Abstract
Whelks Neptunea arthritica cumingi Crosse and Neverita didyma were processed by hot air drying and changes of thei lipids and the mechanism involved were evaluated by analyzing peroxide value, thiobarbituric acid-reactive substances, total oxidation value, fatty acid composition, activities of lipases and lipoxygenase (LOX), as well as contents of triacylglycerol (TAG), free fatty acid (FFA), phosphatidylcholine (PC) and phosphatidylethanolamine (PE). The processing significantly decreased the contents of PC, PE and TAG but increased the content of FFA. The presence of acid lipase and phospholipase in whelk tissues and their activity preservation during processing suggest that the enzymes may help hydrolyze lipids. By contrast, the reduction of PC, PE and TAG was more pronounced than the increase in FFA in whelk tissues upon processing, indicating the oxidative degradation of FFA. LOX may play a role in lipid oxidation due to the stability of the starting components during processing.
Keywords: Whelk, Hot air drying, Lipid quality, Oxidation, Hydrolysis
Introduction
Fresh aquatic products easily undergo spoilage due to their high moisture content and nutrient-rich status that make them suitable for microbial growth and reproduction (Vega-Gálvez et al. 2011). Therefore, timely preservation and processing after harvest is essential. Drying is an important processing method of aquatic products to prolong the shelf-life. The aquatic products after the dehydration have rich nutrient, unique flavor and easy transportation, and are welcomed by customers at large. Drying technologies including sun drying, hot air drying, freeze drying, microwave drying as well as hybrid drying were utilized to decrease water activity and extend shelf-life of seafood (Wang et al. 2011). Among them, hot air drying is the most commonly used drying method of aquatic products because of a better quality of the resultant products, simple operation and low investment cost, and has been widely used in processing of abalone, squid and Atlantic salmon (Jia et al. 2012; Vega-Gálvez et al. 2011).
Generally, lipids in aquatic products have proportions of polyunsaturated fatty acids (PUFA), which are oxidizable matters (Selmi et al. 2010). Moreover, aquatic products are abundant in the active endogenous enzymes, especially phospholipases and lipases, which can hydrolyze the lipids and then release free fatty acids (FFA) that can easily contribute to oxidation (Aubourg et al. 2010; Hoehne-Reitan et al. 2007). Therefore, hydrolysis and oxidation of lipids occur during the drying of seafood which will impact nutritional value and products quality (Chaijan et al. 2017; Shah et al. 2009). Previous studies about the effects of drying process on lipid oxidation of fish species mainly focused on the changes of fatty acids and some oxidative indexes. For example, Shah et al. (2009) reported that drying treatment of herring fillet increased the acid value and peroxide value (POV), while decreased the PUFA content; Ortiz et al. (2013) reported that drying treatment of Atlantic salmon (Salmo salar L.) fillets increased the thiobarbituric acid-reactive substances (TBARS) and anisidine value, while decreased the EPA and DHA contents. Compare to marine fish, of which previous studies of effects on hot air drying concentrated on, the shellfish contains a higher level of n-3 LC-PUFA in the phospholipid (PL) form (Shah et al. 2009; Boselli et al. 2012). However, little research is so far available on the effect of drying on the lipids in univalve shellfish.
Whelk is one of the most important edible marine univalves, the production of which is about 400,230 tonnes according to the statistics of China Fisheries Yearbook (2016). Whelks are generally consumed and considered as a delicacy in Europe, China, Japan and Korea (Woodcock and Benkendorff 2008). It has been suggested in previous studies that whelks are abundant in n-3 LC-PUFA in the forms of PL (Gang et al. 2018; Lee and Lim 2005). Thus, the objective in this study was to evaluate the hydrolysis and oxidation of lipids in whelks Neptunea arthritica cumingi Crosse and Neverita didyma upon hot air drying. To fulfill this goal, PV, TBARS, total oxidation (TOTOX), fatty acid composition, the TAG, FFA, phosphatidylcholine (PC) and phosphatidylethanolamine (PE) contents of as well as activities of lipases and lipoxygenase (LOX) in the whelk tissues subjected to different treatments were determined.
Materials and methods
Materials
Fresh whelks Neptunea arthritica cumingi Crosse and Neverita didyma with mean length of about 3 cm and 5.5 cm, respectively was purchased in September, 2017 from a local market in Dalian, Liaoning, China. The whelks were mature produced in aquaculture from the Yellow Sea near Dalian.
Drying process
The meat were removed from the shell and the average weights of each meat from whelk Neptunea arthritica cumingi Crosse and Neverita didyma was about 7 g and 16 g, respectively. The whelk samples were divided into three parts, which were fresh sample, 5-min boiled sample and 10-min boiled sample. The 5-min boiled sample and 10-min boiled sample were separated into three groups, respectively, of which the two groups of each boiled samples were dried at 50 and 70 °C (BD-5-50, BD-5-70, BD-10-50, BD-10-70, respectively), respectively, while the rest was left as the control groups. Samples were dried under different temperatures with the PH-050(A) hot air oven (Yiheng Science and Technology Co., Ltd., Shanghai, China), and the drying process was terminated when the moisture content was below 22% according to Chinese national standard (NY/T 1712-2009) about the aquatic processing. Thus, the drying time of samples dried under 50 °C was 48 h, while the time of samples dried under 70 °C was 36 h.
Lipid extraction
Bligh and Dyer (1959) method with a modified version was employed for the total lipids. Briefly, 100 g whelk homogenate were poured into an Erlenmeyer flask, and then 100 ml chloroform and 200 ml methanol were added. After stirring at 30 °C for 60 min, 100 ml chloroform and 100 ml deionized water were added. Following complete mixing, the mixture was centrifuged at 7800g for 10 min using a CR22N high-speed refrigerated centrifuge (Hitachi Koki Co., Ltd., Tokyo, Japan). The organic layer was collected and rotary evaporated to a small volume, then dried with nitrogen at 35 °C to obtain the final lipids. The extracted lipids were sealed in nitrogen and stored at − 30 °C away from light for < 2 weeks until further analysis.
Determination of peroxide value (PV)
The PV was measured by the first method of Chinese standard GB5009.227 (2016). The PV were expressed as mmol/kg reactive oxygen in lipid sample.
Determination of thiobarbituric acid reactive substances (TBARS)
The TBARS was determined by an Infinite M200 microplate reader (Tecan Group Ltd., Männedorf, Switzerland) according to Lee et al. (1999). Briefly, 0.5 g whelk homogenate was mixed with 2.5 ml of reaction solution containing 0.375% 2-thiobarbituric acid (TBA), 15% trichloroacetic acid (TCA) and 0.25 N HCl. The mixture was heated for 10 min in a boiling water bath to develop a pink color, cooled with tap water, centrifuged at 5500g for 25 min. The absorbance of the supernatant was measured at 532 nm using an Infinite M200 microplate reader (Tecan Group Ltd., Männedorf, Switzerland). The TBARS, expressed as mg malondialdehyde (MDA)/kg sample, was obtained from a standard curve constructed by using stock solutions of MDA.
Total oxidation (TOTOX) value
As an indicative of both primary and secondary oxidation products, the TOTOX value was calculated in accordance with the following equation (Wanasundara and Shahidi 1995):
Fatty acid composition analysis
The determination of the FA composition was performed using an Agilent 7890A Gas Chromatograph/5977A Mass Spectrometer (GC–MS) system (Palo Alto, CA, USA) equipped with an Agilent HP-5-MS capillary column (30 m × 0.25 mm, 0.25 μm), according to the method reported by Yin et al. (2015). Lipid sample (100 mg) was mixed with 2.5 ml of KOH (500 g/l) and 5 ml of 95% ethanol in a tightly closed Teflon lined screw-capped vial. Incubate the mixture in a water bath at 60 °C for 2 h then cooled quickly to room temperature. The lipid phase containing the unsaponifiables was extracted six times with 3 ml of hexane each time and removed. Then the remaining aqueous phase was adjusted to pH 2 with 6 mol/L HCl and extracted for six times with 3 ml of hexane each time. The hexane extracts were combined and flushed with nitrogen at 35 °C. The residue was dissolved in hexane to 10 mg/ml, and then 2.5 ml of this solution was mixed with 2.0 ml of freshly prepared methylation reagent (10 ml/l H2SO4 in HPLC-grade methanol) in a vial. After incubation in a water bath at 70 °C for 1 h, the mixture was cooled, and 1 ml of distilled water was added. The upper layer containing FA methyl esters was collected and transferred into another new vial with the bottom covered by anhydrous sodium sulphate. After storage at 4 °C overnight, the upper layer was collected, filtered through a 0.22 μm filter membrane and analysed by GC–MS. Helium was used as the carrier gas, and the pressure was kept constant at 7.1 psi. The column temperature profile was the initial temperature of 50 °C maintained for 1 min, raised to 170 °C at a rate of 50 °C/min, raised to 300 °C at a rate of 4 °C/min, raised to 320 °C at a rate of 40 °C/min and kept at 320 °C for 3.6 min. The injection volume was 1 μL with a split ratio of 50:1. The MS was in EI mode (70 eV) with a 2.0 scan/s interval over a 50–550 m/z range. Solvent delay was 4 min. The identification of fatty acid methyl esters was performed by comparison of relative retention times and mass spectra with authentic standards and NIST08 mass spectral database. The composition of fatty acids was expressed as percentage of total fatty acids.
Thrombogenicity and atherogenicity indices
The equation of thrombogenicity index (IT) and atherogenicity index (IA) were calculated according to Ulbricht and Southgate (1991).
Index of atherogenicity (IA):
Index of thrombogenicity (IT):
PUFA = Polyunsaturated fatty acids and MUFA = Monounsaturated fatty acids.
Triacylglycerols (TAG) and free fatty acid (FFA) content analysis
The TAG and FFA were quantitatively analyzed by an Iatroscan MK-6S thin layer chromatography-flame ionization detection (TLC–FID) Analyzer (Iatron Inc., Tokyo, Japan) according to Yin et al. (2015). Lipids were dissolved in chloroform to 10–20 mg/ml. Using a 10 µL syringe, 1 µL of lipid sample was spotted onto silica-coated quartz rods, and the elution was performed with n-heptane/diethyl ether/formic acid (v/v/v, 42:28:0.3). After development, the Chromarod was dried at 60 °C for 5 min and transferred into the Iatroscan where each Chromarod was scanned with the FID to detect and quantify the compounds separated on silica. The hydrogen flow rate was 160 ml/min, the air flow rate was 2 L/min and the scanning speed was 30 s per Chromarod burned. Data acquisition and processing were performed using SIC480 II integration software (Iatron Inc.). The TAG and FFA contents were expressed as mg/g tissue (on a dry weight basis), which were calculated by using a standard curve method using purified TAG and docosahexaenoic acid (DHA, C22:6 n-3), respectively, as standards.
Phosphatidylcholine (PC) and phosphatidylethanolamine (PE) content analysis
The PC and PE were quantitatively detected by a Shimadzu LC-20AVP system (Shimadzu Co. Tokyo, Japan) with an Alltech ELSD 6000 detector (Alltech, Deerfield, IL, USA) described by our previous study Zhou et al. (2019). PC and PE were separated on an Agilent Zorbax RX-SIL (4.6 × 250 mm, 5 μm) column. Solvent A [methanol: water: glacial acetic acid: triethylamine = 85:15:0.45:0.05, v/v/v/v] and solvent B [hexane: isopropanol: solvent A = 20:48:32, v/v/v] were used as mobile phases at a flow rate of 1 ml/min. The gradient program was conducted as follows: 0–20 min, 90–70% B; 21–35 min, 70–5% B; 36–41 min, 90% B. The lipid sample concentration was 2 mg/ml, and the injection volume was 10 µL. The PC and PE contents were expressed as mg/g tissue (on a dry weight basis), which were calculated by standard curve method with PC (16:0/18:1) and PE (16:0/18:1) as standards.
Lipase extraction and activities assay
The extraction of crude lipases and LOX, as well as the determination of lipase, phospholipase and LOX activities were all carried out by the method of Jin et al. (2010).
4-methylumbelliferyl oleate was used as the substrate to measure the aforementioned lipase activities. The substrate will liberate fluorescent 4-methylumbelliferone after lipase hydrolysis, which was determined by using an Infinite F200 Pro microplate reader (Tecan Group Ltd., Männedorf, Switzerland). For determination of different lipase activity, different reaction buffers were used (0.1 M disodium phosphate/0.05 M citric acid [pH 5.0, containing 150 mM sodium fluoride, 0.05% (w/v) Triton X-100 and 0.8 mg/ml bovine serum albumin (BSA)] for phospholipase; 0.22 M tris/HCl (pH 7.5, containing 0.05% (w/v) Triton X-100) for neutral lipase; 0.1 M disodium phosphate/0.05 M citric acid (pH 5.0, containing 0.05% (w/v) Triton X-100 and 0.8 mg/ml BSA) for acid lipase). One unit (U) of lipase or phospholipase activity was defined as the nmol of released 4-methylumbelliferone per minute at 37 °C.
Crude LOX was extracted and determined by using the method of Jin et al. (2010). The activities were determined spectrophotometrically at 234 nm with a linoleic acid substrate solution. The LOX catalyzes the formation of conjugated diene hydroperoxide from linoleic acid while the conjugated double bonds have characteristic absorption at 234 nm. One unit (U) of LOX activity was defined as an increase absorbance of 0.001 per minute at 234 nm.
Statistical analysis
All experiments were carried out in triplicate. The statistical analyses were compared by the one-way analysis of variance (Student–Newman–Keuls post hoc test) by using SPSS 16.0 software (SPSS Inc., Chicago, IL, USA). P values of < 0.05 were considered statistically significant.
Results
Change of moisture content
As listed in Table 1, the water contents of fresh whelk, 5 min-boiled whelk, 10 min-boiled whelk, BD-5-50, BD-5-70, BD-10-50 and BD-10-70 were 65.78 ± 0.80a, 26.63 ± 0.30b, 24.02 ± 0.20b, 0.29 ± 0.01c, 0.21 ± 0.01c, 0.27 ± 0.02c and 0.19 ± 0.01c %, respectively for Neptunea arthritica cumingi Cross. As for Neverita didyma, the corresponding values were 61.34 ± 0.90A, 25.56 ± 0.60B, 21.21 ± 0.51B, 0.21 ± 0.02C, 0.14 ± 0.01C, 0.21 ± 0.01C and 0.17 ± 0.01C %, respectively.
Table 1.
The moisture content (%) of whelks Neptunea arthritica cumingi Crosse and Neverita didyma upon different treatments
| Whelks species | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Neptunea arthritica cumingi Crosse | 65.78 ± 0.80a | 26.63 ± 0.30b | 24.02 ± 0.20b | 0.29 ± 0.01c | 0.21 ± 0.01c | 0.27 ± 0.02c | 0.19 ± 0.01c |
| Neverita didyma | 61.34 ± 0.90A | 25.56 ± 0.60B | 21.21 ± 0.50B | 0.20 ± 0.02C | 0.14 ± 0.01C | 0.21 ± 0.01C | 0.17 ± 0.01C |
Sample 1 represents fresh whelk, sample 2 represents 5 min-boiled whelk, sample 3 represents 10 min-boiled whelk, sample 4 represents 50 °C-dried whelk after 5 min-boiled, sample 5 represents 70 °C-dried whelk after 5 min-boiled, sample 6 represents 50 °C-dried whelk after 10 min-boiled and sample 7 represents 70 °C-dried whelk after 10 min-boiled. Values of different groups with different lower case letters (a–c) and upper case letters (A–C) are significantly different at p < 0.05
Changes of PV, TBARS and TOTOX during processing
The PV, TBARS and TOTOX values for the two whelk species all increased after boiling (Fig. 1). For TBARS and TOTOX, the 5 min-boiled samples had significant higher values than the 10 min-boiled samples (p < 0.05), while for PV, there was no significant difference for the 5 min and 10 min-boiled samples. The hot air drying decreased the PV, TOTOX and TBARS for the two whelk species. In addition, the 50 °C treatment resulted in significant higher values than the 70 °C treatment (p < 0.05).
Fig. 1.
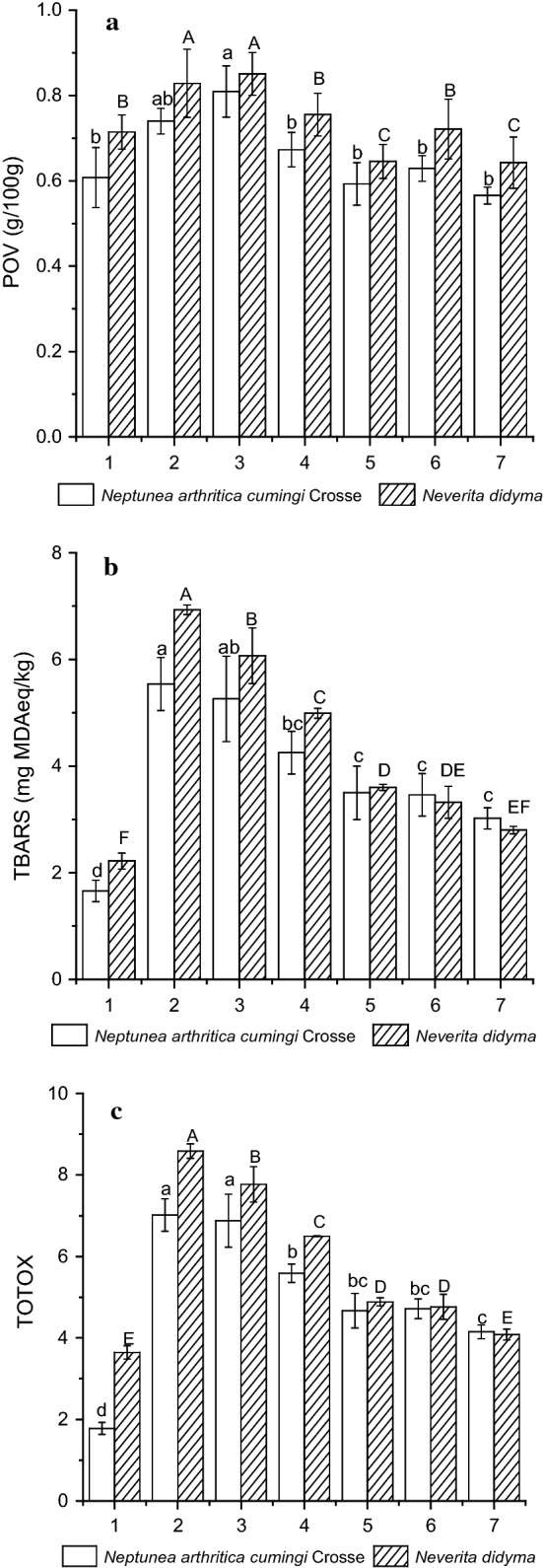
Changes of POV (a), TBARS (b) and TOTOX (c) of whelks Neptunea arthritica cumingi Crosse and Neverita didyma upon different treatments. Sample 1 represents fresh whelk, sample 2 represents 5 min-boiled whelk, sample 3 represents 10 min-boiled whelk, sample 4 represents 50 °C-dried whelk after 5 min-boiled, sample 5 represents 70 °C-dried whelk after 5 min-boiled, sample 6 represents 50 °C-dried whelk after 10 min-boiled and sample 7 represents 70 °C-dried whelk after 10 min-boiled. Values of different groups with different lower case letters (a–d) and upper case letters (A–F) are significantly different at p < 0.05
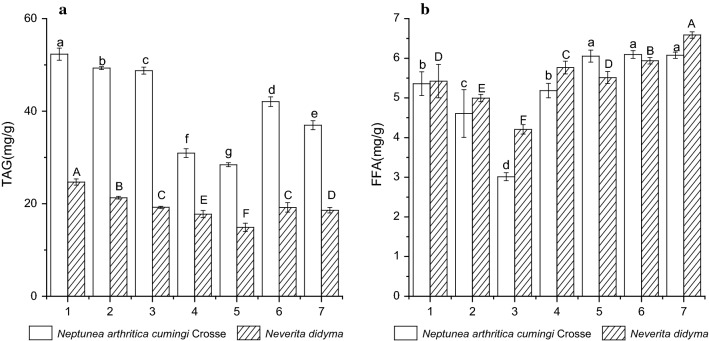
Changes of TAG and FFA contents
The fresh whelk Neptunea arthritica cumingi Crosse and Neverita didyma contained TAG of 52.32 ± 1.32a and 24.68 ± 0.68A mg/g (on a dry weight basis), respectively. The TAG contents of the aforementioned two whelk species showed a time-dependent decrease during boiling process, and dropped to 49.32 ± 0.32b and 21.29 ± 0.29B mg/g, respectively, after 5 min, and dropped to 48.76 ± 0.76c and 19.22 ± 0.22C mg/g, respectively, after 10 min (Fig. 2a). The FFA contents of the aforementioned two whelk species for fresh samples were 5.36 ± 0.31a and 5.43 ± 0.42A mg/g (on a dry weight basis), respectively. The FFA contents also time-dependently decreased during boiling process, and dropped to 4.61 ± 0.62b and 4.99 ± 0.89B mg/g, respectively, after 5 min, and dropped to 3.01 ± 0.13c and 4.20 ± 0.12C mg/g, respectively, after 10 min (Fig. 2b).
Fig. 2.
Changes of the contents of TAG (a) and FFA (b) in whelks Neptunea arthritica cumingi Crosse and Neverita didyma (on a dry basis) upon different treatments. Sample 1 represents fresh whelk, sample 2 represents 5 min-boiled whelk, sample 3 represents 10 min-boiled whelk, sample 4 represents 50 °C-dried whelk after 5 min-boiled, sample 5 represents 70 °C-dried whelk after 5 min-boiled, sample 6 represents 50 °C-dried whelk after 10 min-boiled and sample 7 represents 70 °C-dried whelk after 10 min-boiled. Values of different groups with different lower case letters (a–f) and upper case letters (A–F) are significantly different at p < 0.05
The drying process further decreased the contents of TAG significantly in all the samples (p < 0.05) (Fig. 2a). For the samples with the same boiling time, the 70 °C treatment caused a higher reduction in TAG content than the 50 °C treatment. While for the samples under the same drying temperature, the 10 min-boiled samples retained more TAG than the 5 min-boiled samples. The FFA contents of the two whelk species increased after hot drying treatment (Fig. 2b). However, there was no correlation between FFA contents and drying temperature.
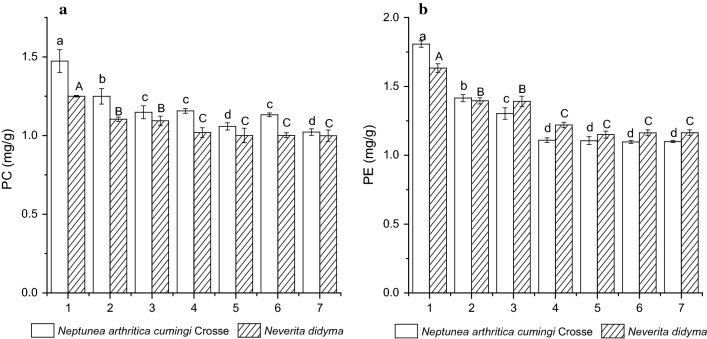
Changes of PC and PE contents
The PC contents of fresh whelks Neptunea arthritica cumingi Crosse and Neverita didyma were 1.47 ± 0.08a and 1.25 ± 0.01A mg/g (on a dry weight basis), respectively, while the corresponding values for PE were 1.81 ± 0.02a and 1.63 ± 0.03A mg/g, respectively. The PC and PE contents of the two aforementioned whelk species showed a time-dependent decrease during boiling process (Fig. 3a, b). For PC, its contents in Neptunea arthritica cumingi Crosse and Neverita didyma dropped to 1.25 ± 0.05b and 1.10 ± 0.01B mg/g, respectively, after 5 min, and dropped to 1.15 ± 0.04c and 1.09 ± 0.03B mg/g, respectively, after 10 min. While for PE, the corresponding values dropped to 1.42 ± 0.03b and 1.40 ± 0.02B mg/g, respectively, after 5 min, and dropped to 1.30 ± 0.04c and 1.39 ± 0.04B mg/g, respectively, after 10 min. The drying process further decreased the PC and PE contents significantly in all the samples (p < 0.05). For the samples with the same boiling period, the 70 °C treatment brought about a significant higher reduction in PC content than the 50 °C treatment (p < 0.05), while the phenomenon was not present in PE. Moreover, the drying treatment caused a bigger reduction in PE contents than PC contents.
Fig. 3.
Changes of the contents of PC (a) and PE (b) in whelks Neptunea arthritica cumingi Crosse and Neverita didyma (on a dry basis) upon different treatments. Sample 1 represents fresh whelk, sample 2 represents 5 min-boiled whelk, sample 3 represents 10 min-boiled whelk, sample 4 represents 50 °C-dried whelk after 5 min-boiled, sample 5 represents 70 °C-dried whelk after 5 min-boiled, sample 6 represents 50 °C-dried whelk after 10 min-boiled and sample 7 represents 70 °C-dried whelk after 10 min-boiled. Values of different groups with different lower case letters (a–d) and upper case letters (A–C) are significantly different at p < 0.05
Changes of fatty acid compositions
As listed in Tables 2 and 3, the lipids recovered from the fresh whelks Neptunea arthritica cumingi Crosse and Neverita didym contained a high proportion of PUFA (46.68–54.86% of total FA), followed by monounsaturated fatty acids (MUFA, 25.11–33.36% of total FA) and saturated fatty acids (SFA, 19.96–20.77% of total FA). Palmitic acid (C16:0, 8.14–9.92% of total FA) and stearic acid (C18:0, 6.62–9.59% of total FA) were the most prevalent SFA, and oleic acid (C18:1n-9, 4.09–10.62% of total FA) and paullinic acid (C20:1n-9, 18.01–18.54%) were the most prevalent MUFA, while eicosapentaenoic acid (EPA, 20:5n-3) (11.92–12.36% of total FA) and DHA (8.71–11.55% of total FA) were the dominant PUFA. Obviously, EPA and DHA were abundant in lipids recovered from whelks Neptunea arthritica cumingi Crosse and Neverita didyma, indicating that they may serve as potential sources of EPA and DHA. By contrast, lipids from the both species have similar proportion of EPA, while Neverita didyma contained a higher proportion of DHA.
Table 2.
Fatty acid composition (%) of lipids recovered from Neptunea arthritica cumingi Crosse upon different treatments
| Fatty acid | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| C14:0 | 1.71 ± 0.04bc | 1.38 ± 0.23c | 1.50 ± 0.10bc | 2.21 ± 0.16a | 1.81 ± 0.01abc | 1.94 ± 0.21ab | 1.56 ± 0.12bc |
| C15:0 | 0.46 ± 0.04 | 0.47 ± 0.02 | 0.39 ± 0.03 | 0.44 ± 0.03 | 0.48 ± 0.04 | 0.45 ± 0.02 | 0.43 ± 0.07 |
| C16:1 n-7 | 3.71 ± 0.02bc | 3.69 ± 0.31bc | 3.42 ± 0.25c | 5.95 ± 0.19a | 4.35 ± 0.15b | 2.84 ± 0.16d | 3.81 ± 0.25bc |
| C16:0 | 9.92 ± 0.75b | 11.51 ± 0.29ab | 11.12 ± 0.07ab | 12.36 ± 0.29ab | 13.74 ± 0.49a | 12.55 ± 0.63ab | 12.32 ± 0.24ab |
| C17:0 | 1.25 ± 0.42 | 0.93 ± 0.01 | 0.79 ± 0.05 | 0.81 ± 0.00 | 0.91 ± 0.10 | 1.03 ± 0.08 | 0.89 ± 0.06 |
| C18:4 n-3 | 1.05 ± 0.03a | nd | 1.18 ± 0.04a | 1.37 ± 0.01a | 0.97 ± 0.22a | 0.69 ± 0.12b | 0.75 ± 0.14ab |
| C18:2 n-6 | 2.01 ± 0.00 | 1.36 ± 0.01 | 1.95 ± 0.11 | 2.26 ± 0.01 | 1.58 ± 0.59 | 1.21 ± 0.19 | 1.85 ± 0.39 |
| C18:1 n-9 | 10.62 ± 0.02ab | 10.62 ± 0.12ab | 11.03 ± 0.32ab | 12.47 ± 0.11a | 9.60 ± 0.69b | 10.70 ± 0.51ab | 10.62 ± 0.07ab |
| C18:0 | 6.62 ± 0.03c | 6.56 ± 0.04d | 5.86 ± 0.01e | 7.81 ± 0.06a | 7.50 ± 0.53ab | 7.30 ± 0.35ab | 7.13 ± 0.10b |
| C20:4 n-6 | 6.61 ± 0.01a | 5.77 ± 0.08b | 3.62 ± 0.25c | 3.76 ± 0.16c | 4.07 ± 0.24c | 5.25 ± 0.48b | 4.38 ± 0.23c |
| C20:5 n-3 | 11.92 ± 0.50 | 11.72 ± 0.50 | 10.49 ± 0.97 | 10.15 ± 0.13 | 11.63 ± 0.56 | 10.45 ± 0.25 | 11.74 ± 0.62 |
| C20:2 | 1.49 ± 0.05 | 0.54 ± 0.01 | 1.10 ± 0.02 | 1.10 ± 0.00 | 0.44 ± 0.03 | 0.79 ± 0.02 | 0.66 ± 0.02 |
| C20:1 n-9 | 18.54 ± 0.4d | 19.06 ± 0.22c | 19.24 ± 0.20c | 17.83 ± 0.32e | 20.00 ± 2.14b | 22.22 ± 1.52a | 20.05 ± 0.44b |
| C22:6 n-3 | 8.71 ± 0.17a | 8.09 ± 0.12ab | 7.49 ± 0.15b | 7.29 ± 0.95b | 7.83 ± 0.60ab | 5.62 ± 0.78d | 6.29 ± 0.10c |
| C22:4 n-6 | 1.22 ± 0.12ab | 1.30 ± 0.07a | 0.81 ± 0.11c | 0.76 ± 0.10c | 0.95 ± 0.01bc | 1.02 ± 0.13abc | 1.07 ± 0.00abc |
| C22:5 n-6 | 8.23 ± 0.42a | 7.81 ± 0.19a | 6.78 ± 0.47b | 5.25 ± 0.12c | 6.17 ± 0.04b | 6.07 ± 0.32b | 6.50 ± 0.15b |
| C22:2 n-9 | 5.44 ± 0.61c | 8.83 ± 0.08a | 8.57 ± 0.17a | 7.62 ± 0.05b | 8.97 ± 0.33a | 8.84 ± 0.08a | 8.96 ± 0.23a |
| C22:1 n-9 | 0.49 ± 0.12c | 0.36 ± 0.19d | 0.67 ± 0.03b | 0.55 ± 0.03bc | nd | 1.03 ± 0.13a | 0.62 ± 0.06b |
| SFA | 19.96 ± 0.44b | 21.85 ± 0.49ab | 20.66 ± 0.23ab | 21.63 ± 0.53ab | 23.43 ± 0.84a | 23.28 ± 2.08a | 21.82 ± 0.39ab |
| MUFA | 33.36 ± 0.29b | 34.73 ± 0.21ab | 34.36 ± 0.80b | 36.8 ± 0.43a | 33.96 ± 0.60b | 36.78 ± 0.72a | 35.59 ± 9.67ab |
| PUFA | 46.68 ± 0.73a | 42.43 ± 0.29c | 44.98b ± 1.03b | 41.57 ± 0.96d | 42.6c ± 1.44c | 39.94 ± 2.80e | 42.59c ± 10.06c |
| IA | 0.16 ± 0.02b | 0.16 ± 0.02b | 0.16 ± 0.03b | 0.18 ± 0.02ab | 0.23 ± 0.04a | 0.22 ± 0.02ab | 0.20 ± 0.02ab |
| IT | 0.20 ± 0.03b | 0.17 ± 0.01b | 0.15 ± 0.02b | 0.17 ± 0.01b | 0.26 ± 0.01a | 0.28 ± 0.04a | 0.25 ± 0.03a |
Sample 1 represents fresh whelk, sample 2 represents 5 min-boiled whelk, sample 3 represents 10 min-boiled whelk, sample 4 represents 50 °C-dried whelk after 5 min-boiled, sample 5 represents 70 °C-dried whelk after 5 min-boiled, sample 6 represents 50 °C-dried whelk after 10 min-boiled and sample 7 represents 70 °C-dried whelk after 10 min-boiled. Values of different groups with different lower case letters (a–f) are significantly different at p < 0.05
Table 3.
Fatty acid composition (%) of lipids recovered from Neverita didyma upon different treatments
| Fatty acid | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| C14:0 | 1.21 ± 0.01a | 0.96 ± 0.00ab | 0.80 ± 0.00b | 1.04 ± 0.04ab | 0.33 ± 0.01c | 0.81 ± 0.00b | 1.06 ± 0.00ab |
| C15:0 | 0.62 ± 0.00ab | 0.50 ± 0.00b | 0.52 ± 0.00b | 0.69 ± 0.06a | 0.32 ± 0.00c | 0.53 ± 0.00b | 0.60 ± 0.00ab |
| C16:1 n-7 | 3.01 ± 0.02a | 3.19 ± 0.01a | 2.14 ± 0.00c | 2.96 ± 0.03a | 2.99 ± 0.03a | 2.65 ± 0.02b | 2.47 ± 0.00b |
| C16:0 | 8.14 ± 0.04d | 10.40 ± 0.20a | 10.13 ± 0.14ab | 9.87 ± 0.30b | 10.11 ± 0.04ab | 10.19 ± 0.02ab | 9.32 ± 0.01c |
| C17:0 | 1.21 ± 0.01 | 0.99 ± 0.07 | 1.08 ± 0.01 | 1.14 ± 0.00 | 1.05 ± 0.00 | 0.99 ± 0.16 | 1.01 ± 0.00 |
| C18:4 n-3 | 1.02 ± 0.00ab | 0.99 ± 0.02ab | 0.90 ± 0.00b | 1.17 ± 0.07a | 0.90 ± 0.01b | 0.86 ± 0.03b | 1.06 ± 0.00ab |
| C18:2 n-6 | 2.38 ± 0.02c | 2.04 ± 0.00d | 2.34 ± 0.02c | 2.88 ± 0.09a | 1.12 ± 0.01e | 2.19 ± 0.02c | 2.62 ± 0.01b |
| C18:1 n-9 | 4.09 ± 0.05b | 4.84 ± 0.01a | 3.89 ± 0.06bc | 4.94 ± 0.05a | 3.61 ± 0.03c | 3.69 ± 0.03c | 4.68 ± 0.00a |
| C18:0 | 9.59 ± 0.06c | 9.16 ± 0.13d | 10.15 ± 0.13b | 9.20 ± 0.22d | 9.33 ± 0.00 cd | 11.73 ± 0.01a | 9.36 ± 0.01 cd |
| C20:4 n-6 | 6.17 ± 0.04c | 4.69 ± 0.08e | 6.94 ± 0.05a | 6.47 ± 0.17b | 5.66 ± 0.04d | 6.76 ± 0.03a | 6.02 ± 0.03c |
| C20:5 n-3 | 12.36 ± 0.03a | 12.08 ± 0.04b | 11.77 ± 0.18c | 11.44 ± 0.10d | 11.3 ± 0.01d | 11.35 ± 0.00d | 11.47 ± 0.03d |
| C20:2 | 2.41 ± 0.01a | 2.28 ± 0.06a | 1.55 ± 0.02c | 1.37 ± 0.02c | 1.93 ± 0.00b | 1.93 ± 0.14b | 2.23 ± 0.01a |
| C20:1 n-9 | 18.01 ± 0.08c | 19.23 ± 0.01a | 18.02 ± 0.24c | 14.81 ± 0.74f | 15.62 ± 0.19e | 18.54 ± 0.01b | 17.44 ± 0.61d |
| C22:6 n-3 | 11.55 ± 0.10a | 11.04 ± 0.10b | 10.64 ± 0.08b | 10.72 ± 0.34b | 9.55 ± 0.02c | 7.92 ± 0.08d | 7.64 ± 0.93d |
| C22:4 n-6 | 2.01 ± 0.01a | 1.67 ± 0.02ab | 1.91 ± 0.03ab | 1.68 ± 0.01ab | 1.54 ± 0.01b | 1.73 ± 0.00ab | 1.09 ± 0.03c |
| C22:5 n-6 | 5.21 ± 0.03a | 4.56 ± 0.06b | 5.19 ± 0.02a | 5.17 ± 0.30a | 3.24 ± 0.01c | 4.37 ± 0.03b | 3.32 ± 0.01c |
| C22:2 n-9 | 11.96 ± 0.12b | 11.38 ± 0.04c | 12.01 ± 0.13b | 10.92 ± 0.12d | 10.37 ± 0.05e | 13.77 ± 0.02a | 12.11 ± 0.09b |
| C22:1 n-9 | nd | nd | nd | 0.54 ± 0.01b | 1.04 ± 0.00a | nd | 0.50 ± 0.03b |
| SFA | 20.77 ± 0.14b | 22.01 ± 0.40b | 22.68 ± 0.29b | 21.93 ± 0.42b | 21.13 ± 0.05b | 24.25 ± 0.13a | 21.35 ± 0.03b |
| MUFA | 25.11 ± 0.08b | 27.25 ± 0.01a | 23.05 ± 0.81c | 23.25 ± 0.65c | 26.25 ± 0.13ab | 24.88 ± 0.05b | 25.09 ± 1.58b |
| PUFA | 54.86 ± 0.07a | 50.74 ± 0.39d | 54.27 ± 0.52ab | 51.82 ± 1.07 cd | 52.62 ± 0.08bcd | 50.87 ± 0.08d | 53.56 ± 1.61abc |
| IA | 0.14 ± 0.01 | 0.18 ± 0.02 | 0.17 ± 0.02 | 0.17 ± 0.01 | 0.18 ± 0.00 | 0.18 ± 0.02 | 0.18 ± 0.02 |
| IT | 0.20 ± 0.02b | 0.22 ± 0.02b | 0.22 ± 0.01b | 0.22 ± 0.02b | 0.23 ± 0.01b | 0.28 ± 0.02a | 0.24 ± 0.01b |
Sample 1 represents fresh whelk, sample 2 represents 5 min-boiled whelk, sample 3 represents 10 min-boiled whelk, sample 4 represents 50 °C-dried whelk after 5 min-boiled, sample 5 represents 70 °C-dried whelk after 5 min-boiled, sample 6 represents 50 °C-dried whelk after 10 min-boiled and sample 7 represents 70 °C-dried whelk after 10 min-boiled. Values of different groups with different upper case letters (a–f) are significantly different at p < 0.05
The boiling and drying treatments slightly changed the FA compositions for the two whelk species. Overall, the percentages of PUFA decreased slightly while that of SFA slightly increased. However, the percentages of PUFA and SFA fluctuated within a narrow range with no correspondence between these values and boiling time as well as processing temperature.
In this study, the IA value for fresh Neptunea arthritica cumingi Crosse and Neverita didyma was 0.16 and 0.14, respectively, while the corresponding value for IT was 0.20 and 0.20, respectively. Overall, the hot air drying processing increased the IA and IT values for the two whelk species (Tables 2, 3).
Changes of lipolytic enzymes and LOX activities
The activities for lipolytic enzymes and LOX in the two whelk tissues during processing are present in Fig. 4. The enzymatic activities for acid lipase, phospholipase and LOX in the fresh samples of whelk Neptunea arthritica cumingi Crosse were 193.93 ± 9.10a, 189.69 ± 7.91a and 11.94 ± 0.92a U/g dry tissue, respectively, while for Neverita didyma, the corresponding values were 222.44 ± 8.72A, 215.03 ± 8.62A and 12.32 ± 1.31A U/g dry tissue, respectively.
Fig. 4.
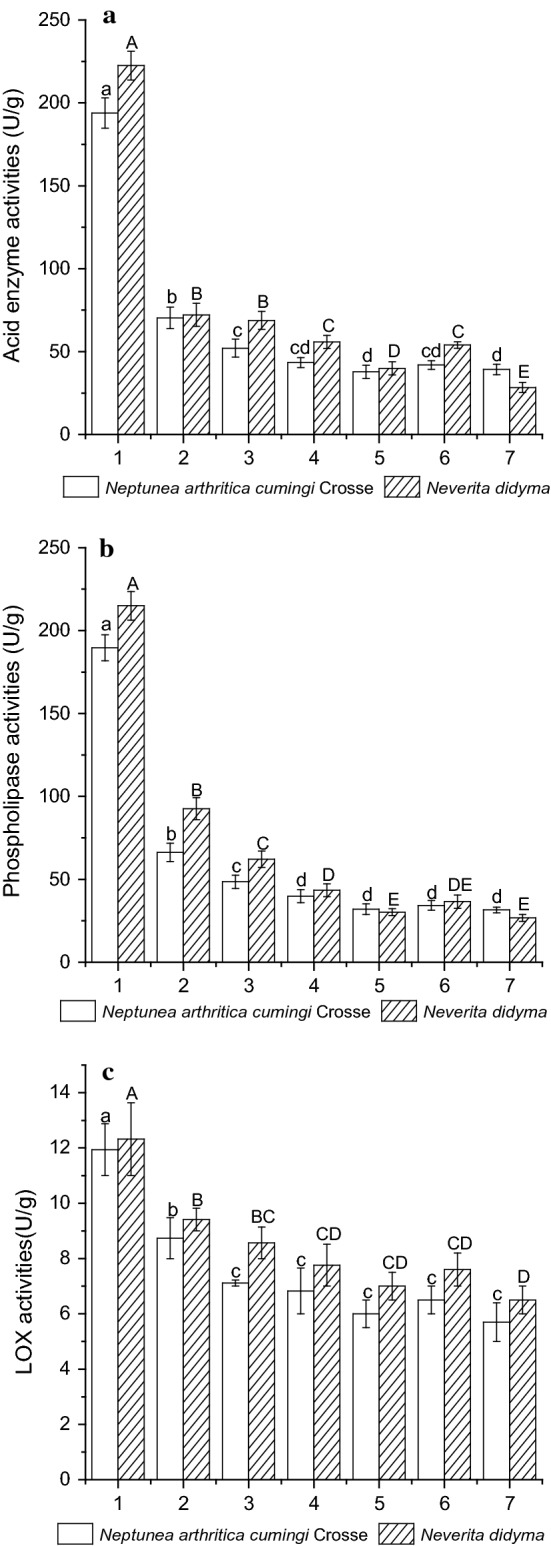
Changes of the activities for acid lapse (a), phospholipase (b) and LOX (c) in whelks Neptunea arthritica cumingi Crosse and Neverita didyma (on a dry basis) upon different treatments. Sample 1 represents fresh whelk, sample 2 represents 5 min-boiled whelk, sample 3 represents 10 min-boiled whelk, sample 4 represents 50 °C-dried whelk after 5 min-boiled, sample 5 represents 70 °C-dried whelk after 5 min-boiled, sample 6 represents 50 °C-dried whelk after 10 min-boiled and sample 7 represents 70 °C-dried whelk after 10 min-boiled. Values of different groups with different lower case letters (a–f), upper case letters (A–F) and roman numeral (I–V) are significantly different at p < 0.05
The enzymatic activities for lipolytic enzymes and LOX in Neptunea arthritica cumingi Crosse and Neverita didyma showed a significant decrease upon the boiling process (p < 0.05). Obviously, the longer boiling time resulted in a higher reduction in enzymatic activities. After 10 min of boiling, the Neptunea arthritica cumingi Crosse tissue retained 27.22 ± 2.86c% of acid enzyme, 25.57 ± 0.02c% of phospholipase and 63.02 ± 7.52c% of LOX, respectively, while for Neverita didyma the corresponding values were 30.87 ± 2.56B%, 28.89 ± 0.83C% and 66.19 ± 6.69BC%, respectively. The activities of all three enzymes were further decreased in the drying process. For samples with the same boiling time, the 70 °C processing caused a significant higher reduction in the activities of enzymes than the 50 °C processing (p < 0.05).
Discussion
As an energy-saving and efficient drying method, hot air drying has the advantages of being simple as well as have low equipment investment and maintenance costs. It is widely used in food drying process, especially for shellfish, which has a small particle size and a large processing capacity (Duan et al. 2013). Shellfish, such as abalone and scallop after drying have been well received by consumers due to their low nutritional loss, good flavor and good taste (Jia et al. 2012).
A boiling treatment is usually required before drying of aquatic products to deactivate microorganisms and enzymes, thus improving the stability and quality of the final products (Wang et al. 2011). In the present study, the boiling treatment time-dependently decreased the TAG, PC and PE contents in whelk tissues, indicating the hydrolysis of TAG and PL. Toyes-Vargas et al. (2016) also reported that the boiling treatment on viscera from squid and scallop caused the reduction in TAG and PL. Our results indicated the containing of acid lipase and phospholipase in whelk tissues, and more than 25% of their activities were retained after 10 min of boiling treatment. Therefore, it was deduced that the two enzymes may conduce to the hydrolysis of TAG and PL in whelk tissues during the boiling treatment. Kaneniwa et al. (2000) also reported that lipolytic enzymes play an essential role in lipid hydrolysis of silver carp muscle during boiling treatment. Overall, the present study indicated that the reduction in PC and PE percentages were more obvious than that of TAG. Furthermore, Toyes-Vargas et al. (2016) showed a similar phenomenon in boiling treatment of viscera from scallop and squid. Meanwhile, the boiling treatment brought about a time dependent reduction in FFA contents in whelk tissues. Similar results have been observed upon boiling treatment of shrimp, which could be explained by the leaching of FFA into hot water during processing (Pérez-Santín et al. 2013).
In present study, the boiling treatment significantly increased the PV, TBARS and TOTOX in whelk tissues (p < 0.05), indicating the oxidation of lipids present. Accordingly, the boiling treatment caused a slight reduction in PUFA content in total FAs (p < 0.05). Previous studies have also demonstrated the occurrence of slight lipid oxidation during the boiling treatment of rainbow trout fillets, Atlantic mackerel and Baltic sprats (Asghari et al. 2013; Stołyhwo et al. 2006). However, Asghari et al. (2013) did not observe any obvious change in FA composition of shrimp upon boiling (Asghari et al. 2013). Additionally, our results indicated that the whelk tissues contained LOX activity, which was retained about 60% after 10 min of boiling. Therefore it was concluded that LOX may play a part in lipid oxidation. Analogously, D’souza and Prabhu (2006) reported that the oxidation in mackerels (Scomber scombrus) during boiling treatment was contributed to the LOX.
The hot air drying further reduced the TAG, PC and PE contents while increased that of FFA. Toyes-Vargas et al. (2016) also reported that drying of viscera from scallop and squid brought about a reduction in TAG and PL. According to our results, some 20% activities of acid lipase and phospholipase were retained after hot air treatment. Therefore, the two enzymes may further hydrolyse TAG and PL in whelk tissues and liberate FFA during hot air treatment. Murueta and Carreño (2007) also reported that hydrolytic enzyme played an essential role in lipid hydrolysis of fish Synodus scituliceps muscle during drying processing. Meanwhile, for samples under the same drying temperature, the 10 min-boiled samples retained more TAG than the 5 min-boiled samples, indicating that the prolonged boiling time contributed to the preservation of TAG in hot drying treatment by inactivating more lipases.
By contrast, the reduction in total TAG, PC and PE contents was more pronounced than the increase in content of FFA in whelk tissues upon hot air drying treatment, indicating the occurrence of oxidative degradation of FFA. It is widely accepted that FFA are more prone to oxidation than their ester counterparts (Toyes-Vargas et al. 2016). While, our results showed that the hot air drying decreased the PV, TOTOX and TBARS significantly for the two whelk species (p < 0.05). Moreover, the 50 °C treatment resulted in the higher values for the aforementioned oxidation indices than the 70 °C treatment. These abnormal results have been observed in hot drying of several kinds of aquatic products such as grass carp, baby clam and sardine (Zhang et al. 2013; Lee et al. 1998). Zhang et al. (2013) suggested that the drying process accompanying by high temperatures could accelerate the decomposition of peroxides into their carbonyl components, and so the peroxide value may remain low. Lee et al. (1998) suggested that the decreased TBARS value during drying could be attributed to the volatilization and loss of aldehydes during hot air drying. In addition, the aldehydes so produced can react with free amine groups of proteins and form Schiff bases under relatively high temperatures (Harkouss et al. 2015).
It has been suggested that whelk may serve as a potential source rich in n-3 LC-PUFA in the forms of PL and TAG (Gang et al. 2018). The n-3 LC-PUFA in the PL form have recently attracted a lot of attention due to their high bioavailability, high tissue-delivery capacity and health promotion (Cook et al. 2016; Liu et al. 2014; Rossmeisl et al. 2012). Our results demonstrated that the PL and TAG in whelk tissues were significantly reduced upon hot air processing due to the hydrolysis and oxidation of lipids.
The values of IA and IT for the two whelk species increased after the hot air drying processing (p < 0.05). IA indicates the relationship between the sum of the main saturates which being considered pro-atherogenic and the sum of the main anti-atherogenic unsaturated (Senso et al. 2007). IT is defined as the relationship between the saturated (pro-thrombogenetic) and the unsaturated (anti-thrombogenetic) FA (MUFA, PUFA-n6 y PUFA-n3) which indicates the tendency to form clots in the blood vessels (Senso et al. 2007). The higher the values of IT and IA mean the greater the harm to human health (Rueda et al. 1997). The IA and IT levels were low in fresh whelks, indicating a good fatty acid ratio for human consumption. The increase in the two indices after hot air processing represents the reduction of the nutritional value of the two whelk species.
Conclusion
Lipid hydrolysis and oxidation in whelks Neptunea arthritica cumingi Crosse and Neverita didyma upon hot air drying were reflected in a reduction of TAG, PC and PE as well as percentage of PUFA but an increase in FFA. The presence of acid lipase, phospholipase and LOX in whelk tissues and their stability during processing suggests that they may promote the hydrolysis and oxidation of lipids. A significant reduction in PL and TAG during hot air drying arising from the hydrolysis and oxidation leaded to a minor decrease in the nutrition value of lipid components in the whelks.
Acknowledgements
This work was financially supported by “National Key R&D Program of China (2018YFD0901002)”, “The National Natural Science Foundation of China (U1808203, 31871759)”, “Public Science and Technology Research Funds Projects of Ocean (201505029)” and “Project of Distinguished Professor of Liaoning Province (2015-153)”.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kai-Qi Gang and Zi-Xuan Wu have contributed equally to this paper.
References
- Asghari L, Zeynali F, Sahari MA. Effects of boiling, deep-frying, and microwave treatment on the proximate composition of rainbow trout fillets: changes in fatty acids, total protein, and minerals. J Appl Ichthyol. 2013;29:847–853. doi: 10.1111/jai.12212. [DOI] [Google Scholar]
- Aubourg SP, Tabilo-Munizaga G, Reyes JE, Rodríguez A, Pérez-Won M. Effect of high-pressure treatment on microbial activity and lipid oxidation in chilled coho salmon. Eur J Lipid Sci Technol. 2010;112:362–372. [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/y59-099. [DOI] [PubMed] [Google Scholar]
- Boselli E, Pacetti D, Lucci P, Frega NG. Characterization of phospholipid molecular species in the edible parts of bony fish and shellfish. J Agric Food Chem. 2012;60:3234–3245. doi: 10.1021/jf205159a. [DOI] [PubMed] [Google Scholar]
- Chaijan M, Panpipat W, Nisoa M. Chemical deterioration and discoloration of semi-dried tilapia processed by sun drying and microwave drying. Dry Technol. 2017;35:642–649. doi: 10.1080/07373937.2016.1199565. [DOI] [Google Scholar]
- Chinese Standard GB 5009.227 . Determination of peroxide value in food. Beijing: China Standards Press of China; 2016. [Google Scholar]
- Cook CM, Hallaråker H, Sæbø PC, Innis SM, Kelley KM, Sanoshy KD, Maki KC. Bioavailability of long chain omega-3 polyunsaturated fatty acids from phospholipid-rich herring roe oil in men and women with mildly elevated triacylglycerols. Prostaglandins Leukot Essential Fatty Acids (PLEFA) 2016;111:17–24. doi: 10.1016/j.plefa.2016.01.007. [DOI] [PubMed] [Google Scholar]
- D’souza HP, Prabhu HR. In vitro inhibition of lipid peroxidation in fish by turmeric (Curcuma longa) Indian J Clin Biochem. 2006;21:138–141. doi: 10.1007/BF02912929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan W, Luo W, Duan Z, Lei X, Feng A. Advances on the research in the processing and exploitation of mussel. Fish Mod. 2013;3:012. [Google Scholar]
- Gang KQ, Zhou DY, Lu T, Liu ZY, Zhao Q, Xie HK, Song L, Shahidi F. Direct infusion mass spectrometric identification of molecular species of glycerophospholipid in three species of edible whelk from Yellow Sea. Food Chem. 2018;245:53–60. doi: 10.1016/j.foodchem.2017.10.077. [DOI] [PubMed] [Google Scholar]
- Harkouss R, Astruc T, Lebert A, Gatellier P, Loison O, Safa H, Portanguen S, Parafita E, Mirade PS. Quantitative study of the relationships among proteolysis, lipid oxidation, structure and texture throughout the dry-cured ham process. Food Chem. 2015;166:522–530. doi: 10.1016/j.foodchem.2014.06.013. [DOI] [PubMed] [Google Scholar]
- Hoehne-Reitan K, Økland SN, Reitan KI. Neutral lipase and phospholipase activities in scallop juveniles (Pecten maximus) and dietary algae. Aquac Nutr. 2007;13:45–49. doi: 10.1111/j.1365-2095.2007.00452.x. [DOI] [Google Scholar]
- Jia M, Cong HH, Xue CH, Xue Y, Sun ZM, Li JZ. Drying kinetics and mathematical modeling of abalone during the hot-air drying process. Sci Technol Food Ind. 2012;3:72–80. [Google Scholar]
- Jin GF, Zhang JH, Yu XA, Zhang YP, Lei YX, Wang JM. Lipolysis and lipid oxidation in bacon during curing and drying-ripening. Food Chem. 2010;123:465–471. doi: 10.1016/j.foodchem.2010.05.031. [DOI] [Google Scholar]
- Kaneniwa M, Miao S, Yuan C, Lida H, Fukuda Y. Lipid components and enzymatic hydrolysis of lipids in muscle of Chinese freshwater fish. J Am Oil Chem Soc. 2000;77:825. doi: 10.1007/s11746-000-0132-3. [DOI] [Google Scholar]
- Lee SM, Lim TJ. Effects of dietary protein and energy levels on growth and lipid composition of juvenile snail (Semisulcospira gottschei) J Shellfish Res. 2005;24:99–102. doi: 10.2983/0730-8000(2005)24[99:EODPAE]2.0.CO;2. [DOI] [Google Scholar]
- Lee KH, Cho TY, Cho HS, Lee JH, Shim KH. Lipid oxidation in shellfish under the different conditions of drying. Korean J Fish Aquat Sci. 1998;31:143–148. [Google Scholar]
- Lee BJ, Hendricks DG, Cornforth DP. A comparison of carnosine and ascorbic acid on color and lipid stability in a ground beef pattie model system. Meat Sci. 1999;51:245–253. doi: 10.1016/S0309-1740(98)00121-1. [DOI] [PubMed] [Google Scholar]
- Liu L, Bartke N, Van Daele H, Lawrence P, Qin X, Park HG, Brenna JT. Higher efficacy of dietary DHA provided as a phospholipid than as a triglyceride for brain DHA accretion in neonatal piglets. J Lipid Res. 2014;55:531–539. doi: 10.1194/jlr.M045930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murueta JHC, Carreño FG. Concentrates of fish protein from bycatch species produced by various drying processes. Food Chem. 2007;100:705–711. doi: 10.1016/j.foodchem.2005.10.029. [DOI] [Google Scholar]
- Ortiz J, Lemus-Mondaca R, Vega-Gálvez Antonio, Ah-Hen K, Puente-Diaz L, Zura-Bravo L, et al. Influence of air-drying temperature on drying kinetics, colour, firmness and biochemical characteristics of atlantic salmon (salmo salar 1.) fillets. Food Chem. 2013;139(1–4):162–169. doi: 10.1016/j.foodchem.2013.01.037. [DOI] [PubMed] [Google Scholar]
- Pérez-Santín E, Calvo MM, López-Caballero ME, Montero P, Gómez-Guillén MC. Compositional properties and bioactive potential of waste material from shrimp cooking juice. LWT Food Sci Technol. 2013;54:87–94. doi: 10.1016/j.lwt.2013.05.038. [DOI] [Google Scholar]
- Rossmeisl M, Jilkova ZM, Kuda O, Jelenik T, Medrikova D, Stankova B, Kristinsson B, Haraldsson GG, Svensen H, Stoknes I, Sjövall P, Magnusson Y, Balvers MG, Verhoeckx KC, Tvrzicka E, Bryhn M, Kopecky J. Metabolic effects of n-3 PUFA as phospholipids are superior to triglycerides in mice fed a high-fat diet: possible role of endocannabinoids. PLoS ONE. 2012;7:e38834. doi: 10.1371/journal.pone.0038834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda FM, Lopez JA, Martinez FJ, Zamora S, Divanach P, Kentouri M. Fatty acids in muscle of wild and farmed red porgy, Pagrus pagrus. Aquac Nutr. 1997;3:161–165. doi: 10.1046/j.1365-2095.1997.00088.x. [DOI] [Google Scholar]
- Selmi S, Bouriga N, Cherif M, Toujani M, Trabelsi M. Effects of drying process on biochemical and microbiological quality of silverside (fish) Atherina lagunae. Int J Food Sci Technol. 2010;45:1161–1168. doi: 10.1111/j.1365-2621.2010.02249.x. [DOI] [Google Scholar]
- Senso L, Suárez MD, Ruiz-Cara T, García-Gallego M. On the possible effects of harvesting season and chilled storage on the fatty acid profile of the fillet of farmed gilthead sea bream (Sparus aurata) Food Chem. 2007;101:298–307. doi: 10.1016/j.foodchem.2006.01.036. [DOI] [Google Scholar]
- Shah AKMA, Tokunaga C, Kurihara H, Takahashi K. Changes in lipids and their contribution to the taste of migaki-nishin (dried herring fillet) during drying. Food Chem. 2009;115(3):1011–1018. doi: 10.1016/j.foodchem.2009.01.023. [DOI] [Google Scholar]
- Stołyhwo A, Kołodziejska I, Sikorski ZE. Long chain polyunsaturated fatty acids in smoked Atlantic mackerel and Baltic sprats. Food Chem. 2006;94:589–595. doi: 10.1016/j.foodchem.2004.11.050. [DOI] [Google Scholar]
- Toyes-Vargas E, Robles-Romo A, Méndez L, Palacios E, Civera R. Changes in fatty acids, sterols, pigments, lipid classes, and heavy metals of cooked or dried meals, compared to fresh marine by-products. Anim Feed Sci Technol. 2016;221:195–205. doi: 10.1016/j.anifeedsci.2016.09.004. [DOI] [Google Scholar]
- Ulbricht TLV, Southgate DAT. Coronary heart disease: seven dietary factors. The Lancet. 1991;338:985–992. doi: 10.1016/0140-6736(91)91846-M. [DOI] [PubMed] [Google Scholar]
- Vega-Gálvez A, Miranda M, Clavería R, Quispe I, Vergara J, Uribe E, Paeza H, Di Scala K. Effect of air temperature on drying kinetics and quality characteristics of osmo-treated jumbo squid (Dosidicus gigas) LWT Food Sci Technol. 2011;44:16–23. doi: 10.1016/j.lwt.2010.06.012. [DOI] [Google Scholar]
- Wanasundara UN, Shahidi F. Storage stability of microencapsulated seal blubber oil. J Food Lipids. 1995;2:73–86. doi: 10.1111/j.1745-4522.1995.tb00032.x. [DOI] [Google Scholar]
- Wang Y, Zhang M, Mujumdar AS. Trends in processing technologies for dried aquatic products. Dry Technol. 2011;29:382–394. doi: 10.1080/07373937.2011.551624. [DOI] [Google Scholar]
- Woodcock SH, Benkendorff K. The impact of diet on the growth and proximate composition of juvenile whelks, Dicathais orbita (Gastropoda: Mollusca) Aquaculture. 2008;276:162–170. doi: 10.1016/j.aquaculture.2008.01.036. [DOI] [Google Scholar]
- Yin FW, Liu XY, Fan XR, Zhou DY, Xu WS, Zhu BW, Murata Y. Extrusion of Antarctic krill (Euphausia superba) meal and its effect on oil extraction. Int J Food Sci Technol. 2015;50:633–639. doi: 10.1111/ijfs.12673. [DOI] [Google Scholar]
- Zhang J, Wu D, Liu D, Fang Z, Chen J, Hu Y, Ye X. Effect of cooking styles on the lipid oxidation and fatty acid composition of grass carp (Ctenopharynyodon idellus) fillet. J Food Biochem. 2013;37:212–219. doi: 10.1111/j.1745-4514.2011.00626.x. [DOI] [Google Scholar]
- Zhou X, Zhou DY, Liu ZY, Yin FW, Liu ZQ, Li DY, Shahidi F. Hydrolysis and oxidation of lipids in mussel Mytilus edulis during cold storage. Food Chem. 2019;272:109–116. doi: 10.1016/j.foodchem.2018.08.019. [DOI] [PubMed] [Google Scholar]