Abstract
Many indigenous fermented foods and beverages consumed throughout the world are produced at home or in crafts enterprises. The production of fermented beverages on a large commercial or industrial scale requires clearly established technical and technological requirements. This study shows a novel way to investigate the optimal process parameters of the Kyrgyz traditional fermented beverage Bozo using rotational rheological parameters. Five significant process parameters were investigated like cooking of millet porridge, the mashing temperature, the mashing time under conditions of mixing and viscosity changes of the end product during storage. According to the gelatinization temperature of millet porridge, cooking parameters were recommended at T = 79 °C and t = 30 min. The optimum mashing temperature of millet porridge was determined to be 65 °C and mashing time under stirring conditions of millet porridge was found to be 10 min. The viscosity of the beverage Bozo was investigated after 7, 14 and 21 days of storage at 5, 10, 20, and 30 °C. The effective viscosity of Bozo was calculated using the Casson model, which increased from 39.67 to 51.25 Pa·s after 21 days of storage. The effect of temperature on effective viscosity of Bozo and the activation energy was calculated using an Arrhenius-type equation. The parameters obtained make it possible to provide food manufacturers useful information for boiling, mashing and storage parameters after fermentation as well as quality control of Bozo.
Keywords: Boza/Bozo, Fermented beverage, Gelatinization, Mashing time, Rheological methods
Introduction
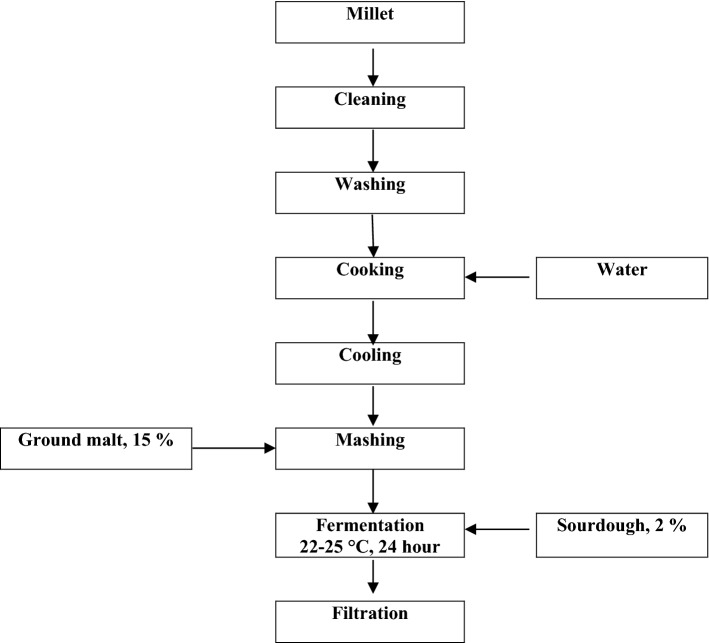
Fermentation is one of the oldest and most economical methods for the production of preserved food (Chavan et al. 1989). In addition, fermented foods, containing lactic acid bacteria, are considered as probiotic products and they are of great significance for human health (Todorov et al. 2008). According to Blandino et al. (2003), Bouza/Boza is a thick, sweet, slightly sour beverage produced from cereals, for example, millet, maize, and wheat, fermented with yeast and lactic acid bacteria and is known to be produced in Egypt, Albania, Turkey, Bulgaria, and Romania. The production method of Kyrgyz traditional beverage Bozo, in contrast, has its own characteristics. According to the typical traditional production process (Fig. 1), the Bozo production can be summarized in several steps: (1) preparation of the cereals, (2) boiling, (3) cooling and mashing by addition of ground malt, (4) fermentation, (5) filtration, and (6) storage. During the production of Bozo, malt produced from barley is usually used as a source for α-amylase and glucoamylase. The resulting amount of fermentable sugars is a significant factor, which is finally a decisive factor for the resulting amount of ethanol that is produced during the fermentation process of Bozo. Bozo can be classified in categories like “mild” or “strong” Bozo, depending on its ethanol content (Iskakova et al. 2017). Respective to our former research work, the alcohol content of Bozo was found as 4% (v/v) and the energy value was 146.43 kJ/100 mL. This indigenous fermented beverage, which is widely consumed in Central Asia and China is generally produced at home in villages and in crafts enterprises only by empirical knowledge. Opposite to that the production of industrial fermented beverages like Bozo requires clearly established technological parameters (Campbell-Platt 1994).
Fig. 1.
Production scheme of Bozo
The fermentation of starchy sources is more complex compared to that of low molecular sugars such as glucose or sucrose because the starch in general at first has to be converted into fermentable sugars before a microbial conversion into ethanol is possible. To achieve almost complete degradation of starch, two main types of amylolytic enzymes are required. An enzyme for liquefaction of the starch polymer like thermostable α-amylase is needed, and saccharifying glucoamylases are helpful (Senn and Pieper 2001). According to Power (2003), the preliminary process is a boiling process of the cereal flour in water at high temperatures between 90 and 95 °C to gelatinize and liquefy the starchy feedstock. During the mashing process, the starch polymer is hydrolyzed by the α-amylase to produce shorter chains consisting of several glucose units, namely dextrins. In a further degradation step, a glucoamylase of malt converts dextrins to fermentable sugars, like glucose, maltose, and maltotriose (Power 2003). All the process steps mentioned above are leading to a change in viscosity of the medium. Numerous methods have been described to measure the changes in viscosity, including optical, calorimetric and rheological methods, and each of these methods records a slightly different aspect of the gelatinization, malting and fermentation processes (Genc et al. 2002). Common techniques used in the food industry to determine the gelatinization temperature, swelling capacity, shear/thermal stability and the extent of retrogradation of starch are Brabender Viscoamylography (BV), Rapid Visco-Analysis (RVA) and Differential Scanning Calorimetry (DSC) (Qian and Kuhn 1999; Singh et al. 2002; Schirmer et al. 2015).
Currently, there are more sensitive rheological methods for material characterization (Fischer and Windhab 2011) available. The comparison of a Rotational Rheometer (RR) and a Brabender Viscograph (BV) for the measurement of gelatinization properties of rice flours revealed that the RR is reflecting the changes in chemical composition of rice better compared to the determination of a BV (Smanalieva et al. 2015). Therefore, the aim of this study was to investigate the changes in the viscosity during processing using absolute rheometry to optimize the domestic technological processes of Kyrgyz traditional beverage Bozo production, which has been described by Iskakova et al. (2017). The technological parameters, such as boiling temperature of millet porridge, malt addition, mashing temperature of millet porridge, mashing time of millet porridge with the addition of malt, viscosity changes during the storage were determined precisely by using rheological techniques.
Materials and methods
Millet (Panicum miliaceum), purchased from local markets in Bishkek, Kyrgyzstan, was used for the preparation of millet flour. Millet (W = 14.2%) was milled using a laboratory blender (Waring Laboratory, Torrington, USA) and sieved through a test sieve with an aperture diameter of 710 μm (Armfield, Hampshire, UK).
Barley (Hordeum vulgare) was used for the preparation of malt, which was purchased from local markets in Bishkek, Kyrgyzstan. Malt was prepared according to a traditional method. Barley grains were selected, weighed, washed, and steeped in water (grain/water ratio 1:2 w/v) for 24 h and allowed to germinate under water up to 4 days at 20–22 °C. The germinated grains were subsequently dried without forced ventilation at 20–22 °C to a moisture content of 14% up to 3 days. The height of the grain bed during germination was 2 cm and 0.5 cm during the drying process. The dried malt was milled using a laboratory blender (Waring Laboratory, Torrington, USA) and sieved through a test sieve with an aperture diameter of 710 μm (Armfield, Hampshire, UK).
Gelatinization profile of millet porridge
The gelatinization profile of millet flour was determined using rotational rheological methods as described by Smanalieva et al. (2015), but with some modifications. A mixture of 25 g water and 2.5 g of milled millet was placed in the concentric cylinder CC27 of the rheometer MCR 302 (Anton Paar, Graz, Austria). The following thermal program was used: (1) pre-equilibration at a constant shear rate of 100 1/s and T = 45 °C for 15 min; (2) heating up to 95 °C at a rate of 2 °C/min, (3) holding temperature at T = 95 °C, (4) cooling down to 55 °C at a rate of 2 °C/min; (5) holding temperature at T = 50 °C. The applied shear rate was 96.75 1/s, which is equivalent to the standard rotational speed of n = 75 1/min used in the BV.
The data were recorded using the supporting rheometer software Rheoplus 32 Multi 6 version 3.40.
Mashing temperature and mixing time of millet porridge
In order to determine the temperature of mashing, the porridge was prepared by the following procedure: 500 g of water was added to 70 g of millet flour and heated in a water bath at 80 °C for 30 min under continuous mixing. Furthermore, 25 g of the millet porridge was placed in the cylinder of the rheometer MCR 302 (Anton Paar, Graz, Austria) and according to the recipe 0.75 g of malt was added at 55, 65, 75 and 85 °C and mixed. The monitoring of the viscosity at a constant shear rate of 5 1/s was started immediately after the addition of malt.
For the determination of the optimum mixing time of the cooked millet porridge after the addition of malt, it was stirred at 100 rpm for 1, 3, 5, 7, 10 and 15 min at 65 °C. In order to stop the enzymatic process (mashing) and inactivation of amylases, the mixture was heated up to 95 °C immediately and held for 1 min and then cooled down to room temperature. The apparent viscosity was measured at a constant shear rate of 5 1/s and at a constant temperature of 25 °C. As a reference, the viscosity of the control porridge without the addition of malt was analyzed.
Flow behaviors of Bozo during the storage period
Mashed Bozo wort was fermented using yeast species like S. cerevisiae and lactic acid bacteria as L. plantarum, P. parvulus, L. casei at 22–25 °C for 24 h as described by Iskakova et al. (2019). After fermentation, it was filtered through a filter with a pore size of 500 µm and was stored at 4°–6°. The following rotational measurement condition of the rheometer was used to obtain the flow behavior of Bozo by measuring shear viscosity (()) and shear stress (τ()) at 5, 10, 20 and 30 °C. The measurements were performed during three different intervals to determine the thixotropic behavior of the fermented beverage: (1) the shear rate was progressively increased linearly from 0.1 to 100 1/s over a span of 90 s; (2) the shear rate was constant at a shear rate 100 1/s; (3) the shear rate was progressively decreased from 100 to 0.1 1/s.
In order to describe the rheological behavior of the beverage, the flow curves were modeled using classic equations such as Herschel–Bulkley, Casson, and Ostwald–de-Waele.
Herschel–Bulkley model:
| 1 |
where is yield stress, K is the consistency index, is the shear rate, n is the flow behavior index.
Casson model:
| 2 |
where is the Casson’s coefficient of viscosity.
Ostwald–de-Waele (or Power-law) model:
| 3 |
The effect of temperature on effective viscosity of millet porridge and the activation energy was calculated using an Arrhenius-type equation:
| 4 |
where, η is the effective viscosity, A is the pre-exponential factor, Ea is the activation energy of flow, R is the universal gas constant and T is the absolute temperature. The activation energy Ea is the energy barrier that must be overcome before the flow process is initiated (Mezger 2011).
Statistical analysis
Each rheological measurement was carried out for two times. All data were analyzed by the IBM Statistic SPSS 22 software (SPSS Inc., Chicago, IL) using the One-way ANOVA test and Tukey post hoc tests with the 95% confidence interval. A p-value less than 0.05 was considered statistically significant.
Results and discussion
Determination of cooking temperature by gelatinization profile of millet porridge
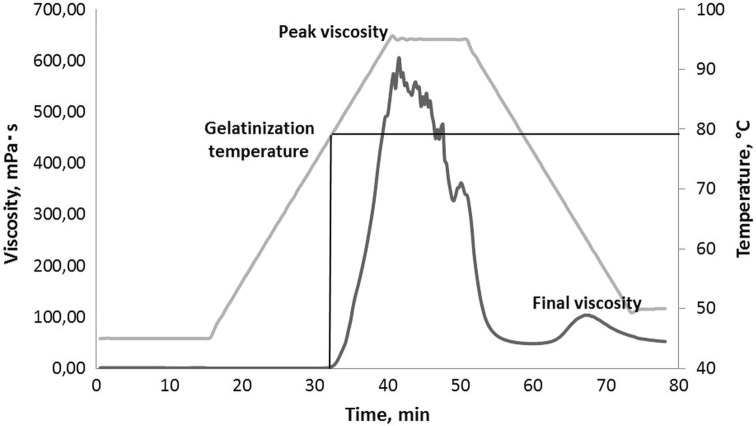
The RR was used to measure the gelatinization properties with the determination of typical parameters such as gelatinization temperature, peak viscosity, breakdown, setback, and final viscosity, as described in AACC Method 76-21.01.
The principal enzyme reaction involved in mashing is the hydrolysis of starch to sugars by α- and β-amylases. Before enzyme hydrolysis can occur it is necessary to exceed the starch gelatinization temperature of millet (Okafor 2007). During the gelatinization, the granular changes that occur can be reflected in a viscosity profile (also known as a pasting curve) as shown in Fig. 2. At the initial heating stage, where T = 45 °C for 15 min and between the temperature range of 45 °C and 79 °C the viscosity of the suspension remains constant, this means millet starch suspension achieves a homogenous and uniform structure. During the onward heating at a temperature starting at 79 °C up to 93 °C, a rise in viscosity can be observed due to swelling of the granules and leaching of amylose from the granules. The temperature at which starch begins to undergo these changes is referred to as the gelatinization temperature or pasting temperature (Whistler et al. 1984; Singh et al. 2002; Schirmer et al. 2015). The peak viscosity occurs when the majority of the granules is fully swollen and indicates the maximum viscosity, which is reached during heating and holding cycle. It is an indication of water holding capacity of starch (Park et al. 2007). In our study, the millet porridges reached the maximum viscosity of 608 mPa·s at a temperature of 93 °C. This finding is in accordance with the results of Siroha and Sandhu (2018), who found peak viscosities of modified starch from pearl millet cultivars at 299–1164 mPa·s at 87.4–90.7 °C. Smanalieva et al. (2015) reported that the peak viscosity of Kyrgyz variety rice flour using rotational rheometry was determined at 107–372 mPa·s between 91.4–95.2 °C. Park et al. (2007) reported that peak viscosities can be found in the range of 420 to 860 mPa·s at peak temperatures in the range of 70.6–93.0 °C for different rice starches, measured by a rotational rheometer. The breakdown viscosity is normally regarded as a measure of the disintegration of the starch granules when they are heated due to the rupture of granules within the shear field of the instrument and the release of soluble amylose (Agu et al. 2006). The breakdown viscosity was calculated as the difference between the maximum and minimum viscosity and was determined at 54.5 mPa·s. These results are in agreement with the study of Siroha and Sandhu (2018), who have reported, that breakdown viscosity (BV) of modified starches from millet in the range of 15 to 470 mPa·s. The final viscosity of the investigated millet paste was 107 mPa·s, which was determined at the end of the cooling phase. Many researchers have been observed that the final viscosity increases during cooling due to the aggregation of the amylose molecules (Miles et al. 1985; Singh et al. 2010).
Fig. 2.
Gelatinization properties of millet flour
In this work, the boiling time was calculated as the difference from the start of gelatinization and final gelatinization time and was recorded as 30 min. Accordingly, for preparation of the millet porridge the following regimen is recommended: soaking millet flour for 15 min at 45 °C, then heating for 30 min at 45–93 °C. It is necessary to select the optimum conditions for the saccharifying enzymes to operate (Yoo et al. 1987). Therefore, in the following experiments the effect of temperature on the α-amylase activity of malt was determined.
Effect of temperature on mashing of millet porridge
The next technological step during Bozo production is the mashing process, which consists of the enzymatic degradation of the polysaccharides. Since non-germinated cereals are generally poor in enzymes, especially endo-enzymes like α-amylase, they can be developed by the malting process. Therefore, malt contains much higher amylase activity. This is the reason to use barley malt for the production of Bozo to improve starch degradation. This is also a fundamental step in the production of beer since the composition of wort after the mashing process determines the formation of alcohol in the final product. To provide Bozo manufacturers with precise mashing parameters, the malt adding temperature to millet porridge was determined. According to Ghumman et al. (2016), the effect of germination duration, type of grain and their interactive effect are significant parameters on pasting properties. As reported by Iskakova et al. (2017), the mashing temperature depends on the malt’s origin. Therefore, in order to find the optimum temperature for mashing with barley malt, measurements were carried out at the following temperatures: 55, 65, 75, and 85 °C. The change of viscosity vs. time during mashing is shown in Fig. 3. At each temperature the decrease in viscosity of millet porridge with malt was different. As reported by Okafor (2007), that malt contains α-amylase and β-amylase with optimum temperatures 70 °C and 60–65 °C, respectively. Therefore, at each temperature, due to the different enzymatic activities of malt, the decrease in the viscosity of millet porridge with the addition of malt was found different. An increase in temperature leads to structural changes as a result of the activation of α-amylase and the millet starch is degraded slower or faster into dextrins. The optimum temperature for the hydrolysis of millet porridge’s starch with the addition of malt is defined at this temperature at which the highest reduction of viscosity during measurement is observed. According to the viscosity curve, after 17 min the apparent viscosity of millet porridge at 65 °C was the lowest i.e. 1.36 Pa·s and at 85 °C it was the highest at 8.72 Pa·s, which means that above 65 °C a deactivation of β-amylase enzymes occurs. These findings are in accordance with the study of Durand et al. (2009), who have detected the decrease of amylase activity at temperatures higher than 63 °C and the mashing process at temperatures close to 67 °C that favor the production of fermentable matter, which is a desirable objective of the beer production process (Durand et al. 2009). According to Piggot and Conner (2003), for preparing a whiskey mash it was useful to cool it down to 60–65 °C before the addition of malted barley. In our study at 85 °C, the decrease of viscosity was lower than at 65 °C, this is due to the inhibition of both enzymes, the β-amylase, and α-amylase at higher temperatures. The α-amylase is said to have a temperature optimum between 70 and 75 °C, whereas, β-amylase has its optimum between 60 and 65 °C (Okafor 2007). Thus, at 65 °C the enzyme activity of barley malt seems to be best for preparing a Bozo mash. For each viscosity curve, the relationship between viscosity and time was found using the power equation type. The correlation coefficients ranged between 0.9093 and 0.9917. The germination process leads to structural modification and the synthesis of new compounds with high bioactivity, which increases the nutritional value (Kaukovirta-Norja et al. 2004; Pal et al. 2016). Therefore, malt is used not only to hydrolyze the starch in millet, it also enriches the product with bioactive compounds.
Fig. 3.
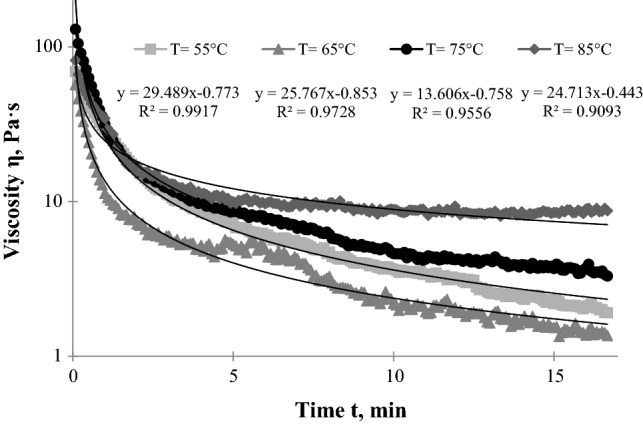
Change of viscosity versus time after addition of malt at 55, 65, 75 and 85 °C
Effect of mashing time on the rheology of millet porridge
To increase the mashing kinetics, the malt (enzyme source) and the millet porridge (substrate) must be mixed thoroughly. The mixing time is defined as the time required for achieving a certain low degree of viscosity of millet porridge mashing millet and malt together in a unit operation vessel. It has been used as a key parameter for assessing the efficiency of the mixing process. Figure 4 shows the changes in viscosity at 65 °C after different mixing times (for 1, 3, 5, 7, 10 and 15 min) of millet porridge in combination with malt (15% w/w). After mashing the porridge with malt for 1 min the final value of viscosity was 190 mPa·s, whereas, after mixing for 3, 5, 7 min the viscosity decreased to values between 100–102 mPa·s. After 10 and 15 min of mixing, viscosity curves overlayed and the final viscosity was determined at 70 mPa·s. Therefore, the results clearly show that there is no necessity to mix the millet porridge in combination with barley malt for more than 10 min.
Fig. 4.
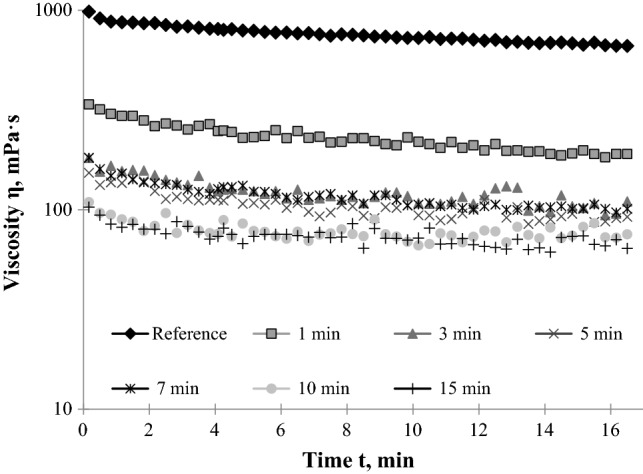
Viscosity versus hydrolysis time of millet porridge with malt
Effect of storage time on the flow behavior of the beverage Bozo
The rheological characteristics of fermented Bozo during the storage at 4–6 °C were investigated after 7, 15 and 21 days. The flow curves: relativity shear rate () versus shear stress (τ) were observed at 5, 10, 20 and 30 °C. As can be seen in Fig. 5, with an increase in shear rate, shear stress increased proportionally with shear stress values and has yield stress. This type of shear thinning behavior is a typical characteristic of starch pastes and starch-rich food matrices (Singh et al. 2010). Shear-thinning behavior occurs as a result of the breakdown of structural units (hydrocolloids) due to the hydrodynamic forces generated during shearing. Repetition of the experiments resulted in an equilibrium hysteresis loop (area). The thixotropic behavior of Bozo at 5 °C became stronger with increasing storage time. These findings are in accordance with Genc et al. who showed that beverage Bozo behaves as a non-Newtonian shear-thinning fluid (Genc et al. 2002).
Fig. 5.

Time-dependent shear-thinning (thixotropic) behavior of Bozo at 5 °C
The curves were fitted to the Herschel–Bulkley, Casson, and Ostwald–de-Waele models. Generally, Bozo was assumed to be a non-Newtonian fluid as reported by many scientists and the Ostwald-de Waele model was frequently used to describe the flow curves of Bozo (Hayta et al. 2001; Genc et al. 2002). In contrast to previous studies, in this work, the Casson model was found the most appropriate to fit the flow curves of the experimental beverage with a correlation coefficient R2 = 0.9995–0.9999. The yield stress () and the Casson’s coefficient of viscosity () values were obtained by fitting the shear rate versus apparent viscosity data to the Casson model (Eq. 2), which are presented in Table 1.
Table 1.
Adjustment of experimental rheological data of beverage Bozo to the Casson model n = 0.5. R = 0.9995–0.9999
| Days | T (°C) | (Pa) | (Pa·s)n | A (Pa/s) | ηeff (Pa·s), = 50 s−1 |
|---|---|---|---|---|---|
| 7 | 5 | 0.71 Ca | 0.08 Ba | 11.26 Ca | 39.67 Ba |
| 10 | 0.66 Bb | 0.08 Aa | 6.65 Cb | 37.98 Bb | |
| 20 | 0.62 Bc | 0.07 Ab | 5.23 Cc | 32.89 Bc | |
| 30 | 0.59 Bd | 0.06 Ac | 3.88 Bd | 28.44 Bd | |
| 14 | 5 | 0.74 Ba | 0.08 Ba | 30.09 Ba | 40.66 Ba |
| 10 | 0.66 Bb | 0.07 Bb | 11.49 Bb | 34.18 Cb | |
| 20 | 0.63 Bc | 0.07 Ab | 6.37 Bc | 33.21 Bc | |
| 30 | 0.55 Cd | 0.06 Ac | 3.40 Cd | 27.19 Bd | |
| 21 | 5 | 0.93 Aa | 0.09 Aa | 49.50 Aa | 51.25 Aa |
| 10 | 0.84 Ab | 0.08 Ab | 15.08 Ab | 43.94 Ab | |
| 20 | 0.77 Ac | 0.07 Ac | 10.16 Ac | 37.67 Ac | |
| 30 | 0.71 Ad | 0.06 Ad | 6.69 Ad | 32.10 Ad |
For each sample, mean value with different letters within each column are significantly different (p < 0.05, small letters within temperatures, capital letters within storage days)
*The rheological parameters are reported as the mean and standard deviation (SD = 0.01) of three independent measurements
The results show that the effective viscosity () of Bozo has increased from 39.67 to 51.25 Pa·s after 21 days of storage. The yield stress () increased from 0.71 to 0.93 Pa proportionally to storage time (after the 14th day) with the significance p < 0.05. The reason for the change of the flow behavior is a physicochemical reaction (such as gelatinization) in the sample. ANOVA analysis showed that there is no significant difference in the Casson’s coefficient of viscosity () of beverage Bozo during storage time.
The yield stress values (), effective viscosity () and hysteresis area (A) of the samples decreased with increasing temperature. ANOVA analysis showed that these values of Bozo samples were significantly different (p < 0.05) at each temperature studied. In contrast, the Casson’s coefficient of viscosity () values of Bozo did not vary very much with change in the temperature. Hayta et al. (2001) and Genc et al. (2002) reported similar results for fermented and unfermented Turkish Boza samples.
An Arrhenius-type relationship was employed to estimate activation energy for Bozo samples. The activation energy was calculated for temperatures between 5 and 30 °C of Bozo samples during storage after 7, 14 and 21 days. The results were 9.50, 9.89, 12.62 kJ/mol, respectively. The correlation coefficients ranged between 0.9013 and 0.9918. Higher values of activation energy (24.83–34.13 kJ/mol) at 35–85 °C were reported for wheat porridge by Sai Manohar et al. (1998) and 18.27 kJ/mol for fermented finger millet paste at 20–55 °C by Ojijo and Shimoni (2004).
Conclusion
The temperature and preparation time of the millet porridge were determined using fundamental rotational rheological measurement. As a result of the investigations, gelatinization temperature of millet starch was found as 79 °C and the peak viscosity was determined to be 93 °C. Effect of temperature on mashing of millet porridge was highest at 65 °C and this temperature is recommended for mashing the millet porridges. Therefore, for the preparation of millet porridge following regimen are recommended: soaking 15 min at 45 °C, cooking 30 min at 45–93 °C and cooling temperature 65 °C. The effect of mixing time on the hydrolyzing degree of millet porridge revealed that the optimum mixing time at a temperature of 65 °C was found to be 10 min. The flow behavior of the beverage Bozo during the storage period at 4–6 °C changed significantly after the 14th day. The possible reason for the change of the flow behavior is an increase of yeast cell concentration in a sample, which has bigger cell morphology (size, shape, and mass) than lactic acid bacteria. Storage in the fridge doesn’t stop microbiological growth. Therefore, further microbiological investigations are required. The possible reason for the change of the flow behavior is an increase of branching yeast cell concentration in a sample, which has bigger cell morphology (size, shape, and mass) than lactic acid bacteria. Storage in the fridge doesn’t stop microbiological growth. Therefore, further microbiological investigations are required. Obtained parameters provide useful information for optimization heating, mashing, and storage parameters, as well as quality control of Bozo.
Acknowledgements
The authors thank Prof. Dr. Coskan Ilicali for valuable comments on the manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- AACC International. Approved methods of analysis, 11th edn. Method 76-21.01 general pasting method for wheat or rye flour or starch using the rapid visco analyser. AACC International, St. Paul, MN, USA. 10.1094/AACCIntMethod-76-21.01
- Agu RC, Bringhurst TA, Brosnan JM. Production of grain whisky and ethanol from wheat, maize, and other cereals. J Inst Brew. 2006;112:314–323. doi: 10.1002/j.2050-0416.2006.tb00737.x. [DOI] [Google Scholar]
- Blandino A, Al-Aseeri ME, Pandiella SS, Cantero D, Webb C. Cereal-based fermented foods and beverages. Food Res Int. 2003;36:527–543. doi: 10.1016/S0963-9969(03)00009-7. [DOI] [Google Scholar]
- Campbell-Platt G. Fermented foods: a world perspective. Food Res Int. 1994;27:253–257. doi: 10.1016/0963-9969(94)90093-0. [DOI] [Google Scholar]
- Chavan JK, Kadam SS, Beuchat LR. Nutritional improvement of cereals by fermentation. Crit Rev Food Sci Nutr. 1989;28:349–400. doi: 10.1080/10408398909527507. [DOI] [PubMed] [Google Scholar]
- Durand GA, Corazza ML, Blanco AM, Corazza FC. Dynamic optimization of the mashing process. Food Control. 2009;20:1127–1140. doi: 10.1016/j.foodcont.2009.03.004. [DOI] [Google Scholar]
- Fischer P, Windhab JE. Rheology of food materials. Curr Opin Colloid Interface. 2011;16:36–40. doi: 10.1016/j.cocis.2010.07.003. [DOI] [Google Scholar]
- Genc M, Zorba M, Ova G. Determination of rheological properties of boza by using physical and sensory analysis. J Food Eng. 2002;52:95–98. doi: 10.1016/S0260-8774(01)00092-9. [DOI] [Google Scholar]
- Ghumman A, Kaur A, Singh N. Impact of germination on flour, protein and starch characteristics of lentil (Lens culinari) and horsegram (Macrotyloma uniflorum L.) lines. LWT Food Sci Technol. 2016;65:137–144. doi: 10.1016/j.lwt.2015.07.075. [DOI] [Google Scholar]
- Hayta M, Alpaslan M, Köse E. The effect of fermentation on viscosity and protein solubility of boza, a traditional cereal-based fermented Turkish beverage. Eur Food Res Technol. 2001;213:335–337. doi: 10.1007/s002170100385. [DOI] [Google Scholar]
- Iskakova J, Smanalieva J, Kulmyrzaev A, Fischer P, Methner F-J. Comparison of rheological and colorimetric measurements to determine α-amylase activity for malt used for the beverage Bozo. Int J Food Prop. 2017;20(9):2060–2070. doi: 10.1080/10942912.2016.1230869. [DOI] [Google Scholar]
- Iskakova J, Hutzler M, Kemelov K, Grothusheitkamp D, Michel M, Methner F-J. Screening a Bozo starter culture for potential application in beer fermentation. J Am Soc Brew Chem. 2019;77(1):54–61. [Google Scholar]
- Kaukovirta-Norja A, Wilhelmsson A, Poutanen K. Germination: a means to improve the functionality of oat. Agric Food Sci. 2004;13:100–112. doi: 10.2137/1239099041838049. [DOI] [Google Scholar]
- Mezger GT. The rheology handbook. 4. Hannover: Vincentz Network; 2011. [Google Scholar]
- Miles MJ, Morris VJ, Orford PD, Ring SG. The roles of amylose and amylopectin in the gelation and retrogradation of starch. Carbohydr Res. 1985;135:271–281. doi: 10.1016/S0008-6215(00)90778-X. [DOI] [Google Scholar]
- Ojijo NK, Shimoni E. Rheological properties of fermented finger millet (Eleucine coracana) thin porridge. Carbohydr Polym. 2004;57:135–143. doi: 10.1016/j.carbpol.2004.02.011. [DOI] [Google Scholar]
- Okafor N. Modern industrial microbiology and biotechnology. Boca Raton: SRS Press; 2007. [Google Scholar]
- Pal P, Singh N, Kaur P, Kaur A, Virdi AS, Parmar N. Comparison of composition, protein, pasting, and phenolic compounds of brown rice and germinated brown rice from different cultivars. Cereal Chem. 2016;93(6):584–592. doi: 10.1094/CCHEM-03-16-0066-R. [DOI] [Google Scholar]
- Park I-M, Ibanez AM, Zhong F, Shoemaker CF. Gelatinization and pasting properties of waxy and non-waxy rice starches. Starch/Stärke. 2007;59:388–396. doi: 10.1002/star.200600570. [DOI] [Google Scholar]
- Piggot JR, Conner JM. Whiskey, whiskey and bourbon: products and manufacture. In: Caballero B, editor. Encyclopedia of food sciences and nutrition. 2. London: Academic Press; 2003. pp. 6171–6177. [Google Scholar]
- Power RF. Enzymatic conversion of starch to fermentable sugars. In: Jacques KA, editor. The alcohol textbook. 4. Nottingham: Nottingham University Press; 2003. pp. 23–32. [Google Scholar]
- Qian J, Kuhn M. Evaluation on gelatinization of buckwheat starch: a comparative study of Brabender viscoamylography, rapid visco-analysis, and differential scanning calorimetry. Eur Food Res Technol. 1999;209(3–4):277–280. doi: 10.1007/s002170050493. [DOI] [Google Scholar]
- Sai Manohar R, Manohar B, Haridas Rao P. Rheological characterization of wheat porridge (cooked dalia), a semi-liquid breakfast food. J Cereal Sci. 1998;27:103–108. doi: 10.1006/jcrs.1997.9999. [DOI] [Google Scholar]
- Schirmer M, Jekle M, Becker T. Starch gelatinization and its complexity for analysis. Starch/Stärke. 2015;67:30–41. doi: 10.1002/star.201400071. [DOI] [Google Scholar]
- Senn T, Pieper HJ. Part 1. Classical Methods. In: Roehr M, editor. The biotechnology of ethanol: classical and future applications. Weinheim: Wiley-Vch Verlag GmbH; 2001. pp. 1–87. [Google Scholar]
- Singh N, Singh J, Sodhi NS. Morphological, thermal, rheological and noodle-making properties of potato and corn starch. J Sci Food Agric. 2002;82(12):1376–1383. doi: 10.1002/jsfa.1194. [DOI] [Google Scholar]
- Singh S, Singh N, Isono N, Noda T. Relationship of granule size distribution and amylopectin structure with pasting, thermal, and retrogradation properties in wheat starch. J Agric Food Chem. 2010;58:1180–1188. doi: 10.1021/jf902753f. [DOI] [PubMed] [Google Scholar]
- Siroha AK, Sandhu KS. Physicochemical, rheological, morphological, and in vitro digestibility properties of cross-linked starch from pearl millet cultivars. Int J Food Prop. 2018;21(1):1371–1385. doi: 10.1080/10942912.2018.1489841. [DOI] [PubMed] [Google Scholar]
- Smanalieva J, Salieva K, Borkoev B, Fischer P, Windhab E. Investigation of changes in chemical composition and rheological properties of Kyrgyz rice cultivars (Ozgon rice) depending on long-term stack-storage after harvesting. Lebensm Wiss Technol. 2015;63:626–632. doi: 10.1016/j.lwt.2015.03.045. [DOI] [Google Scholar]
- Todorov SD, Botes M, Guigas C, Schillinger U, Wiid I, Wachsman MB, Holzapfel WH, Dicks LMT. Boza, a natural source of probiotic lactic acid bacteria. J Appl Microbiol. 2008;104:465–477. doi: 10.1111/j.1365-2672.2007.03558.x. [DOI] [PubMed] [Google Scholar]
- Whistler RL, BeMille JN, Paschall EF. Starch: chemistry and technology. New York: Academic Press; 1984. [Google Scholar]
- Yoo YJ, Hong J, Hatch RT. Comparison of α-amylase activities from different assay methods. Biotechnol Bioeng. 1987;30(1):147–151. doi: 10.1002/bit.260300120. [DOI] [PubMed] [Google Scholar]