Abstract
Background
There is an ongoing debate about the concept of restricted phenotypes of amyotrophic lateral sclerosis (ALS), including progressive bulbar palsy (PBP).
Objective
The study was designed to investigate specific white matter alterations in diffusion tensor imaging (DTI) data from PBP patients using a hypothesis-guided tract-of-interest-based approach (compared with ‘classical’ ALS patients and controls) to identify in vivo microstructural changes according to the neuropathologically defined ALS-related corticoefferent tract pathology.
Methods
DTI-based white matter mapping was performed both by an unbiased voxel-wise statistical comparison and by a hypothesis-guided tract-wise analysis of fractional anisotropy (FA) maps according to the ALS-staging pattern for 23 PBP and 23 ALS patients vs 23 matched controls.
Results
The analysis of white matter integrity demonstrated regional FA reductions along the CST and also in frontal and prefrontal brain areas both in PBP patients and ALS patients with additional regional FA reduction in the pons of the PBP group. In the tract-specific analysis according to the neuropathological ALS-staging pattern, PBP and ALS patients showed identical significant alterations of ALS-related tract systems when compared with controls.
Conclusions
The DTI study including the tract-of-interest-based analysis showed the same microstructural corticoefferent involvement patterns in PBP patients as in ALS, which supports the hypothesis that PBP is a phenotypical variant of ALS.
Keywords: Diffusion tensor imaging, Amyotrophic lateral sclerosis, Progressive bulbar palsy, Magnetic resonance imaging, Motor neuron diseases
Highlights
-
•
Neuropathological ALS-stages can be mapped in vivo in PBP.
-
•
PBP but not classical ALS patients show regional FA reduction in the pons.
-
•
This study supports the hypothesis that PBP is a phenotypical variant of ALS.
1. Introduction
Beyond the ‘classical’ clinical presentation of amyotrophic lateral sclerosis (ALS) with involvement of the upper and the lower motor neuron according to established diagnostic criteria, there is the concept of `restricted` phenotypes, including primary lateral sclerosis (PLS), progressive muscular atrophy (PMA)/lower motor neuron disease, and progressive bulbar paresis (PBP) (Ludolph et al., 2015). This concept is currently controversially discussed (de Vries et al., 2019; Finegan et al., 2019), but is of utmost importance to the patients with these clinical presentations inasmuch as they are currently excluded from ALS clinical trials and novel pharmacological and other treatment approaches if they are not regarded as ALS sub-phenotypes.
The neuropathological demonstration of cerebral TDP43 pathology according to the neuropathological staging concept of ALS (Brettschneider et al., 2013; Braak et al., 2013; Braak et al., 2017) is still lacking within the complete spectrum of restricted phenotypes because of the limited number of autopsies. However, an in vivo approach exists that uses a tract of interest (TOI)-based magnetic resonance imaging (MRI) analysis technique to demonstrate ALS-specific corticoefferent tract pathology (Kassubek et al., 2014, Kassubek et al., 2018), supported by the results of multi-centre diffusion tensor imaging (DTI) analysis (Müller et al., 2016) and a meta-analysis (Gorges et al., 2018). This technique has been applied to the MRI data of patients from different sites with PLS (Müller et al., 2018A; Müller et al., 2018B) and with rapidly progressive lower motor neuron disease (Müller et al., 2018C), demonstrating the same tract involvement patterns as those seen in ALS (Kassubek and Müller, 2016). Here, we performed a DTI- and TOI-based MRI analysis in patients with another restricted phenotype, i.e., PBP, in comparison with disease controls (ALS) and controls to see if PBP patients also show ALS-like cerebral tract changes in accordance with the neuropathological propagation pattern.
2. Methods
2.1. Subjects and patient characteristics
Twenty-three PBP patients were included, who met the diagnostic criteria for PBP. All patients showed an isolated bulbar onset with a progressive affection of the lower cranial nerves causing dysarthria and/or dysphagia, tongue wasting and fasciculation before they developed spinal symptoms of motor neuron disease. All patients in the study had isolated bulbar symptoms (without spinal symptoms) over 6 months according to the definition both by Chiò and colleagues (Chiò et al., 2011; original text: “Bulbar phenotype: These patients had a bulbar onset with dysarthria and/or dysphagia, tongue wasting, fasciculation and no peripheral spinal involvement for the first 6 months after symptoms onset.”) and by Burrell and colleagues (Burrell et al., 2011). None of the patients had any bulbar upper motor neuron signs, neither at onset nor at time of scanning.
Clinically, all patients had an isolated bulbar onset and a prominent progressive bulbar syndrome at the time of MRI scanning, but most of the patients already showed beginning spinal symptoms with fasciculations and pareses which had started after the first six months after symptom onset - this is compatible with the diagnosis PBP.
To be eligible, subjects had to fulfill the following criteria: no family history of MND, no clinical diagnosis of frontotemporal dementia (FTD), no mutations of major genes related to hereditary spastic paraparesis (if known), no other major systemic, psychiatric or neurological illnesses, no history of substance abuse. Further mandatory criteria for inclusion were negative tests for other neuromuscular diseases and for infections of the central nervous system, and routine MRI scans excluded any brain abnormalities indicating a different etiology of the clinical symptoms. Disease duration in the PBP group was 10 ± 3 months (range 6 to 20 months), and age of onset of the motor disorder was 71 ± 11 years (all data are given as arithmetic mean ± standard deviation (SD)). All patients underwent standardized clinical, neurological, and routine laboratory examinations. Clinically, all patients had an isolated bulbar onset and a prominent progressive bulbar syndrome at the time of MRI scanning, but most of the patients already showed beginning spinal symptoms with fasciculations and pareses and not a fully ‘pure’ bulbar phenotype. PBP patients presented a revised ALS functional rating scale (ALS-FRS-R) (Cedarbaum et al., 1999) of 43 ± 3 in average (minimum 39, maximum 47).
A group of 23 ALS patients were selected to match for age and gender to the PBP group. The diagnosis of all cases was made according to the El Escorial diagnostic criteria (Ludolph et al., 2015). All of these patients displayed clinical involvement of the upper and the lower motor neuron according to the ‘classical’ (Charcot's) clinical phenotype. ALS patients presented with an ALS-FRS-R of 42 ± 5 in average (minimum 30, maximum 47) and a disease duration of 12 ± 7 months (range 5 to 30 months). Thus, there were no differences in ALS-FRS-R and disease duration between the PBP and ALS groups.
PBP and ‘classical’ ALS patients were compared with a group of 23 age- and gender-matched controls. Gross brain pathology, including vascular brain alterations, was excluded by conventional MRI. All control individuals lacked a family history of neuromuscular disease and had no history of neurologic, psychiatric, or other major medical illnesses and were recruited from among spouses of patients and by word of mouth.
All participants provided written informed consent for the study protocol according to institutional guidelines which had been approved by the Ethics Committee of Ulm University, Germany (No. 19/12).
A summary of the participants' characteristics is given in Table 1. The group comparison concerning age by Kruskal-Wallis test showed 0.62, concerning gender by Kruskal-Wallis test revealed 0.95, indicating no significant differences.
Table 1.
Subjects' characteristics. ALS-FRS-R – revised ALS functional rating scale.
| PBP (n = 23) | ALS (n = 23) | Controls (n = 23) | p | |
|---|---|---|---|---|
| Male/female | 10/13 | 10/13 | 11/12 | Kruskal-Wallis: 0.95 |
| Age/years (mean ± std. dev.) | 71 ± 11 | 66 ± 7 | 66 ± 9 | Kruskal-Wallis: 0.62 |
| ALS-FRS-R | 43 (39–47) | 42 (30–47) | – | t-test: 0.51 |
| Disease duration/months (mean ± std. dev.) | 10 ± 3 | 12 ± 7 | – | t-test: 0.58 |
2.2. MRI acquisition
MRI scanning was performed on a 1.5 Tesla Magnetom Symphony (Siemens Medical, Erlangen, Germany); the DTI study protocol consisted of 52 volumes (64 slices, 128 × 128 pixels, slice thickness 2.8 mm, pixel size 2.0 mm × 2.0 mm), representing 48 gradient directions (b = 1000 s/mm2) and four scans with b = 0. TE and TR were 95 ms and 8000 ms; 40 T2-weighted coronar slices (Fluid Attenuated Inversion Recovery/FLAIR, TR/TE 6180/112 ms) of 3.0 mm thickness, 0.45 mm × 0.45 mm in-plane resolution and 512 × 448 voxels matrix dimension were scanned; T1-weighted imaging (MPRAGE) consisted of 144 sagittal slices of 1.2 mm thickness, 1.0 mm × 1.0 mm in-plane resolution and 256 × 248 voxels matrix dimension.
2.3. Data analysis
The analysis of the DTI data was performed by use of the software Tensor Imaging and Fiber Tracking (TIFT – Müller et al., 2007a) which has already been applied to the analysis of multicenter studies (Müller et al., 2016). The algorithms used in this study have previously been described in detail (Müller and Kassubek, 2013; Kassubek et al., 2014, Kassubek et al., 2018). Stereotaxic normalization to the Montreal Neurological Institute (MNI) space was performed iteratively by use of study-specific templates (Müller et al., 2009). From the stereotaxically normalized DTI data sets of all subjects, fractional anisotropy (FA) maps were quantitatively calculated to map white matter microstructure (Le Bihan et al., 2001). A Gaussian filter of 8 mm full width at half maximum was applied for smoothing of FA maps for a good balance between sensitivity and specificity (Unrath et al., 2010); finally, FA maps were corrected for the covariate age.
Statistical comparison by Student's t-test was performed voxel-wise for FA values to detect changes between the subject groups (whole brain-based spatial statistics, WBSS). Voxels with FA values below 0.2 were not considered for statistical comparison, since cortical grey matter shows FA values up to 0.2 (Kunimatsu et al., 2004). Statistical results were corrected for multiple comparisons using the false-discovery-rate (FDR) algorithm at p < .05 (Genovese et al., 2002). Further reduction of the alpha error was performed by a spatial correlation algorithm that eliminated isolated voxels or small isolated groups of voxels in the size range of the smoothing kernel leading to a threshold cluster size of 256 voxels.
Defined tract systems according to the ALS-staging system (Braak et al., 2013; Ludolph and Brettschneider, 2015) were identified with the tract-of interest (TOI) approach (Kassubek et al., 2014; Kassubek et al., 2018; Müller et al., 2018a, Müller et al., 2018b, Müller et al., 2018c). TOIs for the four ALS stages were the corticospinal tract (CST, representative or stage 1), the corticorubral and corticopontine tracts (stage 2), the corticostriatal pathway (stage 3), the proximal perforant path (stage 4). As a reference path, a tract originating from the corpus callosum (CC) area V was used where no involvement in ALS-associated neurodegeneration could be anticipated. Tract-wise fractional anisotropy statistics (TFAS – Müller et al., 2007b) was performed by statistically comparing the FA values in a respective tract system between two subject groups (Student's t-test, not considering FA-values <0.2). The use of Student's t-test was justified as the subject groups were large enough to show a Gaussian distribution of FA values.
3. Results
3.1. Whole brain-based spatial statistics of FA maps
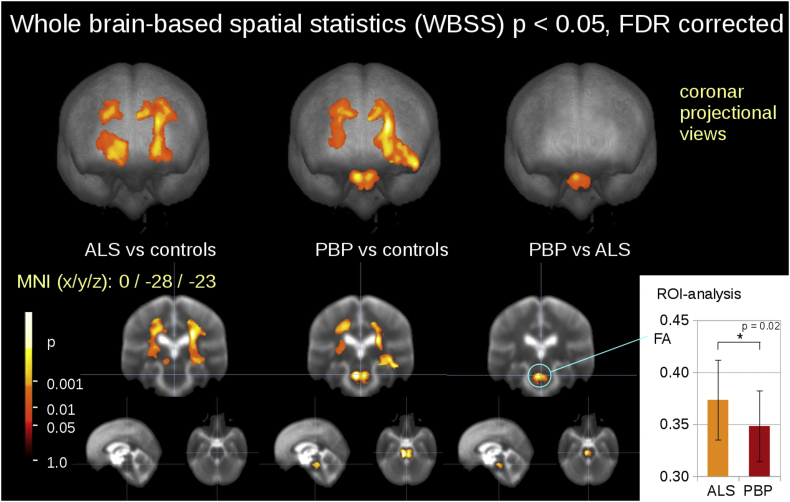
The comparison at the group level by WBSS for PBP patients, ALS patients, and controls demonstrated several clusters of regional alterations at p < .05 (corrected for multiple comparisons). Maps of FA reductions are depicted in Fig. 1 for the group comparisons. When comparing ALS patients and controls, FA reduction was observed mainly along the CST (corresponding to neuropathological stage 1 of ALS (Kassubek et al., 2018)). PBP patients also showed a FA reduction pattern along the CST compared to controls, similar to the comparison between ALS patients and controls, with an additional focus in the lower pons while the comparison between ALS patients and PBP patients showed only one significant cluster localized in the pons. Although the pontine alterations were significant between ALS patients and PBP patients, there is a certain overlap in FA values, as shown by ROI analysis (Fig. 1).
Fig. 1.
Whole brain-based spatial statistics (WBSS) of FA maps of ALS patients, PBP patients, and controls. WBSS of FA maps (p < .05, False-discovery-rate (FDR) corrected) demonstrated multiple clusters of regional FA reductions for ALS patients vs controls along the corticospinal tract (left), for PBP patients vs controls along the corticospinal tract and in the pons (center) and ALS patients vs PSP patients in the pons (right). Inlay in the lower right corner: ROI analysis of the pontine region: FA analysis of a ROI (r = 15 mm) at MNI 0/−28/−23 revealed a significant FA reduction of PBP patients compared with ALS patients.
3.2. Differences of FA in the specific tract systems
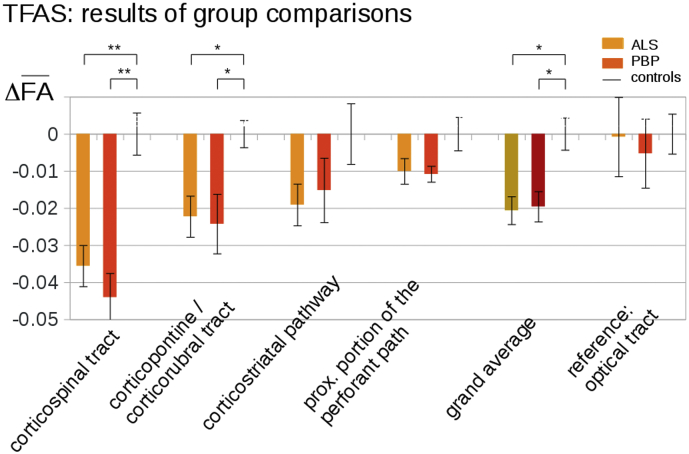
The hypothesis-guided analysis of the FA differences in the ALS-related tract systems by use of TFAS showed significant differences of the averaged FA values between the ALS and control groups as well as between the PBP and the control groups; both the ALS and PBP groups vs. the control group showed the most prominent FA alterations in the CST, followed by FA reductions in the corticorubral and corticopontine tracts (i.e., tracts related to ALS stages 1 and 2) (Fig. 2). Further FA reductions that were, however, not significant at the group level could be observed for the corticostriatal pathway and for the proximal portion of the perforant path (i.e., the tracts related to ALS stages 3 and 4) in PBP and ALS patients each compared to controls. For the grand average of the stage-related tract systems, significant FA reductions were observed for PBP patients and ALS patients compared to controls. No significant FA alterations were found for any group comparison in the reference path. A summary of all alterations in the tract systems at the group level is provided in Table 2.
Fig. 2.
Tractwise fractional anisotropy statistics (TFAS) of FA maps at the group level for ALS patients, PBP patients, and controls. TFAS demonstrated significant regional FA reductions in ALS-related tract systems and in the grand average between ALS patients and controls as well as between PBP patients and controls. No significant alterations between groups were observed in the reference tract. * p < .05, ** p < .001.
Table 2.
Cluster results of WBSS (thresholded at FDR-corrected p < .05). MNI, Montreal Neurological Institute brain atlas; FDR, false discovery rate; CST, corticospinal tract.
| No. | Size/mm3 | Mni of maximum (x y z) | Hemi-sphere | Average p (fdr-corrected) | Anatomical localization |
|---|---|---|---|---|---|
| ALS vs. controls | |||||
| 1 | 15,982 | 24 -24 28 | R | <0.000001 | CST |
| 2 | 9529 | -19 -16 -1 | L | <0.000001 | lower CST |
| 3 | 4906 | -26 -23 35 | L | <0.000001 | upper CST |
| PBP vs. controls | |||||
| 4 | 20,773 | 25 -18 30 | R | <0.000001 | CST |
| 5 | 8946 | -15 -14 36 | L | <0.000001 | CST |
| 6 | 4917 | -5 -29 -5 | R | <0.000001 | pons |
| PBP vs. ALS | |||||
| 7 | 2556 | -4 -28 -24 | R/L | <0.00001 | pons |
3.3. ALS staging at the individual level
ALS staging categorization was performed for the PBP and ALS patients: 87% of the PBP patients were stageable and 91% of the ALS patients were categorised into ALS stages (similar to previous studies (Kassubek et al., 2018)). Of the ALS patients, 30% were in ALS stage 1, 13% in ALS stage 2, 35% in ALS stage 3, and 13% in ALS stage 4. The distribution of ALS stages for PBP patients was similar, i.e., 23% were in ALS stage 1, 14% in ALS stage 2, 9% in ALS stage 3, and 41% in ALS stage 4.
4. Discussion
This study used DTI to investigate patients with a MND with the clinical phenotype of PBP. An unbiased whole brain-based approach demonstrated alterations (i.e., FA reductions) in multiple cerebral areas with a focus on the corticospinal tracts in these patients – similar to patients with ALS, but remarkably with an additional cluster in the pons, most probably as a correlate of the clinically prominent brainstem involvement. In vivo neuropathological staging by TOI-based DTI analysis (Kassubek et al., 2014; Kassubek et al., 2018; Müller et al., 2018a, Müller et al., 2018b, Müller et al., 2018c) demonstrated that patients with PBP showed a pattern of microstructural alterations in corticofugal tracts identical with those seen in ALS. In summary, patients with MND of the PBP phenotype showed the same tract involvement as ALS according to the proposed neuropathological staging in support of the hypothesis that PBP shares the cerebral involvement pattern with ALS and is, as such, an ALS sub-phenotype.
In this study, we used the definition of PBP according to the definition of Chiò and colleagues (Chiò et al., 2011) with an isolated bulbar onset (dysarthria, dysphagia, tongue wasting with fasciculation) over 6 months at symptom onset. These rather strict inclusion criteria are in accordance with the definition by Burrell, Vucic and Kiernan as a bulbar onset presentation with an absence of limb progression over an initial six-month period (Burrell et al., 2011). There is a general consensus in the ALS community that in PBP, the lowest motor neurons of the brain stem are most affected, causing slurred speech and difficulty in chewing and swallowing.
The results of the current DTI study are in accordance with the generally accepted clinical view that PBP patients, who have been properly diagnosed, almost universally turn out to develop ALS (Agosta et al., 2015). In detail, the unbiased WBSS analysis of all significant changes in the brain at group level demonstrated a pattern in PBP that included the core elements of the white matter areas shown to be associated with ALS patients in previous studies with the CST as the most prominent anatomical structure corresponding to ALS-stage 1, in agreement with a recent meta-analysis of DTI studies (Gorges et al., 2018). In addition, the group analysis of the patients with PBP revealed a separate cluster of regional FA reduction that was not observed in the control group of ‘classical’ ALS patients and was the only region that differed when both groups were compared, i.e., a distinct area of white matter damage in the lower pons. To the best of our knowledge, such a regional focus of white matter alterations has not been reported in MND patients with prominent bulbar symptoms until now and seems to indicate that the neurodegenerative process in this clinical phenotype might have a focus in the lower brainstem. This finding can be regarded as a hint to the focality of onset of PBP and should be further investigated in future longitudinal studies.
The aim of the present study was to test the hypothesis that PBP shows the pattern of affected specific cortico-efferent white matter tracts corresponding to the ALS neuropathological spreading pattern (Kassubek et al., 2018), thereby supporting the assumption that PBP is a variant of ALS with a specific clinical phenotype. Indeed, it could be demonstrated that PBP and ‘classical’ ALS shared the same pattern of tract involvement by the application of the hypothesis-guided TOI-based in vivo transfer of the neuropathologically-defined cerebral propagation scheme for ALS (Braak et al., 2013; Kassubek et al., 2014). This result is consonant with the clinical observation that PBP patients turn out to have ALS in the vast majority of cases (Agosta et al., 2015). The sequential involvement of the tract systems according to the ALS-associated pTDP-43 pathology pattern in the brain could not be directly assessed in this cross-sectional study, but it could be shown in a tract-by-tract view that the FA differences in the tracts corresponding to the stages 1 and 2 are higher when compared to controls than those in the tracts corresponding to stages 3 and 4.
On the basis of this neuroimaging study, we believe that the proposed staging scheme for ALS (Braak et al., 2013) is valid also for PBP patients, who should, as a result, receive an appropriate therapy, including the same access to health care systems and the opportunity to be enrolled in clinical trials as ALS patients. Our present findings are an analogy of the demonstration of corticofugal tract involvement in other restricted ALS phenotypes, namely, both fast progressive lower motor neuron disease (Rosenbohm et al., 2016; Müller et al., 2018a) and PLS (Müller et al., 2018b; Müller et al., 2018c).
This study was not without limitations. First, the neuropathological confirmation of the neuroimaging transfer of the ALS propagation scheme in the brain by autopsy results was not available for the patients in this study. Second, all patients had a prominent bulbar syndrome with progressive motor cranial nerve dysfunction and associated dysphagia and bulbar dysarthria when the MRI data were acquired; however, most of the patients then already showed beginning spinal symptoms with fasciculations and paresis and not still an isolated bulbar phenotype at the time of scanning. However, (mild) spinal symptoms after six months are compatible with the clinical characteristics of PBP. We consider this clinical constellation not to be a restriction of the conclusions since the aim of the study was not to differentiate between etiologies of bulbar syndromes (Manole et al., 2014) – although the technique might have some potential for a classification of a given individual patient with a bulbar syndrome as having ALS and thus excluding other etiologies, this (clinical) application has to await future studies on its sensitivity and specificity in larger (multi-centre) studies. Finally, the MRI acquisitions were performed on a 1.5 Tesla scanner. Imaging by use of a scanner with a higher field strength would result in a better signal-to-noise ratio. However, previous studies have shown that a higher signal-to-noise ratio (by use of 3 T data) did not modify the results of tract classification (Kassubek et al., 2014).
In summary, the tract-specific DTI analysis demonstrated the same alterations of ALS-related tract systems for PBP and ALS when compared with controls and showed no differences for the comparison between each other. It seems safe to conclude that the TOI-based imaging of the neuropathologically proposed sequential progression of ALS in PBP supported the hypothesis that PBP, in accordance with the latest revision of the El Escorial criteria, is a restricted phenotype of ALS (Ludolph et al., 2015). The stereotypical pathoanatomy in the brain on the one hand and the distinct clinical phenotypes on the other hand, like also in primary lateral sclerosis or in the lower motor neuron disease variant, might guide in our understanding of potential modifiers of the clinical presentation of ALS in the future.
Author contributions
Hans-Peter Müller: Study concept and design, data analysis and interpretation of data, critical revision of manuscript for intellectual content.
Martin Gorges: Data collection, data analysis, critical revision of manuscript for intellectual content.
Kelly del Tredici: Interpretation of data, critical revision of manuscript for intellectual content.
Albert Ludolph: Interpretation of data, critical revision of manuscript for intellectual content.
Jan Kassubek: study concept and design, interpretation of data, study supervision, drafting of manuscript.
Author disclosures
All authors: none for this study.
Acknowledgments
Acknowledgements
Sonja Fuchs is thankfully acknowledged for her great help in the acquisition of MRI data. The authors would like to thank the Ulm University Center for Translational Imaging MoMAN for its support.
Funding
This study was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG Grant Number LU 336/15-1) and the German Network for Motor Neuron Diseases (BMBF 01GM1103A).
Statement
All human studies have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
References
- Agosta F., Al-Chalabi A., Filippi M. The El Escorial criteria: strengths and weaknesses. Amyotroph Lateral Scler. Frontotemporal Degener. 2015;16:1–7. doi: 10.3109/21678421.2014.964258. [DOI] [PubMed] [Google Scholar]
- Braak H., Brettschneider J., Ludolph A.C. Amyotrophic lateral sclerosis - a model of corticofugal axonal spread. Nat. Rev. Neurol. 2013;9:708–714. doi: 10.1038/nrneurol.2013.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H., Neumann M., Ludolph A.C. Does sporadic amyotrophic lateral sclerosis spread via axonal connectivities? Neurol. Int. Open. 2017;1:E136–E141. [Google Scholar]
- Brettschneider J., Del Tredici K., Toledo J.B. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann. Neurol. 2013;74:20–38. doi: 10.1002/ana.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell J.R., Vucic S., Kiernan M.C. Isolated bulbar phenotype of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 2011;12:283–289. doi: 10.3109/17482968.2011.551940. [DOI] [PubMed] [Google Scholar]
- Cedarbaum J.M., Stambler N., Malta E. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III) J. Neurol. Sci. 1999;169:13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- Chiò A., Calvo A., Moglia C., Mazzini L., Mora G., PARALS Study Group Phenotypic heterogeneity of amyotrophic lateral sclerosis: a population based study. J. Neurol. Neurosurg. Psychiatry. 2011;82:740–746. doi: 10.1136/jnnp.2010.235952. [DOI] [PubMed] [Google Scholar]
- Finegan E., Chipika R.H., Shing S.L.H., Hardiman O., Bede P. Primary lateral sclerosis: a distinct entity or part of the ALS spectrum? Amyotroph Lateral Scler. Frontotemporal Degener. 2019;20:133–145. doi: 10.1080/21678421.2018.1550518. [DOI] [PubMed] [Google Scholar]
- Genovese C.R., Lazar N.A., Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gorges M., Del Tredici K., Dreyhaupt J. Corticoefferent pathology distribution in amyotrophic lateral sclerosis: in vivo evidence from a meta-analysis of diffusion tensor imaging data. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-33830-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassubek J., Müller H.-P. Computer-based magnetic resonance imaging as a tool in clinical diagnosis in neurodegenerative diseases. Expert. Rev. Neurother. 2016;16:295–306. doi: 10.1586/14737175.2016.1146590. [DOI] [PubMed] [Google Scholar]
- Kassubek J., Müller H.P., Del Tredici K. Diffusion tensor imaging analysis of sequential spreading of disease in amyotrophic lateral sclerosis confirms patterns of TDP-43 pathology. Brain. 2014;137:1733–1740. doi: 10.1093/brain/awu090. [DOI] [PubMed] [Google Scholar]
- Kassubek J., Müller H.P., Del Tredici K. Imaging the pathoanatomy of amyotrophic lateral sclerosis in vivo: targeting a propagation-based biological marker. J. Neurol. Neurosurg. Psychiatry. 2018;89:374–381. doi: 10.1136/jnnp-2017-316365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunimatsu A., Aoki S., Masutani Y. The optimal trackability threshold of fractional anisotropy for diffusion tensor tractography of the corticospinal tract. Magn. Reson. Med. Sci. 2004;3:11–17. doi: 10.2463/mrms.3.11. [DOI] [PubMed] [Google Scholar]
- Le Bihan D., Mangin J.F., Poupon C. Diffusion tensor imaging: concepts and applications. J. Magn. Reson. Imaging. 2001;13:534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- Ludolph A.C., Brettschneider J. TDP-43 in amyotrophic lateral sclerosis - is it a prion disease? Eur. J. Neurol. 2015;22:753–761. doi: 10.1111/ene.12706. [DOI] [PubMed] [Google Scholar]
- Ludolph A., Drory V., Hardiman O. A revision of the El Escorial criteria - 2015. Amyotroph Lateral Scler. Frontotemporal Degener. 2015;29:1–2. doi: 10.3109/21678421.2015.1049183. [DOI] [PubMed] [Google Scholar]
- Manole A., Fratta P., Houlden H. Recent advances in bulbar syndromes: genetic causes and disease mechanisms. Curr. Opin. Neurol. 2014;27:506–514. doi: 10.1097/WCO.0000000000000133. [DOI] [PubMed] [Google Scholar]
- Müller H.P., Kassubek J. Diffusion tensor magnetic resonance imaging in the analysis of neurodegenerative diseases. J. Vis. Exp. 2013;(77) doi: 10.3791/50427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller H.P., Unrath A., Ludolph A.C. A, preservation of diffusion tensor properties during spatial normalisation by use of tensor imaging and fibre tracking on a normal brain database. Phys. Med. Biol. 2007;52:N99–109. doi: 10.1088/0031-9155/52/6/N01. [DOI] [PubMed] [Google Scholar]
- Müller H.P., Unrath A., Sperfeld A.D. Diffusion tensor imaging and tractwise fractional anisotropy statistics: quantitative analysis in white matter pathology. Biomed. Eng. Online. 2007;6:42. doi: 10.1186/1475-925X-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller H.P., Unrath A., Riecker A., Pinkhardt E.H., Ludolph A.C., Kassubek J. Intersubject variability in the analysis of diffusion tensor images at the group level: fractional anisotropy mapping and fiber tracking techniques. Magn. Reson. Imaging. 2009;27:324–334. doi: 10.1016/j.mri.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Müller H.P., Turner M.R., Grosskreutz J. A large-scale multicentre cerebral diffusion tensor imaging study in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry. 2016;87:570–579. doi: 10.1136/jnnp-2015-311952. [DOI] [PubMed] [Google Scholar]
- Müller H.P., Agosta F., Gorges M. C, Cortico-efferent tract involvement in primary lateral sclerosis and amyotrophic lateral sclerosis: a two-centre tract of interest-based DTI analysis. Neuroimage Clin. 2018;20:1062–1069. doi: 10.1016/j.nicl.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller H.P., Gorges M., Kassubek R., Dorst J., Ludolph A.C., Kassubek J. Identical patterns of cortico-efferent tract involvement in primary lateral sclerosis and amyotrophic lateral sclerosis: a tract of interest-based MRI study. Neuroimage Clin. 2018;18:762–769. doi: 10.1016/j.nicl.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller H.P., Agosta F., Riva N. Fast progressive lower motor neuron disease is an ALS variant: a two-centre tract of interest-based MRI data analysis. Neuroimage Clin. 2018;17:145–152. doi: 10.1016/j.nicl.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbohm A., Müller H.P., Hübers A. Corticoefferent pathways in pure lower motor neuron disease: a diffusion tensor imaging study. J. Neurol. 2016;263:2430–2437. doi: 10.1007/s00415-016-8281-2. [DOI] [PubMed] [Google Scholar]
- Unrath A., Müller H.P., Riecker A. Whole brain-based analysis of regional white matter tract alterations in rare motor neuron diseases by diffusion tensor imaging. Hum. Brain Mapp. 2010;31:1727–1740. doi: 10.1002/hbm.20971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries B.S., Rustemeijer L.M.M., Bakker L.A. Cognitive and behavioural changes in PLS and PMA:challenging the concept of restricted phenotypes. J. Neurol. Neurosurg. Psychiatry. 2019;90:141–147. doi: 10.1136/jnnp-2018-318788. [DOI] [PubMed] [Google Scholar]