Abstract
This work aimed to investigate the effects of the microwave-assisted extraction (MAE) on the hempseed (Cannabis sativa L.) oil yield, oxidation stability, and antioxidant activity. Power (300, 450, and 600 W) and time (5, 10, and 15 min) were independent variables while oil extraction yield, peroxide value (PV), p-anisidine value (AV), TOTOX value (TV), and DPPH scavenging activity were considered as dependent ones. Optimization was conducted by response surface methodology where the optimum point was 450 W and 7.19 min. In this point, the extraction yield obtained 33.91% w/w and the oil showed acceptable oxidation quality (PV of 2.5 meq/kg, AV of 0.67, and TV of 5.67) and antioxidant activity with the IC50 value of 30.82 mg/mL. The Soxhlet extraction (SE) method was carried out to be compared with MAE. It showed relatively higher oil extraction yield (37.93% w/w) but lower oil oxidation stability with PV of 6.4 meq/kg, AV of 3.69, TV of 16.49, and higher amount of IC50 32.47 mg/mL which showed lower antioxidant activity. Any significant difference between fatty acid compositions was not observed with the dominant amounts of linoleic acid and α-linolenic acid. Also, the tocopherol contents and thermal properties were studied by HPLC and DSC, respectively. MAE showed higher total tocopherol content (929.67 mg/kg) than SE (832.61 mg/kg) and γ-tocopherol was dominant. Moreover, DSC analysis showed that both profiles (crystallization and melting transitions) are likely influenced mostly by the triglyceride compositions and crystals structure.
Keywords: Microwave-assisted extraction (MAE), Ultrasound-assisted extraction (UAE), Hempseed oil, Oxidation stability, Tocopherol content
Introduction
Hempseed (Cannabis sativa L.) has about 28–35% oil depending on the seed variety, climatic condition and the year of cultivation (Kostić et al. 2013; Rezvankhah et al. 2018). Its fatty acid composition among other seeds is unique because of its high amounts of omega-6 and omega-3 which are accounted as essential fatty acids. In more details, hempseed oil composed of 70-80% polyunsaturated fatty acids (PUFAs). More accurately, approximately linoleic acid (LA, 18:2n-6) constitutes about 50–70%, and α-linolenic acid (ALA, 18:3n-3) is 15–25% of the total fatty acid content. It is the while saturated fatty acids are about 10%. The proportion of 3:1 in terms of n-6 and n-3 has been claimed to have medicinal effects on human body, including treatment of glaucoma and cancer, reducing cholesterol and high blood pressure, and providing an anti-inflammatory effect (Rezvankhah et al. 2018; Wall et al. 2010). Generally, omega fatty acids are believed to treat the cardiovascular diseases (Callaway 2004; Wall et al. 2010; Da Porto et al. 2012b; Jiao et al. 2014). Other important fatty acids in hempseed oil are γ-linolenic acid (GLA,18:3n-6) and stearidonic acid (SDA, C18:4n-3), which positively affect patients with rheumatoid arthritis, atopic dermatitis, and allergies (Da Porto et al. 2012b; Dunford 2015). Another prominent and specific property of the hempseed oil is its high tocopherol content so that these antioxidant components are remarkably responsible to prevent the intensive oil oxidation (Rezvankhah et al. 2018; Chouaibi et al. 2019). Among them, γ-tocopherol is the most abundant antioxidant component (Dunford 2015; Da Porto et al. 2012a; Rezvankhah et al. 2018). Oomah et al. (2002) found that about 800 mg/kg tocopherols exist in hempseed oil, mostly in the form of γ-tocopherol (about 85%), which has higher antioxidant activity than α and β tocopherol, but less than δ tocopherol. It was consistent with those found by Rezvankhah et al. (2018).
There are multiple methods to extract oil from hemp seeds. Cold-pressing is a convenient and frequently method which has some advantages and disadvantages. Extracting oil without applying high temperatures and no requirement for any organic solvent hazardous to human health and the environment are two of these benefits (Matthäus and Brühl 2008; Latif and Anwar 2009). Nevertheless, as a disadvantage, it should be noted that a relatively large amount of oil is left in the seed cake resulting in low extraction efficiency and subsequently, the economic drawback is predictable (Aladić et al. 2015). Moreover, high amounts of chlorophyll are co-extracted with the oil (due to intensive mechanical destruction of hempseed cells and releasing pigments from chloroplasts), which can inherently lead to oil photooxidation due to chlorophyll’s role as a sensitizer, and thus reduce the oil quality and shelf life (Choe and Min 2006; Aladić et al. 2015). On the contrary, common maceration method (the seed powder is submerged in chemical solvents) and Soxhlet extraction are considered prevalent solvent methods with relatively low cost and high oil extraction efficiency (Kostić et al. 2014). Despite these, they take much more time to extract the oil and also need chemical solvents, leading to hazardous conditions for both workers and the environment.
To combat the above-mentioned problems associated with cold-press and solvent methods, some novel methods have emerged. Microwave and ultrasound-assisted extractions (MAE and UAE) along with supercritical fluid extraction (SFE) are methods that have been widely used recently as alternative methods for hempseed oil extraction and other seeds oil (Samaram et al. 2015; Gai et al. 2013; Jiao et al. 2014; Da Porto et al. 2016; Aladić et al. 2015; Rezvankhah et al. 2018).
These methods offer high amounts of oil yield (especially in terms of microwave and ultrasound) and also significantly lower extraction time comparing to conventional Soxhlet and solvent extraction methods (Rezvankhah et al. 2018). Indeed, they facilitate the oil extraction by the remarkable effects on the cell walls where the oil entrapped (Rezvankhah et al. 2018). Further, these methods can extract nutraceutical components through less deteriorative effects. It is mainly attributed to their short process time and also the extraction mechanisms, particularly in terms of microwave and ultrasound which destruct the plant tissue. Existing high nutritional value components will cause the extracted oil to be qualified for health-promoting effects. On the other side, efficient extraction of nutraceuticals including Vit E, β-carotene, a high proportion of PUFAs, and also other antioxidant ingredients will increase the oil oxidation stability (Tian et al. 2013; Delfan-Hosseini et al. 2017; Rezvankhah et al. 2018).
Regarding the microwave-assisted extraction, Azadmard-Damirchi et al. (2010) studied the effects of microwave pretreatment (prior to cold-pressing) on oxidative stability and nutraceuticals content of oil extracted from the rapeseed (in comparison with solely cold-pressing). They found that pretreatment with microwaves (applied in versatile power conditions) prior to cold-press extraction could improve the oil yield and increase the oxidative stability of the oil. In another study, Wroniak et al. (2016) investigated the microwave pretreatment effects on the changes in the microstructure of rapeseeds, as well as the chemical composition and oxidative stability of the extracted rapeseed oil. They reported that microwave pretreatment could improve total tocopherol extraction and increase oxidative stability (comparable with the non-microwave condition). No significant changes were found (p > 0.05) in fatty-acid composition with microwave pretreatment.
Overall, all recent studies have shown that applying microwave could facilitate oil extraction and also raised the oil yield. Lower extraction time results in higher oxidative stability which is directly related to less deteriorative effects (in comparison with the Soxhlet extraction with accelerating temperature condition) and also efficient extraction of nutraceuticals such as antioxidant components (Li et al. 2013; Gai et al. 2013; Jiao et al. 2014; Taghvaei et al. 2014).
The objectives of this study were to extract hempseed oil by the microwave-assisted method and evaluate the oil extraction yield along with the oxidative stability of the obtained oils. Power (300, 450, and 600 W) and time (5, 10, and 15 min) were considered as independent variables. While oil yield % (w/w), peroxide value (PV, meq/kg oil), p-anisidine value (AV), TOTOX value (TV), and 2,2-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity were selected as dependent variables. No previous studies have examined these factors by employing a response surface methodology (RSM). In addition, the effects of this novel extraction method on extraction efficiency and oxidation stability were compared with Soxhlet extraction. Further, the extracted oils’ fatty-acid composition, tocopherol contents, and thermal properties were determined by gas chromatography (GC-FID), high-performance liquid chromatography (HPLC), and differential scanning calorimetry (DSC), respectively.
Materials and methods
Materials
Hempseeds (Cannabis sativa L., Tetrahydrocannabinol (THC) 0.2%) were obtained from a local market in Karaj (the seeds were approved by the seed and plant improvement institute, Karaj-Iran). The moisture content of the hempseeds was determined according to the related standard (Horwutz 2000). Samples were cleaned to remove impurities and ground with a laboratory mill (IKA Mills M 20 Universal mill), then sieved with a mesh-20 mm to separate particles based on their size.
Solvents and reagents
Hexane as an extraction solvent was supplied by Neutron-Iran Co. All solvents and reagents used in analytical determinations were purchased from Sigma-Aldrich Co. (Milan, Italy) and Merck Co. (Darmstadt, Germany), pro-analysis types. The chemicals used were all of the analytical reagent grades; these were included p-anisidine and potassium iodide prepared by and purchased from Sigma-Aldrich Co. (Milan, Italy). To determine the antioxidant activity, 2,2-diphenyl-2-picrylhydrazyl (DPPH – 90% purity, Sigma-Aldrich Co., Milano, Italy) was used.
Extraction methods
Soxhlet extraction (SE)
Soxhlet extraction (SE) was done according to Da Porto et al. (2016). In this method, we weighed 10 g ground hempseeds and transferred them to filter-paper-wrapped extraction thimbles for transfer to the Soxhlet apparatus (Buchi, Switzerland). Extraction was completed after 8 h at a maximum temperature of 70 °C (the boiling point of n-hexane). After the extraction process, the residual of the solvent was removed at 50 °C under reduced pressure using a rotary evaporator (Heidolph, Germany). To further reduce the solvent amount, the extracted oil was transferred to an oven with reduced pressure at a temperature of 50 °C until reaching a constant weight. Finally, the resulting oil was weighed, and the oil yield was calculated (Eq. 1). The determination was done in triplicate.
Microwave-assisted extraction (MAE)
The microwave oven apparatus used in this study was manufactured by Samsung Co. (Model: CE32260F, Korea, 900 W output power with 2450 MHz frequency). For each run of the experiment, 15 g ground hemp seeds were weighed in a funnel followed by the addition of n-hexane at a constant solvent-to-seed powder ratio of 10:1 in every run; the mixture was then transferred to the microwave system. After that, the power and time of the extraction process were adjusted. After the operation, the funnel was cooled to condense the n-hexane vapors. The contents of each funnel were transferred to centrifuge tubes and centrifuged for 15 min at 15,000×g. After centrifugation, n-hexane and its dissolved oil were filtered through filter paper by applying a vacuum pump. To deplete the solvent, the filtrate was evaporated with a rotary evaporator under reduced pressure at 50 °C. To further reduce the solvent in the extracted hempseed oil, the oil was placed in a vacuum oven that set at 50 °C until reaching a constant weight (2 h).
Determination of extraction yield
The extraction yield was computed by dividing the amount of extracted oils to the initial amount of seed powder (15 g):
| 1 |
Physiochemical characteristics and antioxidant activity
Physiochemical properties
Physiochemical features of the extracted oils were determined by using the standard methods provided by American Oil Chemists’ Society (AOCS) and the International Union of Pure and Applied Chemistry (IUPAC) (Firestone 2009; Paquot 2013).
The refractive indices and iodine values (IV) were measured according to AOCS recommended practices Cc 7-25 and Cd 1-25, respectively (Firestone 2009). The refractive indices were computed by laboratory refractometer (Bellingham, England).
PV (meq/kg oil) and AV were measured as indices for the oxidative stability of hempseed oils using IUPAC methods (Paquot 2013).
TV for implying overall oxidative stability was calculated based on the following equation (Samaram et al. 2014):
| 2 |
Antioxidant activity determination
The antioxidant activities of the extracted oils were determined based on DPPH radical scavenging activity (2,2-diphenyl-2-picrylhydrazyl radical) with a slight modification according to Jiao et al. (2014). In this experiment, a DPPH solution with a determined concentration (0.2 mM) was prepared. Oil samples were dissolved in ethyl acetate at various concentrations (3.125–50 mg/mL). Then, 2 mL of DPPH solution was mixed with 2 mL of oil solutions and shaken vigorously, then left in darkness. The absorbance was read using a UV-spectrophotometer (SP-UV 500DB spectrophotometer, Spectrum instruments, Canada) after 60 min at 517 nm. A blank sample was also prepared, and its absorbance compared with the main samples. DPPH scavenging activity (%) was calculated for each sample according to following the equation:
| 3 |
To determine the IC50 value, which is the least amount of the oil that can scavenge 50% of the free radicals of DPPH comparing to a blank control, the inhibition rate (DPPH scavenging activity %) was plotted against the sample concentration. A logarithmic regression curve was established (not shown) to calculate the IC50 (Long et al. 2011; Hu et al. 2017).
Determination of fatty acid profile by GC-FID analysis
The fatty acid compositions of extracted hempseed oils (optimum point of MAE and the oil achieved by Soxhlet apparatus) were analyzed using gas chromatography (GC); the gas chromatograph (Agilent 9890, America) was equipped with FID and a capillary column (CPSill-88, Varian, America, with a length and internal and external diameter of 100 m, 0.22 mm, and 0.33 mm, respectively). To separate the fatty acid components, the following temperature program was undertaken: the injection temperature was 270 °C, and the detector temperature was 260 °C. For the column, the temperature program was as follows: 5 min at 165 °C, and temperature elevation of the column at the rate of 2 °C/min to 235 °C (35 min). Eventually, the area under the peaks of mixed standard fatty acids was compared with the area under the peaks of the sample. In order to obtain the profile of the samples’ fatty acid contents, the concentration of fatty acids was also determined.
Determination of total tocopherols (TT) by the HPLC analytical method
The tocopherol contents of the obtained oils were analyzed using HPLC (Korean YOUNGLIN Co.) with a pump of SP930D, equipped with the UV730D detector, a handle injection system with a loop of 20 μL, and AutoChrom 3000 as connector software. The analytical column of this system was a reverse phase with a particle size of 4.6 mm and a column length of 250 mm. A 90:1 ratio of water to methanol was used as the mobile phase, with a flow of 1 mL/min at 30 °C, and the volume of injection was 20 μL.
Determining the thermal properties of the extracted oil
To study the thermal properties of the extracted oil, differential scanning calorimetry (Mettler Toledo DSC, Switzerland) assay was performed for each method of extraction (optimum point of MAE and SE). Aluminum pans, sealed tightly to withstand the internal pressure produced by temperature fluctuations, were used to weigh 6.2–7.5 mg of the extracted hempseed oil. The temperature program of the cooling process started at 100 °C and finished at − 60 °C with a rate of 10 °C/min to obtain a crystallization curve. To obtain the melting point of the extracted oils, the oil samples were heated from − 60 to 100 °C with the rate of 10 °C/min.
Statistical analysis
Statistical analysis of experimental data was performed by Design-expert® software version 11 (Stat-Ease Inc., Minneapolis, MN, USA). The extraction optimization was implemented using RSM with central composite design (CCD) and two independent variables for MAE: irradiation power (X1, W) and process time (X2, min). Finally, 13 runs were accomplished, with 5 center points and 8 non-center points. As mentioned, CCD was used to survey the effects of independent variables at three levels on the five dependent variables (oil yield, PV, AV, TV, and DPPH scavenging activity; Y1, Y2, Y3, Y4, and Y5, respectively) (Table 1). Extraction condition was numerically optimized based on the mentioned responses as follows: maximum extraction yield, minimum PV, AV, TOTOX value, and maximum DPPH scavenging activity as a measurement of antioxidant activity. A second-order polynomial equation was used to express all dependent variables as a function of the two independent variables. Data were analyzed by analysis of variance (ANOVA). Data mean comparison was conducted by one sample T test using SPSS software (version 24, IBM® SPSS Statistics®, USA).
Table 1.
Results of CCD for the oil yield, PV, AV, TV, and DPPH scavenging activity during the MAE process
| Run | Power (W), (X1) | Time (min), (X2) | Yield (%w/w) (Y1) | PV (meq/kg) (Y2) | AV (Y3) | TV (Y4) | DPPH scavenging activity (%) (Y5) |
|---|---|---|---|---|---|---|---|
| Microwave-assisted extraction (MAE) | |||||||
| 1 | 300 (−1) | 5 (−1) | 25.70 | 2.78 | 0.10 | 5.66 | 53.40 |
| 2 | 600 (+1) | 5 (−1) | 34.06 | 3.60 | 1.60 | 8.80 | 70.65 |
| 3 | 300 (−1) | 15 (+1) | 29.68 | 2.98 | 0.46 | 6.42 | 65.90 |
| 4 | 600 (+1) | 15 (+1) | 34.75 | 4.80 | 1.94 | 11.54 | 50.97 |
| 5 | 300 (−1) | 10 (0) | 27.57 | 3.20 | 0.40 | 6.80 | 63 |
| 6 | 600 (+1) | 10 (0) | 35.04 | 3.75 | 1.80 | 9.30 | 68.50 |
| 7 | 450 (0) | 5 (−1) | 33.46 | 2.38 | 0.50 | 5.26 | 67.30 |
| 8 | 450 (0) | 15 (+1) | 35.77 | 3.36 | 0.91 | 7.63 | 70.10 |
| 9 | 450 (0) | 10 (0) | 34.45 | 2.65 | 0.80 | 6.10 | 75.96 |
| 10 | 450 (0) | 10 (0) | 34.61 | 2.46 | 0.70 | 5.62 | 72.33 |
| 11 | 450 (0) | 10 (0) | 34.46 | 2.31 | 0.76 | 5.38 | 73.24 |
| 12 | 450 (0) | 10 (0) | 35.05 | 2.60 | 0.78 | 5.98 | 71.90 |
| 13 | 450 (0) | 10 (0) | 35.03 | 2.73 | 0.80 | 6.26 | 68.70 |
Results of CCD for the oil yield, PV*, AV*, TV*, and DPPH scavenging activity of the MAE process
*PV peroxide value, AVp-anisidine value, TV TOTOX value
Results and discussion
Effects of independent variables on extraction oil yield
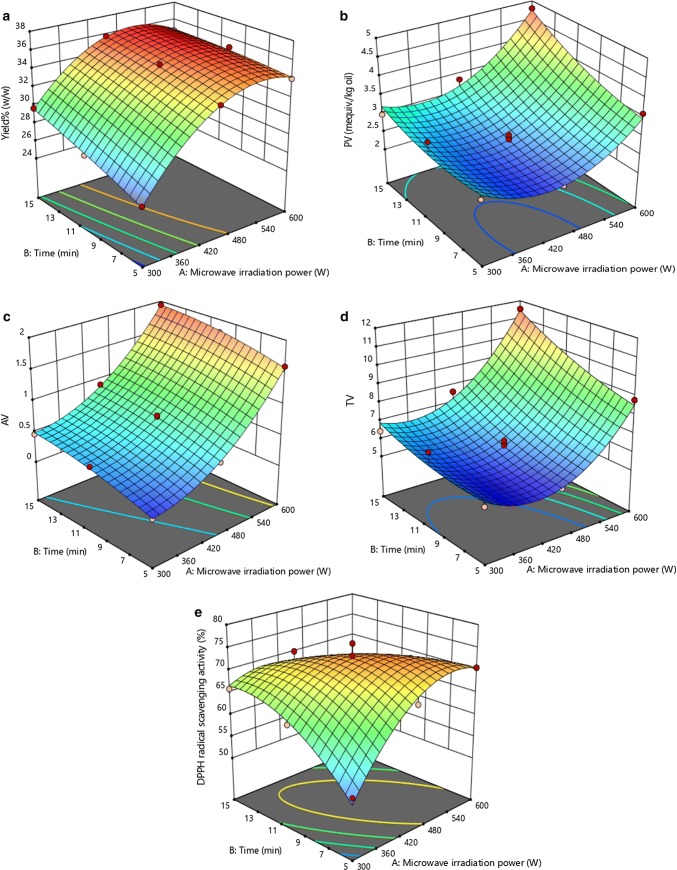
Hempseed powder used for extraction had about 4% w/w moisture content (based on dry basis weight) (Rezvankhah et al. 2018). The effects of two independent factors, power and time, were investigated for MAE in order to study the oil extraction yield and oil quality (Table 1). Regarding the ANOVA analysis, the quadratic model of oil extraction yield became significant (p < 0.05) (with a high coefficient determination of R2 = 0.99), and both power and time showed a significant effect with respect to their linear terms (Table 2). Further, the interaction effect was also significant (p < 0.05). Increases in power and time at a constant solvent-to-seed powder ratio of 10:1 following temperature elevation led to a movement in media and reduction of solvent viscosity and as a consequence, oil diffusion into surroundings was facilitated and therefore, the oil yield was improved (Kostić et al. 2013). Generally, the application of microwaves can rupture and damage cell walls as well as the lipoprotein membrane surrounding individual lipid bodies (Wroniak et al. 2016; Hu et al. 2017). This structural change enhanced the release of oil from the cell membrane (Delfan-Hosseini et al. 2017). As shown in Fig. 1a, with increases in power and time, oil extraction also increased. To elucidate the effects, as presented in Table 1, the oil yield was 25.70% w/w for 300 W and 5 min. While it was increased to 27.57 and 29.68% w/w, respectively for 10 and 15 min. However, this increase was limited to 450 W and 15 min. By increasing the power to 600 W (5 min), although it was observed an increase in oil yield (34.06% w/w), it was not a highlight shift comparing to 33.46% w/w obtained for 450 W (5 min) (Table 1). Indeed, there was not revealed the notable difference between the amounts obtained for 450 W (10 min) and 600 W (10 min). The only effect of 600 W at the prolonged time (especially at 15 min) was the fast elevations of the temperature, resulting in partial thermal decomposition of oil in some extent (Chen et al. 2016; Hu et al. 2017). Seemingly, about 95% w/w of the hempseed oil was extracted (the oil recovery; efficiency) at the initial time of extraction which showed the significant quadratic effect of power and insignificant effect of time (Table 1, 2 and Fig. 1a). It was concluded that microwave could extract the oil more efficiently than using the supercritical CO2 extraction method (with a maximum oil yield of 21.50%) studied by Da Porto et al. (2012b). Also, it was relatively higher than the amount of the obtained by solvent extraction (maximum yield of 30%) (Kostić et al. 2014). Da Porto et al. (2016) remarked that microwave irradiation could swell and destroy the cell walls. In this state, the respective microscopic structure would be changed and subsequently, rupturing of cell walls would occur and oil diffusion would be accelerated (Chen et al. 2016).
Table 2.
ANOVA analysis of the quadratic models for the oil yield, PV, AV, TV, and DPPH scavenging activity of the MAE process
| Response | Modela | X 1 | X 2 | X 1 X 2 | R 2 | Lack of fit | ||
|---|---|---|---|---|---|---|---|---|
| MAE | ||||||||
| Yield (Y1, %w/w) | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | 0.3304 | 0.0007 | 0.9955 | 0.5683 |
| PV (Y2, meq/kg oil) | 0.0005 | 0.0008 | 0.0042 | 0.0007 | 0.2026 | 0.0688 | 0.9353 | 0.1430 |
| AV (Y3) | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | 0.0230 | 0.8070 | 0.9971 | 0.5657 |
| TV (Y4) | < 0.0001 | 0.000 | 0.0019 | 0.0003 | 0.3121 | 0.0861 | 0.9587 | 0.1526 |
| DPPH scavenging activity (Y5, %) | 0.0008 | 0.2687 | 0.5226 | 0.0022 | 0.0239 | 0.0005 | 0.9250 | 0.4529 |
PV peroxide value, AVp-anisidine value, TV TOTOX value, DPPH antioxidant activity, X1 power, X2 time. X1 and X2 represent the single effects of variables; and represent the quadratic effects of variables, X1X2 represents the interaction between variables
aStatistically significant at the confident level of 95% (p < 0.05)
Fig. 1.
The 3D-surface plots of parameters affecting extraction efficiency (a), oil oxidation stability (b, c, d), and DPPH radical scavenging activity (e) of MAE hempseed oil
Effects of independent variables on physiochemical properties
Oxidation stability of MAE hempseed oil
Oil oxidation stability is an important property in determining oil quality. It mainly depends on the extraction conditions such as temperature, pressure, oxygen, and also the extraction procedure. Peroxides and hydroperoxides are the first products that are produced during autoxidation; these are unstable and being degraded at a relatively high temperature into stable products such as aldehydes and ketones (Choe and Min 2006; Taghvaei et al. 2014). Hempseed oil constituted with 75% PUFAs, which were unstable due to their sensitivity to high temperature. Based on the statistical results presented in Table 2, the obtained quadratic model for PV was significant (p < 0.05). Besides this, according to the respective high coefficient of determination (R2 = 0.93), it could be claimed that the experimental data had a good-fitting state with the obtained model. As shown in Fig. 1b, the terms for both linear effects of power and time, had significant effects on peroxide production (p < 0.05). It was the while that the quadratic terms of power and time obtained significant and not significant, respectively. Also, the linearity interaction effect became insignificant (p > 0.05). The experiments showed that with increasing power (300–450 W) at a constant time process (5 min), firstly the PV was decreased (2.78–2.38 meq/kg oil) due to the decomposition of oxidation initial products (hydroperoxides) into secondary products such as malondialdehydes. It could be explained that applying high microwave irradiation power have elevated the solvent temperature. At first, the PV was decreased (attributed to decomposition effect) but then, at 600 W (5 min) reached 3.60 meq/kg oil. By applying 600 W, PV was increased because of occurring intensive oil oxidation. Additionally, the prolongation led to higher PV at constant irradiation power (Table 1). According to Fig. 1b, the PV was increased linearly by the time was increasing. The longtime process could lead to the promoted oxidation. It could be remarked that although there was not a statistically significant relation between power and time, high power applying and prolong processing induced the oxidation more intensively. With respect to the 600 W and 15 min conditions, the PV reached 4.80 meq/kg oil. Microwave at higher powers can drastically elevate the solvent temperature which is so considerable factor in oxidation. However, as mentioned, a decline at PV was observed when the power shifted to 450 W. It could be communicated with the complexity of the oxidation process. Regarding the time effects, the quadratic term became insignificant (p > 0.05). It could be interpreted that microwave immediately increased the media temperature and applying higher microwave power intrinsically led to elevated temperature. Thus, PV was decreased and increased by the irradiation power alteration, regardless of the time change. Therefore, PV was not a reliable factor in determining oil quality and it was necessary to use a more reliable factor such as AV, which is the index for determination of secondary products of oxidation such as aldehydes and ketones. Accordingly, as the power of the microwave was increased followed by the temperature elevation, the first products were degrading into secondary products, and thus, the respective secondary oxidation index increased (Fig. 1c). Based on the ANOVA statistical analysis, it was shown that power and time both had significant effects (p < 0.05) on the associated values of AV with regards to the quadratic model with a high coefficient of determination (R2 = 0.99) (Table 2): when power and time were increased, AV was also increased (Table 1). The reason for the effect of power and time independently was that increased power elevated the temperature, and the long-time process exposed the oil more to oxidation condition which both could intensify the oxidation severity. In addition, the interaction term was insignificant (p > 0.05) which might be attributed to that simultaneous increasing power and time could result in higher antioxidants extraction (Choe and Min 2006). This fact could prevent oxidation which could be induced again by the intensive temperature elevation due to the higher power and time levels. The quadratic terms of both power and time for AV were significant (p < 0.05), but that was negative for the time which can be observed that AV was more influenced by power in respect to its slope shown in Fig. 1c. Power had a rising slope and the AV was more significantly influenced by the power alteration.
To assess total oil oxidation stability, TV was computed by the equation stated above (eq. 2). According to the statistical analysis, the obtained quadratic model for TV was significant (p < 0.05) and a high coefficient of determination was obtained (R2 = 0.95) which showed the model good-fitting data (Table 2). Increasing power and time levels resulted in higher TV as describing significantly the linear effects of respective factors (p < 0.05). Further, the interaction term in the model was insignificant (p > 0.05) which was similar to that obtained for PV. Moreover, the quadratic term effect of power was significant (Fig. 1d). However, as similar as obtained for PV, the quadratic effect was insignificant for the time factor (p > 0.05). This means that temperature elevation with employing high microwave power was remarkably effective. By using of high power, temperature elevation could occur in a shorter time and resulted in increasing TOTOX values. At a constant process time duration, total oxidation stability index was initially decreased by changing the power to 450 W but then, it was increased by applying 600 W. Although the time did not have a significant effect regarding the quadratic effect, at 600 W and 15 min, the maximum TV (11.54) was measured.
Overall, the oxidation process is considered as a complicated process which is dominantly affected by the extraction conditions. Temperature elevation can strongly decrease the oxidation stability. Higher microwave power and prolonged time lead to developed stages of oxidation which are responsible for the production of products causing oil off-odor.
Antioxidant activity of MAE hempseed oil
Free radicals are produced as a result of losing electrons from the double bonds in unsaturated triglycerides. It occurs especially when the oil extraction is performed at an elevated temperature or the extracted oil is exposed to oxygen, light, and temperature. In this condition, the oxidation would be accelerated and electrons would separate from double bonds resulting in free radical’s formation which propagates the oxidation (Choe and Min 2006). Hempseed oil contains natural antioxidants that interact with these radicals and initial oxidation products (hydroperoxides). Tocopherols which are the dominant antioxidants in hempseed oil, protect the oil from oxidation. DPPH scavenging activity measures antioxidant activity. It implies that how much of DPPH free radicals react with antioxidants and being scavenged.
Oomah et al. (2002) reported that microwave treatment could improve oil yield and efficiently extracted many of oil-soluble nutraceutical components. According to our study, ANOVA variance analysis showed that the obtained quadratic model for DPPH scavenging activity was significant (p < 0.05) with a high coefficient of determination (R2 = 0.92), but neither time nor power had a significant effect on DPPH scavenging activity in respect to linear terms, based on statistical analysis (Table 2). Both quadratic terms of power and time had significant effects on DPPH scavenging activity (p < 0.05) (Fig. 1e). Moreover, the interaction term showed a significant effect (p < 0.05). The results showed that relatively high microwave power (350 and 450 W) with temperature elevation could extract high amounts of tocopherols which can interact with DPPH free radicals (Duvernay et al. 2005; Jiao et al. 2014). Microwave irradiation can change the cell membrane proteins and phospholipids which lead to altering the membrane diffusivity. It would inherently give rise to rupturing the cell walls and consequently, releasing the oil-soluble components (with the dominant amounts of tocopherols). It was observed that raising the power (300–450 W, time of 10 min) resulted in the higher DPPH radical scavenging activity (63–75.96%) (Table 1). Higher antioxidant activity caused higher oxidation stability. Although the oil produced in these conditions showed high stability against oxidation, applying 600 W (at a constant time of 10 min) gave rise to a decrease at DPPH scavenging activity (68.50%). This decrease was attributed to degradation of antioxidants at elevated temperature. In addition, longer exposure time (particularly at 600 W) caused also unfavorable results so that the oxidation was developed and respective values of DPPH scavenging activity were remarkably decreased (50.97%). It was revealed that DPPH scavenging activity was also significantly influenced by the quadratic effect of time which was not prevalent with respect to the oxidation indices. Indeed, antioxidant ingredients were more extracted by increasing power and time. On the other side, PUFAs due to their several double bonds were undergone severe oxidation by rising irradiation power. Insignificant quadratic effects of time in respect to the PV and TV could be ascribed to more antioxidant extraction occurring at the prolonged time (limited to 300 and 450 W) and also decomposition of initial unstable oxidation products. Thus, antioxidants can interact with the hydroperoxides and prevent the intensive oxidation. However, DPPH radical scavenging activity was decreased at the prolonged time of 600 W. As mentioned, sthe imultaneous increase of power and time values had synergistic effects and increased the DPPH scavenging activity. It was more highlighted at 300 W (53.40, 63, and 65.90%) so that applying 450 W resulted in less increase at antioxidant activity (67.30 to ~ 72%) and deterioration of antioxidants occurred at 600 W (5–10 and 15 min), largely because of the increases in temperature (Aladić et al. 2015).
Physical and chemical properties of extracted hempseed oils
Initially, the refractive indices were measured for extracted methods and results were presented in Table 3. The indices were closely grouped with an identical value of measurements (~ 1.47). In addition, the iodine value was also determined for extraction methods (Table 3). Based on the fatty acid profiles, the obtained oil by MAE showed relatively higher iodine value (148.78 g I2/100 g oil) due to its high linoleic acid extraction (55.50% w/w). For SE, it was measured about 148.47 g I2/100 g oil. The obtained values for IV were lower than the values found in previous studies (Kostić et al. 2013; Latif and Anwar 2009). In general, no superior significant difference was observed between SE and MAE methods (Hu et al. 2017).
Table 3.
Physical and chemical properties of the obtained oils with different extraction methods and data validation
| SE | MAE* | ||
|---|---|---|---|
| Experimental | Predicted | ||
| Refractive index (25 °C) | 1.47 ± 0.05a | 1.465 ± 0.05a | – |
| Iodine value (g I2/100 g oil) | 148.47 ± 0.1a | 148.78 ± 0.1b | – |
| Oil yield (%w/w) | 37.93 ± 0.95a | 33.91 ± 0.07b | 34.03b |
| Peroxide value (PV) (meq/kg oil) | 6.40 ± 0.55a | 2.50 ± 0.05b | 2.42b |
| p-Anisidine value (AV) | 3.69 ± 0.15a | 0.67 ± 0.02b | 0.64b |
| TOTOX value (TV) | 16.49 ± 0.66a | 5.67 ± 0.09b | 5.49b |
| DPPH scavenging activity (%) | 65.70 ± 0.3a | 71.83 ± 1.35b | 71.63b |
*The optimized point (450 W and 7.19 min). Different superscripts represent statistically significant differences between experimental and predicted values (p < 0.05)
a,bShow the significantly different data which were provided with mean and SD
Response surface optimization of MAE conditions and data validation
By employing CCD, 13 treatments (with 5 center points) were done for MAE. Numerical optimization was implemented based on the maximum oil yield and antioxidant activity achievements and obtaining minimum oxidation stability indices (PV, AV, and TV). On this basis, the optimum point was estimated under the condition of 450 W and 7.19 min with the desirability of 0.87. As abovementioned, power and time significantly affected the oil extraction yield as well as the oxidation quality of the oil (as shown by its oxidation factors). At the determined optimum point, the predicted oil yield, PV, AV, TV, and antioxidant scavenging activity were 34.03% w/w, 2.42, 0.64, 5.49, and 71.63%, respectively (Table 3). These values were predicted by the software and to verify them, they were all experimentally measured in three duplications (Table 3). Overall, the validation study revealed that the experimental results did not significantly differ from the predicted values (p > 0.05) (Table 3).
Comparison of extraction yield and oxidation stability of SE with MAE
As shown in Table 3, SE showed relatively higher extraction yield (37.93% w/w) and lower oxidation stability determined by PV, AV, and TV which were 6.4 meq/kg oil, 3.69, and 16.49, respectively. Extraction yield of SE was significantly higher than the amount obtained for MAE. Furthermore, the oxidation stability of the oil obtained by SE was significantly lower than that of MAE (p < 0.05). It could be explained by the elevated temperature of SE and also its long process time (8 h) (Moghadas and Rezaei 2017). On the contrary, MAE showed lower oil extraction yield (33.91% w/w) and higher oxidation stability (2.5 meq/kg oil, 0.67, and 5.67, respectively). In fact, applying MAE (450 W and 7.19 min) was so beneficial due to a short process time in which less oxidation inducing condition occurred (Hu et al. 2017). Also, the obtained oil by the optimum point of MAE had higher DPPH scavenging activity (71.83% versus 65.70%). It could be mainly related to the efficient extraction of bioactive components (tocopherols) and also the shortened extraction time that maintained the oil quality (Moghadas and Rezaei 2017; Hu et al. 2017). Tocopherols and other bioactive components extracted by MAE were maintained more than those were extracted in SE (Chouaibi et al. 2019). Elevated temperature at SE resulted in lower DPPH radical scavenging activity which was attributed to degradation reactions. Also, the determined values of IC50 for the extraction methods showed a higher amount for SE (32.47%) than for MAE (30.82%) (Table 4), which exhibited the high quality of oil extracted by MAE, as a lower amount of oil was needed to interact with DPPH free radicals (Jiao et al. 2014; Hu et al. 2017). Rezvankhah et al. (2018).
Table 4.
Fatty acid profiles and antioxidant contents obtained by GC-FID and HPLC for extraction methods (SE, MAE(
| Extraction methods | SE | MAE* |
|---|---|---|
| Fatty acids % | ||
| Myristic acid (C14:0) | 0.03 ± 0.00a | 0.02 ± 0.00a |
| Myristoleic acid (C14:1) | 0.02 ± 0.00a | 0.01 ± 0.00a |
| Palmitic acid (C16:0) | 6.17 ± 0.1a | 5.78 ± 0.13b |
| Palmitoleic acid (C16:1) | 0.13 ± 0.01a | 0.04 ± 0.00b |
| Margaric acid (C17:0) | N.D | 0.03 ± 0.00 |
| Ginkgolic acid (C17:1) | N.D | 0.02 ± 0.01 |
| Stearic acid (C18:0) | 2.63 ± 0.1a | 2.56 ± 0.06a |
| Oleic acid (C18:1) | 15.78 ± 0.05a | 15.98 ± 0.08b |
| Linoleic acid (C18:2, tr) | 0.03 ± 0.01 | N.D |
| Linoleic acid (C18:2, Cis ω6) | 55.07 ± 0.1a | 55.50 ± 0.15b |
| α-Linolenic acid (C18:3ω3) | 18.5 ± 0.15a | 18.13 ± 0.07b |
| γ -Linolenic acid (C18:3 ω6) | 0.6 ± 0.02a | 0.63 ± 0.03a |
| Arachidic acid (C20:0) | 0.53 ± 0.02a | 0.50 ± 0.05a |
| Godonic acid (C20:1) | 0.18 ± 0.01a | 0.34 ± 0.02b |
| Behenic acid (C22:0) | 0.23 ± 0.01a | 0.14 ± 0.00b |
| Erucic acid (C22:1) | N.D | 0.02 ± 0.01 |
| Lignoceric acid (C24:0) | 0.1 ± 0.00a | 0.21 ± 0.02b |
| Nervonic acid (C24:1) | N.D | 0.04 ± 0.00 |
| PUFA sum | 74.2 | 74.26 |
| Monounsaturated | 16.11 | 16.45 |
| Saturated | 9.69 | 9.24 |
| ω-6/ω-3 ratio | 2.97 | 3.06 |
| PUFA/SFA ratio | 7.65 | 8.03 |
| Tocopherol (mg/kg) | ||
| α-tocopherol | 7.90 ± 1.5a | 47.12 ± 5.4b |
| γ-tocopherol | 792.86 ± 20a | 841.05 ± 17.4b |
| δ-tocopherol | 31.85 ± 3.4a | 41.5 ± 4.4b |
| Total tocopherol content | 832.61 | 929.67 |
| IC50 (mg/mL) | 32.47 ± 0.4a | 30.82 ± 0.2b |
a,bDifferent superscripts represent statistically significant differences between two different extraction methods values (p < 0.05)
−The optimized point
The fatty acid composition of extracted oils
The fatty acid contents of the extracted oils were measured using GC-FID (Table 4). As shown in Table 4, most of the fatty acid contents of oil obtained by MAE were PUFAs (74.26%). This was a similar fatty acid profile to that obtained by SE (74.2%) (Hu et al. 2017; Rezvankhah et al. 2018). Monounsaturated fatty acids were 16.45 and 16.11% with the dominant amounts of oleic acid with 15.98 and 15.78%, respectively for MAE and SE. Predominant fatty acids were linoleic acid and α-linolenic acid. They were 55.50% and 18.13% for MAE and 55.07% and 18.5% were for SE, respectively. Also, γ-linolenic acid was detected in these two extracted oils (0.6 and 0.63% for SE and MAE, respectively). It has been claimed to have positive effects on patients with rheumatoid arthritis, and atopic dermatitis (Da Porto et al. 2012b). In terms of saturated fatty acids, 9.69 and 9.24% were achieved for SE and MAE, respectively. Although the extraction process took a shorter time (7.19 min) for MAE, the fatty acid profiles were similar. Nevertheless, MAE can be accounted for as a beneficial alternative extraction procedure for the plants seed oil, because in shorter extraction time yields the oils with similar fatty acid compositions comparing to conventional extractions. The high ratio of PUFA to SFA (7.65 for SE and 8.03 for MAE) and the optimal ratio of omega-6 to omega-3 (2.97 for SE and 3.06 for MAE) has been confirmed to have medicinal effects on the human body (Senanayake and Shahidi 2000; Remans et al. 2004). Other studies have shown a significant difference in fatty acid profiles; for example, Da Porto et al. (2012b) reported about 81.08% PUFAs extracted by supercritical CO2 extraction. This difference might be related to the variety of hempseed and the extraction method. So, as a result, no highlight a significant difference in fatty acid composition was observed between the SE and MAE methods (Hu et al. 2017). Rezvankhah et al. (2018) extracted the hempseed oil by exploiting UAE method. Their results in terms of fatty acid components were similar to those found in this study (55.3 and 18.09%, respectively for linoleic acid and α-linolenic acid.
TT contents of extracted oils
Vitamin E is composed of tocopherols (α, β, γ, δ) and tocotrienols (α, β, γ, δ) which are antioxidant components naturally found in plant seeds oil (Jiao et al. 2014). In hempseed oil, tocopherols are the dominant antioxidant components that can stabilize the oil against oxidation. Based on the literature review, α, γ, and δ-tocopherol are the main antioxidants found in hempseed oil and the greatest amount is γ-tocopherol (Rezvankhah et al. 2018). Callaway (2004); Oomah et al. (2002); Da Porto et al. (2012b); Latif and Anwar (2009) were found 800 mg/kg tocopherols mostly in the form of γ-tocopherol (about 85%). The current study investigated the effects of SE and MAE on tocopherol extraction. The tocopherol contents were determined by reverse phase-HPLC and the results were presented in Table 4. It was shown that MAE could facilitate the oil extraction (in a short time) and oil-soluble components such as antioxidants were efficiently extracted and fewer amounts were degraded (Jiao et al. 2014). The important point is short extraction time in this novel method which can maintain the oil quality. It was the while SE took longer extraction process and thus, resulted in the propagation of oxidation and also deterioration of the antioxidants present in the extracted oil (Duvernay et al. 2005). In fact, the total tocopherol content for SE (832.61 mg/kg) was less than that obtained for MAE (929.67 mg/kg) (Table 4). Especially, γ-tocopherol dominated and obtained 792.86 mg/kg for SE and 841.05 for MAE. Also, α-tocopherol was detected 47.12 and 7.90 mg/kg and δ-tocopherol contents were 41.5 and 31.85 mg/kg, respectively for MAE and SE (Table 4). These results might be related to the long extraction time of SE (Aladić et al. 2015). Moreover, SE required a high extraction temperature of about 70 °C for 8 h, whereas, in the MAE method, the extraction temperature reached 59 °C (at the optimum point). Thus, MAE did not have an intensive deterioration effect on antioxidant contents. According to the previous studies, the oil extracted by MAE showed higher tocopherol contents than the enzyme-assisted cold-press method investigated by Latif and Anwar (2009). They reported about 724.4–788.8 mg/kg oil. In general, hemp seed oil is considered a good source of strong antioxidant components particularly due to its high γ-tocopherol content. It was also consistent with those found by Rezvankhah et al. (2018).
Thermal properties of extracted oils
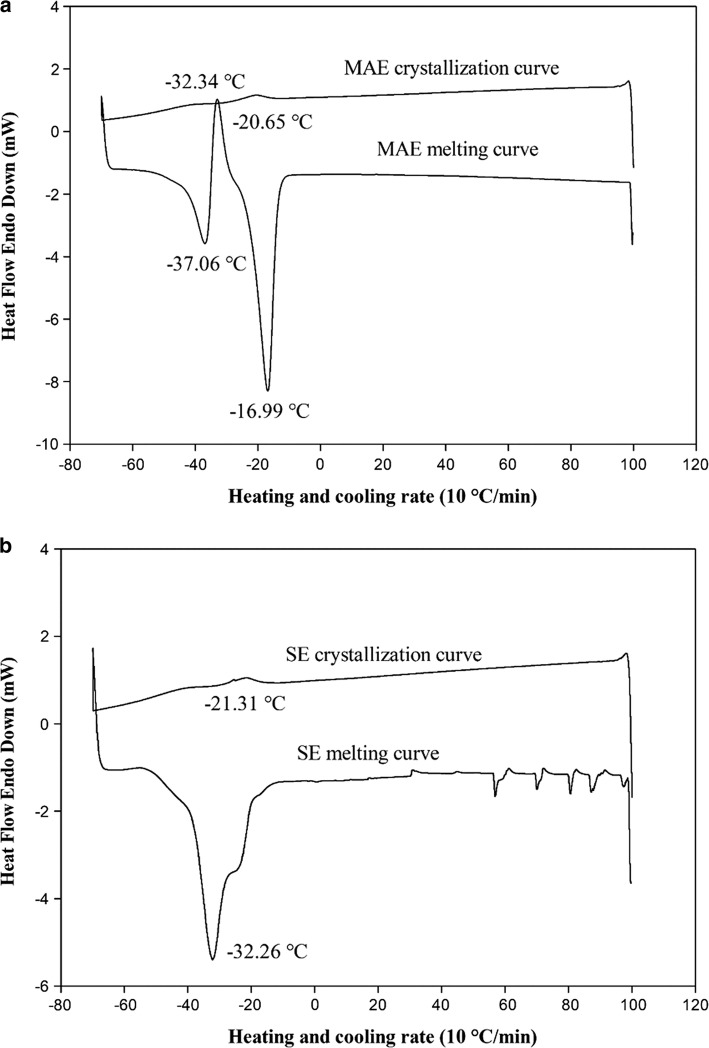
According to the melting curves shown in Fig. 2a-b, the oil obtained by MAE illustrated two sharp peaks with the transition of − 16.99 and − 37.06 °C having normalized ΔH endotherm of − 40.58 and − 12.82 J/g, respectively (Fig. 2a). For SE, a sharp peak was observed at − 32.26 °C with the normalized ΔH endotherm of − 49.80 J/g (Fig. 2b). These peaks showed that not only the fatty acid profiles can affect the melting curve but also triglyceride compositions and crystals structures can influence it (Teh and Birch 2013). According to the fatty acid contents (presented in Table 4), the MAE showed a high ratio of PUFAs to SFA (8.03) while for SE it was obtained less amount (7.65). On the other hand, SE hempseed oil showed 74.20% α-linolenic acid and linoleic acid which was partially less than 74.26% for MAE. In more details, the fatty acid profile for MAE showed higher linoleic acid (55.50%) and less α-linolenic acid (18.13%) than SE which was determined as 55.07% and 18.5%, respectively. Also, the chosen heating rate of 10 °C/min displayed a region of polymorphic transition indicative of recrystallization of metastable forms that occurred at maxima of − 32.34 °C with exotherm enthalpy of 0.8 J/g for MAE (Fig. 2a). The results showed that the use of microwave caused in a lower oil melting point due to its more unsaturated fatty acid content (Teh and Birch 2013; Oomah et al. 2002). Despite, there was no superior significant difference between the fatty acids profiles (Table 4), the DSC assay showed that a slight difference between fatty acid profiles and triglyceride compositions, can result in different melting curves and subsequently melting points.
Fig. 2.
DSC crystallization and melting thermographs of SE (a), MAE (b), extracted hempseed oil
The crystallization curve was depicted in Fig. 2a-b by the cooling process. It has been demonstrated that fatty-acid profiles and triglyceride compositions can affect the crystallization point. Specifically, the oils with higher saturated fatty acids have a higher crystallization point. On the other side, the oils with higher unsaturated fatty acids (especially in terms of omega-3 and omega-6 fatty acids) would have lower crystallization point. SE yielded oil with higher amounts of saturated fatty acids (9.69%) than the oil obtained by MAE (9.24%). In addition, the obtained oil by SE had 74.2% PUFAs while the one obtained by MAE had 74.26%. It was not observed a prominent difference between the fatty acid profiles. However, the crystallization point for MAE obtained at − 20.65 °C, but for SE achieved at − 21.31 °C (Fig. 2a, b). It could be attributed to the complex behavior of fatty acids in the crystallization process. The higher amount of α-linolenic acid (18.5%) in oil extracted by the SE method possibly could have affected the crystallization point (Fig. 2b).
Conclusion
MAE is the method that has been suggested for oil extraction due to its benefits in the production of high-quality oil. The hempseeds used in this study had about 75% PUFAs. This amount makes the oil so susceptible and prone to oxidation. MAE extracted the oil in significant lower time and presented higher oxidation stability comparing to conventional extraction (SE). The optimized conditions based on RSM were 450 W and 7.19 min, in which the extraction yield, oxidation stability responses, and DPPH radical scavenging activity were 33.91% w/w, PV of 2.5 meq/kg oil, AV of 0.67, TV of 5.67, and 71.83%. On the contrary, SE yielded higher oil amount (37.93%) but lower oil oxidation stability with PV of 6.4 meq/kg oil, AV of 3.69, TV of 16.49, and DPPH scavenging activity of 65.70%. GC profiles showed that there was no superior significant difference between MAE and SE. Moreover, HPLC analysis showed that MAE could extract higher levels of tocopherols (929.67 mg/kg) than SE (832.61 mg/kg), with less damage to the associated bioactive components. It was attributed to lower extraction temperature and a shorter process time of MAE. The IC50 value of the obtained oil by SE (32.47 mg/mL) was higher than that obtained for MAE (30.82 mg/mL). The oil obtained by MAE had a lower melting point (37.06 °C) which was related to fatty acid contents (higher amount of PUFAs to SFA ratio).
The presence of high amounts of omega-3 and omega-6 in hempseed oil makes it unique among the plant seeds oil with respect to its health beneficial effects. Hence, it should be taken into account to use in an appropriate way. Microencapsulation of hempseed oil can be a good approach which is so promising in the protection of sensitive bioactive compounds.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aladić K, Jarni K, Barbir T, Vidović S, Vladić J, Bilić M, Jokić S. Supercritical CO2 extraction of hemp (Cannabis sativa L.) seed oil. Ind Crops Prod. 2015;76:472–478. doi: 10.1016/j.indcrop.2015.07.016. [DOI] [Google Scholar]
- Azadmard-Damirchi S, Habibi-Nodeh F, Hesari J, Nemati M, Achachlouei BF. Effect of pretreatment with microwaves on oxidative stability and nutraceuticals content of oil from rapeseed. Food Chem. 2010;121(4):1211–1215. doi: 10.1016/j.foodchem.2010.02.006. [DOI] [Google Scholar]
- Callaway JC. Hempseed as a nutritional resource: an overview. Euphytica. 2004;140(1):65–72. doi: 10.1007/s10681-004-4811-6. [DOI] [Google Scholar]
- Chen F, Xinqi D, Yuangang Z, Yang L, Wang F. Microwave-assisted method for distillation and dual extraction in obtaining essential oil, proanthocyanidins, and polysaccharides by one-pot process from Cinnamomi Cortex. Sep Purif Technol. 2016;164:1–11. doi: 10.1016/j.seppur.2016.03.018. [DOI] [Google Scholar]
- Choe E, Min DB. Mechanisms and factors for edible oil oxidation. Compr Rev Food Sci Food Saf. 2006;5(4):169–186. doi: 10.1111/j.1541-4337.2006.00009.x. [DOI] [Google Scholar]
- Chouaibi M, Rezig L, Hamdi S, Ferrari G. Chemical characteristics and compositions of red pepper seed oils extracted by different methods. Ind Crops Prod. 2019;128:363–370. doi: 10.1016/j.indcrop.2018.11.030. [DOI] [Google Scholar]
- Da Porto C, Decorti D, Tubaro F. Fatty acid composition and oxidation stability of hemp (Cannabis sativa L.) seed oil extracted by supercritical carbon dioxide. Ind Crops Prod. 2012;36(1):401–404. doi: 10.1016/j.indcrop.2011.09.015. [DOI] [Google Scholar]
- Da Porto C, Voinovich D, Decorti D, Natolino A. Response surface optimization of hemp seed (Cannabis sativa L.) oil yield and oxidation stability by supercritical carbon dioxide extraction. J Supercrit Fluids. 2012;68:45–51. doi: 10.1016/j.supflu.2012.04.008. [DOI] [Google Scholar]
- Da Porto C, Decorti D, Natolino A. Microwave pretreatment of Moringa oleifera seed: effect on oil obtained by pilot-scale supercritical carbon dioxide extraction and Soxhlet apparatus. J Supercrit Fluids. 2016;107:38–43. doi: 10.1016/j.supflu.2015.08.006. [DOI] [Google Scholar]
- Delfan-Hosseini S, Nayebzadeh K, Mirmoghtadaie L, Kavosi M, Hosseini SM. Effect of extraction process on composition, oxidative stability and rheological properties of purslane seed oil. Food Chem. 2017;222:61–66. doi: 10.1016/j.foodchem.2016.11.150. [DOI] [PubMed] [Google Scholar]
- Dunford NT (2015) Hemp and flaxseed oil: properties and applications for use in food. Specialty Oils and Fats in Food and Nutrition: Properties, Processing, and Applications 39
- Duvernay WH, Assad JM, Sabliov CM, Lima M, Xu Z. Microwave extraction of antioxidant components from rice bran. Pharm Eng. 2005;25(4):126. [Google Scholar]
- Firestone D (2009) Official methods and recommended practices of the AOCS: AOCS
- Gai Q-Y, Jiao J, Pan-Song M, Wang W, Luo M, Li C-Y, Yuan-Gang Z, Wei F-Y, Yu-Jie F. Microwave-assisted aqueous enzymatic extraction of oil from Isatis indigotica seeds and its evaluation of physicochemical properties, fatty acid compositions, and antioxidant activities. Ind Crops Prod. 2013;45:303–311. doi: 10.1016/j.indcrop.2012.12.050. [DOI] [Google Scholar]
- Horwutz W (2000) Official methods of analysis of AOAC International. In: AOAC International, 17th edn. vol 1. Gaithersburg, MD
- Hu B, Li C, Zhang Z, Zhao Q, Zhu Y, Zhao S, Chen Y. Microwave-assisted extraction of silkworm pupal oil and evaluation of its fatty acid composition, physicochemical properties, and antioxidant activities. Food Chem. 2017;231:348–355. doi: 10.1016/j.foodchem.2017.03.152. [DOI] [PubMed] [Google Scholar]
- Jiao J, Li Z-G, Gai Q-Y, Li X-J, Wei F-Y, Yu-Jie F, Ma W. Microwave-assisted aqueous enzymatic extraction of oil from pumpkin seeds and evaluation of its physicochemical properties, fatty acid compositions, and antioxidant activities. Food Chem. 2014;147:17–24. doi: 10.1016/j.foodchem.2013.09.079. [DOI] [PubMed] [Google Scholar]
- Kostić MD, Joković NM, Stamenković OS, Rajković KM, Milić PS, Veljković VB. Optimization of hempseed oil extraction by n-hexane. Ind Crops Prod. 2013;48:133–143. doi: 10.1016/j.indcrop.2013.04.028. [DOI] [Google Scholar]
- Kostić MD, Joković NM, Stamenković OS, Rajković KM, Milić PS, Veljković VB. The kinetics and thermodynamics of hempseed oil extraction by n-hexane. Ind Crops Prod. 2014;52:679–686. doi: 10.1016/j.indcrop.2013.11.045. [DOI] [Google Scholar]
- Latif S, Anwar F. Physicochemical studies of hemp (Cannabis sativa) seed oil using enzyme-assisted cold-pressing. Eur J Lipid Sci Technol. 2009;111(10):1042–1048. doi: 10.1002/ejlt.200900008. [DOI] [Google Scholar]
- Li J, Yuan-Gang Z, Luo M, Cheng-Bo G, Zhao C-J, Efferth T, Yu-Jie F. Aqueous enzymatic process assisted by microwave extraction of oil from yellow horn (Xanthoceras sorbifolia Bunge.) seed kernels and its quality evaluation. Food Chem. 2013;138(4):2152–2158. doi: 10.1016/j.foodchem.2012.12.011. [DOI] [PubMed] [Google Scholar]
- Long J-j, Yu-jie F, Yuan-gang Z, Li J, Wang W, Cheng-bo G, Luo M. Ultrasound-assisted extraction of flaxseed oil using immobilized enzymes. Biores Technol. 2011;102(21):9991–9996. doi: 10.1016/j.biortech.2011.07.104. [DOI] [PubMed] [Google Scholar]
- Matthäus B, Brühl L. Virgin hemp seed oil: an interesting niche product. Eur J Lipid Sci Technol. 2008;110(7):655–661. doi: 10.1002/ejlt.200700311. [DOI] [Google Scholar]
- Moghadas HC, Rezaei K. Laboratory-scale optimization of roasting conditions followed by aqueous extraction of oil from wild almond. J Am Oil Chem Soc. 2017;94:1–10. doi: 10.1007/s11746-016-2934-2. [DOI] [Google Scholar]
- Oomah BD, Busson M, Godfrey DV, Drover JC. Characteristics of hemp (Cannabis sativa L.) seed oil. Food Chem. 2002;76(1):33–43. doi: 10.1016/S0308-8146(01)00245-X. [DOI] [Google Scholar]
- Paquot C. Standard methods for the analysis of oils, fats, and derivatives. New York: Elsevier; 2013. [Google Scholar]
- Remans PHJ, Sont JK, Wagenaar LW, Wouters-Wesseling W, Zuijderduin WM, Jongma A, Breedveld FC, Van Laar JM. Nutrient supplementation with polyunsaturated fatty acids and micronutrients in rheumatoid arthritis: clinical and biochemical effects. Eur J Clin Nutr. 2004;58(6):839–845. doi: 10.1038/sj.ejcn.1601883. [DOI] [PubMed] [Google Scholar]
- Rezvankhah A, Emam-Djomeh Z, Safari M, Askari G, Salami M. Investigation on the extraction yield, quality, and thermal properties of hempseed oil during ultrasound-assisted extraction: a comparative study. J Food Process Preserv. 2018 [Google Scholar]
- Samaram S, Mirhosseini H, Tan CP, Ghazali HM. Ultrasound-assisted extraction and solvent extraction of papaya seed oil: crystallization and thermal behavior, saturation degree, color, and oxidative stability. Ind Crops Prod. 2014;52:702–708. doi: 10.1016/j.indcrop.2013.11.047. [DOI] [Google Scholar]
- Samaram S, Mirhosseini H, Tan CP, Ghazali HM, Bordbar S, Serjouie A. Optimisation of ultrasound-assisted extraction of oil from papaya seed by response surface methodology: oil recovery, radical scavenging antioxidant activity, and oxidation stability. Food Chem. 2015;172:7–17. doi: 10.1016/j.foodchem.2014.08.068. [DOI] [PubMed] [Google Scholar]
- Senanayake SN, Shahidi F. Lipid components of borage (Borago officinalis L.) seeds and their changes during germination. J Am Oil Chem Soc. 2000;77(1):55–61. doi: 10.1007/s11746-000-0009-5. [DOI] [Google Scholar]
- Taghvaei M, Jafari SM, Assadpoor E, Nowrouzieh S, Alishah O. Optimization of microwave-assisted extraction of cottonseed oil and evaluation of its oxidative stability and physicochemical properties. Food Chem. 2014;160:90–97. doi: 10.1016/j.foodchem.2014.03.064. [DOI] [PubMed] [Google Scholar]
- Teh S-S, Birch J. Physicochemical and quality characteristics of cold-pressed hemp, flax, and canola seed oils. J Food Compos Anal. 2013;30(1):26–31. doi: 10.1016/j.jfca.2013.01.004. [DOI] [Google Scholar]
- Tian Y, Zhenbo X, Zheng B, Martin Lo Y. Optimization of ultrasonic-assisted extraction of pomegranate (Punica granatum L.) seed oil. Ultrason Sonochem. 2013;20(1):202–208. doi: 10.1016/j.ultsonch.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Wall R, Paul Ross R, Fitzgerald GF, Stanton C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev. 2010;68(5):280–289. doi: 10.1111/j.1753-4887.2010.00287.x. [DOI] [PubMed] [Google Scholar]
- Wroniak M, Rękas A, Siger A, Janowicz M. Microwave pretreatment effects on the changes in seeds microstructure, chemical composition and oxidative stability of rapeseed oil. LWT-Food Sci Technol. 2016;68:634–641. doi: 10.1016/j.lwt.2016.01.013. [DOI] [Google Scholar]