Abstract
During treatment with protein therapeutics, such as monoclonal antibodies, the development of anti-drug antibodies is a serious side-effect of modern pharmacology. Anti-drug antibodies are produced as the number and exposure to therapeutic proteins increase. In this context, less immunogenic responses could diminish these noxious effects. Biophysical characterization of antigens, that is size, chemical composition, physical form, and degrability, are known to influence the outcome of immune responses. Here, using chemical modification, we have prepared oligomers of hen egg lysozyme (HEL), 3- to 5-mer, as a typical antigen in immunology and evaluated the efficacy as a tolerogen in HEL-specific antibody responses. Our results clearly demonstrated that pre-exposed the HEL-oligomers into mice effectively suppressed HEL-specific IgG responses regardless of the cross-linking mode. Therefore, the oligomerization is a method to induce tolerogenicity of proteins and may emerge as a promising strategy to control the production of undesirable anti-protein drug antibodies.
Keywords: Anti-drug antibodies, Hen egg white lysozyme, Immune tolerance, Oligomerization
Highlights
-
•
Pre-exposure of protein oligomers induced specific humoral immune tolerance.
-
•
Specific humoral immune tolerance was independent of cross-linking mode.
-
•
Immune tolerance may be involved in the high solubility of protein oligomers.
1. Introduction
Over the years, therapeutic proteins, such as monoclonal antibodies, cytokines, growth factors, hormones, and enzymes, have been used in usual clinical settings and are now considered to offer important treatment options for many diseases [1,2]. Recently, therapeutic immunoglobulins with engineered variable regions have been produced in order to improve antigen binding properties [3], pharmacokinetics [4] and pharmaceutical properties [5,6]. However, repeated administration of protein therapeutics, whether natural or recombinant, often leads to the induction of undesirable anti-drug antibodies (ADAs), which often neutralize the effect of the drug [[7], [8], [9], [10], [11], [12]]. A recent review paper reported from 2018 indicated that the number of reports on immunogenicity have more than doubled in the last 5 years [13], whereas protein therapeutics are increasingly developed in order to treat disease with unmet medical needs. However, so far, there are few reports describing strategies to depress ADAs.
The form of protein antigens can profoundly affect the quality of the ensuing immune responses, which can range from tolerance to lasting immunity. In general, purified soluble proteins are less immunogenic, whereas particulate, aggregated, or denatured proteins are more immunogenic. It has been demonstrated that oligomerized or polymerized peptide/protein antigens have different capacities to induce immune responses. Polymerization/oligomerization of allergens into higher molecular weight polymers can improve the potential for immunotherapy by reducing side effects while retaining immunogenicity [14]. Goodnow and his colleagues demonstrated that soluble hen egg lysozyme (HEL) and membrane-bound HEL induce anergy and clonal deletion, respectively, in an anti-HEL BCR expressing transgenic B cell [15], suggesting that the extent of BCR ligation determines the outcome of tolerance status in B cells. Stienekemeier et al. reported that a polypeptide oligomer harboring 16 repeats of the neuritogenic T cell epitope is highly effective for suppression of experimental autoimmune neuritis (EAN) mediated by autoantigen-specific T cells [16]. Moreover, it is also reported that a 4-mer self-peptide protects animals from experimental autoimmune diabetes by targeting pathogenic autoreactive CD4 T cell responses and inducing regulatory T cells [17], and an amino acid copolymer, glatiramer acetate [poly-(Y,E,A,K)n], ameliorates experimental autoimmune encephalomyelitis by inducing the copolymer-specific IL-10-producing regulatory T cells [18], suggesting that oligomerization of T cell epitope is also effective to control the undesirable T cell responses. Although it is clear that repetitive epitopes in a single molecule or a cell play a critical role for tolerizing anitgen-specific lymphocyte responses, it is still unclear how we can prepare an oligomerized/polymerized protein antigen retaining original T cell and B cell epitopes, which could effectively suppress humoral immunity.
In this study, we have prepared HEL-oligomers consisting of three to five HEL molecules, to examine the tolerogenicity for antigen-specific antibody responses. We demonstrate that pre-exposure of HEL-oligomers into mice effectively suppresses anti-HEL humoral responses.
2. Materials and methods
2.1. Animals
Female BALB/c mice were obtained from Japan SLC, inc. (Shizuoka, Japan) and were immunized at 7 weeks of age. All animal experiments were conducted according to relevant national and international guidelines ‘Act on Welfare and Management of Animals’ (Ministry of Environment of Japan) and ‘Regulation of Laboratory Animals’ (Kyushu University), and under the protocols approved by the Institutional Animal Care and Use committee review panels at Kyushu University (Permit Number: A20-023-0).
2.2. Materials
Five-times-recrystallized HEL was a gift from QP (Tokyo, Japan). Ovalbumin (OVA) was purchased from Sigma (Mo, USA). 2,2′-Dithiodipyridine was obtained from Tokyo Kasei Kogyo (Tokyo, Japan). 1-Ethyl-3-[3-(dimethylamino)propyl]-carbodiimide hydrochloride (EDC) was purchased from Nakalai Tesque (Kyoto, Japan). N-succinimidyl-3-(2-pyridyldithiol)propionate (SPDP) and disuccinimidyl suberate (DSS) were purchased from Thermo Scientific (Rockford, USA). BioGel P4 was a product of Bio-Rad Laboratories (Richmond, CA). CM-toyopearl was obtained from Tosoh (Kyoto, Japan). Sephadex G-100 was purchased from GE Healthcare Biosciences. All other chemicals used were of the highest quality commercially available.
2.3. Preparation of Asp 101-ss-py HEL
First, 2-(2-pyridyldithio) ethylamine was prepared according to a previous report [19]. Briefly, 300 mg of 2,2′-dithiodipyridine and 140 mg of 2-mercaptoethylamine hydrochloride were dissolved in a mixture of 20 ml of H2O and 20 ml of methanol, and stirred for 30 min. After evaporation of methanol, the remaining aqueous solution was washed with 20 ml of chloroform five times to remove unreacted 2,2′-dithiodipyridine and the undesired product, pyridine 2-thione. The aqueous layer was neutralized by addition of a small amount of ammonium bicarbonate and then extracted with 30 ml of chloroform twice. The chloroform extracts were combined and extracted with 50 ml of 0.01 M HCl. The HCl extract was lyophilized to give 54 mg of 2-(2-pyridyldithio) ethylamine dihydrochloride as a slightly yellow solid.
Second, the β-carboxyl group of Asp 101 in HEL was selectively modified with 2-(2-pyridyldithio) ethylamine (Asp 101-ss-py HEL) in the presence of EDC [19]. Briefly, 200 mg of HEL in 20 ml of H2O containing 54 mg of 2-(2-pyridyldithio) ethylamine was stirred with 10 mg of EDC at pH 5.0 and at room temperature for 2.5 h. The mixture was thoroughly dialyzed against distilled water, and then the dialysate was applied to a cation-exchange column, CM toyopearl, equilibrated in 20 mM phosphate buffer, pH 7.0. The column was eluted with a linear gradient of 500 ml of 20 mM phosphate buffer, pH 7.0, containing 0.1 M NaCl and 500 ml of the same buffer containing 0.4 M NaCl. The peak of Asp 101-ss-py HEL appeared after the peak of unreacted HEL consistent with a previous report [19]. The fraction of Asp 101-ss-py HEL was collected and lyophilized.
2.4. Preparation of SPDP-HEL
Amino groups of HEL were modified with SPDP disulfide-containing linkages that can be cleaved later with reducing agents. SPDP (10.9 mg) dissolved in 1.75 ml of dimethyl sulfoxide (DMSO) was added to 14 ml of PBS solution containing HEL (100 mg) and stirred for 60 min at room temperature. The mixture was thoroughly dialyzed against 10% aqueous acetic acid and lyophilized.
2.5. Preparation of HEL-oligomers with Asp 101-ss-py HEL and SPDP-HEL
HEL-oligomers were prepared by covalent linkages between Asp 101-ss-py HEL and SPDP-HEL, namely, HEL-oligomers (PDE + SPDE). Asp 101-ss-py HEL (30 mg) was dissolved in 1.5 ml of 0.1 M sodium acetate buffer, pH 4.5, containing 0.1 M of NaCl and the protein was reduced with 5 mg of dithiothreitol (DTT) for 5 min at room temperature. The reaction products of low-molecular weight were removed by gel-filtration on a BioGel P-4 column equilibrated in 0.1 M potassium phosphate buffer, pH 7.5. The protein fraction was mixed with 6 mg SPDP-HEL and reacted for 24 h at room temperature. After the protein fraction was concentrated with 65% saturated ammonium sulfate, the protein was loaded to a gel-filtration column of Sephadex G-100 (1.5 × 150 cm) equilibrated with 10% aqueous acetic acid.
2.6. Preparation of HEL-oligomers with DSS
HEL-oligomers were prepared with DSS according to the method of Montesano et al. [20], that we termed HEL-oligomers (DSS). Briefly, 30 mg of HEL were dissolved in 6 ml of 20 mM sodium phosphate buffer, pH 7, and 53 μl of 20 mM DSS in DMSO was added to the HEL solution every 30 min for total 10 times at room temperature. After the final addition of DSS, the mixture was stirred for additional 2 h and precipitated with 65% saturated ammonium sulfate. The precipitated protein was separated with a Sephadex G-100 column (1.5 cm × 150 cm) equilibrated with 10% aqueous acetic acid.
2.7. Tolerance induction and immunization
Prior to immunization, mice were given an intraperitoneal (i.p.) injection of 100 μg of HEL-oligomers, HEL or saline on day −7. On day 0, 7, 14, 21, and 28, all mice were immunized i.p. with 50 μg of native HEL emulsified in complete Freund's adjuvant (CFA). Blood samples were drawn from the orbital sinus into capillary tubes every week. Serum total IgG levels to each protein antigen were determined by enzyme-linked immunosorbent assay (ELISA), as the previously described [21].
2.8. Quantification of the amount of HEL-oligomers in spleen cells
Mice were injected i.p. with 100 μg of HEL-oligomers on day −7 and were immunized with HEL in CFA on day 0, as described above. On day 1 and 15, spleen cells were harvested from mice and the residual amount of HEL-oligomers in spleen cells was measured by competitive ELISA according to a previously described method [22]. The same experiments were carried out in triplicate, i.e. by employing three mice.
2.9. Statistical analysis
P-values were calculated using Student's t-test, and statistical significance was set at P < 0.05. Results were expressed as mean ± SE.
3. Results
3.1. Preparation of HEL-oligomers (PDE + SPDP) by intermolecular disulfide bonds
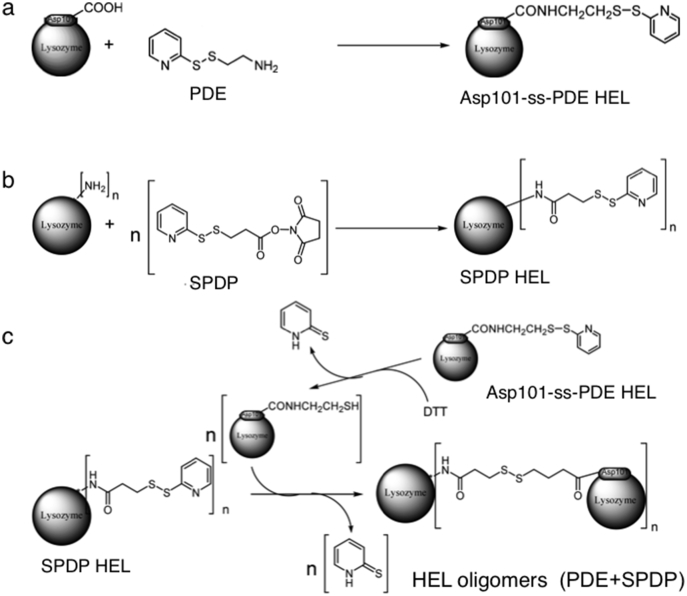
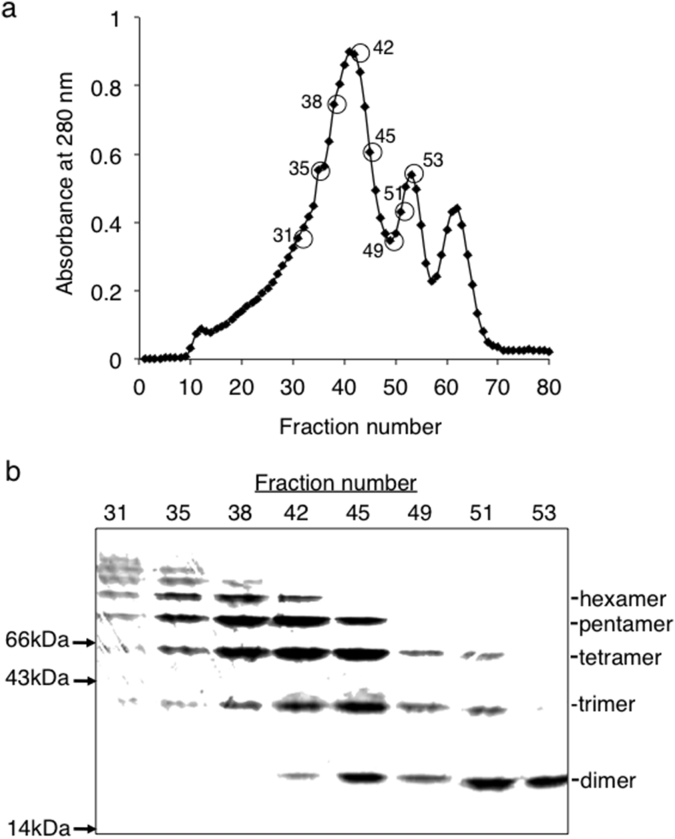
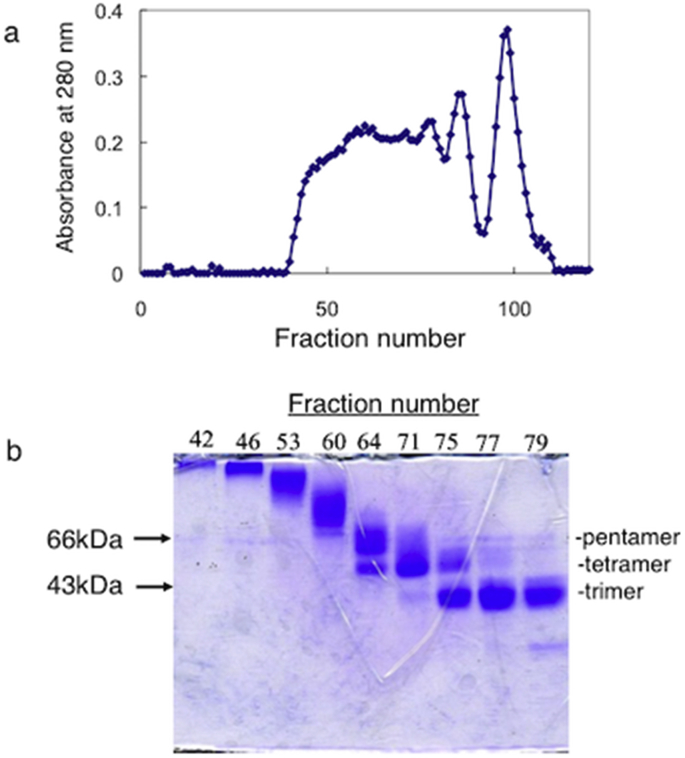
Oligomerization of HEL by artificial intermolecular disulfide bonds was carried out according to the scheme in Fig. 1. Asp101 in HEL was modified with PDE in the presence EDC, resulting in the production of Asp101-ss-PDE-HEL. Amino groups in HEL were separately modified with SPDP, generating SPDP-HEL. After the partial reduction of Asp101-ss-PDE-HEL using a low concentration of DTT, the freed thiol group linked to Asp101 in HEL reacted with SPDP-HEL under weak acidic conditions. The reaction mixture was dialyzed against distilled water and the sample subsequently applied to a Sephadex G-100 column (1.5 × 150 cm). The elution was monitored at 280 nm (Fig. 2a). The molecular species in fractions 31, 35, 38, 42, 45, 49, 51 and 53 were examined by SDS-PAGE (Fig. 2b). We collected and pooled fractions 35–42, which mainly contained trimer, tetramer and pentamer forms of HEL, but not monomer, and dialyzed then against distilled water followed by lyophilization. The product was termed HEL-oligomers (PDE + SPDP). We obtained about 7 mg of HEL-oligomers (PDE + SPDP), sufficient for the experiments carried out below.
Fig. 1.
Preparation of chemically modified HELs. a. Asp101-SS-PDE-HEL, b. SPDP-HEL, c. HEL-oligomers.
Fig. 2.
Purification of HEL-oligomers by gel-filtration chromatography. After the reaction of Asp101-ss-PDE-HEL with SPDP-HEL, the mixture was applied to the column of Sephadex G-100. a. Elution profile of HEL-oligomers. b. A SDS-PAGE profile of HEL-oligomers in the indicated of the fractions in gel-filtration chromatography. Fractions from 35 to 42 were pooled, lyophilized, and used as HEL-oligomers.
3.2. Pre-exposure with HEL-oligomers (PDE + SPDP) effectively tolerizes HEL-specific humoral responses
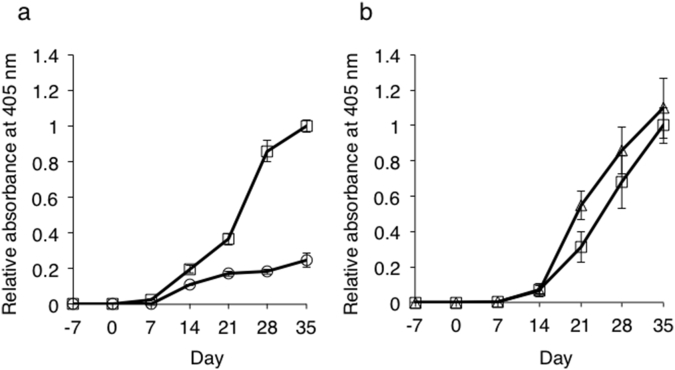
We previously demonstrated that unmodified native HEL does not possess tolerogenic capabilities to suppress HEL-specific antibody responses [20]. To determine whether HEL-oligomers (PDE + SPDP) elicit different tolerogenic responses and produce more effective induction of antigen-specific tolerance, mice were pre-exposed to HEL-oligomers (PDE + SPDP) or saline solution, followed by challenge-immunization with HEL in CFA every 7 days to determine anti-HEL serum IgG responses. Pre-exposure with HEL-oligomers (PDE + SPDP) significantly suppressed HEL-specific IgG responses (Fig. 3a), indicating that HEL-oligomers (PDE + SPDP) act as a tolerogen controlling HEL-specific humoral responses. Moreover, pre-exposure with the same amount of HEL than that employed for HEL-oligomers (PDE + SPDP) did not suppress HEL-specific IgG responses (Supplemental Fig. 1). To confirm the antigen specificity, mice pre-exposed with HEL-oligomers (PDE + SPDP) or with saline were challenge-immunized with OVA in CFA every 7 days. Anti-OVA IgG levels in the sera of mice pre-exposed with HEL-oligomers (PDE + SPDP) were almost comparable to those in mice pre-exposed with saline (Fig. 3b), suggesting that the tolerogenic effect elicited by HEL oligomers is specific for HEL.
Fig. 3.
Tolerogenic activity of HEL-oligomers (PDE + SPDP). Two groups of five BALB/c mice were pre-exposed with saline or HEL-oligomers (PDE + SPDP) on day −7 and challenged with native HEL (a) or OVA (b) on day 0, 7, 14, 21, and 28. a. Serum anti-HEL IgG levels in mice pre-exposed with HEL-oligomers (○) or saline (□). a. Serum anti-OVA IgG levels in mice pre-exposed with HEL oligomers (△) or saline (□). Serum IgG antibody levels to HEL (a) and OVA (b) were measured by ELISA. The average concentration of IgG was above 10 mg/ml in sera of mice pretreated with saline on day 35. To detect the antibody response, the sera was diluted between 400- and 6400-fold. P-values were calculated using Student's t-test. Statistical significance was set at P < 0.05.
3.3. Suppression of humoral responses by HEL-olimomers prepared with DSS
Disulfide bonds in proteins were reduced by a gamma interferon-inducible lysosomal thiol reductase (GILT) before degradation by proteases in endosomes [23]. Since the HEL-oligomers composed of PDE and SPDP are held together by intermolecular disulfide bonds, we examined how the intermolecular disulfide bonds affect the tolerogenic property of HEL-oligomers. To examine the question, we employed a different chemical crosslinker termed DSS. DSS crosslinks amino groups between HELs generating HEL-oligomers with a profile similar to that of disulfide-based oligomers (Fig. 4a). Reaction mixtures of HEL with DSS were applied to a Sephadex G-100 column, and HEL-oligomers (DSS), mainly containing trimers, tetramers and pentamers of HEL, but not monomer, were obtained (Supplementary Fig. 2). To determine whether the tolerance established by HEL-oligomers (DSS) was similar to that elicited by HEL-oligomers (PDE + SPDP), mice pre-exposed with HEL-oligomers (DSS) or saline were challenge-immunized with HEL in CFA every 7 days. Anti-HEL IgG levels were monitored to examine the tolerogenicity of HEL-oligomers (DSS) (Fig. 4). This result indicates that HEL-oligomers (DSS) has a comparable tolerogenic capacity to that of HEL-oligomers (PDE + SPDP) (Fig. 3, Fig. 4) and that the degree of oligomerization, rather than the chemical linkages between HELs, plays a critical role in developing tolerogenicity.
Fig. 4.
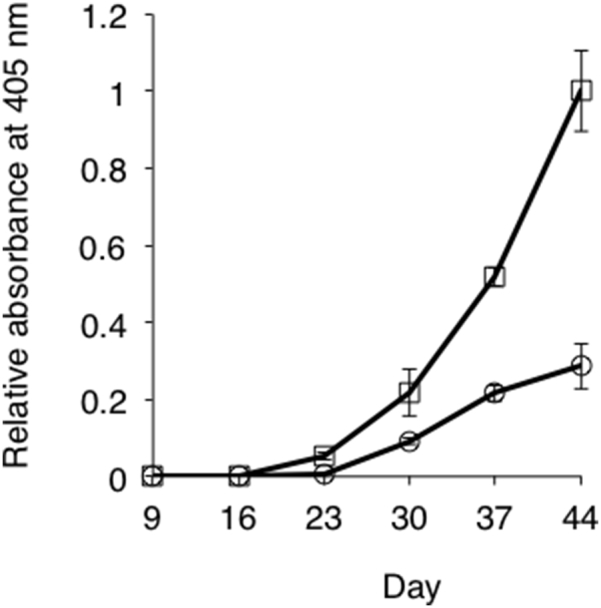
Tolerogenic activity of HEL-oligomers (DSS). Two groups of five BALB/c mice were pre-exposed with saline or HEL-oligomers (DSS) and challenged with native HEL as in Fig. 3. Serum anti-HEL IgG levels in mice pretreated with HEL oligomers (DSS) (○) or with saline (□). P-values were calculated using Student's t-test. Statistical significance was set at P < 0.05.
3.4. Stability of HEL-oligomers (PDE + SPDP) in vivo
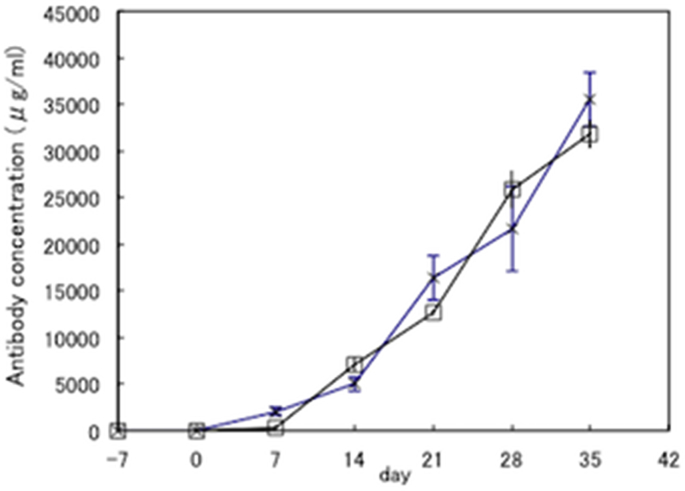
Our previous report demonstrated that monomethoxypolyethylene glycol-conjugated HEL (mPEG-HEL) acts as a potent tolerogen, which is mediated by the long half-life in vivo, (10−8 M of mPEG-HEL in serum is detected at 28 days after injecting 50 μg of mPEG-HEL into mice [22]). Therefore, we examined whether the tolerogenic capacity of HEL-oligomers used in this study was derived from its higher stability or long half-life in vivo. To examine this question, the amount of HEL-oligomers (PDE + SPDP) in spleen cells was determined by competitive ELISA (Fig. 5). HEL-oligomers (PDE + SPDP) were detected on day 1 but not on day 15, suggesting that the tolerance induced by HEL-oligomers (PDE + SPDP) does not originate from their long circulation half-life.
Fig. 5.
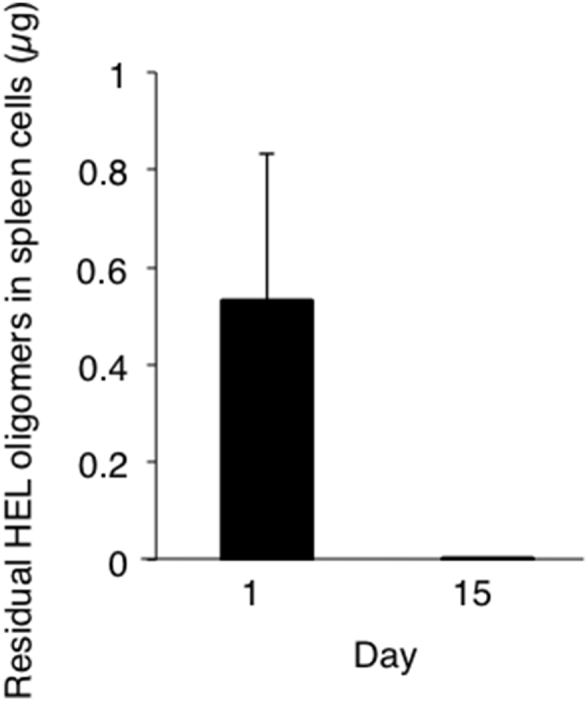
Blood concentration of HEL-oligomers (PDE + SPDP). Sera were obtained from mice given a single administration with 100 μg HEL-oligomers (PDE + SPDP) on day −7 followed by immunization with HEL in CFA on day 0. On day 1 and 15, spleen cells were harvested from mice and the residual amount of HEL-oligomers in spleen cells were determined by competitive ELISA. Each point represents the mean concentration (M) and standard deviation of 3 mice.
4. Discussion
A quarter of century ago, De Wit et al. demonstrated that the balance of type 1/type 2 helper T cell responses changed after removing deaggregated gamma globulins by centrifugation at 150,000 g × 180 min [24]. Aggregation states altered by the expression and purification procedures [25] or by mutation of cysteine residues in vaccine candidate proteins [26] resulted in different immunogenic properties. Moreover, a recent report examining hydrodynamic diameter of hepatitis B surface antigen in the presence of different salts demonstrated that the oligomerization state had a significant impact in antigenicity: aggregated antigen was significantly more antigenic than monomer or low oligomerization state [27]. To overcome protein aggregation or antigenicity, so far, numerous efforts have been undertaken to reduce them by chemical modification with low immunogenic polymers such as polyethylene glycol (PEG) [21,22,[28], [29], [30], [31]].
In this study, we have showed a tolerogenic capacity of oligomerized HEL that is effective to suppress humoral responses, in contrast to previous reports in which protein aggregation was closely related to greater immune responses [[25], [26], [27]]. Since HEL has a high pKa 10–11, the oligomerized HELs employed here are likely to remain soluble after cross-linking, resulting in no formation of aggregated HELs below pH 7 under the immune experimental conditions. HEL-oligomers, both (PDE + SPDP) and (DSS), showed a similar tolerogenic activity in antibody responses, indicating that the mode of chemical crosslink between proteins may not be critical for the tolerogenicity. Thus, the HEL-oligomers (PDE + SPDP) may trigger BCR on the B cell surface before entering into cellular compartments containing GILT, resulting in the induction of anergy or clonal deletion in HEL-specific B cells, as is demonstrated by Goodnow and his colleagues [15].
We have previously shown that antigen-specific tolerance is induced by pre-exposure of pegylated proteins into mice [21,22,30,31]. Those observations are likely to the result of the extended blood half-life of pegylated proteins, but not suppressive cell populations, plays a crucial role for the tolerance based on our paper [22]. This time, the HEL-oligomers (PDE + SPDP) used in this study disappeared from the spleen within 15 days after injection (Fig. 5), indicating that the tolerance mechanism induced by HEL-oligomers is different from that induced by pegylated HEL. The method shown here to induce HEL-specific immune tolerance is novel, although the mechanism remains to be elucidated. As shown in supplementary material, pre-exposure of oligomers of HEL was necessary to develop immune tolerance of HEL-specific humoral response in mice. Therefore, our experiments demonstrate a method to induce tolerogenic function of HEL-specific humoral responses by pre-exposure with oligomeric forms of the antigen.
Recently, a review about the mitigation of immunogenicity of therapeutic proteins were published [32]. For example, the therapeutic potential of antibodies employed in cancer therapy such as pembrolizumab and nivolumab [33] could be compromised by the development of ADAs. Therefore, mitigating the appearance of ADAs is an urgent medical problem that should be resolved as soon as possible. Because of the proliferation of numerous studies citing candidate residues for the preparation of antibody-drug conjugates, we propose that these sites could also be employed to generate oligomers by chemical modification. Using this class of protein oligomers, as described herein, could reduce the development of ADAs and benefit therapeutic outcomes.
Funding
This work was in part supported by a JSPS-KAKENHI (18K19406) (to T.U.).
Acknowledgements
We thank Dr. Jose Caaveiro of Kyshu University for critical readership of the manuscript. We also thank Dr. Abe of our laboratory for giving us useful comments.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2019.100679.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
References
- 1.Chan A.C., Carter P.J. Therapeutic antibodies for autoimmunity and inflammation. Nat. Rev. Immunol. 2010;10:301–316. doi: 10.1038/nri2761. [DOI] [PubMed] [Google Scholar]
- 2.Weiner L.M., Surana R., Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat. Rev. Immunol. 2010;10:317–327. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajpal A., Beyaz N., Haber L., Cappuccilli G., Yee H., Bhatt R.R., Takeuchi T., Lerner R.A., Crea R. A general method for greatly improving the affinity of antibodies by using combinatorial libraries. Proc. Natl. Acad. Sci. U.S.A. 2005;102:8466–8471. doi: 10.1073/pnas.0503543102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Igawa T., Ishii S., Tachibana T., Maeda A., Higuchi Y., Shimaoka S., Moriyama C., Watanabe T., Takubo R., Doi Y., Wakabayashi T., Hayasaka A., Kadono S., Miyazaki T., Haraya K., Sekimori Y., Kojima T., Nabuchi Y., Aso Y., Kawabe Y., Hattori K. Antibody recycling by engineered pH-dependent antigen binding improves the duration of antigen neutralization. Nat. Biotechnol. 2010;28:1203–1207. doi: 10.1038/nbt.1691. [DOI] [PubMed] [Google Scholar]
- 5.Cacia J., Keck R., Presta L.G., Frenz J. Isomerization of an aspartic acid residue in the complementarity-determining regions of a recombinant antibody to human IgE: identification and effect on binding affinity. Biochemistry. 1996;35:1897–1903. doi: 10.1021/bi951526c. [DOI] [PubMed] [Google Scholar]
- 6.Nakano K., Ishiguro T., Konishi H., Tanaka M., Sugimoto M., Sugo I., Igawa T., Tsunoda H., Kinoshita Y., Habu K., Orita T., Tsuchiya M., Hattori K., Yamada-Okabe H. Generation of a humanized anti-glypican 3 antibody by CDR grafting and stability optimization. Anti Canccer Drugs. 2010;21:907–916. doi: 10.1097/CAD.0b013e32833f5d68. [DOI] [PubMed] [Google Scholar]
- 7.Schellekens H. Immunogenicity of therapeutic proteins: clinical implications and future prospects. Clin. Ther. 2002;24:1720–1740. doi: 10.1016/s0149-2918(02)80075-3. [DOI] [PubMed] [Google Scholar]
- 8.Schellekens H. Factors influencing the immunogenicity of therapeutic proteins. Nephrol. Dial. Transplant. 2005;20:3–9. doi: 10.1093/ndt/gfh1092. [DOI] [PubMed] [Google Scholar]
- 9.Porter S. Human immune response to recombinant human proteins. J. Pharm. Sci. 2001;90:1–11. doi: 10.1002/1520-6017(200101)90:1<1::aid-jps1>3.0.co;2-k. AID-JPS1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 10.Kromminga A., Schellekens H. Antibodies against erythropoietin and other protein-based therapeutics: an overview. Ann. N. Y. Acad. Sci. 2005;1050:257–265. doi: 10.1196/annals.1313.027. [DOI] [PubMed] [Google Scholar]
- 11.De Groot A.S., Scott D.W. Immunogenicity of protein therapeutics. Trends Immunol. 2007;28:482–490. doi: 10.1016/j.it.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Harding F.A., Stickler M.M., Razo J., DuBridge R.B. The immunogenicity of humanized and fully human antibodies: residual immunogenicity resides in the CDR regions. mAbs. 2010;2:256–265. doi: 10.4161/mabs.2.3.11641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenberg A.S., Sauna Z.E. Immunogenicity assessment during the development of protein therapeutics. J. Pharm. Pharmacol. 2018;70:584–594. doi: 10.1111/jphp.12810. [DOI] [PubMed] [Google Scholar]
- 14.Larché M., Akdis C.A., Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat. Rev. Immunol. 2006;10:761–771. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- 15.Goodnow C.C. Transgenic mice and analysis of B-cell tolerance. Annu. Rev. Immunol. 1992;10:489–518. doi: 10.1146/annurev.iy.10.040192.002421. [DOI] [PubMed] [Google Scholar]
- 16.Stienekemeier M., Falk K., Rötzschke O., Weishaupt A., Schneider C., Toyka K.V., Gold R., Strominger J.L. Vaccination, prevention, and treatment of experimental autoimmune neuritis (EAN) by an oligomerized T cell epitope. Proc. Natl. Acad. Sci. U.S.A. 2001;98:13872–13877. doi: 10.1073/pnas.241504598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.E1 Piaggio, Mars L.T., Cassan C., Cabarrocas J., Hofstätter M., Desbois S., Bergereau E., Rötzschke O., Falk K., Liblau R.S. Multimerized T cell epitopes protect from experimental autoimmune diabetes by inducing dominant tolerance. Proc. Natl. Acad. Sci. U.S.A. 2007;104:9393–9398. doi: 10.1073/pnas.0610423104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stern J.N., Keskin D.B., Zhang H., Lv H., Kato Z., Strominger J.L. Amino acid copolymer-specific IL-10-secreting regulatory T cells that ameliorate autoimmune diseases in mice. Proc. Natl. Acad. Sci. U.S.A. 2008;105:5172–5176. doi: 10.1073/pnas.0712131105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ueda T., Yamada H., Sakamoto N., Abe Y., Kawano K., Terada Y., Imoto T. Preparation and properties of a lysozyme derivative in which two domains are cross-linked intramolecularly between Trp 62 and Asp101. J. Biochem. 1991;110:719–725. doi: 10.1093/oxfordjournals.jbchem.a123647. [DOI] [PubMed] [Google Scholar]
- 20.Montesano L., Cawley D., Herschman H.R. Disuccinimidyl suberate cross-linked ricin does not inhibit cell-free protein synthesis. Biochem. Biophys. Res. Commun. 1982;109:7–13. doi: 10.1016/0006-291x(82)91558-3. [DOI] [PubMed] [Google Scholar]
- 21.Ito H.O., So T., Hirata M., Koga T., Ueda T., Imoto T. Tolerogenic activity of polyethylene glycol-conjugated lysozyme distinct from that of the native counterpart. Immunology. 1998;93:200–207. doi: 10.1046/j.1365-2567.1998.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.So T., Ito H.O., Hirata M., Ueda T., Imoto T. Extended blood half-life of monomethoxypolyethylene glycol-conjugated hen lysozyme is a key parameter controlling immunological tolerogenicity. Cell. Mol. Life Sci. 1999;55:1187–1194. doi: 10.1007/s000180050365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maric M., Arunachalam B., Phan U.T., Dong C., Garrett W.S., Cannon K.S., Alfonso C., Karlsson L., Flavell R.A., Cresswell P. Defective antigen processing in GILT-free mice. Science. 2001;294:1361–1365. doi: 10.1126/science.1065500. [DOI] [PubMed] [Google Scholar]
- 24.De Wit D., Van Mechelen M., Ryelandt M., Figueiredo A.C., Abramowicz D., Goldman M., Bazin H., Urbain J., Leo O. The injection of deaggregated gamma globulins in adult mice induces antigen-specific unresponsiveness of T helper type 1 but not type 2 lymphocytes. J. Exp. Med. 1992;175:9–14. doi: 10.1084/jem.175.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gurramkonda C., Zahid M., Nemani S.K., Adnan A., Gudi S.K., Khanna N., Ebensen T., Lünsdorf H., Guzmán C.A., Rinas U. Purification of hepatitis B surface antigen virus-like particles from recombinant Pichia pastoris and in vivo analysis of their immunogenic properties. J. Chromatogr. B. 2013;940:104–111. doi: 10.1016/j.jchromb.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 26.Seid C.A., Jones K.M., Pollet J., Keegan B., Hudspeth E., Hammond M., Wei J., McAtee C.P., Versteeg L., Gutierrez A., Liu Z., Zhan B., Respress J.L., Strych U., Bottazzi M.E., Hotez P.J. Cysteine mutagenesis improves the production without abrogating antigenicity of a recombinant protein vaccine candidate for human chagas disease. Hum. Vaccines Immunother. 2017;13:621–633. doi: 10.1080/21645515.2016.1242540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y., Zhang Y., Quan C., Luo J., Yang Y., Yu M., Kong Y., Ma G., Su Z. Aggregation and antigenicity of virus like particle in salt solution--A case study with hepatitis B surface antigen. Vaccine. 2015;33:4300–4306. doi: 10.1016/j.vaccine.2015.03.078. [DOI] [PubMed] [Google Scholar]
- 28.Abuchowski A., Kazo G.M., Verhoest C.R., Jr., Van Es T., Kafkewitz D., Nucci M.L., Viau A.T., Davis F.F. Cancer therapy with chemically modified enzymes. I. Antitumor properties of polyethylene glycol-asparaginase conjugates. Cancer Biochem. Biophys. 1984;7:175–186. [PubMed] [Google Scholar]
- 29.Hershfield M.S. PEG-ADA replacement therapy for adenosine deaminase deficiency: an update after 8.5 years. Clin. Immunol. Immunopathol. 1995;76:S228–S232. doi: 10.1016/s0090-1229(95)90306-2. [DOI] [PubMed] [Google Scholar]
- 30.Ito H.O., So T., Ueda T., Imoto T., Koga T. Prevention of collagen-induced arthritis (CIA) by treatment with polyethylene glycol-conjugated type II collagen; distinct tolerogenic property of the conjugated collagen from the native one. Clin. Exp. Immunol. 1997;108:213–219. doi: 10.1046/j.1365-2249.1997.3721269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.So T., Ito H.O., Tsujihata Y., Hirata M., Ueda T., Imoto T. The molecular weight ratio of monomethoxypolyethylene glycol (mPEG) to protein determines the immunotolerogenicity of mPEG proteins. Protein Eng. 1999;12:701–705. doi: 10.1093/protein/12.8.701. [DOI] [PubMed] [Google Scholar]
- 32.Sauna Z.E., Lagsse D., Pedras-Vasconcelos J., Golding B., Rosenberg A.S. Evaluating and mitigating the immunonegenicity of therapeutic proteins. Trends Biotechnol. 2018;S0167–7799(18):30143–30144. doi: 10.1016/j.tibtech.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 33.Syn L.N., Teng M.W.L., Mok T.S.K., Soo R.A. De-novo and acqui resistance to immune checkpoint targeting. Lancet Oncol. 2017;18:e731–e741. doi: 10.1016/S1470-2045(17)30607-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.