Abstract
This study investigated the effects of shallot (Allium ascalonicum L.) supplementation on improved nutritional quality and browning index of apple juice, specifically by comparing between heated (96 °C, 30 min) and unheated apple juice. The results showed that total soluble solid, phenolic content, flavonoid content, as well as antioxidant activities of heated and unheated apple juice, significantly (p < 0.05) increased with the increase in shallot supplementation (0.5%, 1.0%, 1.5%, and 2.0%). The outcomes indicated that the nutritional parameters values for heated apple juice supplemented with shallot were greater than those of unheated apple juice. The unheated and heated apple juice supplemented with shallot showed inhibition of browning, while shallot supplementation exhibited higher functionality upon being heated. The heated apple juice appeared to be an efficient way to reduce browning and to increase antioxidant activities, irrespective of shallot supplementation. Shallot supplementation inhibited browning and improved the nutritional quality of unheated apple juice. These results proved that heated apple juice supplemented with shallot exhibited maximum inhibition of browning and increased nutritional quality. Therefore, heating and shallot supplementation can massively improve the quality of apple juice.
Keywords: Allium ascalonicum, Antioxidant, Apple, Browning, Juice, Phenolic content, Shallot
Introduction
Apple (Malus pumila) is one of the main fruit crops available worldwide and consumed daily by millions of people. The world apple production for 2017/2018 was 76.2 million tonnes. The five top countries that produce apples are China, USA, European Union, Turkey, and India with production rates at 56.4%, 15.9%, 6.0%, 3.5%, and 2.5%, respectively (USDA 2017). Processed apple products in the food industry, such as juice, apple sauce, and fermented juice or cider, are high in demand. However, the major problem faced during handling, processing, and storage after harvest refers to enzymatic browning (Oms-Oliu et al. 2010). This effect degrades the quality of apples and processed apples. Enzymatic browning leads to deteriorated safety, off flavours, and lower nutritional value (Toivonen and Brummell 2008). Normally, the yellowish shade is the most important property of apple juice that indicates freshness. Apple juices are extremely vulnerable to enzymatic browning, as they can be catalysed by polyphenol oxidase (PPO) and peroxidase (POD) (Jang and Moon 2011). PPO refers to a copper-containing enzyme, which is also called tyrosinase that catalyses hydroxylation of monophenols to ortho-diphenols (cresolase or monophenolase) and oxidation of ortho-dihydroxyphenols to ortho-quinones (reactive electrophiles) using molecular oxygen (Oms-Oliu et al. 2010). The reactions form undesirable enzymatic browning in apple juice, post-harvest physiology of fruit and vegetable products, and other food processing (Guerrero-Beltrán et al. 2005). Therefore, appropriate control of enzymatic browning reaction in apple juice has been narrowed to PPO and POD within the food processing industries (Aydemir 2004). Sulfites are commercially available substances and have been widely used as an effective inhibitor for PPO. These substances, however, have been limited by the Food and Drug Administration (USFDA) as they can deteriorate the human health, particularly amongst asthmatic patients. Besides, these substances can react with other compounds in the food system and cause adverse health effects (Stohs and Miller 2014).
At present, several researchers have studied the extraction of natural products as food additives that can inhibit enzymatic browning and improve nutrient quality (Espley et al. 2014; Fante et al. 2013; Zocca et al. 2011; Sudha et al. 2011). Lee et al. (2002) reported that onion extracts effectively inhibited PPO and accordingly inhibited enzymatic browning of potato. This is similar to a report published by Kim et al. (2005), who found that onion extract repressed enzymatic browning in pear. Hence, onion extract may be applied as food additive, especially for beverages. The strong odour and flavour of uncooked onion can be reduced via heat treatment (Lee et al. 2016). Heating treatment increases polyphenol and flavonoid contents, antioxidant activities, and metal chelating capacity of onion (Lee et al. 2016; Woo et al. 2007).
The literature depicts that shallot extract is a good source of polyphenol, such as gallic acid, eriodictyol, apigenin, isoquercetin, kaempferol, quercetin, and tannic acid, which possesses antifungal property (Patcharawan et al. 2018; Mnayer et al. 2014; Alves et al. 2014). The application of natural extracts as natural food additive for food products, as Lee et al. (2016) had asserted, heated apple juice supplemented with onion extracts exhibited anti-browning reaction and improved nutrient value. Eissa et al. (2014) discovered that natural products, such as cucumber, squash, pepper, and oyster mushroom extracts, can substitute synthetic chemical compounds to prevent browning of apple juices by inhibiting PPO activity. Eissa and Salama (2002) also reported that some leafy vegetables were efficient in inhibiting browning of dried apple rings. Raeisi et al. (2016) reported about shallot bulb extracts had antioxidants, antimicrobial properties, and effectively delayed the shelf life of rainbow trout fillets during storage.
Since 1988s, shallot has been reckoned to be a good source of polyphenol that displays antioxidant activity and possesses antifungal property. To date, many researchers have reported on onion extraction. However, the effect of shallot on enzymatic browning of fruit juice product as a natural food additive has yet to be reported. Hence, this study examined the effects of shallot supplementation on improved quality parameters of apple juice, such as browning and antioxidant activities. This study specifically compared heated and unheated apple juice supplemented with shallot with respect to nutritional quality and browning index.
Materials and methods
Materials
Standard gallic acid, standard quercetin, 2, 2-Diphenyl-picrylhydrazyl (DPPH), Folin–Ciocalteu reagent, and 2, 4, 6-Tripyridyl-s-triazine (TPTZ) were obtained from Sigma-Aldrich (St. Louis, Mo, USA). Sodium nitrite and aluminum chloride were obtained from J.T. Baker (Phillipsburg, USA). Other reagents, such as hydrochloric acid, sulphuric acid, sodium carbonate, sodium hydroxide, and ethanol 95%, were of analytical reagent grade.
Apple juice supplemented with shallot preparation and heat treatment
Fuji apples (Malus pumila cv. Fuji) were purchased from Makro wholesaler supermarket (Ubon Ratchathani, Thailand) and shallots (Allium ascalonicum L.) were obtained from a local market (Ubon Ratchathani, Thailand). The shallots were peeled, washed, chopped into smaller pieces, and dried at 50 °C until the weight of the dried shallot was constant. The moisture content of the sample was 1.25 ± 0.02% in weight. Next, the dried shallot was blended into fine powder by using a mechanical blender and was passed through a pore-size sieve (80 mesh) before it was kept in a dark airtight bottle at − 18 1 °C until further experimental analysis. Apple juice was prepared by adhering to the procedure used by Lee et al. (2016) with minor modifications. Briefly, the pulp of apples (30 g) was homogenised with 100 mL of distilled water using a blender at speed 4 for 1 min. Next, the mixture was centrifuged (5000 g) at ambient temperature for 40 min and the supernatant was filtrated by using No. 4 Whatman filter paper. The filtrate was collected as pure apple juice. The apple juice supplemented with shallot was prepared by adding and homogenising apple pulps with dried shallots in varied concentrations [0%, 0.5%, 1.0%, 1.5%, and 2.0% (w/v)], and the apple juice samples for heat treatment were heated at 96 °C for 30 min. For preliminary study, the heated apple juice prepared using this method resulted in inactivation of both polyphenol oxidase and peroxidase. The unheated apple juice supplemented with shallot emitted a slight sulphur odour. However, the heated apple juice with shallot supplementation had better flavour attributes, when compared with unheated samples, since organosulfur compounds were transformed to thiosulfinate derivatives and these compounds were not detected.
Colour measurement
The colour of the apple juice samples was determined by using a colorimeter (Hunter Lab, Model colour Flex, Reston, VIRG, USA). The colorimeter was calibrated on the CIE lab colour system using a calibration plate (L* = 96.68, a* = − 0.16, and b* = 1.65), whereby the samples were evaluated for their colour parameters at ambient temperature (28 ± 2 °C). The L*, a*, and b* values represent lightness, redness (+a) or greenness (−a), and blueness (−b) or yellowness (+b), respectively.
Determination of browning index
The browning index of the sample was measured based on the method recommended by Supapvanich et al. (2011) by using a spectrophotometer (Genesys20, Thermo Fisher Scientific, Watham, MA, USA) at 420 nm. Low absorbance value corresponds to lower browning index. The percentage of browning index (%) was calculated as follows:
β0 = Browning of sample was evaluated as absorbance at 420 nm (at t = 0 min).
β = Browning of sample was evaluated as absorbance at 420 nm (at t = 10 min).
Determination of total soluble solid
The total soluble solid of sample was determined by using a refractometer (Master Refractometer, Atago, Tokyo, Japan) following a procedure used by AOAC method 970.59 (AOAC 2006), whereby a unit of measurement for total soluble solid is expressed in terms of °Brix. The total soluble solids (%) equivalent to the solids (%) was measured by refractometer × (100 − b)/100, where b = % H2O-insoluble solids.
Determination of total phenolic content (TPC)
The TPC of apple juice was carried out in accordance to a method reported by Bahukhandi et al. (2013) using a modified colorimetric method via UV–VIS spectrophotometric assay. Briefly, a sample (100 µL) was added into a test tube containing 1 mL of Folin–Ciocalteu reagent (diluted with deionised water 1:10) and was left for reaction up to 4 min. Next, the mixture was combined with 800 µL of 7.5% (w/v) aqueous sodium carbonate and kept in the dark at ambient temperature (28 ± 2 °C) for 2 h. A blank solution was prepared using the same procedure, but by replacing the sample with 100 µL of deionised water. Absorbance of blank solution and the apple juice mixture were analysed by using UV–VIS spectrophotometer at 765 nm. The final results were compared to a calibration curve of standard gallic acid solutions within the range of 20–100 μg/mL (y = 0.0121x + 0.0988, R2 = 0.9994). The results of apple juice sample are expressed as mg of gallic acid equivalent (GAE) per g dry weight (mg GAE g−1 DW).
Determination of flavonoid content
The flavonoid content of the sample was determined by adhering to a method reported by Eghdami and Sadeghi (2010), who used the colorimetric approach. Briefly, 100 µL of sample was added into a test tube containing 300 µL of deionised water, and 30 µL of 5% aqueous sodium nitrite solution. After 5 min, 30 µL of 10% aluminum chloride was added. The mixture was shaken vigorously and incubated for 5 min, later mixed with 200 µL of 1 mM sodium hydroxide solution, and the volume was adjusted to 1 mL with deionised water. The content was placed in the dark at ambient temperature (28 ± 2 °C) for 30 min and the absorbance value was recorded by using a UV–VIS spectrophotometer at 510 nm. The total flavonoid content of the apple juice is expressed as mg of quercetin equivalent (QE) per g dry weight (mg QE g−1 DW), which was calculated based on a calibration curve of quercetin concentrations within the range of 20–200 µg/mL (y = 0.0009x + 0.0025, R2 = 0.9948).
Estimation of free radical scavenging activity
DPPH radical scavenging activity
The DPPH radical scavenging activity of the sample was analysed as described by Plank et al. (2012) by using DPPH radical scavenging assay with minor modifications. Briefly, 100 µL of apple juice (apple juice sample concentration was at 300 µg/mL) was added to 100 µL of deionised water, and combined with 3.9 mL of 60 µM stable DPPH radical solution in 95% of ethanol for a period of 30 min. The absorbance of remaining DPPH radical of the mixture was recorded at 517 nm in the dark at ambient temperature (28 ± 2 °C) by using a UV–VIS spectrophotometer. The percentage of radical scavenging activity was calculated as follows:
A0 = absorbance of blank, Ae = absorbance of apple juice sample.
Ferric reducing antioxidant power (FRAP)
FRAP in the sample was carried out based on the procedure proposed by Tundis et al. (2013) with minor modifications. Ferrous sulfate solution with a 1–4 mM range in deionised water was prepared as standard. The FRAP reagent was freshly prepared before use by mixing 100 mL of 300 mM (pH 3.6) sodium acetate buffer, 10 mL of 10 mM TPTZ solution, and 10 mL of 20 mM ferric chloride solution at a ratio of 10:1:1 (v/v/v), and then, warmed at 37 °C before further analysis. The reaction mixture that contained 100 µL of apple juice, 1 mL of deionised water, and 1 mL of FRAP reagent was incubated to stand for reaction up to 4 min. The absorbance of the mixture at 593 nm was measured in the dark at room temperature (29 ± 1 °C) by using a UV–VIS spectrophotometer. The FRAP value was calculated as mM of ferrous sulfate equivalent per g dry weight of sample, which had been calculated based on a calibration curve of ferrous sulfate concentrations (y = 0.2389x + 0.1163, R2 = 0.9919).
Statistical analysis
All experimental data were performed at least in triplicate and are presented as mean values ± standard deviation. The two-way analysis of variance (ANOVA) was carried out to test the effects of shallot supplementation, heating treatments, and their interaction. The Tukey’s test was performed by using the Minitab software (Version 16.1.1.0) (State College, PA, USA). The significant level of p value < 0.05 was statistically significant.
Results and discussion
Effects of shallot supplementation and heating on colour quality of apple juice
The colour quality of apple juice is degraded by browning reaction. Hence, this study investigated if shallot supplementation and/or heating had an impact upon browning of apple juice by using a colorimeter. The increase of a* value indicates higher red colour, while the decrease of L* value denotes a darker colour. Hence, the increase of a* value and the decrease of L* value signify high browning colour (Subhashree et al. 2017). The L* value of heated and unheated apple juice samples significantly (p < 0.05) increased with increment in shallot supplementation (0.5%, 1.0%, 1.5%, and 2.0%) (Fig. 1a). The heated and unheated apple juice samples without shallot supplementation exhibited significantly (p < 0.05) lower L* values, when compared to apple juice samples supplemented with shallot. The L* value was lower in unheated apple juice, as compared to that of heated. These data present that heating and shallot supplementation can inhibit browning of apple juice. The a* value of unheated apple juice showed significant (p < 0.05) difference between shallot supplementation (Fig. 1b). Interestingly, a* value of heated apple juice increased gradually with the increase of shallot supplementation. However, the effect of shallot supplementation (0%, 0.5%, 1.0%, 1.5%, and 2.0%) in heated apple juice was insignificant (p > 0.05). The data showed that heating itself could successfully control the a* values of apple juice to be stable. The b* values of heated and unheated apple juice samples significantly (p < 0.05) increased with the increase in shallot supplementation (Fig. 1c). The unheated apple juice supplemented with shallot had b* value lower than that of heated. Apple juice was heated at 96 °C for 30 min in order to inactivate enzyme polyphenol oxidase and peroxidase, which could avoid the formation of coloured substances. Moreover, natural sulphur compounds, such as thiosulfinates and S-alk(en)yl-l-cysteine sulfoxides, in shallot could have inhibited the enzymatic browning enzyme (Barbagallo et al. 2012). The results suggest that shallot supplementation can prohibit browning of apple juice, irrespective of heating, but the apple juice can greatly improve its colour quality if shallot supplementation and heating are embedded.
Fig. 1.

Effect of shallot supplementations (%) and heating on colour (L*, a* and b*) of apple juice. The L* values are represented by using representative data; (n = 3), while error bars are indicated as ± standard deviation; (n = 9). Statistically significant (p < 0.05) differences are showed by different letters above the bars. Different capital letters present significant differences among the shallot supplementations at a given heat/unheat treatments, while different lowercase letters present significant differences between heat and unheat treatments for similar shallot concentration
Effects of shallot supplementation and heating on browning index of apple juice
The browning index value of heated and unheated apple juice samples significantly (p < 0.05) decreased with increase in shallot supplementation (Fig. 2). The heated apple juice had browning index lower than that of unheated with significant (p < 0.05) difference. The heated apple juice with shallot supplementation (2%) had the highest inhibition for browning index of apple juice. The data showed that shallot supplementation can prohibit browning index of apple juice, irrespective of heating. In addition, heating (boiled at 96 °C for 30 min) and shallot supplementation can greatly inhibit browning of apple juice. These results are in agreement with those reported by Lee et al. (2016) who found unheated apple juice supplemented with onion extracts decreased the browning index, while heating treatment (boiled at 96 °C for 1 h) of apple juice with addition of onion extracts had mostly suppressed the enzyme activities.
Fig. 2.
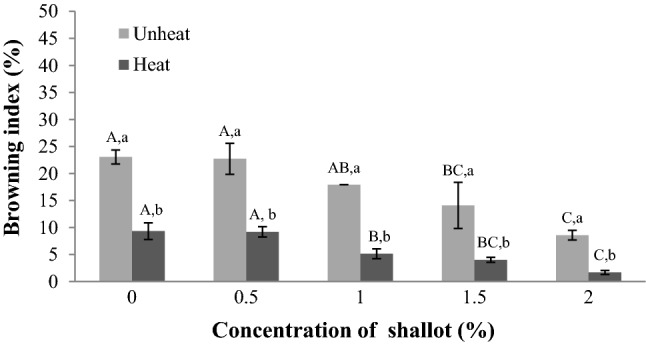
Effect of shallot supplementations (%) and heating on browning index (%) of apple juice. Browning index values are represented by using representative data; (n = 3), while error bars are indicated as ± standard deviation; (n = 9). Statistically significant (p < 0.05) differences are showed by different letters above the bars. Different capital letters present significant differences among the shallot supplementations at a given heat/unheat treatments, while different lowercase letters present significant differences between heat and unheat treatments for similar shallot concentration
Effects of shallot supplementation and heating on total soluble solid of apple juice
The amount of total soluble solids in apple juice is referred as Brix value and had been determined by using a refractometer. Normally, the Brix of apple juice should have adequate concentration. The total soluble solid of heated and unheated apple juice significantly (p < 0.05) increased gradually with increase in shallot supplementation and the heated apple juice had total soluble solid higher than that of the unheated (see Fig. 3). The apple juice supplemented with shallot had total soluble solid higher than the heated and unheated apple juice without shallot. Therefore, the results exhibited that apple juice supplemented with shallot can increase the total soluble solid, while heated apple juice supplemented with shallot can generate higher total soluble solid than that recorded for the unheated sample.
Fig. 3.
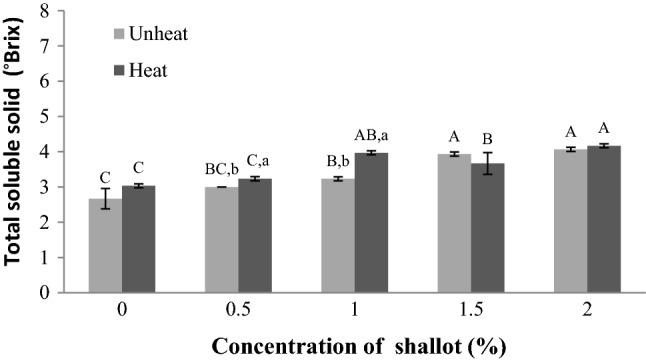
Effect of shallot supplementations (%) and heating on total soluble solid (°Brix) of apple juice. Total soluble solid values are represented by using representative data; (n = 3), while error bars are indicated as ± standard deviation; (n = 9). Statistically significant (p < 0.05) differences are showed by different letters above the bars. Different capital letters present significant differences among the shallot supplementations at a given heat/unheat treatments, while different lowercase letters present significant differences between heat and unheat treatments for similar shallot concentration
Effects of shallot supplementation and heating on TPC and flavonoid content of apple juice
Apples display strong antioxidant properties, such as polyphenols and flavonoid, as functional compounds that can prevent oxidative stress and several degenerative diseases in human (Di Pietro et al. 2007). Shallot extract is a good source of polyphenols, such as gallic acid, apigenin, kaempferol, and quercetin, whereby these compounds have been proven to possess antioxidant, antimicrobial, and antifungal properties (Mnayer et al. 2014; Alves et al. 2014; Patcharawan et al. 2018). As for TPC and flavonoid contents of apple juice, this study determined if shallot supplementation and/or heating can increase the TPC and flavonoid content in apple juice. The results revealed that the TPC and flavonoid content of heated and unheated apple juice significantly (p < 0.05) increased with increment in shallot supplementation, whereas heated apple juice supplemented with shallot recorded the highest TPC and flavonoid values (Fig. 4a and b). These results indicated that shallot supplementation and heating had essential effects on increasing the TPC and flavonoid content in apple juice. These results are in agreement with those reported by Mnayer et al. (2014), Woo et al. (2007), and Lee et al. (2016), whereby heating treatment had been found to increase polyphenol and flavonoid concentrations in apple juice. Moreover, the organosulfur compounds, such as diallyl thiosulfinate, in heated apple juice supplemented with shallot were changed to dipropyl disulfide, dipropyl trisulfide, methyl propyl disulfide and methyl propyl trisulfide, which later exhibited higher functionality (Tocmo et al. 2015). Some researchers revealed that phenolic compounds and flavonoids, such as gallic acid, apigenin, kaempferol, protocatechuic acid, flavones, and quercetin, were effective in preventing enzymatic browning in apple (Momtaz et al. 2008; Brewer 2011).
Fig. 4.
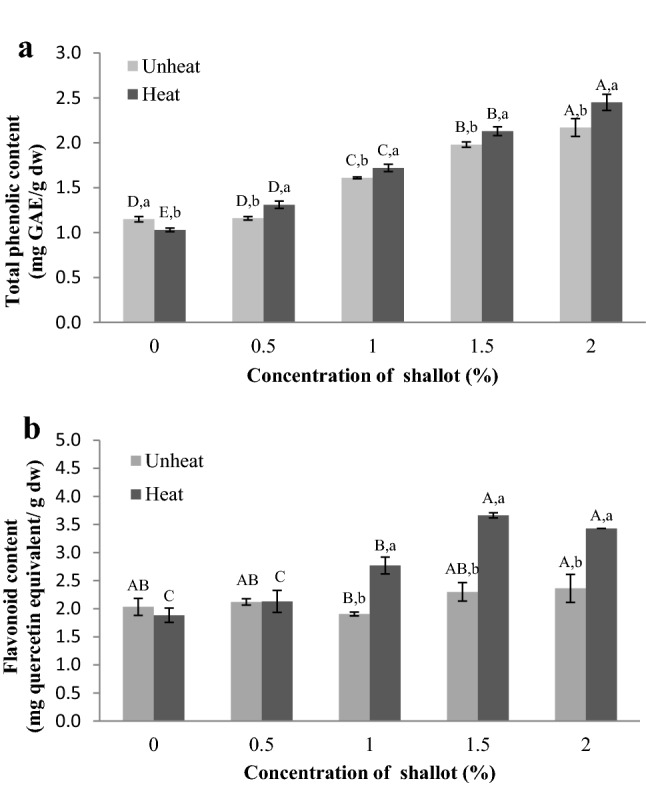
TPC (a) and flavonoid content (b) of apple juices supplemented with different concentrations of shallot and heating. The TPC and flavonoid content are represented by using representative data; (n = 3). The error bars are indicated as ± standard deviation; (n = 9). Statistically significant (p < 0.05) differences are showed by different letters above the bars. Different capital letters present significant differences among the shallot supplementations at a given heat/unheat treatments, while different lowercase letters present significant differences between heat and unheat treatments for similar shallot concentration
Effects of shallot supplementation and heating on antioxidant activities of apple juice
As for antioxidant activities of apple juice, the study investigated if shallot supplementation and/or heating affected DPPH radical scavenging activity and FRAP in apple juice. The results exhibited that DPPH radical scavenging activity of heated and unheated apple juice significantly (p < 0.05) increased with increment in shallot supplementation, in which heated apple juice supplemented with shallot (0%, 0.5%, 1.5%, and 2.0%) displayed higher DPPH radical scavenging activity than that of unheated apple juice (Fig. 5a). The ferric-reducing antioxidant power of heated apple juice significantly (p < 0.05) increased with increment in shallot supplementation, but the unheated apple juice exhibited significant (p < 0.05) increment with shallot supplementation of up to 1% and it steadily retained at 2% (Fig. 5b). Insignificant (p > 0.05) difference was noted between heated and unheated apple juice. The results indicated that shallot supplementation and heating increased DPPH radical scavenging activity and FRAP of apple juice. Shallot supplementation (0.5%, 1.0%, 1.5%, and 2.0%) and heating (boiled at 96 °C for 30 min) may synergistically strengthen the antioxidant activities of apple juice. These results are similar to those reported by Lee et al. (2016), who discovered that heating (boiled at 96 °C for 1 h), together with the addition of onion, can increase radical scavenging activities and metal chelating capacity of apple juice. Other studies also reported that the total phenolic compounds in apples are strongly correlated with antioxidant activities. The flavonoids in shallot affect antioxidant activity especially quercetin, isorhamnetin and their glycosides (Lu et al. 2011). These compounds display a mechanism of action, including chelation of transition of metal ions, free radical scavenging, and inhibition of oxidation (Zielinska et al. 2008). Additionally, thiosulphinates and S-alk(en)yl-l-cysteine sulfoxides in shallot could also contribute to antioxidant activity (Lu et al. 2011). Apples with plenty of polyphenols display the tendency to possess higher radical scavenging activities (Kalinowska et al. 2014).
Fig. 5.

DPPH radical scavenging activity (a) and FRAP (b) of apple juices supplemented with different concentrations of shallot and heating. DPPH radical scavenging and FRAP are represented by using representative data; (n = 3). The error bars are indicated as ± standard deviation; (n = 9). Statistically significant (p < 0.05) differences are showed by different letters above the bars. Different capital letters present significant differences among the shallot supplementations at a given heat/unheat treatments, while different lowercase letters present significant differences between heat and unheat treatments for similar shallot concentration
Conclusion
The results demonstrated that shallot extract has a potential to be used as natural food additive to improve the quality parameters of apple juice. The total soluble solid, TPC, the flavonoid content, and the antioxidant activities of both heated and unheated apple juice significantly (p < 0.05) increased with increment in shallot supplementation. The nutritional parameters values of heated apple juice supplemented with shallot had been higher than that of unheated. Although both unheated and heated apple juice supplemented with shallot showed inhibition of browning, that with shallot supplementation exhibited higher functionality when heated. The heated apple juice appeared to be an efficient way to decrease browning values, apart from increasing DPPH radical scavenging activity and FRAP, irrespective of shallot supplementation. Shallot supplementation inhibited browning values and improved the nutritional quality of unheated apple juice. The results clearly showed that heated apple juice supplemented with shallot displayed maximum anti-browning and greatly increased nutritional quality. These empirical outcomes provide important reference data for subsequent studies, a basis for new product development in industrial application, and a potential for apple juice to meet consumer demand for nutritious and functional foods.
Acknowledgements
The author would like to thank the Faculty of Agriculture, Ubon Ratchathani Rajabhat University (UBRU) for providing the necessary facilities.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alves CT, Ferreira IC, Barros L, Silva S, Azeredo J, Henriques M. Antifungal activity of phenolic compounds identified in flowers from North Eastern Portugal against Candida species. Future Microbiol. 2014;9:139–146. doi: 10.2217/fmb.13.147. [DOI] [PubMed] [Google Scholar]
- Association of Official Analytical Chemists (AOAC) (2006) Solids (soluble) in tomato products. Official methods of analysis, 18th edn. Association of Official Analytical Chemists, Gaithersburg
- Aydemir T. Partial purification and characterization of polyphenol oxidase from artichoke (Cynara scolymus L.) heads. Food Chem. 2004;87:59–67. doi: 10.1016/j.foodchem.2003.10.017. [DOI] [Google Scholar]
- Bahukhandi A, Rawat S, Bhatt ID, Rawal RS. Influence of solvent types and source of collection on total phenolic content and antioxidant activities of Acorus calamus L. Natl Acad Sci Lett. 2013;36:93–99. doi: 10.1007/s40009-012-0109-8. [DOI] [Google Scholar]
- Barbagallo RN, Riggi E, Avola G, Patanè C. Biopreservation of ‘Birgah’ eggplant from polyphenol oxidase activity assayed in vitro with onion (Allium Cepa L.) by-products. Chem Eng Trans. 2012;27:43–48. [Google Scholar]
- Brewer MS. Natural antioxidants: sources, compounds, mechanisms of action, and potential applications. Compr Rev Food Sci Food Saf. 2011;10:221–247. doi: 10.1111/j.1541-4337.2011.00156.x. [DOI] [Google Scholar]
- Di Pietro PF, Medeiros NI, Vieira FGK, Fausto MA, Bell-Klein A. Breast cancer in southern Brazil: association with past dietary intake. Nutr Hosp. 2007;22:565–572. [PubMed] [Google Scholar]
- Eghdami A, Sadeghi F. Determination of total phenolic and flavonoids contents in methanolic and aqueous extract of Achillea Millefolium. Org Chem J. 2010;2:81–84. [Google Scholar]
- Eissa HA, Salama MF. Inhibition of enzymatic browning by natural leafy vegetables extracts and keeping quality of fresh and dried apple rings. Pol J Food Nutr Sci. 2002;3(11/52):27–32. [Google Scholar]
- Eissa HA, Mostafa BM, Bareh GF, Shouk AA. Effect of extraction method of some natural extracts on enzymatic browning of apple juice. Int J Food Nutr Sci. 2014;3(6):54–62. [Google Scholar]
- Espley RV, Butts CA, Laing WA, Martell S, Smith H, McGhie TK, Zhang J, Paturi G, Hedderley D, Bovy A, Schouten HJ, Putterill J, Allan AC, Hellens RP. Dietary flavonoids from modified apple reduce inflammation markers and modulate gut microbiota in mice. J Nutr. 2014;144(2):146–154. doi: 10.3945/jn.113.182659. [DOI] [PubMed] [Google Scholar]
- Fante L, Scher CF, Noreña CPZ, Rios AO. Study of enzyme inactivation using steam in yacon (Smallanthus sonchifolius) roots. J Food Process Preserv. 2013;37(1):16–24. doi: 10.1111/j.1745-4549.2011.00609.x. [DOI] [Google Scholar]
- Guerrero-Beltrán JA, Swanson BG, Barbosa-Cánovas GV. Inhibition of polyphenol oxidase in mango puree with 4-hexylresorcinol, cysteine and ascorbic acid. LWT-Food Sci Technol. 2005;38(6):625–630. doi: 10.1016/j.lwt.2004.08.002. [DOI] [Google Scholar]
- Jang JH, Moon KD. Inhibition of polyphenol oxidase and peroxidase activities on fresh-cut apple by simultaneous treatment of ultrasound and ascorbic acid. Food Chem. 2011;124(2):444–449. doi: 10.1016/j.foodchem.2010.06.052. [DOI] [Google Scholar]
- Kalinowska M, Bielawska A, Lewandowska-Siwkiewicz H, Priebe W, Lewandowski W. Apples: content of phenolic compounds vs. variety, part of apple and cultivation model, extraction of phenolic compounds, biological properties. Plant Physiol Biochem. 2014;84:169–188. doi: 10.1016/j.plaphy.2014.09.006. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Kim CY, Park I. Prevention of enzymatic browning of pear by onion extract. Food Chem. 2005;89(2):181–184. doi: 10.1016/j.foodchem.2004.02.018. [DOI] [Google Scholar]
- Lee MK, Kim YM, Kim NY, Kim GN, Kim SH, Bang KS, Park I. Prevention of browning in potato with a heat-treated onion extract. Biosci Biotechnol Biochem. 2002;66(4):856–858. doi: 10.1271/bbb.66.856. [DOI] [PubMed] [Google Scholar]
- Lee B, Seo JD, Jk Rhee, Kim CY. Heated apple juice supplemented with onion has greatly improved nutritional quality and browning index. Food Chem. 2016;201:315–319. doi: 10.1016/j.foodchem.2016.01.092. [DOI] [PubMed] [Google Scholar]
- Lu X, Wang J, Al-Qadiri HM, Ross CF, Powers JR, Tang J, Rasco BA. Determination of total phenolic content and antioxidant capacity of onion (Allium cepa) and shallot (Allium oschaninii) using infrared spectroscopy. Food Chem. 2011;129:637–644. doi: 10.1016/j.foodchem.2011.04.105. [DOI] [PubMed] [Google Scholar]
- Mnayer D, Fabiano-Tixier AS, Petitcolas E, Hamieh T, Nehme N, Ferrant C, Fernandez X, Chemat F. Chemical composition, antibacterial and antioxidant activities of six essentials oils from the Alliaceae family. Molecules. 2014;19:20034–20053. doi: 10.3390/molecules191220034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momtaz S, Mapunya BM, Houghton PJ, Edgerly C, Hussein A, Naidoo S, Lall N. Tyrosinase inhibition by extracts and constituents of Sideroxylon inerme L. stem bark, used in South Africa for skin lightening. J Ethnopharmacol. 2008;119(3):507–512. doi: 10.1016/j.jep.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Oms-Oliu G, Rojas-Graü A, González L, Varela P, Soliva-Fortuny R, Hernando M, Pérez Munuera I, Fiszman S, Martín-Belloso O. Recent approaches using chemical treatments to preserve quality of fresh-cut fruit: a review. Postharvest Biol Technol. 2010;57:139–148. doi: 10.1016/j.postharvbio.2010.04.001. [DOI] [Google Scholar]
- Patcharawan S, Yossan S, Prasertsan P (2018) Antifungal property of chili, shallot and garlic extracts against pathogenic fungi, Phomopsis spp., isolated from infected leaves of para rubber (Hevea brasiliensis Muell. Arg.). Agric Nat Res: 1–7. (in press)
- Plank DW, Szpylka J, Sapirstein H, Woodllard D, Zapf CM, Lee V, Chen C-YO, Liu RH, Tsao R, Dusterloh A, Baugh S. Determination of antioxidant activity in foods and beverages by reaction with 2,2′-diphenyl-1-picrylhydrazyl (DPPH): collaborative study first action. J AOAC Int. 2012;95(6):1562–1569. doi: 10.5740/jaoacint.CS2012_04. [DOI] [PubMed] [Google Scholar]
- Raeisi S, Sharifi-Rad M, Quek SY, Shabanpour B, Sharifi-Rad J. Evaluation of antioxidant and antimicrobial effects of shallot (Allium ascalonicum L.) fruit and ajwain (Trachyspermum ammi (L.) Sprague) seed extracts in semi-fried coated rainbow trout (Oncorhynchus mykiss) fillets for shelf-life extension. LWT-Food Sci Technol. 2016;65:112–121. doi: 10.1016/j.lwt.2015.07.064. [DOI] [Google Scholar]
- Stohs SJ, Miller MJS. A case study involving allergic reactions to sulfur-containing compounds including, sulfite, taurine, acesulfame potassium and sulfonamides. Food Chem Toxicol. 2014;63:240–243. doi: 10.1016/j.fct.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Subhashree SN, Sunoj S, Xue J, Bora GC. Quantification of browning in apples using colour and textural features by image analysis. Food Qual Saf. 2017;1(3):221–226. doi: 10.1093/fqsafe/fyx021. [DOI] [Google Scholar]
- Sudha G, Priya MS, Shree RI, Vadivukkarasi S. In vitro free radical scavenging activity of raw pepino fruit (Solanum muricatum aiton) Int J Curr Pharm Res. 2011;3(2):137–140. [Google Scholar]
- Supapvanich S, Pimsaga J, Srisujan P. Physiochemical changes in fresh-cut wax apple (Syzygium samarangenese Blume Merrill & L.M. Perry) during storage. Food Chem. 2011;127:912–917. doi: 10.1016/j.foodchem.2011.01.058. [DOI] [PubMed] [Google Scholar]
- Tocmo R, Liang D, Lin Y, Huang D. Chemical and biochemical mechanisms underlying the cardioprotective roles of dietary organopolysulfides. Front Nutr. 2015;2:1–18. doi: 10.3389/fnut.2015.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toivonen PMA, Brummell DA. Biochemical bases of appearance and texture changes in fresh-cut fruit and vegetables. Postharvest Biol Technol. 2008;48(1):1–14. doi: 10.1016/j.postharvbio.2007.09.004. [DOI] [Google Scholar]
- Tundis R, Menichini F, Bonesi M, Conforti F, Statti G, Menichini F, Loizzo MR. Antioxidant and hypoglycaemic activities and their relationship to phytochemicals in Capsicum annuum cultivars during fruit development. LWT-Food Sci Technol. 2013;53:370–377. doi: 10.1016/j.lwt.2013.02.013. [DOI] [Google Scholar]
- USDA . Fresh deciduous fruit: world markets and trade (apples, grapes, and pears) Washington, D.C.: Foreign Agricultural Service, United States Department of Agriculture; 2017. [Google Scholar]
- Woo KS, Hwang IG, Kim TM, Kim DJ, Hong JT, Jeong HS. Changes in the antioxidant activity of onion (Allium cepa) extracts with heat treatment. Food Sci Biotechnol. 2007;16(5):828–831. [Google Scholar]
- Zielinska D, Wiczkowski W, Piskula MK. Determination of the relative contribution of quercetin and its glucosides to the antioxidant capacity of onion by cyclic voltammetry and spectrophotometric methods. J Agric Food Chem. 2008;56(10):3524–3531. doi: 10.1021/jf073521f. [DOI] [PubMed] [Google Scholar]
- Zocca F, Lomolino G, Lante A. Dog rose and pomegranate extracts as agents to control enzymatic browning. Food Res Int. 2011;44(4):957–963. doi: 10.1016/j.foodres.2011.02.010. [DOI] [Google Scholar]


