Abstract
Background
Mild Cognitive Impairment (MCI) is an intermediate state between normal cognition and dementia that is associated with twice the risk of falls. It is unknown whether white matter integrity (WMI) is associated with increased risk of falls in MCI. The purpose of this study was to evaluate if early changes in WMI were associated with gait impairment and falls.
Methods
Forty-three participants with MCI from the Gait and Brain Study underwent standardized assessment of cognition, gait performance under single and dual-task conditions (walking while talking), and WMI using 3 Tesla diffusion tensor imaging (DTI). Macro-structural imaging characteristics (white and grey matter morphology) as well as microstructural WMI parameters were examined for associations with falls and gait performance. Significantly associated WM tracts were then used to test the interplay between WMI and history of falls, after adjusting for other important covariates.
Results
Multiple WM tracts (corpus callosum, forceps minor, and the left inferior fronto-occipital fasciculus) were significantly associated with history of falls and lower dual-task gait performance. A multivariable regression model showed that fall history was associated with the radial diffusivity in the forceps minor, even after adjusting for education, sex, BMI, MMSE scores, comorbidities, gait velocity and WMH volume as covariates.
Conclusions
Multiple WM tracts that are known to be involved in executive and visuospatial functions were preferentially affected in MCI individuals with history of falls. Our preliminary findings support the notion that WMI in key brain regions may increase risk of falls in older adults with MCI.
Keywords: Diffusion tensor imaging, Falls, Gait, Executive function, White matter, Microstructure, MCI
Abbreviations: MCI, Mild Cognitive Impairment; DTI, Diffusion Tensor Imaging; WMI, White Matter Integrity; BMI, Body Mass Index; MMSE, Mini Mental State Exam
Highlights
-
•
43 older adults with MCI were assessed for falls, gait, and WMI using 3 T DTI.
-
•
Multiple WM tracts were associated with falls and lower dual-task gait performance.
-
•
Tracts affected are known to be involved in executive and visuospatial functions.
1. Introduction
Older adults with Mild Cognitive Impairment (MCI), a transitional stage between normal aging and dementia,(Ritchie, 2004) are not only at higher risk of progression to dementia but also at higher risk of falls.(Tinetti et al., 1997; Tinetti et al., 1988) Individuals with MCI have twice the fall risk when compared with age matched cognitively normal older adults.(Tinetti et al., 1988) Falls in this population have been associated with serious adverse events including injuries, disability, nursing home placement and even mortality(Tinetti et al., 1997). Thus, a better understanding of the neuro-mechanism contribution to the high risk of falls seen in MCI will eventually help to detect modifiable risk factors.
The interplay between falls, cognition, and gait is complex (Montero-Odasso et al., n.d.) and not coincidentally related, but rather it may reflect damage in shared brain regions and networks that are affected by aging, neurodegeneration, and microvascular mechanisms.(Montero-Odasso et al., n.d.; Montero-Odasso and Hachinski, 2014; Montero-Odasso et al., 2012a) Indeed, brain macro-structural changes, including grey matter volume loss(Whitwell et al., 2007) and white matter hyperintensities (WMH) burden, are common in individuals with MCI.(Smith et al., 2008; Annweiler et al., 2012; Annweiler et al., 2013) WMH burden has been shown to be associated with poor balance, slowing gait speed(Starr et al., 2003), and falls history in community-dwelling older adults.(Zheng et al., 2012; Koo et al., 2012) However, these associations have not been thoroughly explored in individuals with MCI Moreover, it remains unclear whether white matter integrity (WMI) in specific brain regions is related to a greater risk of falls or slow gait seen in older adults with MCI.
Diffusion Tensor Imaging (DTI) is an MRI technique that can predict the formation of WMH de Groot et al., 2013 and has been increasingly used to investigate WMI with cognitive changes and aging. DTI provides microstructural information about WM health by measuring several diffusion parameters of water(Bennett et al., 2010) in nerve fibers such as fractional anisotropy (FA) which is sensitive to fiber structural integrity, myelination degree, fiber diameter, density, and tract coherence.(Le Bihan et al., 2001; Mori and Zhang, 2006) Mean diffusivity (MD) can also be derived from DTI, a measure of isotropic average diffusion (in all directions of the fiber), which indicates density.(Beaulieu, 2002) In addition, diffusion in the primary direction, axial diffusivity (AD) and average diffusion in the perpendicular direction, radial diffusivity (RD) can also be used for interpretation of myelin and axon pathologies. Generally, reduced FA implies reduced structural integrity by damage to axons, myelin or loss of coherence, increased MD implies loss of axons or myelination, low AD indicates damage to axons, and high RD denotes myelin damage.(Song et al., 2005; Sun et al., 2006)
An analytic processing tool to investigate DTI parameters called tract-based spatial statistics (TBSS) involves aligning data into a template, keeping only white matter tract information to be collected and saved onto DTI parametric maps. The TBSS approach improves sensitivity, objectivity and interpretability of multi-subject DTI analysis by using carefully tuned nonlinear registration of patient data followed by the projection of the WM DTI parameters onto an alignment-invariant “skeleton” tract.(Smith et al., 2006) WM tracts provide the infrastructure for inter- and intra-brain network communications,(Fields, 2008) some of which may be involved synergistically in natural gait and cognitive function.(Scherder et al., 2011; Snijders et al., 2007) Therefore, specific WM fiber tract-based regions of interest (ROI) may be used to examine the skeletonized data of specific regions.
The goal of this study was to investigate the cross-sectional association between WM microstructure changes, history of falls, and gait characteristics in an MCI population. We hypothesize that examination of microstructural white matter abnormalities in older adults with MCI may reveal early changes in brain regions associated with falls and slow gait.
2. Materials and methods
2.1. Participants
This analysis includes baseline data from 43 participants with MCI who completed a 3 T MRI exam including DTI in the Gait and Brain Study, a prospective cohort study designed to determine whether quantitative gait impairments can predict cognitive and mobility decline, and progression to dementia and falls among community-dwelling older adults. Study design and logistics have been described in detail elsewhere.(Montero-Odasso et al., 2009a; Montero-Odasso et al., 2014; Robertson et al., 2014) and more details can be found at clinicaltrials.gov (NCT: 03020381).
MCI was diagnosed as follows: i) presence of subjective memory complaints from the patient and family, ii) objective memory impairment in cognitive tests (below 1.5 standard deviation under the expected performance in cognitive tests for age and education), iii) preserved activities of daily living(Lawton and Brody, 1969), and iv) absence of clinical dementia (established using DSM-IV-TR criteria). Participants were required to be able to walk independently for 10 m without any aid, have no Parkinsonism or other residual motor deficits, have no major depression, to be free from the use of neuroleptics and benzodiazepines, and have no musculoskeletal or joint disorder affecting gait performance detected by clinical and physical exam by a skilled clinician.
Participants underwent a comprehensive assessment that included medical questionnaires, cognitive tests, and quantitative gait assessments. Sociodemographic characteristics, chronic medications, history of falls, and comorbidities were collected using standardized questionnaires in face-to-face interviews. Cognition was assessed using the Mini-Mental State Examination (MMSE) (Mori and Zhang, 2006) and the Montreal Cognitive Assessment (MoCA) (Beaulieu, 2002).
In order to investigate whether WM changes were associated with a higher number of falls, participants were stratified to two groups based on history of falls; those with ≥1 fall in the past 12 months were categorized as “fallers” while those with no falls were categorized as “non-fallers”.
2.2. Quantitative gait measurements and falls ascertainment
Gait performance under simple and dual-tasks was assessed using an electronic walkway (GAITRite System, 600 cm long, CIR Systems, Inc. NJ, USA) which provides data for both spatial and temporal gait parameters. Start and end points were marked on the floor 1 m from either walkway end to avoid recording acceleration and deceleration phases. Each participant performed one practice trial walking on the mat. The single-task trial consisted of walking the length of the mat at the participant's usual pace. For the dual-task trials, participants walked at their usual pace with no instruction to prioritize the gait or cognitive task; while doing the following cognitive tasks aloud – one at a time, (i) counting backwards from one hundred by ones (ii) subtracting serial sevens from one hundred, and (iii) naming animals out loud. The rationale for the selection of these dual-task conditions has been described elsewhere.(Montero-Odasso et al., n.d.) Allowing both gait and cognitive tasks to vary provides a better representation of daily living activities. To balance and minimize the effects of learning and fatigue, the order of the simple and dual-tasks was randomized. Reliability for this gait assessment protocol has been previously established in MCI populations.(Montero-Odasso et al., 2009b) Gait speed was expressed in meters per second (m/s).
A fall was defined as an event in which in a person comes to a rest unintentionally on a lower level (e.g. ground). The number of falls was reported as the total number of fall incidents within and no later than a year prior to the imaging schedule. This methodology to record falls has been previously validated (Montero-Odasso et al., 2014).
2.3. Imaging acquisition
MRI was performed on a 3 T Siemens Magnetom Prisma Fit scanner (Siemens, Germany) equipped with an 8-channel phased array head coil at the Centre for Functional and Metabolic Mapping, Robarts Research Institute, London, ON, Canada. Volumetric T1-weighted structural images were acquired using a 3D MPRAGE sequence with matrix = 240 × 256 × 160, TE = 2.9 ms TR = 2.3 s, field of view = 240 mm × 256 mm × 192 mm. DTI was performed with a single-shot echo-planar imaging (EPI) sequence with matrix = 128 × 128, TE = 87 ms, TR = 7.7 s, field of view = 256 mm × 256 mm, 58 slices, slice thickness = 2 mm, gap = 0. Diffusion-sensitizing gradients were applied along 64 noncolinear directions (b value = 1000 s/mm2), together with one b = 0 image. All scans were visually inspected to exclude motion artifacts.
2.4. DTI data processing and interpretation
All DTI processing was performed using FSL 5.0.8 (FMRIB Software Library, http://www.fmrib.ox.ac.uk/fsl) including brain extraction (BET, part of FSL) from DTI (b = 0) and T1-weighted images, correcting for susceptibility, eddy currents, subject head movement (via affine registration; FDT, part of FSL) and gradient directions (applied during diffusion weighted volumes acquisition) as well as fitting diffusion tensor models at each voxel. Then, tract-based spatial statistics (TBSS), a part of FSL was run as previously described (Smith et al., 2006). TBSS involves aligning data into 1 mm × 1 mm Montreal Neurological Institute (MNI) 152 Space and registering each patient's brain into the FMRIB58 template using the FMRIB's Nonlinear Image Registration Tool (FNIRT, part of FSL). Then a mean image of FA values (including pixels with values above 0.2 to eliminate voxels in the grey matter or cerebrospinal fluid from the analysis) was created and thinned to create a mean FA “skeleton” which represents the centers of all tracts within the subject group. Finally, each subject's aligned data was projected onto this skeleton; MD, AD and RD were processed in the same way. In addition, WM fiber tracts' DTI parameters averages were calculated for each tract ROI per patient to allow for further examination if necessary. For masking of WM fiber tract ROIs, we used the Johns-Hopkins University (JHU) DTI-based white-matter probability maps in FSL toolbox(Douaud et al., 2007) with a threshold of 10% (probability of voxels being white matter). These tracts are visualized in Fig. 1 (with either left or right hemispheric tracts shown only for ease of visualization purposes).
Fig. 1.
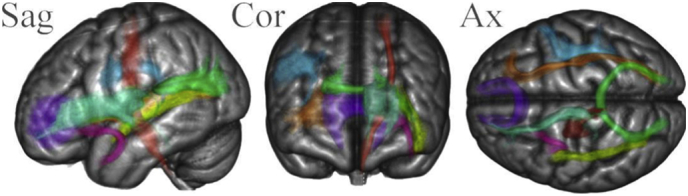
WM fiber tract ROIs used for region specific analysis. Fiber tract probability maps were thresholded at 10% and converted to masks to be used for region-specific analysis of TBSS produced collapsed diffusion parameters. The ROIs are shown in the sagittal (Sag), coronal (Cor) and axial (Ax) direction superimposed on a 152 MNI 1 mm brain template 3D surface render. The tracts are: Superior Longitudinal Fasciculus - Blue, Inferior longitudinal Fasciculus - Yellow, IFOF - Inferior Fronto-Occipital Fasciculus - Brown, Corpus Callosum – not shown, Forceps Minor - Purple, Forceps Major - Green, CS Corticospinal tract - Red, Anterior Thalamic Radiation - Teal, Uncinate Fasciculus – Pink.
2.5. White matter hyperintensities
Image analysis for WMH was performed for each subject with our in-house fully automated pipeline utilizing FSL tools (See Supplemental Figs. 1). In short, after correcting for bias-field, (1) each subject's T1weighted structural images were co-registered to their own T2-FLAIR images; (2) T2-FLAIR and T1-weighted images were spatially normalized to the MNI standard space; (3) Both T1-weighted and T2-FLAIR images were segmented into grey matter (GM), WM and Cerebral Spinal Fluid (CSF); (4) slice-by-slice thresholding and clustering tools were used to separate and delineate hyperintensities. It was necessary to investigate whether WMHs have a potential influence on the DTI measures of the WM tracts. A group probability map of WMHs was generated by summing the binarized WMHs image of each MCI subject in the MNI space and multiplied by the probability map of WM (giving lower weighing factors to any voxels found in regions that were not deemed WM).
2.6. Gray matter volumetric analysis
Structural data were analyzed with FSL-volume based morphometry (VBM; the tool can be found at the FSL website: http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLVBM (Douaud et al., 2007)), an optimized VBM protocol (Good et al., 2001) in the FSL tools (Smith et al., 2004). First, structural images were brain-extracted (Smith, 2002) and grey matter-segmented before being registered to the MNI 152 standard space using linear (Jenkinson and Smith, 2001) and non-linear (Andersson et al., 2007) registration. The resulting images were averaged and flipped along the x-axis to create a left-right symmetric, study-specific grey matter template. Second, all native grey matter images were non-linearly registered to this study-specific template and “modulated” to correct for local expansion (or contraction) due to the non-linear component of the spatial transformation. The modulated grey matter images were then smoothed with an isotropic Gaussian kernel with a sigma of 4 mm. Finally, voxel-wise non-parametric permutation tests were used while correcting for multiple comparisons across space, as has been validated previously (Good et al., 2001).
2.7. Statistical analysis
In order to test for group differences between fallers and non-fallers in demographic, clinical, quantitative gait and cognition data, a one-way analysis of variance (ANOVA) was performed for continuous variables, and Fisher's exact test for binomial data.
Because it is believed that WMH and gait velocity (single- and dual-task) could be used to predict falls, these parameters were tested for differences between fallers and non-fallers with analyses of covariance (ANCOVA) that may reduce bias as a result of covariates, including age, sex, BMI, years of education, and MMSE scores.
Voxel-wise non-parametric permutation tests were used to compare DTI values with gait variables and number of falls. The analysis of the different DTI parameters was performed using the FSL Randomize permutation-based statistical analysis. For this analysis, 5000 permutations(Winkler et al., 2014) corrected for multiple comparisons were tested using the threshold-free cluster enhancement (TFCE) method with family wise-error rate controlled-Corrected P statistic which was further reduce to P ˂ 0.0125 (0.05 divided by 4 for the number of DTI parameters examined) considered significant. The latter was performed with age, sex, BMI, years of education and MMSE scores as covariates of no interest.
Finally, participant-specific DTI average of the WM tract that showed significant differences between fallers and non-fallers was entered in a series of multivariate negative binomial regression models as an independent variable, while including the following covariates: age, sex, BMI, years of education, MMSE scores, number of comorbidities, and gait variables, to determine the association between these measures and previous number of falls.
All non-voxel-wise imaging statistical analyses were performed using SPSS (Chicago, IL, USA). The threshold for statistical significance was P ˂ 0.05 unless described differently.
3. Results
3.1. Demographic, cognitive, and mobility characteristics
Forty-three participants with MCI (74.5 ± 5.9 years of age; 18 (42%) women) were included in these analyses. Table 1 presents the sample characteristics stratified by history of falls. No significant differences in age, number of years of education, gender, BMI, and MMSE or MoCA scores were found across groups. In addition, there were no significant differences in the frequency of comorbidities, balance performance, and in quantitative gait performance parameters in both single-and dual-task gait velocity. However, while controlling for age, sex, BMI, years of education and MMSE scores as confounding variables, quantitative dual-task gait performance (velocity; while counting) proved to depict an observable statistically significant difference (poorer performance by those with history of falls). Seventeen participants were characterized as fallers (with 10, 2, and 5 patients reporting 1, 2, and 3 or more falls respectively in one year). Of the 26 non-fallers, 13, 9, and 4 self-classified themselves as having a vigorously, moderately and seldom active lifestyle, respectively. In contrary, of the 17 fallers, 14, and 3 considered themselves having vigorously, and seldom active lifestyles respectively (having no participants classify themselves as having moderately active lifestyle.
Table 1.
Cross-sectional demographic and clinical characteristics, and gait performance of the Gait and Brain participants included in this study.
| Demographics | Full sample N = 43 | Non-Fallers N = 26 | Fallers N = 17 | p value | p value* |
|---|---|---|---|---|---|
| Age (mean ± SD) | 74.5 ± 5.9 [63–87] | 74.2 ± 5.9 [63–84] | 75 ± 6.1 [66–87] | 0.41 | – |
| Sex, # of females (%) | 18 (41.9%) | 12 (46.2%) | 6 (35.3%) | 0.52 | – |
| BMI | 26 ± 4.1 [19–36] | 25.2 ± 4 [19–33] | 27.2 ± 4.1 [21–36] | 0.35 | – |
| Years of Education | 13.3 ± 2.6 [8–20] | 13.3 ± 1.9 [10–18] | 13.3 ± 3.4 [8–20] | 0.93 | – |
| Cognitive performance | |||||
| MMSE | 27.4 ± 2.5 [20−30] | 26.7 ± 2.7 [20–30] | 28.5 ± 1.7 [24–30] | 0.30 | – |
| MoCA | 23.7 ± 3.7 [12−30] | 23.3 ± 4.1 [12–30] | 24.2 ± 3.1 [19–29] | 0.58 | – |
| Comorbidities | |||||
| Number of Comorbidities | 5.7 ± 2.8 [0−13] | 5.6 ± 3.4 [0–13] | 5.8 ± 2.5 [0−11] | 0.87 | – |
| hypertension | 21 (48.8%) | 14 (53.8%) | 7 (41.2%) | 0.53 | – |
| Diabetes | 7 (16.3%) | 4 (15.4%) | 3 (17.6%) | 0.68 | – |
| Osteoporosis | 7 (16.3%) | 4 (15.4%) | 3 (17.6%) | 0.68 | – |
| Chronic Lung Disease | 3 (7%) | 3 (11.5%) | 0 (0%) | 0.54 | – |
| Osteoarthritis | 13 (30.2%) | 7 (26.9%) | 6 (35.3%) | 0.74 | – |
| Cancer | 15 (34.9%) | 11 (42.3%) | 4 (23.5%) | 0.19 | – |
| Hearing Problems | 19 (44.2%) | 11 (42.3%) | 8 (47.1%) | 1.00 | – |
| Depression | 12 (27.9%) | 7 (26.9%) | 5 (29.4%) | 0.72 | – |
| Stroke | 3 (7%) | 1 (3.8%) | 2 (11.8%) | 0.28 | – |
| Visual Impairment | 40 (93%) | 24 (92.3%) | 16 (94.1%) | 1.00 | – |
| CHF/MI/Angina | 2 (4.7%) | 1 (3.8%) | 1 (5.9%) | 1.00 | – |
| Atrial fibrillation | 2 (4.7%) | 1 (3.8%) | 1 (5.9%) | 1.00 | – |
| WMH | |||||
| Volume of WMH (mL) | 12.3 ± 3.0 | 11.8 ± 2.0 | 13.2 ± 4.3 | 0.437 | 0.28 |
| Quantitative Gait | |||||
| Usual Gait Velocity (mean ± SD) cm/s | 108.2 ± 21.3 [57–165] | 109.6 ± 19.8 [66–151] | 106 ± 23.8 [57–165] | 0.26 | 0.09 |
| Counting gait velocity cm/s | 103.9 ± 26.8 [44–177] | 105.5 ± 26.4 [61–167] | 101.3 ± 28 [44–177] | 0.20 | 0.03 |
| Serial 7 s velocity cm/s | 90.5 ± 30.8 [37–181] | 89.7 ± 31.2 [37–168] | 91.7 ± 31.3 [39–181] | 0.59 | 0.16 |
| Naming animals velocity cm/s | 92.7 ± 27.9 [35–170] | 90.5 ± 28.7 [49–170] | 96.1 ± 27.1 [35–163] | 0.73 | 0.38 |
* indicates p value was obtained by running ANCOVA using age, sex, BMI, years of education and MMSE scores as confounding variables of no interest. Mean ± standard deviation where applicable; (% of N listed at top of column); [range: minimum to maximum].
3.2. White matter hypertensities
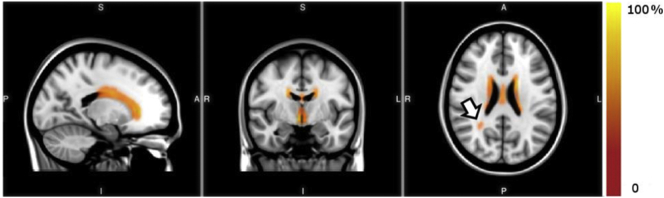
A probability map of WMH was calculated for the study cohort as shown in Fig. 2. As expected, the most common locations for development of WMH in this cohort were along the “rims and caps”(Kertesz et al., 1988) congruent with normal aging and consistent with periventricular edema or disturbed CSF transport.(Fazekas, 1989) WMH volumes were calculated for all subjects (Table 1) and ranged from 7.6 to 28.1 mL with an average of 12.2 mL. The WMH measurement used correlates well (correlation coefficient = 0.72) with a previously published method implemented in the LST Toolbox for SPM (Schmidt et al., 2012), although WMH volumes are systematically underestimated (Supplemental Fig. 2). The spatial distribution of WMH in the current study (Fig. 2) did not differ from other WMH studies.(de Leeuw et al., 2001) ANCOVA did not present significant differences in the volume of WMHs between fallers and non-fallers (Table 1).
Fig. 2.
WMH Probability map in MCI. The probability distribution of WMHs are shown in red-yellow superimposed on the 1 mm resolution MNI T1 template. The color bar denotes the percentage of subjects who had WMHs in each image voxel. WMH near the ventricles are common in MCI patients, with less frequent presentation away from the ventricles and toward the brain periphery. The arrow head points at a location distant from the ventricles with a moderate frequency of WMHs among the cohort studied.
3.3. GM volumetric analysis
GM volume was lower in fallers compared to non-fallers (Supplemental Fig. 3), however, differences were not significant while correcting for age, sex, BMI, years of education and MMSE.
3.4. Quantitative gait measurements
Dual-task walking while counting gait velocity was significantly lower in participants with history of falling compared to those without (p = .03) after adjusting for confounding effect of age, sex, years of education and MMSE scores (Table 1). All other gait variables were not statistically different between fallers and non-fallers.
3.5. Exploratory TBSS voxel-wise analysis
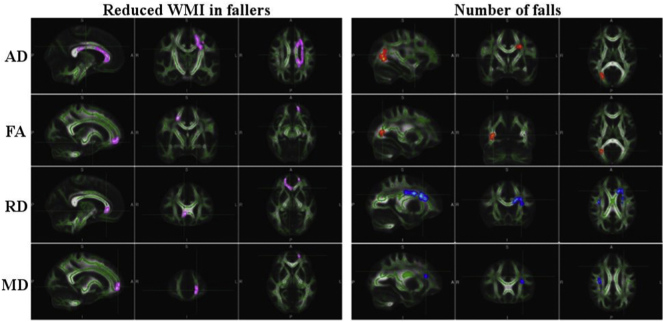
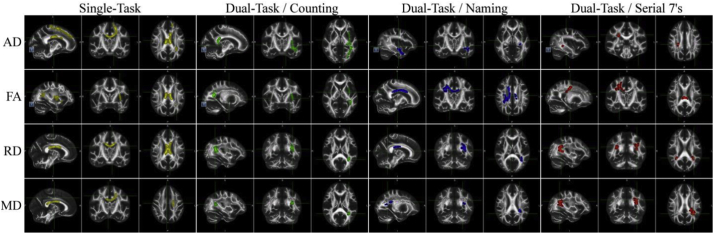
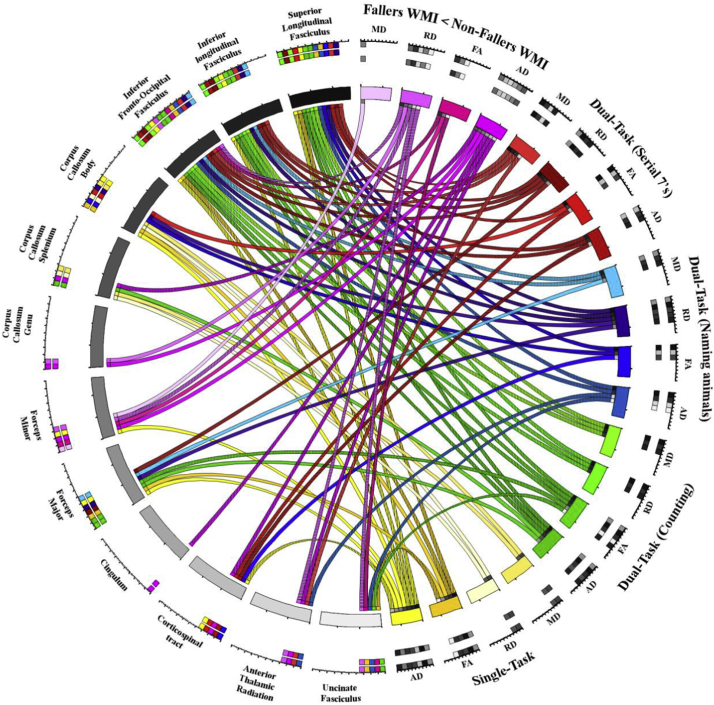
Voxel-wise non-parametric permutation test found that reduced gait performance, propensity to fall, and increased number of falls significantly correlated with high MD or RD values and low FA and AD values as shown at sample loci in Fig. 3 for falls and Fig. 4 for quantitative gait parameters (velocities). Findings are summarized by showing the associations between WM tracts in which significant voxel-wise correlations were found (based on the significant voxels anatomical localization within the JHU WM tractography atlas in MNI space) between DTI parameters (FA, MD, AD and RD) and gait parameters (single-task in yellow; dual-task counting in green; dual-task naming animals in blue; dual-task serial 7's in red), and falls (pink; fallers versus non-fallers differences) in Fig. 5 using a circular layout Krzywinski et al., 2009, CIRCOS, 2019. In Fig. 5. dominant presentation of DTI links (significant association between reduced gait performance and reduced WMI; color coded similar to Fig. 4) can be seen between gait parameters and the superior longitudinal fasciculus, inferior longitudinal fasciculus and the inferior fronto-occipital fasciculus. More importantly, the DTI parameters were indicative of reduced WMI in the group of patients with history of falls in several tracts (Fig. 3& 5; pink), in particular within the left hemispheric forceps minor tract. This was further validated by a specific, tract-base average DTI parameter ANCOVA (with age, sex, BMI, years of education, and MMSE acting as covariates), showing the RD was significantly higher throughout the Forceps minor tract (eta = 0.104, power = 0.511, p = .048) in fallers.
Fig. 3.
Significantly lower WMI clusters (Pink) in patients with history of falling compared to those without (Fallers WMI is compromised compared to non-fallers WMI) shown on the left. Significant correlations between the number of falls reported in the one year time frame prior to the imaging session and different scalar DTI parameters (red-yellow: negative correlation, blue-light blue: positive correlation) shown on the right. All voxel-wise results were acquired by nonparametric permutation inference testing controlling for age, sex, years of education, BMI and MMSE scores. Data is presented overlaid on the MNI 152 1 mm space standard brain, and average skeletonized WM tracts in green.
Fig. 4.
Significantly correlated WM clusters with gait velocity. Significant correlations (p ≤ .0125) between gait velocity and WMI as measured by different scalar DTI parameters (only correlations between reduced WMI and reduced gait performance, ie velocity, are shown). The different gait tests are color coded: yellow: single-task; green: dual-task counting; blue: dual-task naming animals; red: dual-task serial 7's. All voxel-wise results were acquired by nonparametric permutation inference testing controlling for age, sex, years of education, BMI and MMSE scores. Data is presented overlaid FMRIB58 1 mm space standard brain.
Fig. 5.
Circular plot of all WM tracts containing voxels clusters significantly correlated with gait velocity (single- or dual-tasks) as well as associated with reduced integrity in fallers compared with non-fallers. All voxel-wise results were acquired by nonparametric permutation inference testing controlling for age, sex, years of education, BMI and MMSE scores. Colors correspond to DTI parameters and comparisons made in Fig. 2, Fig. 3. Significant correlations are distinguished for left (vertical lines), right (horizontal lines), and bilateral tracts accordingly.
3.6. Relationship between WMI, gait, and number of falls
Negative binomial regression analyses were used to test the association between the RD in the Forceps minor and falls (Table 2), and the model remained significant after adjusting for age, number of years of education, gender, BMI and MMSE scores, as well as further adjusted for number of comorbidities, counting gait velocity and WMH volume (which were otherwise not accounted for by the TBSS analysis). The regression analysis using average DTI values from across the tract provide a level of independence from voxel-wise and cluster-specific extreme DTI values (possibly due to WMH lesions). Furthermore, the utilization of a commonly used statistical test renders the results more readily interpretable by a wider audience.
Table 2.
Association between WMI as a predictor variable and previous number of falls.
| Unadjusted |
Adjusted† |
Adjusted†† |
Adjusted††† |
|
|---|---|---|---|---|
| χ2 (df) p value |
χ2 (df) p value |
χ2 (df) p value |
χ2 (df) p value |
|
| Number of falls (n = 43) | 6.335 (1) 0.012* |
15.676 (7) 0.028* |
19.777 (8) 0.011* |
25.162 (9) 0.003** |
* Significant at the p < 0.05 level; ** significant at the p < 0.01 level; unadjusted model uses the RD of the forceps minor to predict number of falls. † adjusted for age, number of years of education, gender, BMI and MMSE scores and number of comorbidities †† adjusted for age, number of years of education, gender, BMI and MMSE scores, number of comorbidities and counting gait velocity ††† adjusted for age, number of years of education, gender, BMI and MMSE scores, number of comorbidities, counting gait velocity and WMH volume. χ2 - Likelihood Ratio Chi-Square, df - degrees of freedom.
According to the fully adjusted model, a participant's previous number of falls can be described by the combination of the factors listed in Table 3 including the incident rate ratio (IRR) which uses history of falls in the past year as a surrogate to risk of falling. The fit of the full predictor model with RD of the forceps minor, MMSE, counting gait velocity, and WMH, had a statistically significant improvement over the null model (P < .01) uniquely accounting for the variation in the number of falls. The WMI as measured by RD in the forceps minor had a strong effect on number of falls, with 1 standard deviation of the mean leading to a 200% increased incident rate. WMH presented significant associations with the number of falls, despite not having a significant mean difference between fallers and non-fallers. A Spearman's rank-order correlation was run to determine the relationship between predicted and actual number of falls, and found a strong, positive correlation which was statistically significant (rs = 0.641, P < .0001).
Table 3.
Parameters estimates for negative binomial regression with multiple predictors.
| Parameter | Sig. | IRR | 95% Confidence Interval for IRR |
|
|---|---|---|---|---|
| Lower | Upper | |||
| RD ForcepsMin (107 μm2/s) | 0.02⁎ | 1.02 | 1.00 | 1.04 |
| Gender | 0.12 | 3.24 | 0.74 | 14.18 |
| Age (yrs) | 0.16 | 0.89 | 0.75 | 1.05 |
| Body Mass Index (kg/m2) | 0.98 | 1.00 | 0.81 | 1.23 |
| Number of years of education (yrs) | 0.93 | 0.99 | 0.77 | 1.28 |
| T0 MMSE | 0.01⁎ | 1.91 | 1.15 | 3.17 |
| Total number of comorbidities | 0.65 | 0.94 | 0.73 | 1.22 |
| counting gait velocity (m/s) | 0.02⁎ | 0.95 | 0.90 | 0.99 |
| WMH (cm3) | 0.03⁎ | 1.20 | 1.02 | 1.42 |
Significant at the p < .05 level (in bold); IRR: Incident Rate Ratios.
4. Discussion
Changes in tissue microstructure in WM were significantly associated with gait disturbances and falls in older adults with MCI, even after adjusting for WMH and other important covariates. Specifically, we found that several DTI parameters correlated with slow gait and previous falls in the superior longitudinal fasciculus, inferior longitudinal fasciculus, inferior fronto-occipital fasciculus, corpus callosum, forceps minor and major, corticospinal tract, anterior thalamic radiation and uncinate fasciculus. DTI can be used to probe the integrity of WM tissue microstructure. In this study, the analysis of DTI parameters FA, MD, AD and RD provided specific quantitative measures that were associated with gait performance and history of falls.
Previous non-TBSS imaging studies focusing on MCI-related abnormalities found WM alterations in people with MCI in the frontal, parietal, and temporal lobes(Medina and Gaviria, 2008; Rose et al., 2006) as well as the posterior cingulum,(Chua et al., 2009) splenium of corpus callosum,(Cho et al., 2008) long association fascicles,(Bai et al., 2009) and internal capsule.(Chua et al., 2009) TBSS MCI studies found reduced WMI in the splenium of the corpus callosum, crus of fornix, (Zhuang et al., 2010) and para hippocampal WM, uncinate fasciculus, and WM tracts of the brain stem and cerebellum.(Liu et al., 2011) Imaging studies focusing on gait related abnormalities found association between gait performance and WM hyperintensities(Rosano et al., 2006) and WMI in the genu of the corpus callosum and gait in the elderly (Bhadelia et al., 2009) as well as bilateral frontal and periventricular WM lesions-affected voxels corresponding to major anterior projection fibers (thalamic radiations, corticofugal motor tracts) and adjacent association fibers (corpus callosum, superior fronto-occipital fasciculus, short association fibers) (Srikanth et al., 2010). Furthermore, a recent cross-sectional imaging study of normal adults at risk of falls found several clusters of abnormal WM regions that correlated with gait performance (medial frontal. Parietal subcortical pathways, genu and splenium of corpus callosum, posterior cingulum, prefrontal and orbitofrontal pathways, and longitudinal pathways that connect frontal-parietal-temporal lobes), with a smaller portion (medial frontal and parietal subcortical pathways) that also correlated with MMSE scores (Koo et al., 2012). Finally, one study that focused on finding the cross-sectional association between history of falls and the WMI of patients with Cirrhosis. Gómez-Ansón et al. (2015) found significant deteriorations in local WMI within the superior longitudinal fasciculus and corticospinal tract associated with falls in agreement with our TBSS findings.
When our sample was stratified based on history of falls, fallers were found to have significantly reduced WMI in the corpus callosum, uncinate fasciculus, and forceps minor. Using voxel-wise non-parametric permutation test of all four DTI parameters, limited loci within WM tracts were found to be significantly correlated with some gait parameters and falls. Our primary findings indicated that WMI, as measured by AD, RD, MD or FA, was lower in MCI patients with a history of falls, within several WM tracts including but not limited to the inferior fronto-occipital fasciculus, the forceps minor and the uncinate fasciculus. Successful regression models using the RD of the forceps minor showed that poor WMI is significantly associated with history of falls in MCI. The model remained significant after accounting for age, sex, BMI, years of education, MMSE, comorbidities, dual-task gait velocity, and WMH.
The executive network has been previously suggested to be involved with mobility function and falls (Montero-Odasso and Hachinski, 2014). Therefore, it was expected that lower WMI would be found in the forceps minor (a tract that connects the frontal and prefrontal lobes by passing though the genu of the corpus callosum) and the left inferior fronto-occipital fasciculus (a tract that connects the occipital, posterior temporal, and the orbito-frontal areas associated with visuospatial function) WM regions in patients with history of falls since the executive network relies on the integrity of these tracts (Genova et al., 2013). Additionally, the implied disruption in fronto-occipital fasciculus as determined by the reduced WMI (low FA and AD) might indicate disruption in the connectivity between different functional networks (e.g. executive function – in the prefrontal cortex and visual and proprioception in the occipital/parietal lobes). Further implicating the executive network in falls stems from the observation that significant WMI differences between fallers and non-fallers appear to be lateralized predominantly to the left hemisphere, consistent with previous evidence suggesting left-lateralized WM damage is responsible for executive function impairment (Barbey et al., 2012). Finally, executive dysfunction has been associated with reduced dual-task gait velocity in healthy older adults (Coppin et al., 2006) and MCI (Montero-Odasso et al., 2009b).
Clinically, our results highlight the relationship between gait, cognitive dysfunction, and fall risk in older adults with MCI as symptomatic flags to the presence of underlying pathology affecting WM structural connectivity between common brain regions. This may unravel the role of modifiable factors including vascular damage, in addition to the neurodegenerative process (Holtzer et al., 2014). Indeed, it was previously shown that older adults with MCI that had high prevalence of vascular risk factors were more likely to present slowing gait, greater dual-task cost on gait, and executive dysfunction (Hajjar et al., 2011; Montero-Odasso et al., 2012b). Our study provides further evidence that specific tracts are associated with gait characteristics and falls. These results are consistent with recent work evaluating normal older adults that also found a strong association between the forceps minor and gait performance (Poole et al., 2018).
Some limitations of our study should be considered. The cross-sectional design precludes a direct causal interpretation of the associations between differences in WMI and history of falls. Stratification of patients to the two groups assumed singular fall events were not incidental but related to underlying pathology, which may not be correct for all patients. Furthermore, history of falls was used in lieu of- and as a surrogate for- each patient's fall risk. An additional limitation was that WMH volume was not accounted for in the TBSS analysis, which could introduce variability due to the significantly different DTI parameters values within these lesions. However, the patient cohort studied presented with generally small WMHs, and WMH volume was used as a confounding variable in the regression to minimize potential bias. Several other confounding variables that may modify the association between WMI, falls and gait performance were controlled for; however, there may be other un-accounted confounders in this study. For example, it may be important in future studies to control for single-task gait characteristics or at least assess dual-task cost, when testing dual-task performances and associations with WMI, since it is possible baseline single-task performance introduce a bias into the dual-task analysis when not controlled for. A weakness of this study is the exploratory nature of the associations and consequently the relatively large number of statistical tests performed which increased the risk of a Type 1 error (false positives). No corrections were made for multiple comparisons to reduce the likelihood of missing important differences and reduce likelihood of Type 2 errors (Perneger, 1998). Based on the results of the current study, prospective studies can be designed to focus on specific WM tracts, which would reduce these concerns. Finally, our study sample consisted exclusively of MCI older adults and thus results may not generalize well beyond this population. Future studies should explore if WMI can prospectively predict falls in older adults with MCI. Our study strengths include the comprehensive clinical, cognitive and mobility assessment in a very well identified cohort of individuals with MCI. In addition, we applied novel and emerging imaging tools and methodologies to study WMI and its role in mobility-cognitive dysfunction. DTI parameters were investigated using tract-based spatial statistics (TBSS), an automated exploratory approach to investigate the whole brain while circumventing the shortcomings of ROI and volume based morphometry techniques, and is therefore considered more sensitive and less prone to errors (Smith et al., 2006). For regression analysis our measurements of region-specific DTI parameters of the Forceps minor were made using standard brain atlas-derived regions of interest (ROIs) within the skeletonized DTI parametric maps. This further supported the interpretation that the Forceps minor WM tract is critically associated with falls. And finally, the group comparisons and regression models accounted for multiple confounders including age, sex, years of education, BMI, and MMSE scores.
5. Conclusion
Quantitative measures of WMI using voxel-wise TBSS technique was found to be significantly associated with history of falls and poor gait performance in MCI. There was no significant white matter or grey matter macro-structural differences between the groups in this cohort. However, the integrity of WM microstructure in the tracts that are involved in executive and visuospatial functions were found to be preferentially affected in MCI individuals with history of falls and dual-task gait disturbances.
Our findings support the notion that white matter integrity in key brain regions is affected in older adults with MCI and history of falls and are aligned with the hypothesis that early abnormalities in WM play a role in the higher prevalence of falls seen in older adults with MCI. Future studies should explore the potential predictive ability of white matter integrity, incidence of falls and the interplay with onset of dementia.
In summary, we found that older adults with MCI and a recent history of falls may have disrupted structural connectivity. This may be associated with greater decline in both cognition and mobility in this population and subsequently increases their risk of falling.
Acknowledgments
Dr. Montero-Odasso's program in “Gait and Brain Health” is supported by grants from the Canadian Institutes of Health Research (MOP 211220; PJT 153100), the Ontario Ministry of Research and Innovation (ER11–08–101), the Ontario Neurodegenerative Diseases Research Initiative (OBI 34739), the Canadian Consortium on Neurodegeneration in Aging (FRN CNA 137794), and Department of Medicine Program of Experimental Medicine Research Award (POEM 768915), University of Western Ontario. He is the first recipient of the Schulich Clinician-Scientist Award.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2019.101975.
Appendix A. Supplementary data
Supplementary material
References
- Andersson, J., Jenkinson, M. & Smith, S. Non-linear Registration Aka Spatial Normalisation FMRIB Technial Report TR07JA2. 2007 from www.fmriboxacuk/analysis/techrep.
- Annweiler C. Contribution of brain imaging to the understanding of gait disorders in Alzheimer's disease: a systematic review. Am. J. Alzheimers Dis. Dementiasr. 2012;27:371–380. doi: 10.1177/1533317512454710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annweiler C. Motor cortex and gait in mild cognitive impairment: a magnetic resonance spectroscopy and volumetric imaging study. Brain J. Neurol. 2013;136:859–871. doi: 10.1093/brain/aws373. [DOI] [PubMed] [Google Scholar]
- Bai F. Abnormal integrity of association fiber tracts in amnestic mild cognitive impairment. J. Neurol. Sci. 2009;278:102–106. doi: 10.1016/j.jns.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Barbey A.K. An integrative architecture for general intelligence and executive function revealed by lesion mapping. Brain. 2012 doi: 10.1093/brain/aws021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Bennett I.J., Madden D.J., Vaidya C.J., Howard D.V., Howard J.H.J. Age-related differences in multiple measures of white matter integrity: a diffusion tensor imaging study of healthy aging. Hum. Brain Mapp. 2010;31:378–390. doi: 10.1002/hbm.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhadelia R.A. Diffusion tensor imaging, white matter lesions, the corpus callosum, and gait in the elderly. Stroke J. Cereb. Circ. 2009;40:3816–3820. doi: 10.1161/STROKEAHA.109.564765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H. Abnormal integrity of corticocortical tracts in mild cognitive impairment: a diffusion tensor imaging study. J. Korean Med. Sci. 2008;23:477–483. doi: 10.3346/jkms.2008.23.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua T.C. Diffusion tensor imaging of the posterior cingulate is a useful biomarker of mild cognitive impairment. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry. 2009;17:602–613. doi: 10.1097/JGP.0b013e3181a76e0b. [DOI] [PubMed] [Google Scholar]
- CIRCOS Circular Genome Data Visualization Introduction to Circos, Features and Uses. 2006. http://circos.ca/ Available at. (Accessed: 2nd March 2018)
- Coppin A.K. Association of executive function and performance of dual-task physical tests among older adults: analyses from the InChianti study. Age Ageing. 2006;35:619–624. doi: 10.1093/ageing/afl107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot M. Changes in Normal-appearing white matter precede development of white matter lesions. Stroke. 2013;44:1037. doi: 10.1161/STROKEAHA.112.680223. [DOI] [PubMed] [Google Scholar]
- de Leeuw F.E. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam scan study. J. Neurol. Neurosurg. Psychiatry. 2001;70:9–14. doi: 10.1136/jnnp.70.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douaud G. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain J. Neurol. 2007;130:2375–2386. doi: 10.1093/brain/awm184. [DOI] [PubMed] [Google Scholar]
- Fazekas F. Magnetic resonance signal abnormalities in asymptomatic individuals: their incidence and functional correlates. Eur. Neurol. 1989;29:164–168. doi: 10.1159/000116401. [DOI] [PubMed] [Google Scholar]
- Fields R.D. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008;31:361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genova H.M., DeLuca J., Chiaravalloti N., Wylie G. The relationship between executive functioning, processing speed and white matter integrity in multiple sclerosis. J. Clin. Exp. Neuropsychol. 2013;35:631–641. doi: 10.1080/13803395.2013.806649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Ansón B. Alterations in cerebral white matter and neuropsychology in patients with cirrhosis and falls. PLoS One. 2015;10 doi: 10.1371/journal.pone.0118930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good C.D. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Hajjar I. Hypertension, white matter hyperintensities, and concurrent impairments in mobility, cognition, and mood: the cardiovascular health study. Circulation. 2011;123:858–865. doi: 10.1161/CIRCULATIONAHA.110.978114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R., Epstein N., Mahoney J.R., Izzetoglu M., Blumen H.M. Neuroimaging of mobility in aging: a targeted review. J. Gerontol. A Biol. Sci. Med. Sci. 2014;69:1375–1388. doi: 10.1093/gerona/glu052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Smith S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kertesz A. Periventricular and subcortical hyperintensities on magnetic resonance imaging. ‘Rims, caps, and unidentified bright objects. Arch. Neurol. 1988;45:404–408. doi: 10.1001/archneur.1988.00520280050015. [DOI] [PubMed] [Google Scholar]
- Koo B.-B. Clinical prediction of fall risk and white matter abnormalities: a diffusion tensor imaging study. Arch. Neurol. 2012;69:733–738. doi: 10.1001/archneurol.2011.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzywinski M. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19:1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton M.P., Brody E.M. Assessment of older people: self-maintaining and instrumental activities of daily living. The Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- Le Bihan D. Diffusion tensor imaging: concepts and applications. J. Magn. Reson. Imaging JMRI. 2001;13:534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- Liu Y. Diffusion tensor imaging and tract-based spatial statistics in Alzheimer's disease and mild cognitive impairment. Neurobiol. Aging. 2011;32:1558–1571. doi: 10.1016/j.neurobiolaging.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Medina D.A., Gaviria M. Diffusion tensor imaging investigations in Alzheimer's disease: the resurgence of white matter compromise in the cortical dysfunction of the aging brain. Neuropsychiatr. Dis. Treat. 2008;4:737–742. doi: 10.2147/ndt.s3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero-Odasso M., Hachinski V. Preludes to brain failure: executive dysfunction and gait disturbances. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2014;35:601–604. doi: 10.1007/s10072-013-1613-4. [DOI] [PubMed] [Google Scholar]
- Montero-Odasso M. Quantitative gait analysis under dual-task in older people with mild cognitive impairment: a reliability study. J. Neuroengineering Rehabil. 2009;6(35) doi: 10.1186/1743-0003-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero-Odasso M. Dual-tasking and gait in people with mild cognitive impairment. The effect of working memory. BMC Geriatr. 2009;9(41) doi: 10.1186/1471-2318-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero-Odasso M., Verghese J., Beauchet O., Hausdorff J.M. Gait and cognition: a complementary approach to understanding brain function and the risk of falling. J. Am. Geriatr. Soc. 2012;60:2127–2136. doi: 10.1111/j.1532-5415.2012.04209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero-Odasso M. Vascular burden predicts gait, mood, and executive function disturbances in older adults with mild cognitive impairment: results from the gait and brain study. J. Am. Geriatr. Soc. 2012;60:1988–1990. doi: 10.1111/j.1532-5415.2012.04180.x. [DOI] [PubMed] [Google Scholar]
- Montero-Odasso M. The motor signature of mild cognitive impairment: results from the gait and brain study. J. Gerontol. A Biol. Sci. Med. Sci. 2014;69:1415–1421. doi: 10.1093/gerona/glu155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero-Odasso, M., Muir, S. W. & Speechley, M. Dual-task complexity affects gait in people with mild cognitive impairment: the interplay between gait variability, dual tasking, and risk of falls. Arch. Phys. Med. Rehabil. 93, 293–299. [DOI] [PubMed]
- Mori S., Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51:527–539. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Perneger T.V. What's wrong with Bonferroni adjustments. BMJ. 1998;316(7139):1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole V.N. Compromised prefrontal structure and function are associated with slower walking in older adults. NeuroImage. Clin. 2018;20:620–626. doi: 10.1016/j.nicl.2018.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie K. Mild cognitive impairment: an epidemiological perspective. Dialogues Clin. Neurosci. 2004;6:401–408. doi: 10.31887/DCNS.2004.6.4/kritchie. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D.A., Savva G.M., Coen R.F., Kenny R.-A. Cognitive function in the prefrailty and frailty syndrome. J. Am. Geriatr. Soc. 2014;62:2118–2124. doi: 10.1111/jgs.13111. [DOI] [PubMed] [Google Scholar]
- Rosano C., Brach J., Longstreth W.T., Newman A.B. Quantitative measures of gait characteristics indicate prevalence of underlying subclinical structural brain abnormalities in high-functioning older adults. Neuroepidemiology. 2006;26:52–60. doi: 10.1159/000089240. [DOI] [PubMed] [Google Scholar]
- Rose S.E. Diffusion indices on magnetic resonance imaging and neuropsychological performance in amnestic mild cognitive impairment. J. Neurol. Neurosurg. Psychiatry. 2006;77:1122–1128. doi: 10.1136/jnnp.2005.074336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherder E., Eggermont L., Visscher C., Scheltens P., Swaab D. Understanding higher level gait disturbances in mild dementia in order to improve rehabilitation: ‘last in-first out’. Neurosci. Biobehav. Rev. 2011;35:699–714. doi: 10.1016/j.neubiorev.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Smith S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith S.M. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith E.E. Magnetic resonance imaging white matter hyperintensities and brain volume in the prediction of mild cognitive impairment and dementia. Arch. Neurol. 2008;65:94–100. doi: 10.1001/archneurol.2007.23. [DOI] [PubMed] [Google Scholar]
- Snijders A.H., van de Warrenburg B.P., Giladi N., Bloem B.R. Neurological gait disorders in elderly people: clinical approach and classification. Lancet Neurol. 2007;6:63–74. doi: 10.1016/S1474-4422(06)70678-0. [DOI] [PubMed] [Google Scholar]
- Song S.-K. Demyelination increases radial diffusivity in corpus callosum of mouse brain. NeuroImage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Srikanth V. The location of white matter lesions and gait--a voxel-based study. Ann. Neurol. 2010;67:265–269. doi: 10.1002/ana.21826. [DOI] [PubMed] [Google Scholar]
- Starr J.M. Brain white matter lesions detected by magnetic resonance [correction of resosnance] imaging are associated with balance and gait speed. J. Neurol. Neurosurg. Psychiatry. 2003;74:94–98. doi: 10.1136/jnnp.74.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S.-W. Noninvasive detection of cuprizone induced axonal damage and demyelination in the mouse corpus callosum. Magn. Reson. Med. 2006;55:302–308. doi: 10.1002/mrm.20774. [DOI] [PubMed] [Google Scholar]
- Tinetti M.E., Speechley M., Ginter S.F. Risk factors for falls among elderly persons living in the community. N. Engl. J. Med. 1988;319:1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- Tinetti M., Baker D., Dutcher J., Vincent J., Rozett R. Peaceable Kingdom Press; 1997. Reducing the Risk of Falls among Older Adults in the Community. [Google Scholar]
- Whitwell J.L. Patterns of atrophy differ among specific subtypes of mild cognitive impairment. Arch. Neurol. 2007;64:1130–1138. doi: 10.1001/archneur.64.8.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler A.M., Ridgway G.R., Webster M.A., Smith S.M., Nichols T.E. Permutation inference for the general linear model. NeuroImage. 2014;92:381–397. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J.J.J. Brain white matter hyperintensities, executive dysfunction, instability, and falls in older people: a prospective cohort study. J. Gerontol. A Biol. Sci. Med. Sci. 2012;67:1085–1091. doi: 10.1093/gerona/gls063. [DOI] [PubMed] [Google Scholar]
- Zhuang L. White matter integrity in mild cognitive impairment: a tract-based spatial statistics study. NeuroImage. 2010;53:16–25. doi: 10.1016/j.neuroimage.2010.05.068. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material