Abstract
A 32-year-old nonlactational women with a nipple piercing and previous oral-to-breast contact presented with findings consistent with mastitis and abscess, however, the patient failed multiple courses of empiric antimicrobials. Needle aspiration was performed and the culture was positive for N. gonorrhoeae. She was successfully treated with intravenous ceftriaxone and transitioned to oral ciprofloxacin once susceptibilities were known.
N. gonorrhoeae is an uncommon cause of nonlactational mastitis and abscess. A few cases have been reported in the context of sexual contact and nipple piercings. In an era of increasing antimicrobial resistance and with the risk of disseminated gonococcal infection, a high index of suspicion should be maintained within this clinical context.
Keywords: Neisseria gonorrhoeae, Breast abscess
Introduction
Mastitis is inflammation of the breast with or without infection. Mastitis with infection, in a woman, can be divided into lactational and nonlactational. Nonlactational mastitis leading to an abscess is uncommon [1]. Nearly 90% of nonlactational breast abscesses are subareolar and typically occur toward the end of a woman’s reproductive years. Risk factors identified include smoking, trauma, congenital malformations, and diabetes [2]. The most common organism cultured in nonlactational breast abscesses is S. aureus (including Methicillin-resistant S. aureus), followed by Streptococcus spp. and anaerobic organisms [3]. Piercing-related mastitis and abscesses have only been reported in case reports and series in the setting of recent (<1 year) piercing, and no predominant organisms have yet been identified [4].
Neisseria gonorrhoeae is a gram negative diplococcus that primarily infects mucosal surfaces of the body. Abscess formation by N. gonorrhoeae is typically due to local spread from mucosal areas, leading to tubo-ovarian abscesses in women and periurethral abscesses in men. Diagnosing N. gonorrhoeae is of particular importance in view of the emergence of highly antimicrobial-resistant strains. Once a penicillin-susceptible organism, it has developed resistance to penicillin, cefiximine, and, most recently, fluoroquinolones. Ceftriaxone is now recommended as first-line treatment, however, as with penicillins, the recommended dose has increased over time [5].
We report a case of nonlactational mastitis and breast abscess due to N. gonorrhoeae. The patient initially failed empiric treatment for common causes of mastitis as initial antimicrobials did not treat N. gonorrhoeae. Successful treatment of this entity requires a high index of suspicion in patients with recent sexual contact and skin barrier breakdown.
Case report
A 32-year-old Dominican woman with no known medical problems was admitted to the hospital with 4 weeks of induration and erythema of the right breast with purulent discharge from a nipple piercing site. She had a recent history of two sexual encounters with two different partners, both approximately 2 weeks prior to symptom onset. She utilized barrier contraception with condoms in both encounters and both involved direct oral to breast contact. She was unsure of direct penile to breast contact. One partner reported recent negative test results for sexually transmitted infections (STI) and the patient was not aware of the STI status of the other partner. She reported treatment for chlamydia ten years prior and, again, seven months prior. She denied any other history of STIs. Her right nipple was pierced two years prior and had been without infectious complications or inflammation until this episode.
The patient’s history was relevant for a presentation to an urgent care clinic with induration and erythema of the right breast and purulent drainage. She was referred to the emergency department where an ultrasound study demonstrated a hypoechoic focus adjacent to the right nipple. She was prescribed trimethoprim-sulfamethoxazole 160–800 mg twice daily and referred to the breast surgery clinic. She was unable to obtain the antimicrobials and returned to urgent care five days later with worsening pain and induration of the right breast, subjective fevers, and chills. She was prescribed clindamycin 300 mg three times a day and completed a 10-day course of treatment; but the erythema of her right breast continued to expand. She returned to breast surgery clinic and aspiration of the area was performed with 3 mL of fluid removed and sent for cultures. She was prescribed an additional 10 days of clindamycin, which she took for only 1 day. When she returned for follow-up one week following aspiration, she reported no clinical improvement, and was noted to have fever of 38.8 degrees C. Repeat aspiration of the breast was attempted, but was unsuccessful. The wound culture from the initial aspiration resulted as N. gonorrhoeae using matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry, without susceptibilities available at that time. She was given an intramuscular injection of 1 g of ceftriaxone and a prescription for 1 g of azithromycin and was referred to an infectious disease clinic. She took the azithromycin, but reported an episode of emesis immediately following ingestion. Given the potential for resistance, duration and severity of symptoms, and inability to tolerate oral azithromycin, she was referred to the hospital for intravenous (IV) antimicrobials.
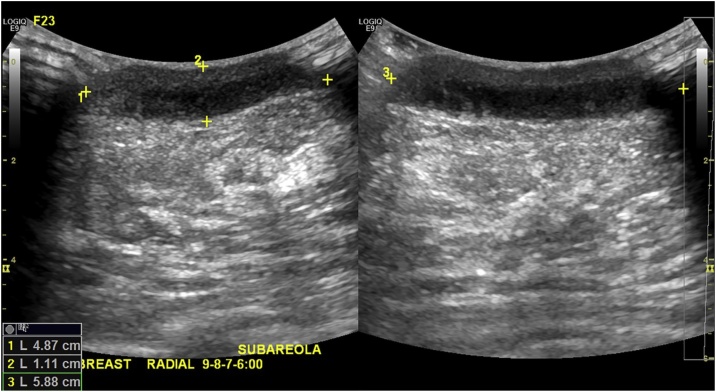
On examination in the emergency department, the patient’s temperature was 36.9 degrees C, blood pressure 111/64 mmHg, heart rate 82 beats per minute, and respiratory rate 18 per minute. Examination of right breast revealed an area of tender, warm, erythematous, periareolar fluctuance with surrounding induration. There was no purulence or drainage. The nipple ring had been removed. The left breast was without erythema, warmth, induration, or drainage. The remainder of the examination revealed no rashes, conjunctivitis, or joint abnormalities. Breast ultrasound revealed a 4.87 cm x 1.11 cm x 5.88 cm retroareolar fluid collection in the right breast (Fig. 1). The patient declined aspiration at that time. Complete blood count revealed WBC 7.7 × 1000/microL with 65% neutrophils. Serum syphilis IgG/IgM antibodies were negative. A 4th generation HIV Ag/Ab Combo Assay (Abbott ARCHITECT) was negative. Urine nuclear acid amplification test (NAAT) was negative for N. gonorrhoeae and C. trachomatis. Throat and rectal swabs were also negative for N. gonorrhoeae and C. trachomatis. The gonococcal isolate obtained at breast surgery clinic was found to be susceptible to ceftriaxone and ciprofloxacin.
Fig. 1.
Emergency department ultrasound revealing 4.87 cm × 1.11 cm × 5.88 cm hypoechoic retroareolar fluid collection in the right breast.
She received 5 days of IV ceftriaxone. During the course of her hospitalization, she agreed to surgical management and underwent needle aspiration of the abscess with removal of 4 mL of serosanguinous fluid which was sent for culture. The aspiration was performed after two doses of IV ceftriaxone, and culture of this fluid was negative. Post-aspiration and following clinical improvement, antimicrobials were changed to oral ciprofloxacin 500 mg every 12 h. In total, she completed a 10-day course of appropriate antimicrobials. At a one month follow-up visit, the patient was asymptomatic, with resolution of the breast abscess. She confirmed that both partners were notified and treated appropriately.
Discussion
Previously, N. gonorrhoeae cutaneous abscesses in nongenital sites have been associated with disseminated gonococcal infection (DGI) [6]. These infections have no pathognomonic features, therefore physicians must maintain vigilance in obtaining proper testing and treatment. Cutaneous N. gonorrhoeae infections in the absence of DGI have been have reported on the abdomen, hand, and fetal scalp and are often proceeded by skin barrier breakdown [7].
To date, however, only three prior cases of gonococcal mastitis and subsequent breast abscess have been reported in the literature. The first was of a 42-year-old man from Australia with mastitis of the left breast due to N. gonorrhoeae. As described in the case report, it was probably acquired as a result of direct mouth-nipple contact [8]. The second case was of a 38-year-old man, also from Australia, who reported direct mouth-nipple contact approximately two weeks prior to presentation [9]. The third case was of a 21-year-old woman who reported direct mouth-nipple contact approximately one week prior to presentation [10]. All of the patients in these cases had prior, healed nipple piercings and no other organisms were isolated. This foreign body disrupts the skin barrier, predisposing to abscess formation upon exposure to N. gonorrhoeae.
In addition, the third case was the only other case to report using MALDI-TOF for identification of the organism. The advantage of MALDI-TOF is the ability to make a quick and accurate diagnosis with minimum growth. Specifically, it has been shown to have a positive predictive value of 99.3% and sensitivity of 99.9% in the identification of N. gonorrhoeae [11]. This case highlights the potential role for MALDI-TOF in abscess identification, particularly when N. gonorrhoeae is suspected.
Conclusion
N. gonorrhoeae should be considered as a potential etiologic agent for mastitis and abscess. Patients with nipple piercings (recent or remote) and mastitis or breast abscess should be asked detailed questions about recent sexual contact. N. gonorrhoeae should be considered particularly if mastitis is refractory to appropriate empiric antimicrobial therapy. Mastitis due to N. gonorrhoeae is a potentially difficult disease to treat given escalating resistance of N. gonorrhoeae and the concern for disseminated disease. In cases of refractory mastitis and abscess, MALDI-TOF may be a powerful tool for the quick identification of unusual organisms. Careful antimicrobial management and source control are imperative.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Declaration of Competing Interest
The authors declare that there are no conflicts of interest.
Acknowledgements
The authors thank Dr. Liise-anne Pirofski for her helpful discussion and critical review of the manuscript. We thank Dr. Wendy Szymczak for her assistance with review of the microbiology guidelines, practices, and findings.
References
- 1.Lam E., Chan T., Wiseman S.M. Breast abscess: evidence based management recommendations. Expert Rev Anti Infect Ther. 2014;12(7):753–762. doi: 10.1586/14787210.2014.913982. [DOI] [PubMed] [Google Scholar]
- 2.Versluijs-Ossewaarde F.N., Roumen R.M., Goris R.J. Subareolar breast abscesses: characteristics and results of surgical treatment. Breast J. 2005;11(3):179–182. doi: 10.1111/j.1075-122X.2005.21524.x. [DOI] [PubMed] [Google Scholar]
- 3.Giamarellou H., Soulis M., Antoniadou A. Periareolar nonpuerperal breast infection: treatment of 38 cases. Clin Infect Dis. 1994;18(1):73–76. doi: 10.1093/clinids/18.1.73. [DOI] [PubMed] [Google Scholar]
- 4.Lee B., Vangipuram R., Petersen E. Complications associated with intimate body piercings. Dermatol Online J. 2018;24(7) [PubMed] [Google Scholar]
- 5.Hook E.W., 3rd, Kirkcaldy R.D. A brief history of evolving diagnostics and therapy for gonorrhea: lessons learned. Clin Infect Dis. 2018;67(8):1294–1299. doi: 10.1093/cid/ciy271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghosn S.H., Kibbi A.G. Cutaneous gonococcal infections. Clin Dermatol. 2004;22(6):476–480. doi: 10.1016/j.clindermatol.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Scott M.J., Jr., Scott M.J., Sr Primary cutaneous Neisseria gonorrhoeae infections. Arch Dermatol. 1982;118(5):351–352. [PubMed] [Google Scholar]
- 8.Bodsworth N.J., Price R., Nelson M.J. A case of gonococcal mastitis in a male. Genitourin Med. 1993;69(3):222–223. doi: 10.1136/sti.69.3.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pendle S., Barnes T. Neisseria gonorrhoeae isolated from an unexpected site. Sex Health. 2016;13(6):593–594. doi: 10.1071/SH16124. [DOI] [PubMed] [Google Scholar]
- 10.Bateman A.C. Unusual cause of a wound infection. J Appl Lab Med. 2017;2(3):444–448. doi: 10.1373/jalm.2017.024000. [DOI] [PubMed] [Google Scholar]
- 11.Buchanan R., Ball D., Dolphin H. Matrix-assisted laser desorption-ionization time-of-flight mass spectrometry for the identification of Neisseria gonorrhoeae. Clin Microbiol Infect. 2016;22(9):815. doi: 10.1016/j.cmi.2016.06.010. e5-e7. [DOI] [PubMed] [Google Scholar]