Abstract
The study investigated the effect of sourdough made from combinations of four Lactobacillus spp. on the physicochemical properties, consumer acceptability, and shelf life of bread made from pearl millet flour. Fermentation based on both single and multiple species reduced the pH of the dough and increased its titratable acidity and H2O2 content. The addition of sourdough increased the elasticity and reduced the stiffness of the pearl millet dough. Sourdough fermented with L. brevis had the greatest effect on loaf height, specific volume, porosity, and moisture content. During storage, the moisture content of the bread crumb decreased, but that of their crust increased. Sourdough-based loaves retained their moisture better than conventional loaves and the sourdough suppressed the development of mold for a longer period. An organoleptic assessment showed that the sourdough-based bread was more palatable than either conventional or chemically acidified ones. The tissue softness, chewiness, and flavor of the pearl millet bread decreased during storage. The use of sourdough based on either L. brevis, L. paralimentarius, or L. brevis + L. paralimentarius is recommended to produce high-quality pearl millet-based bread.
Keywords: Gluten-free bread, Millet, Probiotic starters, Sourdough
Introduction
The quality and quantity of gluten (a mixture of endosperm storage proteins present in grains such as wheat and some other cereals) are critical determinants of the suitability of flour to make bread. To cater for patients with celiac disease (gluten intolerance), bread and other bakery products need to be prepared by gluten-free (GF) flour (Aguilar et al. 2016). However, such flour typically produces bread that is poor in flavor, texture, and shelf life (Arendt et al. 2007). Therefore, alternatives to gluten are sought to produce GF bakery products.
One of the most promising approaches taken to substitute for the absence of gluten is to ferment GF dough with various species of Lactobacillus (LAB). The addition of the so-called “sourdough” to conventional dough promotes the retention of carbon dioxide produced by yeast, thereby supporting the leavening process, prolonging shelf life, improving crumb texture, and enhancing both the nutritional quality and taste of the product (Gobbetti et al. 2017. Fermentation by LAB acidifies the dough, exerting a positive effect on both flavor and yeast activity (Moroni et al. 2011).
GF flour can be prepared from a wide range of cereal grains, including maize (Hager and Arendt 2013), rice (Yano et al. 2017), pearl millet (Romero et al. 2017), sorghum (Ogunsakin et al. 2017), teff (Gebremariam et al. 2014), and oat (Wolter et al. 2014). Non-cereal starch-rich seed can also be used, notably from amaranth, quinoa (Elgeti et al. 2014), and buckwheat (Nogueira Novaes Southgate et al. 2017). The flour can also be prepared from starchy roots, such as those of cassava (Onyango et al. 2011), yam (Nindjin et al. 2011), plantain (Sarawong et al. 2014), and taro (Kaushal and Sharma 2014), as well as potato tubers (Onyango et al. 2011). The grain of pearl millet (Pennisetum glaucum) has been used as human food from prehistoric times. In modern times, it is used in South Asian cuisine to prepare flatbread and in Africa as a base for porridge. It is suggested as a viable source of raw material for GF products. Yet, its suitability for the preparation of GF bread is not rigorously investigated. The current study aimed at exploring the potential of combining LAB-based sourdough with pearl millet flour for the production of acceptable GF bread.
Materials and methods
Raw materials
Pearl millet sourdoughs were constituted by mixing water, pearl millet flour (9.9% moisture, 9.2% protein, 0.9% ash), white sugar, iodized salt, baking powder and lyophilized LAB. The moisture, protein and ash content of the pearl millet flour were determined according to the standard methods (AACC 44-16, 46-10 and 08-01: AACC 2000). Four LAB strains (Table 1) were tested in various combinations; they were isolated from locally based sourdoughs, cultured in de Man Rogosa Sharpe (MRS) medium at 37 °C for 24 h, and conserved in glycerol at − 80 °C.
Table 1.
Signs and abbreviations used instead of treatments titles in sourdough, dough and bread and formulation of GF bread dough preparation
| Signs and abbreviations of used strains in treatments | Formulation of GF bread dough preparation | ||||
|---|---|---|---|---|---|
| Treatments | LAB strains | Raw materials | Control dough | Sourdough dough | Acid-chemical dough |
| 1 M | L. sanfranciscensis | Flour | 100 | 92.5 | 100 |
| 2 M | L. brevis | Salt | 2 | 2 | 2 |
| 3 M | L. paralimentarius | Sugar | 5 | 5 | 5 |
| 4 M | L. plantarum | Yeast | 3 | 3 | 3 |
| 5 M | L. sanfranciscensis + L. brevis | Egg | 12 | 12 | 12 |
| 6 M | L. sanfranciscensis + L. paralimentarius | Sodium caseinate | 1.5 | 1.5 | 1.5 |
| 7 M | L. sanfranciscensis + L. plantarum | Skim milk | 5 | 5 | 5 |
| 8 M | L. brevis + L. paralimentarius | Guar gum | 3 | 3 | 3 |
| 9 M | L. brevis + L. plantarum | Oil | 5 | 5 | 5 |
| 10 M | L. paralimentarius + L. plantarum | Sourdough | – | 15 | – |
| 11 M | L. sanfranciscensis + L. brevis + L. paralimentarius | Acid | – | – | 0.12 |
| 12 M | L. sanfranciscensis + L. brevis + L. plantarum | Water | 90 | 82.5 | 90 |
| 13 M | L. brevis + L. paralimentarius + L. plantarum | Raw materials | Control dough | Sourdough dough | Acid-chemical dough |
| 14 M | L. sanfranciscensis + L. paralimentarius + L. plantarum | ||||
| 15 M | L. sanfranciscensis + L. brevis + L. paralimentarius + L. plantarum | ||||
| 16 M | Chemically acidified (CA) sample | ||||
| 17 M | Control (CO) sample (without starter) | ||||
Sourdough preparation and fermentation
For the preparation of sourdough, LAB was recovered from storage by culturing overnight in MRS broth at 37 °C. Pearl millet flour (92.5 g) was mixed with water (82.5 mL) to which 107 CFU g−1 LAB was added. The quantities of each of these ingredients are given in Table 1. The dough was allowed to ferment at 30 °C for 24 h.
Functional characteristics of sourdough samples
Total titratable acidity (TTA) and pH
The pH of the sourdough was measured with a standard pH meter after soaking a 10-g of sample in 90 mL of distilled water. The TTA was obtained by recording the volume of 0.1 M NaOH needed to raise the same samples’ pH to 8.5 (Paramithiotis et al. 2010).
Diacetyl and hydrogen peroxide (H2O2) content
The content of diacetyl was estimated by homogenizing a 10-g sourdough sample in 90 mL of distilled water. After adding 7.5 mL of 1 M hydroxylamine to 25 mL of the homogenate, the sample was titrated with 0.1 M HCl to reach a pH of 3.4. The diacetyl content (AK, measured in mg mL−1) was calculated following Edema and Sanni (2008) using the below formula:
where R (mL) represents the volume of 0.1 M HCl, S (mL) the volume of 0.1 M HCl required for the titration, E the equivalence factor (21.52 mg), and W the volume of the sample (mL). The H2O2 content was measured by adding 25 mL of 10% H2SO4 to 25 mL of the homogenate; then titrating with 0.1 M potassium permanganate to a point where the pale pink color persisted for 15 s before becoming decolorized. The H2O2 content was calculated based on Edema and Sanni (2008) using the following equation: 100 × (mL KMnO4 × 0.1 × 1.701)/(mL H2SO4 × sample volume).
LAB cell count
A 10-g sample of sourdough was homogenized in 90 mL of 0.15 M NaCl and serial dilutions were prepared in phosphate-buffered saline (PBS). The dilutions were spread in triplicate onto MRS agar and incubated for 48 h at 30 °C.
Dough rheology
The rheological properties of the dough were evaluated following Moroni et al. (2011) and Singh and Singh (2013). Briefly, a stress/strain-controlled rheometer (Antoon Paar MCR 301, Ostfildern, Germany) was used to measure standard rheological parameters using parallel plate geometry. The samples were allowed to rest for 10 min prior to evaluation and were pre-incubated for 1 h at 30 °C and 75% relative humidity. The analyses were carried out at a constant temperature of 30 °C, maintained by attaching a Peltier Plate System to a water circulation unit.
Preparation of GF bread
The recipes used to produce GF loaves are listed in Table 1. The salt was dissolved in warm water (80% of the total volume added) and this was mixed with pearl millet flour for 3 min using a domestic food processor (Brites et al. 2008). After cooling down to room temperature, the other ingredients were added, including the sourdough starter, and the mixture was blended for 5 min. The dough was fermented for 75 min at 30 °C and a relative humidity of 75%. A 150-g portion of each dough sample was baked in an oven using a baking tin (15 × 8.5 × 5.7 cm) at 225 °C for 30 min, followed by cooling down for 1 h before enclosing in a polyethylene bag. The control bread was baked from either a yeast-free mixture (CO bread) or dough without sourdough acidified by the addition of 120 mL of a 4:1 (v/v) mixture of (11.3 M) lactic acid and (17.4 M) acetic acid (CA bread).
Bread physico-chemical attributes
The pH and TTA
The pH of the bread was measured with a standard pH meter after soaking a 10-g of sample in 90 mL of distilled water. TTA values were obtained by recording the volume of 0.1 M NaOH needed to raise the same samples’ pH to 8.5.
Loaf specific volume, height, crumb, crust color, and moisture content
The specific volume of the loaves was measured 1 h after baking using the rapeseed displacement method (AACC10-05). A pair of digital calipers was used to estimate the loaf height. Coloration was quantified using computerized image processing. The loaves were photographed and the indices L*, a*, and b* (measuring lightness, redness–greenness, and yellowness–blueness, respectively) were determined using Adobe Photoshop software (www.adobe.com). The moisture content of the crust and crumb was measured following the AACC44-16 method shortly after baking, then after 2 and 4 days of storage.
Determination of baking loss
The baking loss was calculated from weight measurements taken before and after baking, according to the following formula:
Evaluation of the bread porosity
Porosity was quantified 2 h after baking by analyzing digital images of the cut surface using ImageJ software (www.imagej.nih.gov/ij/).
Evaluation of crumb and crust hardness
A textural profile analysis was performed on the crumb and a penetration test used to quantify crust hardness. Both tests reproduced the methods described by Crowley et al. (2002).
Sensory properties of bread
The sensory attributes of the breads were determined following a standard protocol ISO 8587, 1988 which employed a panel of twelve trained judges. An overall score was based on the individual assessments of crust and crumb colour, porosity, elasticity, acidic smell, texture softness, chewiness and taste. The texture characteristics, chewiness and taste were first evaluated 2 h after baking, then again after 2 and 4 days of storage.
Shelf life evaluation
The bread was enclosed in polyethylene bags after cooling and cutting with a sterile knife. The number of storage days at room temperature required for the appearance of mold was considered as the bread’s shelf life.
Statistical analysis
The data were statistically analysed (one-way analysis of variance) using routines implemented in SAS v9.0 software (SAS Institute, Cary, NC, USA). Means were compared using the Duncan (main effects) and Tukey (interactions) tests.
Results and discussion
Functional properties of the sourdough
There was no significant difference between the various types of starter culture and the sourdough pH, but all developed a significantly lower pH than the control (no LAB) sourdough (Table 2). The sourdough made from the 5 M (L. sanfranciscensis + L. brevis) and 11 M (L. sanfranciscensis + L. brevis + L. paralimentarius) treatments provided the highest TTAs. These results are in accordance with similar experiments described by Corsetti et al. (2000) and Wolter et al. (2014). All of the tested sourdough contained both diacetyl and H2O2 more than the LAB-free sample. The sourdough made from the 8 M treatment had the highest diacetyl content while the one made from the 10 M treatment had the highest H2O2 content. There is little information in the literature regarding variations in the production of diacetyl and H2O2 by LAB in sourdough, although both products are dependent on pH, acidification, and production of antimicrobial compounds, as discussed by Lacaze et al. (2007) and Pepe et al. (2003). LAB-generated H2O2 is thought to exert an antimicrobial effect due to its sulfhydryl group’s oxidation and membrane lipids peroxidation abilities. The production of H2O2 by Lactobacillus spp. and Lactococcus spp. is known to suppress the multiplication of both Pseudomonas spp. and Staphylococcus aureus (Santiago et al. 2016).
Table 2.
Impact of different starters on functional characteristics of sourdough
| Treatment | pH value | Titratable acidity (mL) | Diacetyl (mg mL−1) | Hydrogen peroxide (mmol L−1) |
|---|---|---|---|---|
| 1 M | 4.301 ± 0.006b | 13.17 ± 0.132c | 4.33 ± 0.456fg | 6.749 ± 0.507de |
| 2 M | 4.294 ± 0.019b | 14.30 ± 0.145b | 13.79 ± 1.32bc | 6.882 ± 0.199cd |
| 3 M | 4.334 ± 0.020b | 14.21 ± 0.190b | 12.93 ± 0.575bc | 6.362 ± 0.169def |
| 4 M | 4.290 ± 0.020b | 13.30 ± 0.065c | 14.64 ± 0.754b | 5.541 ± 0.292gh |
| 5 M | 4.312 ± 0.009b | 14.39 ± 0.155b | 12.087 ± 1.15c | 7.531 ± 0.315abc |
| 6 M | 4.292 ± 0.011b | 11.21 ± 0.190fg | 6.88 ± 0.53e | 5.147 ± 0.553h |
| 7 M | 4.313 ± 0.025b | 09.99 ± 0.120h | 3.45 ± 0.164g | 5.371 ± 0.178gh |
| 8 M | 4.306 ± 0.009b | 11.43 ± 0.108f | 21.51 ± 2.47a | 7.761 ± 0.613ab |
| 9 M | 4.332 ± 0.020b | 12.69 ± 0.200d | 5.48 ± 1.13ef | 6.112 ± 0.224efg |
| 10 M | 4.282 ± 0.035b | 10.90 ± 0.230g | 4.27 ± 0.249fg | 8.086 ± 0.390a |
| 11 M | 4.305 ± 0.008b | 15.49 ± 0.230a | 3.44 ± 0.563g | 7.094 ± 0.411bcd |
| 12 M | 4.357 ± 0.081b | 13.36 ± 0.109c | 5.13 ± 0.351efg | 6.862 ± 0.455cd |
| 13 M | 4.331 ± 0.013b | 11.10 ± 0.200fg | 6.86 ± 0.557e | 6.885 ± 0.691cd |
| 14 M | 4.273 ± 0.240b | 11.01 ± 0.230g | 12.14 ± 1.47c | 5.779 ± 0.457fgh |
| 15 M | 4.315 ± 0.021b | 12.23 ± 0.291e | 9.47 ± 1.06d | 7.847 ± 0.252a |
| Control | 5.463 ± 0.255a | 07.70 ± 0.365i | 1.216 ± 0.308h | 2.649 ± 0.388i |
The control sample is millet dough without starter that has been incubated for 24 h at 30 °C
Same letter in each column represent no significant difference in the level of 5% (P < 0.05)
The type of starter culture affected the LAB population size in the ripe sourdough. The most prolific LAB developed in sourdough produced from treatment 11 M: the population density in this case reached 10.14 Log CFU g−1 after a fermentation period of 48 h at 30 °C. According to (Wolter et al. 2014), the LAB titer in sourdough seeded with L. plantarum after a 24 h fermentation reached to 109 CFU g−1. In a study reported by Gül et al. (2005), the density of LAB produced in wheat sourdoughs developed from samples collected from various bakeries was between 5.28 and 9.57 Log CFU g−1.
Physicochemical properties of pearl millet dough and bread
The addition of sourdough to conventional dough decreased the pH of dough and bread and increased their TTA significantly (Table 3). Single-species LAB starters were more effective than starters containing a mixture of species. The lowest pH was attained in sourdough fermented with the 2 M (L. brevis) treatment, and the highest TTA with the 11 M treatment. Non-acidified dough and bread are expected to have higher pH and lower TTA than acidified ones (Komlenić et al. 2010). The present data were consistent with the expectations. Variation in the pH and TTA probably reflects differences in the LAB species involved in these sorts of experiments, but it can also be influenced by the nature of the substrate (flour origin), dough recipe, and fermentation conditions.
Table 3.
Changes in pH and TTA in the dough and gluten-free bread and impact of different starters on traits of gluten-free millet bread
| Treatments | Fermented dough | Bread | Impact of different starters on traits of gluten-free millet bread | |||||
|---|---|---|---|---|---|---|---|---|
| pH | TTA (ml) | pH | TTA (ml) | Height (cm) | Porosity (%) | Specific volume (ml/g) | Baking loss (%) | |
| 1 M | 4.916 ± 0.011i | 8.95 ± 0.06c | 5.715 ± 0.003ef | 3.61 ± 0.085abcd | 5.37 ± 0.06ab | 27.57 ± 1.07bc | 2.991 ± 0.016ab | 16.14 ± 0.4de |
| 2 M | 4.843 ± 0.031j | 10.10 ± 0.21a | 5.673 ± 0.006f | 3.801 ± 0.007ab | 05.50 ± 0.1a | 28.46 ± 1.75abc | 3.062 ± 0.049a | 13.45 ± 0.27i |
| 3 M | 4.868 ± 0.015j | 9.65 ± 0.14b | 5.754 ± 0.009def | 3.366 ± 0.145cdef | 5.38 ± 0.03ab | 30.13 ± 0.378a | 3.032 ± 0.017a | 15.69 ± 0.45ef |
| 4 M | 4.952 ± 0.011hi | 8.25 ± 0.01d | 5.748 ± 0.006def | 3.5 ± 0.008bcde | 5.23 ± 0.02bcd | 27.7 ± 0.985bc | 2.921 ± 0.027abcde | 16.24 ± 0.54de |
| 5 M | 5.017 ± 0.002ef | 9.59 ± 0.22b | 5.793 ± 0.018cdef | 3.31 ± 0.085def | 5.15 ± 0.05cde | 27.43 ± 0.503bc | 3.033 ± 0.052a | 14.64 ± 0.67gh |
| 6 M | 4.968 ± 0.001gh | 7.02 ± 0.13ef | 5.835 ± 0.012bcde | 3.65 ± 0.039abc | 5.12 ± 0.08cde | 27.63 ± 2.16bc | 2.767 ± 0.159e | 17.72 ± 0.7ab |
| 7 M | 5.003 ± 0.013fg | 6.36 ± 0.30hi | 5.795 ± 0.081cdef | 3.352 ± 0.147cdef | 5.02 ± 0.08ef | 24.7 ± 1.73de | 2.808 ± 0.107cde | 18.10 ± 0.50a |
| 8 M | 5.131 ± 0.004b | 6.84 ± 0.20fg | 5.866 ± 0.124bcd | 3.317 ± 0.186def | 5.47 ± 0.25a | 27.03 ± 1.47bc | 2.963 ± 0.038abc | 17.13 ± 0.45bc |
| 9 M | 5.073 ± 0.014cd | 6.40 ± 0.14hi | 5.853 ± 0.021bcd | 3.11 ± 0.085fgh | 5.22 ± 0.1bcd | 24.37 ± 1.69de | 2.950 ± 0.071abcd | 16.61 ± 0.35cd |
| 10 M | 5.013 ± 0.018ef | 9.05 ± 0.34c | 5.860 ± 0.020bcd | 3.320 ± 0.171def | 4.87 ± 0.06f | 23.53 ± 1.08e | 2.790 ± 0.121de | 17.23 ± 0.27bc |
| 11 M | 5.006 ± 0.016fg | 5.90 ± 0.14j | 5.776 ± 0.017def | 3.827 ± 0.38a | 5.13 ± 0.06cde | 27.43 ± 0.41bc | 2.946 ± 0.066abcd | 14.03 ± 0.78hi |
| 12 M | 5.017 ± 0.018ef | 5.52 ± 0.16k | 5.813 ± 0.022cde | 3.106 ± 0.14fgh | 5.25 ± 0.13bcd | 29.13 ± 0.97ab | 2.849 ± 0.015bcde | 13.37 ± 0.45i |
| 13 M | 5.060 ± 0.008cd | 7.21 ± 0.15e | 5.834 ± 0.18bcde | 3.207 ± 0.25efg | 5.13 ± 0.11cde | 27.87 ± 1.45abc | 2.964 ± 0.001abc | 15.2 ± 0.1fg |
| 14 M | 5.083 ± 0.075cd | 6.60 ± 0.25gh | 5.916 ± 0.004bc | 2.95 ± 0.17gh | 5.18 ± 0.03cde | 28.0 ± 0.45abc | 2.754 ± 0.129e | 15.32 ± 0.65fg |
| 15 M | 5.055 ± 0.028de | 8.20 ± 0.07d | 5.806 ± 0.135cde | 3.05 ± 0.04fgh | 5.18 ± 0.08cde | 27.5 ± 1.21bc | 2.853 ± 0.085bcde | 15.26 ± 0.38fg |
| 16 M | 5.100 ± 0.006bc | 6.15 ± 0.19ij | 5.952 ± 0.026b | 2.811 ± 0.286h | 5.17 ± 0.12cde | 26.05 ± 2.64cd | 2.803 ± 0.136cde | 17.57 ± 0.12ab |
| 17 M | 5.447 ± 0.022a | 4.94 ± 0.19l | 6.368 ± 0.019a | 2.041 ± 0.144i | 5.17 ± 0.11cde | 27.3 ± 1.23bc | 2.967 ± 0.123abc | 13.54 ± 0.16i |
Different letters in each column represent significant differences (P < 0.05)
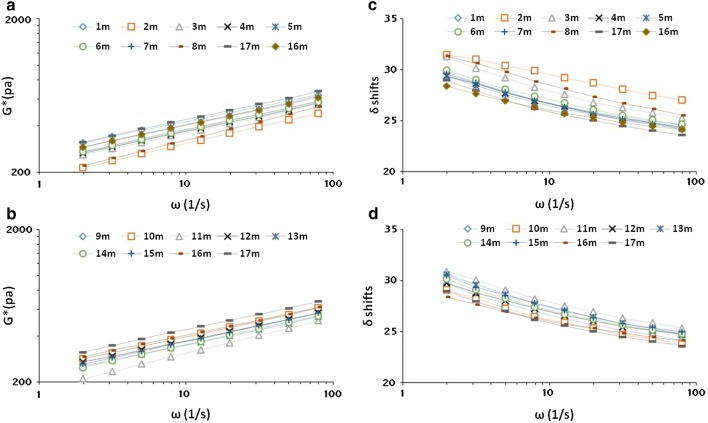
The effect of the various treatments on the rheological properties of the doughs was evaluated. In all treatments, as the frequency (ω) increased, the complex modulus (G*) also increased. Figure 1 illustrates the behavior of the parameters G* and phase angle (δ). The highest G* value was observed in CO dough. Doughs developed following the treatments 2 M, 8 M (L. brevis + L. paralimentarius) and 11 M all generated a particularly low G* value. The addition of sourdough boosted δ. The increase in δ, along with a fall in G*, produced doughs which were less elastic and softer. The basis of the alterations to dough rheology induced by the inclusion of sourdough is likely complex, but a major component is thought to be its influence over enzymatic activity, particularly related to proteolysis and acid production (Schober et al. 2003), and also exopolysaccharide production (Singh et al. 2016). A similar weakening of the dough following sourdough fermentation has been reported in doughs formed from the flour of oat (Hüttner et al. 2010), buckwheat (Moroni et al. 2011), amaranth, rice and sorghum. (Houben et al. 2010), in their analysis of the effect of L. plantarum and L. paralimentarius on the rheology of amaranth-based dough, showed that fermentation caused a pronounced softening of the dough. L. plantarum appeared to have a larger effect on rheology than L. paralimentarius, possibly reflecting species differences in their fermentation efficiency.
Fig. 1.
Comparing the complex modulus (G*, Panels a, b) and phase angle (δ, Panels c, d) of millet dough with increasing frequency (ω)
The effect of sourdough inclusion on loaf quality
The type of starter used to form the sourdough had a significant positive effect on loaf height, specific volume, porosity, and baking loss (Table 3). With respect to crust and crumb color, there was a significant effect on b*, but not on either L* or a* (Table 4). Also, color values of millet based gluten free bread were in accordance with findings reported by of Kaur et al. (2018). The smallest baking loss was associated with loaves made from sourdough fermented with the 12 M treatment (L. sanfranciscensis + L. brevis + L. plantarum). The single-species LAB starters had a greater effect than the multiple species starters on loaf height and specific volume. The largest loaf height and specific volume were related to the bread made from sourdough fermented with the 2 M treatment. With respect to porosity, the bread made from sourdough fermented with the 3 M treatment (L. paralimentarius) performed better than any other formulations (Table 3). With respect to the b* index, the inclusion of sourdough to dough tended to increase crumb yellowness and decrease crust yellowness when compared to the CO bread, but there were marginally significant differences between the treatments.
Table 4.
Impact of different starters on colored indices of millet bread crumb and crust
| Treatments | Millet bread crumb | Millet bread crust | ||||
|---|---|---|---|---|---|---|
| b* | a* | L* | b* | a* | L* | |
| 1 M | 29.77 ± 5.84abc | − 0.304 ± 0.04abc | 78.13 ± 2.52ab | 19.89 ± 2.04bcde | 22.17 ± 3.08abc | 73.04 ± 6.61abc |
| 2 M | 27.86 ± 2.47abcd | − 0.319 ± 0.03abc | 77.41 ± 0.64abc | 18.19 ± 3.59cdef | 22.42 ± 1.57abc | 75.74 ± 4.41abc |
| 3 M | 27.86 ± 2.16abcd | − 0.342 ± 0.10abc | 77.41 ± 2.72abc | 18.67 ± 5.35cdef | 22.42 ± 0.54abc | 71.88 ± 4.02abc |
| 4 M | 31.68 ± 2.47ab | − 0.243 ± 0.09a | 76.4 ± 2.04abc | 17.21 ± 2.52def | 23.86 ± 2.64abc | 72.07 ± 6.46abc |
| 5 M | 30.73 ± 6.93abc | − 0.311 ± 0.03abc | 75.82 ± 3.15abc | 16.73 ± 3.10ef | 21.45 ± 1.37bc | 77.10 ± 2.37a |
| 6 M | 24.28 ± 1.99bcde | − 0.296 ± 0.06abc | 78.56 ± 2.33a | 19.40 ± 2.01bcde | 21.93 ± 2.61abc | 72.84 ± 0.53abc |
| 7 M | 24.76 ± 3.31bcde | − 0.319 ± 0.03abc | 74.24 ± 2.97bc | 18.92 ± 2.00cdef | 22.42 ± 2.32abc | 70.72 ± 3.32bc |
| 8 M | 26.67 ± 3.83abcd | − 0.311 ± 0.04abc | 74.38 ± 1.56bc | 17.46 ± 2.37def | 21.93 ± 2.47abc | 74.20 ± 1.36abc |
| 9 M | 21.41 ± 0.84de | − 0.311 ± 0.06abc | 76.83 ± 1.66abc | 14.53 ± 3.38f | 24.59 ± 2.69ab | 73.23 ± 3.68abc |
| 10 M | 19.03 ± 4.54e | − 0.319 ± 0.05abc | 76.11 ± 3.04abc | 22.08 ± 2.04abcd | 24.35 ± 2.62ab | 77.10 ± 2.56a |
| 11 M | 30.49 ± 8.16abc | − 0.319 ± 0.10abc | 76.11 ± 3.62abc | 23.06 ± 3.38abc | 25.07 ± 3.15a | 69.94 ± 2.86c |
| 12 M | 31.44 ± 5.76ab | − 0.258 ± 0.08abc | 74.81 ± 2.94abc | 21.35 ± 7.43abcde | 20.73 ± 2.16c | 72.46 ± 5.73abc |
| 13 M | 31.20 ± 3.90ab | − 0.304 ± 0.08abc | 75.39 ± 2.31abc | 21.60 ± 4.56abcde | 21.69 ± 2.02abc | 76.52 ± 4.30ab |
| 14 M | 32.39 ± 7.75a | − 0.357 ± 0.05bc | 73.95 ± 3.62c | 24.27 ± 2.64ab | 23.14 ± 1.98abc | 73.42 ± 4.23abc |
| 15 M | 23.32 ± 5.82abc | − 0.357 ± 0.07bc | 78.13 ± 2.07ab | 20.13 ± 3.27bcde | 22.18 ± 3.19abc | 75.94 ± 2.50abc |
| 16 M | 29.29 ± 4.82abc | − 0.365 ± 0.05c | 76.11 ± 3.62abc | 25.97 ± 2.04a | 23.14 ± 1.01abc | 74.20 ± 5.84abc |
| 17 M | 26.19 ± 5.78abcd | − 0.318 ± 0.07abc | 76.26 ± 1.99abc | 26.22 ± 1.59a | 21.45 ± 1.83bc | 76.91 ± 2.50ab |
Same letter in each column represent no significant difference in the level of 5% (P < 0.05)
Baking loss is an economically important factor. Aplevicz et al. (2014) have shown that breads made with just yeast experienced a higher baking loss than those made with a combination of yeast and LAB. Hüttner et al. (2010) observed no significant effect on baking loss of the presence of sourdough in oat-based breads. In the pearl millet breads, the inclusion of sourdough had a positive effect on loaf volume. The inclusion of sourdough in bread formulations can induce marked changes to loaf volume: while in some cases the effect is negative (Armero and Collar 1996), more frequently it is positive (Crowley et al. 2002; Novotni et al. 2013). According to Clarke et al. (2002), the increased capacity of the loaf to expand as a result of the inclusion of sourdough reflects changes induced in the dough’s rheology, specifically it’s greater elasticity and reduced stiffness. It is likely that these changes flow from the proteolytic activity of the LAB altering the nature of the protein network in the dough. Softening the dough has the effect of improving its capacity to retain the carbon dioxide generated during fermentation. Increasing loaf volume is accompanied by a reduction in bread hardness, as pointed out by. The benefit of sourdough inclusion to bread porosity has also been noted in sourdough rye-based bread).
Crust and crumb moisture content
The results showed the significant effect of starter culture and storage (and their interaction) on the moisture content of the pearl millet bread crumb and crust. In fresh loaves, there were no significant differences between various treatments in terms of the crumb moisture content. However, during storage, the moisture content decreased unevenly between various formulations; the bread made from sourdough fermented with the 14 M treatment (L. sanfranciscensis + L. paralimentarius + L. plantarum) retained the most moisture (45.4% ± 0.40) at the end of the storage period (data not shown). On the other hand, the moisture content of the crust increased during the storage period. At the end of storage, the highest moisture content of the crust (35.0% ± 0.24) was attributed to the bread made from sourdough fermented with the 12 M treatment (L. sanfranciscensis + L. brevis + L. plantarum) and the least moist crust (29.6% ± 0.35) was related to the bread made from sourdough fermented with the 6 M treatment (L. sanfranciscensis + L. paralimentarius). The crust moisture levels of the remaining treatments were similar (data not shown). In experiments involving wheat bread, Gül et al. (2005) tested a variety of LAB species in combination with baker’s yeast and showed that crumb moisture did not depend on the formulation of the dough, a conclusion also reached from similar experiments conducted by Sanz-Penella et al. (2011). However, Torrieri et al. (2014) claimed that the type of starter culture does have a significant positive effect on crumb moisture content, suggesting that certain LAB strains capable of producing exopolysaccharides were able to boost moisture retention during storage.
Whether the inclusion of sourdough in the formulation of the dough reduces or enhances the kinetics of staling is controversial: in some cases, the rate is reduced (Crowley et al. 2002), while in others it is accelerated (Armero and Collar 1996). While Thiele et al. (2004) have suggested that the slowing of staling arises as a result of the production of particular LAB metabolites and their proteolytic activity, at least some of the variation in staling rate can also be ascribed to differences in loaf moisture content: in general, the higher a loaf’s moisture content, the longer is its shelf-life. The staling process represents a hardening of the crumb, a combined result of starch retro-gradation and an interaction between starch and protein, in which moisture is transferred from the crumb to the crust (Shin et al. 2010). The levels of crumb and crust moisture recorded for the pearl millet breads was in line with those reported for other breads. A possible explanation for the more stable moisture content of sourdough breads during their storage lies in the interaction of water with organic acids produced by LAB during fermentation.
Crumb and crust hardness
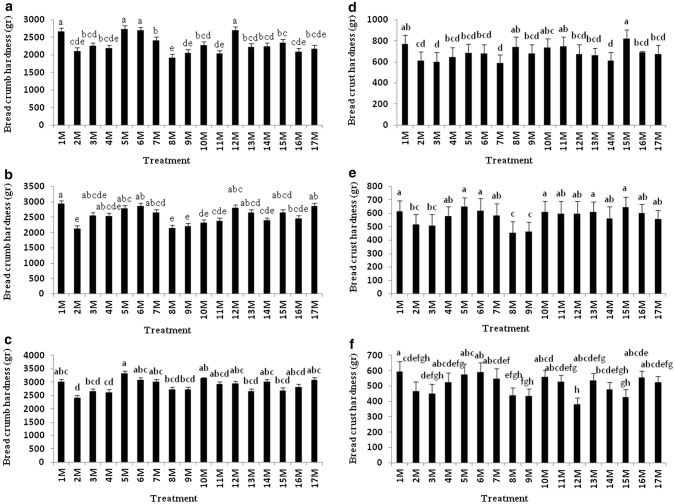
Both the type of starter culture and the storage time had a significant effect on the pearl millet breads’ crumb and crust hardness. Crumb hardness increased gradually during storage (Fig. 2a–c). For fresh loaves, breads prepared with sourdough produced from treatment 8 M developed the least firm crumb, while those produced from treatments 1 M (L. sanfranciscensis), 5 M, 6 M and 12 M developed the firmest crumb. When the breads were compared after 2 days of storage, those prepared with sourdough produced from treatments 2 M, 8 M and 9 M (L. brevis + L. plantarum) were associated with the lowest crumb firmness, while after 4 days of storage, loaves prepared with sourdough produced from treatment 2 M had the softest crumb. Crust hardness fell during storage: for fresh loaves, those prepared with sourdough produced from treatment 1 M formed the hardest and those from 7 M (L. sanfranciscensis + L. plantarum) the softest crusts (Fig. 2d–f). Following storage for 2 days, loaves prepared with sourdough produced from treatment 8 M formed the softest crusts. For loaves sampled after 4 days of storage, the hardest crust was associated with those prepared with sourdough produced from treatment 1 M and the softest from treatment 12 M. The latter formed a softer crust than did CO bread. Aplevicz et al. (2014) have shown that the inclusion of sourdough based on L. plantarum softens the crumb.
Fig. 2.
Comparison of bread crumb hardness at day 1 (a), day 3 (b), day 5 (c), and bread crust hardness at day 1 (d), day 3 (e) and day 5 (f)
Organoleptic assessment of the breads
There were significant differences between the treatments with respect to the organoleptic properties of the pearl millet breads. Notably, breads prepared with sourdough seeded with a combination of three LAB species were considered to be less palatable than the other breads. Loaves prepared with sourdough from treatments 7 M, 8 M and 9 M scored well for organoleptic quality. Tissue softness, chewiness and taste for all of the breads fell over storage time (data not shown). With respect to tissue softness, the addition of sourdough had a significant effect both for fresh loaves and for those stored for 2 days, but after 4 days of storage, there was no longer any significant treatment effect (data not shown). The most favored loaves with respect to tissue softness were those prepared with sourdough produced from treatments 3 M and 8 M. Loaves prepared with sourdough produced from treatments 8 M and 9 M scored highest for tissue softness at the end of the storage period. The chewiness score also varied significantly between treatments and over storage time (data not shown). For this property, breads prepared with sourdough produced from treatments 7 M, 8 M and 9 M scored more highly than did any of the other treatments.
Taste was also influenced by the type of starter culture. Fresh loaves prepared with sourdough produced from treatments 6 M, 7 M and 8 M were significantly superior to the others, but at the end of storage period, these significant effects had all disappeared. The good performance of loaves prepared with sourdough produced from treatment 8 M may have been due to their particularly strong accumulation of diacetyl during fermentation, but the lack of a correlation between diacetyl content and taste implies the influence of additional flavor ingredients. Fermentation is known to accentuate both desirable and undesirable flavors (Thiele et al. 2004). The sourish flavor of sourdough breads reflects the production of lactic and acetic acid during fermentation. The basis of all round flavor is particularly complex, however it is important to avoid excess acidity while bringing out the roasted taste. A number of factors, including the identity of the starter culture, are known to affect flavor (Katina et al. 2005). One of these other factors in sourdough breads is represented by the free amino acids which form as a result of proteolysis during fermentation. These are precursors of iso-alcohols which have a strong effect on bread flavor. Phenolic compounds also make a contribution to bread flavor. The substantial concentration of free phenolic compounds formed in rye-based sourdough is responsible for an intense aftertaste, described as bitterness (Katina et al. 2006). Acetic acid acts as a flavor enhancer when present at a low concentration, but increases pungency at higher concentrations. According to Novotni et al. (2013), the boosts to aroma achieved by incorporating sourdough results from the proteolytic release of free amino acids during fermentation.
The effect of including sourdough on staling
The use of sourdough could delay the development of mold during storage. After 7 days of storage, the bread made from sourdough fermented with 1 M, 3 M, 5 M, 6 M, 7 M, and 8 M treatments showed the lowest extent of mold growth. The antimicrobial activity of sourdough is due to its lactic acid, acetic acid, carbon dioxide, diacetyl, ethanol, and H2O2 contents, as well as various antibiotic compounds produced by LAB during fermentation. An analysis of the time required for mold to grow on sourdough bread based on Kluyveromyces marxianus and Lactobacillus bulgaricus has suggested that these LAB species are superior to those used to make conventional sourdough bread (Plessas et al. 2011).
Conclusion
The study proved the benefits of using various sourdoughs, based on combinations of four LAB species, for the quality and shelf life of GF pearl millet bread. The two most promising LAB species were L. brevis and L. paralimentarius. The inclusion of sourdough in conventional dough had a positive effect on both the shelf life and product quality of the bread. The results of the study may help the bakery industry to produce high-quality GF bread with longer shelf life.
Acknowledgements
The authors would like to thank “Agricultural Biotechnology Research Institute of Iran’’ (ABRII) for financial support of this work.
Funding
This work was supported by the Agricultural Biotechnology Research Institute of Iran (ABRII) (Grant Number 3-05-0551-88020).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aguilar N, Albanell E, Miñarro B, Capellas M. Chestnut flour sourdough for gluten-free bread making. Eur Food Res Technol. 2016;242(10):1795–1802. [Google Scholar]
- Aplevicz KS, da Silva T, Fritzen-Freire CB, Amboni RDMC, Barreto PLM, Sant’Anna ES. Effect of the Incorporation of different freeze-dried cultures on the properties of sourdough bread. J Culin Sci Technol. 2014;12(4):354–367. [Google Scholar]
- Arendt EK, Ryan LAM, Dal Bello F. Impact of sourdough on the texture of bread. Food Microbiol. 2007;24(2):165–174. doi: 10.1016/j.fm.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Armero E, Collar C. Antistaling additives, flour type and sourdough process effects on functionality of wheat doughs. J Food Sci. 1996;61(2):299–303. [Google Scholar]
- Brites C, Trigo MJ, Santos C, Collar C, Rosell CM. Maize-based gluten-free bread: influence of processing parameters on sensory and instrumental quality. Food Bioprocess Technol. 2008;3(5):707–715. [Google Scholar]
- Clarke C, Schober T, Arendt E. Effect of single strain and traditional mixed strain starter cultures on rheological properties of wheat dough and on bread quality. Cereal Chem. 2002;79(5):640. [Google Scholar]
- Corsetti A, Gobbetti M, De Marco B, Balestrieri F, Paoletti F, Russi L, Rossi J (2000) Combined effect of sourdough lactic acid bacteria and additives on bread firmness and staling. J Agric Food Chem 48(7):3044–3051 [DOI] [PubMed]
- Crowley P, Schober T, Clarke C, Arendt E. The effect of storage time on textural and crumb grain characteristics of sourdough wheat bread. Eur Food Res Technol. 2002;214(6):489–496. [Google Scholar]
- Edema MO, Sanni AI. Functional properties of selected starter cultures for sour maize bread. Food Microbiol. 2008;25(4):616–625. doi: 10.1016/j.fm.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Elgeti D, Nordlohne SD, Föste M, Besl M, Linden MH, Heinz V, Becker T. Volume and texture improvement of gluten-free bread using quinoa white flour. J Cereal Sci. 2014;59(1):41–47. [Google Scholar]
- Gebremariam MM, Zarnkow M, Becker T. Teff (Eragrostis tef) as a raw material for malting, brewing and manufacturing of gluten-free foods and beverages: a review. J Food Sci Technol. 2014;51(11):2881–2895. doi: 10.1007/s13197-012-0745-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbetti M, Pontonio E, Filannino P, Rizzello CG, De Angelis M, Di Cagno R. How to improve the gluten-free diet: the state of the art from a food science perspective. Food Res Int. 2017 doi: 10.1016/j.foodres.2017.04.010. [DOI] [PubMed] [Google Scholar]
- Gül H, Özçelik S, Sağdıç O, Certel M. Sourdough bread production with lactobacilli and S. cerevisiae isolated from sourdoughs. Process Biochem. 2005;40(2):691–697. [Google Scholar]
- Hager AS, Arendt EK. Influence of hydroxypropylmethylcellulose (HPMC), xanthan gum and their combination on loaf specific volume, crumb hardness and crumb grain characteristics of gluten-free breads based on rice, maize, teff and buckwheat. Food Hydrocolloid. 2013;32(1):195–203. [Google Scholar]
- Houben A, Götz H, Mitzscherling M, Becker T. Modification of the rheological behavior of amaranth (Amaranthus hypochondriacus) dough. J Cereal Sci. 2010;51(3):350–356. [Google Scholar]
- Hüttner EK, Bello FD, Arendt EK. Rheological properties and bread making performance of commercial wholegrain oat flours. J Cereal Sci. 2010;52(1):65–71. [Google Scholar]
- Katina K, Arendt E, Liukkonen KH, Autio K, Flander L, Poutanen K (2005) Potential of sourdough for healthier cereal products. Trends Food Sci Technol 16(1–3):104–112
- Katina K, Heiniö RL, Autio K, Poutanen K (2006) Optimization of sourdough process for improved sensory profile and texture of wheat bread. LWT-Food Sci Technol 39(10):1189–1202
- Kaur P, Singh N, Pal P, Kaur A. Traditional and improved paddy varieties: composition, protein, pasting and gluten free chapati making properties. Cereal Chem. 2018;95(5):666–678. [Google Scholar]
- Kaushal P, Sharma H. Effect of incorporating taro (Colocasia esculenta), rice (Oryza sativa), and pigeon pea (Cajanus cajan) flour blends on noodle properties. Int J Food Prop. 2014;17(4):765–781. [Google Scholar]
- Komlenić DK, Ugarčić-Hardi Ž, Jukić M, Planinić M, Bucić-Kojić A, Strelec I. Wheat dough rheology and bread quality effected by Lactobacillus brevis preferment, dry sourdough and lactic acid addition. Int J Food Sci Technol. 2010;45(7):1417–1425. [Google Scholar]
- Lacaze G, Wick M, Cappelle S. Emerging fermentation technologies: development of novel sourdoughs. Food Microbiol. 2007;24(2):155–160. doi: 10.1016/j.fm.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Moroni AV, Bello FD, Zannini E, Arendt EK. Impact of sourdough on buckwheat flour, batter and bread: biochemical, rheological and textural insights. J Cereal Sci. 2011;54(2):195–202. [Google Scholar]
- Nindjin C, Amani G, Sindic M. Effect of blend levels on composite wheat doughs performance made from yam and cassava native starches and bread quality. Carbohydr Polym. 2011;86(4):1637–1645. [Google Scholar]
- Southgate ANN, Scheuer PM, Martelli MF, Menegon L, de Francisco A. Quality properties of a gluten-free bread with buckwheat. J Culin Sci Technol. 2017;15(4):339–348. [Google Scholar]
- Novotni D, Čukelj N, Smerdel B, Ćurić D. Quality attributes and firming kinetics of partially baked frozen wholewheat bread with sourdough. Int J Food Sci Technol. 2013 [Google Scholar]
- Ogunsakin AO, Vanajakshi V, Anu-Appaiah KA, Vijayendra SVN, Walde SG, Banwo K, Prabhasankar P. Evaluation of functionally important lactic acid bacteria and yeasts from Nigerian sorghum as starter cultures for gluten-free sourdough preparation. LWT Food Sci Technol. 2017;82:326–334. [Google Scholar]
- Onyango C, Mutungi C, Unbehend G, Lindhauer MG. Modification of gluten-free sorghum batter and bread using maize, potato, cassava or rice starch. LWT Food Sci Technol. 2011;44(3):681–686. [Google Scholar]
- Paramithiotis S, Tsiasiotou S, Drosinos EH. Comparative study of spontaneously fermented sourdoughs originating from two regions of Greece: Peloponnesus and Thessaly. Eur Food Res Technol. 2010;231(6):883–890. [Google Scholar]
- Pepe O, Villani F, Oliviero D, Greco T, Coppola S (2003) Effect of proteolytic starter cultures as leavening agents of pizza dough. Int J food microbiol 84(3): 319–326 [DOI] [PubMed]
- Plessas S, Alexopoulos A, Mantzourani I, Koutinas A, Voidarou C, Stavropoulou E, Bezirtzoglou E. Application of novel starter cultures for sourdough bread production. Anaerobe. 2011;17(6):486–489. doi: 10.1016/j.anaerobe.2011.03.022. [DOI] [PubMed] [Google Scholar]
- Romero HM, Santra D, Rose D, Zhang Y. Dough rheological properties and texture of gluten-free pasta based on proso millet flour. J Cereal Sci. 2017;74:238–243. [Google Scholar]
- Santiago AJ, Ahmed MN, Wang SL, Damera K, Wang B, Tai PC, Derby CD. Inhibition and dispersal of Pseudomonas aeruginosa biofilms by combination treatment with escapin intermediate products and hydrogen peroxide. Antimicrob Agents Chemother. 2016;60(9):5554–5562. doi: 10.1128/AAC.02984-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Penella JM, Tamayo-Ramos JA, Haros M. Application of bifidobacteria as starter culture in whole wheat sourdough breadmaking. Food Bioprocess Technol. 2011;5(6):2370–2380. [Google Scholar]
- Sarawong C, Gutiérrez ZR, Berghofer E, Schoenlechner R. Effect of green plantain flour addition to gluten-free bread on functional bread properties and resistant starch content. Int J Food Sci Technol. 2014;49(8):1825–1833. [Google Scholar]
- Schober TJ, Dockery P, Arendt EK. Model studies for wheat sourdough systems using gluten, lactate buffer and sodium chloride. Eur Food Res Technol. 2003;217(3):235–243. [Google Scholar]
- Shin M, Gang DO, Song JY. Effects of protein and transglutaminase on the preparation of gluten-free rice bread. Food Sci Biotechnol. 2010;19(4):951–956. [Google Scholar]
- Singh S, Singh N. Relationship of polymeric proteins and empirical dough rheology with dynamic rheology of dough and gluten from different wheat varieties. Food Hydrocolloids. 2013;33(2):342–348. [Google Scholar]
- Singh JP, Kaur A, Singh N. Development of eggless gluten-free rice muffins utilizing black carrot dietary fibre concentrate and xanthan gum. J Food Sci Technol. 2016;53(2):1269–1278. doi: 10.1007/s13197-015-2103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele C, Grassl S, Gänzle M. Gluten hydrolysis and depolymerization during sourdough fermentation. J Agric Food Chem. 2004;52(5):1307–1314. doi: 10.1021/jf034470z. [DOI] [PubMed] [Google Scholar]
- Torrieri E, Pepe O, Ventorino V, Masi P, Cavella S. Effect of sourdough at different concentrations on quality and shelf life of bread. LWT Food Sci Technol. 2014;56(2):508–516. [Google Scholar]
- Wolter A, Hager AS, Zannini E, Czerny M, Arendt EK. Impact of sourdough fermented with Lactobacillus plantarum FST 1.7 on baking and sensory properties of gluten-free breads. Eur Food Res Technol. 2014;239(1):1–12. [Google Scholar]
- Yano H, Fukui A, Kajiwara K, Kobayashi I, Yoza KI, Satake A, Villeneuve M. Development of gluten-free rice bread: pickering stabilization as a possible batter-swelling mechanism. LWT Food Sci Technol. 2017;79:632–639. [Google Scholar]