline (Sal) vehicle.(B) Scatter graph of area under the curve (AUC: Mean ± SD) of 3 phases (early: days 0–7, mid: days 7–10, late: days 10–14). The AUCs were compared using One-Way ANOVA, with post hoc comparisons made between AUC of drug vs. saline using Dunnett’s test.) *: p <0.05; **: p<0.01 (C) Scatter graph of the incidence of animals displaying Straub tail during the days 1–4 or 5–7 period. (D) Scatter graph of the incidence of animals showing severe motor dysfunction at any time during the time course.
Continuous IT infusion of DMT-DALDA (0.5μl/hr): Formalin flinching
Following the initiation of IT infusion of DMT DALDA (20 pmol/μL = 10 pmol/hr) there was a significant reduction in Phase 1 and Phase 2 flinching as compared to IT saline infused controls (Fig. 3A,B,C). By day 7, the effects of the IT DMT-DALDA were lost as compared to vehicle and by day 13, Phase 2 flinching was significantly greater than that observed in the vehicle group. Of note, while Phase 1 and Phase 2 flinching in the vehicle treated animals across the three test intervals was comparable (indicating that the three repeated test epochs did not in and of itself lead to a change in phases 1 and 2 flinching), the phase 2 flinching showed a progressive increase in the DMT-DALDA infused animals.
Figure 3:
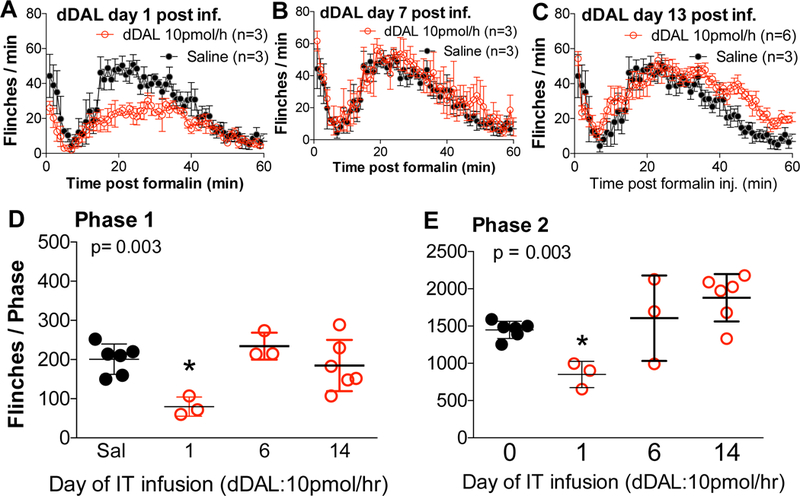
(A, B, C) Minute by minute plot of formalin induced flinching (Flinch counts: mean ± SEM for clarity) in groups after infusion for 1 day or 7 days, and 13 days of intrathecal DMTDALDA (dDAL: 10 pmol/hour). Saline infusion group was for 1 day only. (D, E) Total flinch count of phase 1 (0–10 min) and phase 2 (11–60 min) on day 1, day 6, and day 13 (Mean ± SD). Total Flinch counts for phase 1 and phase 2 were analyzed with One-Way ANOVA and Dunnett’s test and compared to saline (*p<0.05).
Continuous IT infusion of DMT-DALDA and morphine (0.5 μL/hr): Thermal escape/Withdrawal.
Rats were randomly assigned to receive IT infusions of 0.5 μL/hr of saline DMT-DALDA (20 pmol/μL = 10 pmol/hr) or an equi-analgesic concentration of morphine (75 nmol/μL = 37.5 nmol/hr). As indicated (Fig. 4A,C), over 14 days, saline infused animals displayed a stable baseline thermal escape. In contrast, IT infusion of DMT-DALDA or morphine resulted in a similar maximum effect maximum by day 2 (days 1–7), a decline to escape latencies observed with IT infusion of saline (days 7–10) and then below vehicle control (days 10–14).
Figure 4:
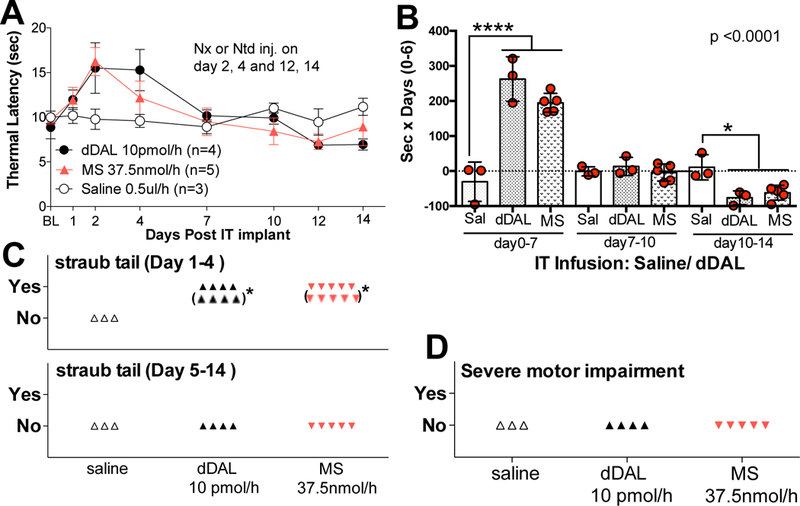
(A) Time effect graph of IT DMT-DALDA (dDAL) infusion 10 pmol/hour (0.5 μl/hour of 20 pmol/μl, 14 days), morphine sulfate (MS) 37.5 nmol/hour (0.5 μl/hour of 75 nmol/μl, 14 days) or saline (Sal) vehicle. Bolus IP injections were made at day 2 or 4 and day 12 or 14 of naloxone or on the alternate days, naltrindole. (Results of effects of antagonists on thermal escape and withdrawal indices are presented in Figure 5). (B) Scatter graph of area under the curve (AUC) of each 3 phases of DMT-DALDA, morphine sulfate and saline vehicle (early: days 0–7, mid: days 7–10, late: days 10–14), excluding in each case the 6 hr window after IP antagonist treatments. These AUCs were compared using One-Way ANOVA followed by designated comparisons between d-DALDA or morphine vs. vehicle during three time windows after initiation of intrathecal infusion, using a t-test with Bonferroni adjustments to prevent alpha build up (*p<0.05 and ***p<0.001). (C) Scatter graph of the observance of Straub tail during the days 1–4 or 5–14 period (* behavior after naloxone and naltrindole administration). (D) Scatter graph of the observance of severe motor dysfunction at any time during the time course.
Antagonist reversal of IT infusion mediated antinociception and motor effect
To define the antagonist pharmacology of IT infusions of morphine and MT-DALDA, rats receiving continuous infusions of these opioids or vehicle received in a counterbalanced order on days 2 and 4 naloxone (1 mg/kg, IP) or naltrindole (1 mg/kg, IP) and this protocol was repeated on days 12 and 14. As indicated (Figure 5A) neither antagonist had any effect upon baseline latencies after IT infusion of vehicle during early and late intervals. In contrast, during the early phase, naloxone resulted in a reduction in the analgesic effect of DMT-DALDA and morphine. Naltrindole had no effect on the anti-nociception associated with DMT-DALDA and unexpectedly had a significant effect upon the effects produced by morphine. As noted previously Straub tail was observed after IT DMT-DALDA or morphine during the first 4 days and which subsequently resolved by 4 days. In this series, the Straub tail present in the days 2–4 interval continued to be observed during the 30 min interval after naloxone and naltrindole delivery.
Figure 5:
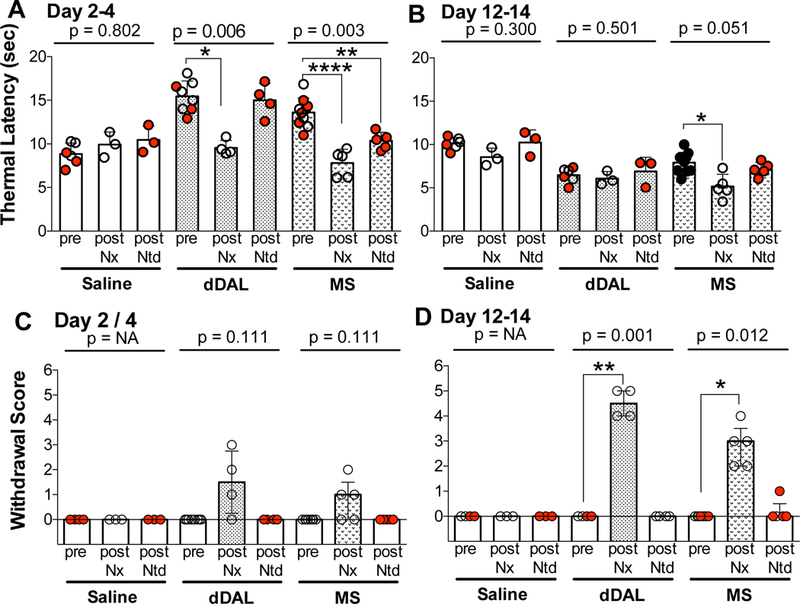
In rats receiving intrathecal infusion of saline, DMT DALDA (dDAL) or morphine (MS), thermal latencies are indicated before, and 15 min after intraperitoneal injection of naloxone or naltrindole during early phase (days 2,4) and late phase (days 12,14) of infusion. One-Way repeated measures ANOVA was performed on each treatment set (saline, dDAL and MS) during early and late phases. Note that for each treatment the Pre-bar scatter plot shows the Pre-escape latencies for each antagonist (open circle for naloxone and closed red for naltrindole). This pairing was used for the repeated measures analysis). Post hoc analysis of each treatment set for comparison of postantagonist treatment during the early (days 2 and 4) and late (days 12 and 14) phases was performed using a Dunnett’s test. *p<0.05, **p<0.01, ****p<0.0001. (C, D) Withdrawal symptom score on early phase (days 2,4) and late phase (days 12,14) after intraperitoneal injection of naloxone and naltrindole scored by 6 signs (diarrhea, agitation, shivering, salivation, vocalization, chromodachyorrhea) was analyzed using the Friedman non-parametric test with designated post hoc comparisons accomplished with a Dunn’s test (*p<0.05; ** p<0.01).
Antagonist evoked withdrawal after IT infusion of DMT-DALDA and morphine (0.5 μl/h)
The delivery of naloxone but not naltrindole resulted in a moderate incidence of withdrawal signs at the 2–4-day time point of infusion, and robust incidence of withdrawal at the 12–14-day interval with a greater withdrawal observed after DMT-DALDA (Fig. 5C,D).
Flare after intradermal injection.
The intradermal injection of compound 48–80 (a mast cell degranulating polymer), morphine sulfate and DMT-DALDA resulted in a flare, the area of which was concentration-dependent. Regression analysis of log data indicated all slopes were statistically significant (p < 0.0001) and not different from each other (p > 0.05). Concentrations of the three drugs producing a fixed flare effect (50 mm2) were determined and found to have overlapping 95% confidence intervals, e.g. were not different (Table 1). Pretreatment with the mast cell stabilizer cromolyn (10 mg/kg) prevented the observed flare formation after all drugs, while naltrexone (1 mg/kg IM) had no effect upon the flare produced by 48–80, morphine or DMT-DALDA) (Fig. 6B). These results suggest that the effects of morphine and DMT-DALDA were mediated by mast cell degranulation and that neither agent acted through an opioid receptor.
Table 1:
Comparison of analgesic and flare producing activity of morphine and DMT-DALDA
| Antinociceptive dose a (Bolus delivery ) |
Antinociceptive Concentration b (Chronic infusion) |
Flare producing Concentration c (Intradermal) |
|
|---|---|---|---|
| 48-80 | - | - | 0.7 mM (0.3-1.6) |
| Morphine | 30,000 pmol/10μL | 37,500 nmol/hr | 1.5 mM (0.4-6.0) |
| DMT-DALDA | 10pmol/10μL | 3 pmol/hr | 2.3 mM (1.2-5.8) |
| Dose ratio (Morphine/ DMT-DALDA) |
3000 | 12,500 | 0.6 |
Data from [6]. Minimum dose in rats producing maximum effect upon thermal escape. Bolus delivery; dose delivered in 10 μL
Data from this paper: Rat 14-day infusion; 0.5 μL/hr.
Data from this paper representing concentration of drug resulting in a standard flare area of 50 mm2
Figure 6:
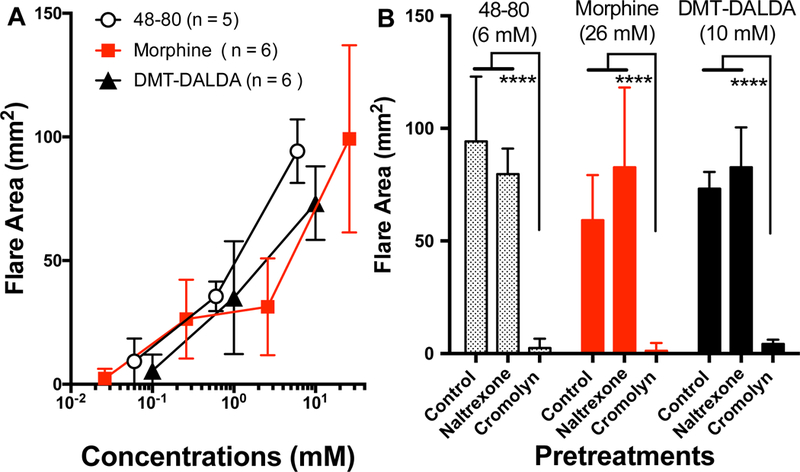
(A ) Concentration dependent increases in flare area after intradermal injection of several concentrations of 48–80, morphine or DMT-DALDA in anesthetized dogs. Regression analysis of log data indicated all slopes were statistically significant ( (p < 0.0001) and not different from each other (p>0.05). Concentrations of the three drugs producing a fixed flare effect were determined and found to have overlapping 95% confidence intervals, e.g. were not different. (see Table 1) (B) Figure shows the flare size observed after a fixed dose of 48–80,morphine or DMT-DALDA was prevented by pretreatment with cromolyn (10 mg/kg, IM), but not naltrexone (1 mg/kg, IM) (2 way ANOVA, Dunnetts post hoc ****; p<0.0001. )
DISCUSSION
Analgesic actions of DMT-DALDA
DMT-DALDA is a mu opioid agonist [4, 10, 15], which, when given intrathecally as a bolus, was observed to be active in the pmol vs. the nmol range, as required by other mu opioid alkaloids [7, 16]. This relative potency was clearly preserved using a chronic infusion paradigm (see Table 1).
Pharmacology of intrathecal DMT-DALDA.
In these studies, naloxone, but not naltrindole, reversed the analgesia associated with DMT-DALDA. The doses employed were based on previous work showing the selectivity of their effect against several intrathecal bolus mu and delta agonists in the rat [3, 17, 18].
Tolerance.
As previously reported [19, 20], intrathecal DMT-DALDA resulted in tolerance and dependency. Interestingly, a 7-day exposure led to a loss of any analgesic activity as measured by thermal escape and by inhibition of formalin evoked flinching. However, at intervals greater than 7–14 days, the thermal thresholds and the formalin induced flinching displayed an enhanced pain behavior, paralleling the phenomena referred to as opiate-induced hyperalgesia. The development of enhanced pain processing secondary to chronic opiate exposure may account in part for the loss of analgesic action of opiates [21]. Importantly, interventions blocking aspects of hyperalgesic processing, as the NMDA [22] or TLR4 receptor [23] have been reported to attenuate the right shift in the dose effect curve otherwise observed with continuous intrathecal opiate infusion.
Motor effects.
At the analgesic doses, the only evident motor effect of DMT-DALDA was Straub tail. This phenomenon, said to be a model of transient spasticity [24], reflects increased motor tone in the sacrococcygeus dorsalis muscle. It is considered based on systemic drug delivery and the action of pan antagonists such as naloxone to be mediated by opiate receptor activation of lumbosacral outflow [25, 26]. Failure to prevent this effect of systemic opioids with spinal transection indicates a spinal action. Electrophysiogical studies have long demonstrated that opiates can result in a block of Renshaw cell function [27], a classic glycine mediated inhibitor of motor neuron excitability. Such inhibition would lead to a bilateral increase in motor outflow leading to the apparent rigidity of the tail. Intrathecal delivery of DMT-DALDA and Morphine resulted in a Straub tail phenotype, as previously reported in mice and rats [28–30]. This action was characterized by two properties: first, that it disappeared after 4 days with both morphine and DMT-DALDA, and second, that it was present after systemic delivery of naloxone at doses which completely reversed a drug induced increase in thermal escape. Previous work has shown tolerance of Straub tail following repeated dosing with systemic morphine [31], and as noted, a reversal by naloxone. Of note, the one comparable study of which we are aware with intrathecal morphine and Straub tail showed that the dose of systemic naloxone required to block the Straub tail was greater than that required to produce parallel rightward shifts in the IT morphine tail flick inhibition curves in the mouse [28]. As only a single dose of naloxone (or naltrindole) were employed here, it is possible that as in the cited study, higher doses of naloxone might have shown efficacy.
Mast cell degranulation
Several opiate analgesics (morphine, hydromorphone) but not others (fentanyl, alfentanil) delivered by intrathecal infusion can result in fibroblast-rich masses embedded in a collagen matrix arising from the meninges [32–34]. These masses are not prevented by opiate receptor antagonism [33]. The common feature of the agents resulting in such masses is their propensity to degranulate mast cells in an opiate receptor independent fashion [8]. In the present studies, we demonstrate that DMT-DALDA and morphine at comparable molar concentrations both produce a robust mast cell activation in the cromolyn sensitive intradermal flare model. As shown in the Table 1 summary, equianalgesic effects of DMT-DALDA were noted at > three orders of magnitude lower concentrations. This raises the possibility that an agent such as DMT-DALDA might display a lower risk of intrathecal mass formation. While systematic histopathology was not performed in these pharmacological studies, we have previously observed that a 14-day infusion of analgesic concentrations of morphine will yield such masses and an attendant motor dysfunction in guinea pigs [35] (not seen in the present studies). In this regard, morphine interacts with Mas-related G protein-coupled receptors (MRGs), and mast cell degranulation is mediated in part by activation of this signaling cascade and that fentanyl, which does not activate MRGs [36], does not degranulate mast cells [8] and has a low risk of producing spinal masses in animals and humans [37, 38].
CONCLUSION:
These data suggest that DMT-DALDA is a potent, spinally active mu agonist with a propensity to produce tolerance, dependence and mast cell degranulation. However, while it was equiactive to morphine in producing mast cell degranulation, it was >10,000 fold more potent in producing analgesia, suggesting a possible lower risk for producing a spinal mass at equianalgesic doses.
Acknowledgments
Source of financial support: This work was supported by NIH grant DA015353 (TY). These results were reported in part in an abstract presented at the annual meeting of the Society for Neuroscience, Washington, 2017.
(Supported by NIH DA015353).
Footnotes
Conflict of Interest statement: Peter Schiller has a patent related to this molecule; no other authors have any conflicts of interest to disclose.
REFERENCES
- 1.Yaksh TL, Jessell TM, Gamse R, Mudge AW, and Leeman SE. Intrathecal morphine inhibits substance P release from mammalian spinal cord in vivo. Nature 1980; 286: 155–7. [DOI] [PubMed] [Google Scholar]
- 2.Aimone LD and Yaksh TL. Opioid modulation of capsaicin-evoked release of substance P from rat spinal cord in vivo. Peptides 1989; 10: 1127–31. [DOI] [PubMed] [Google Scholar]
- 3.Kondo I, Marvizon JC, Song B, Salgado F, Codeluppi S, Hua XY, et al. Inhibition by spinal mu- and delta-opioid agonists of afferent-evoked substance P release. J Neurosci 2005; 25: 3651–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schiller PW, Nguyen TM, Berezowska I, Dupuis S, Weltrowska G, Chung NN, et al. Synthesis and in vitro opioid activity profiles of DALDA analogues. Eur J Med Chem 2000; 35: 895–901. [DOI] [PubMed] [Google Scholar]
- 5.Novoa A, Van Dorpe S, Wynendaele E, Spetea M, Bracke N, Stalmans S, et al. Variation of the net charge, lipophilicity, and side chain flexibility in Dmt(1)-DALDA: Effect on Opioid Activity and Biodistribution. J Med Chem 2012; 55: 9549–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kokubu S, Eddinger KA, Nguyen TM, Huerta-Esquivel LL, Yamaguchi S, Schiller PW, et al. “Characterization of the antinociceptive effects of intrathecal DALDA peptides following bolus intrathecal delivery”. Scand J Pain 2018. [DOI] [PubMed] [Google Scholar]
- 7.Shimoyama M, Shimoyama N, Zhao GM, Schiller PW, and Szeto HH. Antinociceptive and respiratory effects of intrathecal H-Tyr-D-Arg-Phe-Lys-NH2 (DALDA) and [Dmt1] DALDA. J Pharmacol Exp Ther 2001; 297: 364–71. [PubMed] [Google Scholar]
- 8.Schmidt-Rondon E, Wang Z, Malkmus SA, Di Nardo A, Hildebrand K, Page L, et al. Effects of opioid and nonopioid analgesics on canine wheal formation and cultured human mast cell degranulation. Toxicol Appl Pharmacol 2018; 338: 54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yaksh TL and Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav 1976; 17: 1031–6. [DOI] [PubMed] [Google Scholar]
- 10.Schiller PW, Nguyen TMD, Chung NN, and Lemieux C. Dermorphin Analogs Carrying an Increased Positive Net Charge in Their Message Domain Display Extremely High Mu-Opioid Receptor Selectivity. J Med Chem 1989; 32: 698–703. [DOI] [PubMed] [Google Scholar]
- 11.Malmberg AB and Yaksh TL. Voltage-sensitive calcium channels in spinal nociceptive processing: blockade of N- and P-type channels inhibits formalin-induced nociception. J Neurosci 1994; 14: 4882–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dirig DM, Salami A, Rathbun ML, Ozaki GT, and Yaksh TL. Characterization of variables defining hindpaw withdrawal latency evoked by radiant thermal stimuli. J Neurosci Methods 1997; 76: 183–91. [DOI] [PubMed] [Google Scholar]
- 13.Yaksh TL, Ozaki G, McCumber D, Rathbun M, Svensson C, Malkmus S, et al. An automated flinch detecting system for use in the formalin nociceptive bioassay. J Appl Physiol 2001; 90: 2386–2402. [DOI] [PubMed] [Google Scholar]
- 14.Gu G, Kondo I, Hua XY, and Yaksh TL. Resting and evoked spinal substance P release during chronic intrathecal morphine infusion: parallels with tolerance and dependence. J Pharmacol Exp Ther 2005; 314: 1362–9. [DOI] [PubMed] [Google Scholar]
- 15.Neilan CL, Nguyen TM, Schiller PW, and Pasternak GW. Pharmacological characterization of the dermorphin analog [Dmt(1)]DALDA, a highly potent and selective mu-opioid peptide. Eur J Pharmacol 2001; 419: 15–23. [DOI] [PubMed] [Google Scholar]
- 16.Riba P, Ben Y, Nguyen TM, Furst S, Schiller PW, and Lee NM. [Dmt(1)]DALDA is highly selective and potent at mu opioid receptors, but is not cross-tolerant with systemic morphine. Curr Med Chem 2002; 9: 31–9. [DOI] [PubMed] [Google Scholar]
- 17.Svensson CI, Rew Y, Malkmus S, Schiller PW, Taulane JP, Goodman M, et al. Systemic and spinal analgesic activity of a delta-opioid-selective lanthionine enkephalin analog. J Pharmacol Exp Ther 2003; 304: 827–32. [DOI] [PubMed] [Google Scholar]
- 18.Kouchek M, Takasusuki T, Terashima T, Yaksh TL, and Xu Q. Effects of intrathecal SNC80, a delta receptor ligand, on nociceptive threshold and dorsal horn substance p release. J Pharmacol Exp Ther 2013; 347: 258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao GM, Wu D, Soong Y, Shimoyama M, Berezowska I, Schiller PW, et al. Profound spinal tolerance after repeated exposure to a highly selective mu-opioid peptide agonist: role of delta-opioid receptors. J Pharmacol Exp Ther 2002; 302: 188–96. [DOI] [PubMed] [Google Scholar]
- 20.Ben Y, Smith AP, Schiller PW, and Lee NM. Tolerance develops in spinal cord, but not in brain with chronic [Dmt(1)]DALDA treatment. Brit J Pharmacol 2004; 143: 987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roeckel LA, Le Coz GM, Gaveriaux-Ruff C, and Simonin F. Opioid-induced hyperalgesia: Cellular and molecular mechanisms. Neuroscience 2016; 338: 160–182. [DOI] [PubMed] [Google Scholar]
- 22.Dunbar SA and Yaksh TL. Spinal infusion of N-methyl-D-aspartate antagonist MK801 induces hypersensitivity to the spinal alpha-2 agonist ST91 in the rat. J Pharmacol Exp Ther 1997; 281: 1219–25. [PubMed] [Google Scholar]
- 23.Grace PM, Maier SF, and Watkins LR. Opioid-induced central immune signaling: implications for opioid analgesia. Headache 2015; 55: 475–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belozertseva IV, Dravolina OA, Tur MA, Semina MG, Zvartau EE, and Bespalov AY. Morphine-induced Straub tail reaction in mice treated with serotonergic compounds. Eur J Pharmacol 2016; 791: 1–7. [DOI] [PubMed] [Google Scholar]
- 25.Bilbey DL, Salem H, and Grossman MH. The anatomical basis of the straub phenomenon. Br J Pharmacol Chemother 1960; 15: 540–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aceto MD, McKean DB, and Pearl J. Effects of opiates and opiate antagonists on the Straub tail reaction in mice. Br J Pharmacol 1969; 36: 225–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Felpel LP, Sinclair JG, and Yim GK. Effects of morphine on Renshaw cell activity. Neuropharmacology 1970; 9: 203–10. [DOI] [PubMed] [Google Scholar]
- 28.Hylden JL and Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol 1980; 67: 313–6. [DOI] [PubMed] [Google Scholar]
- 29.Hasegawa Y, Kurachi M, and Otomo S. Dopamine D2 receptors and spinal cord excitation in mice. Eur J Pharmacol 1990; 184: 207–12. [DOI] [PubMed] [Google Scholar]
- 30.Kakinohana M, Marsala M, Carter C, Davison K, and Yaksh TL. Neuraxial morphine may trigger transient motor dysfunction after a noninjurious interval of spinal cord ischemia - A clinical and experimental study. Anesthesiology 2003; 98: 862–870. [DOI] [PubMed] [Google Scholar]
- 31.Zarrindast MR, Ghadimi M, Ramezani-Tehrani B, and Sahebgharani M. Effect of GABA receptor agonists or antagonists on morphine-induced Straub tail in mice. Int J Neurosci 2006; 116: 963–73. [DOI] [PubMed] [Google Scholar]
- 32.Yaksh TL, Horais KA, Tozier NA, Allen JW, Rathbun M, Rossi SS, et al. Chronically infused intrathecal morphine in dogs. Anesthesiology 2003; 99: 174–87. [DOI] [PubMed] [Google Scholar]
- 33.Yaksh TL, Allen JW, Veesart SL, Horais KA, Malkmus SA, Scadeng M, et al. Role of meningeal mast cells in intrathecal morphine-evoked granuloma formation. Anesthesiology 2013; 118: 664–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yaksh TL, Steinauer JJ, Veesart SL, and Malkmus SA. Alfentanil: correlations between absence of effect upon subcutaneous mast cells and absence of granuloma formation after intrathecal infusion in the dog. Neuromodulation 2013; 16: 459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eddinger KA, Rondon ES, Shubayev VI, Grafe MR, Scadeng M, Hildebrand KR, et al. Intrathecal Catheterization and Drug Delivery in Guinea Pigs: A Small-animal Model for Morphine-evoked Granuloma Formation. Anesthesiology 2016; 125: 378–94. [DOI] [PubMed] [Google Scholar]
- 36.Lansu K, Karpiak J, Liu J, Huang XP, McCorvy JD, Kroeze WK, et al. In silico design of novel probes for the atypical opioid receptor MRGPRX2. Nat Chem Biol 2017; 13: 529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allen JW, Horais KA, Tozier NA, and Yaksh TL. Opiate pharmacology of intrathecal granulomas. Anesthesiology 2006; 105: 590–8. [DOI] [PubMed] [Google Scholar]
- 38.Deer TR, Pope JE, Hayek SM, Lamer TJ, Veizi IE, Erdek M, et al. The Polyanalgesic Consensus Conference (PACC): Recommendations for Intrathecal Drug Delivery: Guidance for Improving Safety and Mitigating Risks. Neuromodulation 2017; 20: 155–176. [DOI] [PubMed] [Google Scholar]


