Abstract
Olive seeds, a potential food by-product from both table olive and olive oil industries, were examined for their overall proximate composition, oil, protein, mineral and phenolic components. Proximate analysis indicates that olive seeds are an unusually rich source of total dietary fibre (≅ 47% dry weight basis, DWB), as well as lipids (≅ 30%) and proteins (≅ 17%). Oil composition shows high levels of oleic (≅ 62% of total fatty acids) and linoleic (≅ 24%) acids, moderate concentrations of tocopherols (≅ 460 mg/kg) and squalene (≅ 194 mg/kg), and relatively high amounts of several sterols and non-steroidal triterpenoids. Olive seed proteins are a rich source of essential amino acids (about 46% of the total AA content). Olive seeds also contain significant amounts of some essential macro-elements (K, Ca, Mg, Na, P) and micro-elements (Zn, Mn, Cu). Phenolic compounds are present at relatively high quantities (≅ 2.8 mg/g seed, DWB); the most abundant belong to the group of secoiridoid compounds (elenolic acid derivatives) including oleuropein and structurally related substances (demethyloleuropein and ligstroside), and nüzhenide derivatives. Based on the general nutritional profile and nutraceutical components, olive seeds have value-added potential as a source of edible oil, proteins or meal serving as feed supplements.
Keywords: Olive seed, Nutritional profile, Oil composition, Amino acids, Minerals, Phenolic compounds
Introduction
In the last years, the possibility of large-scale production of virgin olive oil (VOO) from de-stoned olives has raised increasing concern. Although this process may decrease industrial olive oil yields, manufacturing plants for stone removal and milling of pulp are being set up. This is on behalf of scientific evidence showing that oils extracted from de-stoned fruits have better quality than oils obtained from the traditional milling of whole fruits (Del Caro et al. 2006; Moghaddam et al. 2012; Ranalli et al. 2012). Production of VOO from de-stoned fruits is expected to increase thus leading to increasing generation of olive pits. Currently, the olive pit is considered an industrial waste with main applications in energy production (Rodríguez et al. 2008). Nowadays, by using specific machines, it is possible to separate the pit whole during the processing of olives and also recover the entire seeds.
Metabolic studies concerning oil and other olive fruit components have been mainly focused on fruit pulp components. Surprisingly, studies on other tissues such those of the seed have been of little concern. Some pioneer’s works have focused on the analysis of proteins and transcripts of interest in olive seeds, like legumins (Alché et al. 2006) and vicilins (Zafra et al. 2018). Cellular and developmental analyses of the olive seed tissues (Zienkiewicz et al. 2014; Zafra et al. 2018) suggest a potential to produce oil, proteins and nutraceutical ingredients from olive seeds. Scientific literature about these topics, however, is still limited.
The profiling of plant metabolites with nutritional/nutraceutical interest includes the well-known primary metabolism components (i.e., lipids, proteins and carbohydrates), and a vast array of bioactive secondary metabolites such as certain vitamins, terpenoid and phenolic compounds, among others. Innumerable scientific evidences indicate that the amounts and types of macro-nutrients and their components (fatty acids, amino acids, fibre, minerals) have a crucial importance from a nutritional standpoint (Primo Yúfera 1998; Belitz et al. 2009). In addition, there are increasing concerns about the role of tocopherols, phytosterols and natural phenolics on prevention of several diseases in which oxidative stress reactions are involved including inflammation and endothelial dysfunction, hypertension, and coronary heart disease (Varadharaj et al. 2017; Sanchez-Rodriguez et al. 2018).
Our general interest is to contribute information to nutrient databanks of underexploited plant resources. The main objective of the current study was to assess the presence of diverse metabolites from olive seeds to provide better insight into their nutritional traits and bioactive components.
Materials and methods
Plant material
Olive seed samples (Olea europaea L. cv ‘Picual’) were provided by Elayo Group S.L. (https://www.elayo.es), an industrial factory located in Castillo de Locubín (Jaén, Spain), proprietary of a novelty industrial procedure to extract clean, whole olive seeds from olive pits. Three independent seed samples were processed for oil extraction and analytical determinations. All chemical analyses were done in triplicate and expressed as mean values ± standard deviation.
Proximate composition
Standard AOCS (2009) official methods were used to determine moisture, oil, protein, ash and fibre contents. In brief, seed samples (200 g each) were ground using a universal cutting mill (Tecno Dalvo, Argentina). Dry matter content was determined after oven drying at 80 °C for 72 h. Lipid extraction for total oil content determination was performed using Soxhlet devices with n-hexane as solvent. Total nitrogen was quantified by the Kjeldahl method and it was converted to seed total protein content by using a conversion factor of 5.3. Ash content was quantified by incineration of seeds samples (10 g each) at 550 °C in a muffle furnace. Crude fibre and insoluble fibre determinations were essentially done following the AOCS procedures.
Oil analyses
For analytical and oxidative stability determinations, oils were extracted at room temperature (22–25 °C) using a manually operated pilot-plant hydraulic press (press load 600 kg/cm2).
Fatty acid (FA) composition was analyzed by gas chromatography (GC) according to protocols for oil sample preparation and GC conditions previously reported (Maestri et al. 2015). Identity was confirmed by means of GC (Clarus 580, Perkin-Elmer, Shelton, USA)–mass spectrometry (MS, Clarus SQ8S) analysis. Separations were performed on a CP Wax 52 CB (Varian, Walnut Creek, USA) fused-silica capillary column using helium (flow rate 1 mL/min) as carrier gas. The GC oven temperature was initially maintained at 180 °C (5 min) and then increased at 2 °C/min to 220 °C. Both injector and detector temperatures were set at 250 °C. The FA components were identified by mass spectra matching using the Wiley mass spectra search library.
Tocopherol composition was analyzed by high performance liquid chromatography (HPLC, Perkin-Elmer, Shelton, USA) following analytical methods used previously (Maestri et al. 2015). Accurately-weighted oil samples (1 g each) were diluted with n-hexane to 10 mL. The solutions were filtered through 0.45 μm pore filters. Aliquots of 20 μL of the filtrated solutions were injected into a Supelcosil LC-NH2-NP column (25 cm × 4.6 mm, Supelco, Bellefonte, USA). The mobile phase was n-hexane/ethyl acetate (70/30 V/V) with a flow rate of 1 mL/min. UV detection at 295 nm was performed. Individual tocopherols were identified by comparing their retention times with those of authentic standards (α-, γ- and δ-tocopherols, ICN Biomedicals, Costa Mesa, USA), and were quantified by the external standard method. The linearity of the response was verified by fitting to line results of each one tocopherol individuals (ten standard solutions with known concentrations) covering the concentration range from 2 to 1000 ppm, with a linearity regression coefficient R2 = 1.
For squalene content determination, oil samples (400 mg) were mixed with 1 mL n-hexane, 1 mL squalane solution (1 mg/mL n-hexane) and 2 mL KOH solution (2 N in methanol). After 1 min of vigorous shaking, the mixture was left to react for 10 min (the time required for hydrolysis of glycerides). After decanting, the upper phase (n-hexane) was extracted and washed twice (5 mL every time) with ethanol/water (50/50 V/V). The n-hexane phase was recovered and used for GC and GC–MS analyses according to conditions previously reported (Maestri et al. 2015). Squalene was identified by comparing its mass spectra data with those of the Wiley mass spectra search library. Squalene concentration was calculated on the basis of the internal standard (squalane) concentration.
Sterols analysis was performed essentially according to Martínez et al. (2006). Briefly, oil samples (1 mL) were subjected to alkaline saponification by reflux (30 min) using 50 mL 2 N KOH in methanol. The unsaponifiable matter was extracted with n-hexane (40 mL × three times). Extracts obtained were pooled, the solvent was removed using a rotary vacuum evaporator at 40 °C, and the residue was fractionated by preparative thin layer chromatography (TLC, 0.5 mm silica gel; Merck, Darmstadt, Germany) using toluene/acetone (95:5, V/V) as developing system. After developing, two separated bands containing sterols and methylsterols were identified, removed from the plates and extracted with ethyl ether. Sterols and methylsterols were analyzed by GC–MS using a HP 5 capillary column and helium (1 mL/min) as carrier gas. Oven temperature was programmed from 250 to 290 °C at 2 °C/min; both injector and detector temperatures were set at 300 °C. The identification was performed on the basis of mass spectral data of reference compounds. The quantification was based on calibration curves using β-sitosterol as external standard for sterols, and lanosterol for methylsterols.
The oil oxidative stability index (OSI) was determined under accelerated oxidation conditions using the Rancimat (Metrohm, Herisau, Switzerland) apparatus. Airflow rate was set at 20 L/h and temperature of the heating block was maintained at 110 °C.
Amino acid analysis
Olive seed meal samples (100 mg) were hydrolyzed following the standard AOCS (2009) procedure. Hydrolyzed samples were filtered using 0.45 μm membrane filters and then submitted to pre-column derivatization with diethyl ethoxymethylenemalonate (Sigma-Aldrich, St. Louis, USA) and reversed-phase HPLC (Perkin-Elmer, Shelton, USA) with UV detection at 280 nm according to conditions previously reported (Alaiz et al. 1992). Individual amino acids (AA) were identified by comparing their retention times with those of authentic standards (Sigma-Aldrich, St. Louis, USA), and were quantified by the external standard method. The linearity of the response was verified by fitting to line results of each one amino acid individuals of ten standard solutions with known concentrations.
The contents of the various identified AA were expressed as mg/g of total protein and compared with the FAO/WHO/UNU (1985) reference pattern. Individual essential amino acids (EAA) scores were determined according to FAO/WHO/UNU (1985) using the formula:
Limiting AA were identified as those having a ratio less than 1.
Mineral composition
Samples of ground seeds (2 g each) were placed into platinum crucibles, dried until constant weight by using a conventional oven (80 °C), and then ground with agate mortar and pestle. Milled samples were digested and mineralized by using HNO3 (sub boiling grade) and 30% (V/V) H2O2 (ultrapure) in closed Teflon tubes at 220 °C for 8 h. Mineralized samples were quantitatively transferred to 10 mL volumetric flasks, completing the volume with HNO3 2% (V/V) in ultrapure water, followed by filtration through 0.45 μm pore filters. The mineral concentration was measured using an Agilent 7500cx inductively coupled plasma–mass spectrometer (ICP–MS) (Agilent Technologies, California, USA) according to conditions reported elsewhere (Griboff et al. 2017). All ICP–MS measurements were performed using Sc, Ge, In and Re as internal standards. Full quantitative analysis was carried out against calibration standards for each element.
Total phosphorus content was determined by ashing dried and milled seed samples (1 g each) in the presence of zinc oxide, followed by spectrophotometric measurement of phosphorous as a blue phosphomolybdic acid complex (AOCS 2009).
Phenolic compounds
Phenolic compounds were extracted from 10 g seed samples previously defatted at room temperature (n-hexane 50 mL × three times). Defatted samples (5 g) were extracted thrice with methanol (80% V/V, 50 mL every time). The extracts were combined and washed twice (50 mL every time) with n-hexane. The hydro-alcoholic phase was recovered, filtrated through 0.45 μm pore filters, concentrated under vacuum below 50 °C to a final volume of 1 mL, and further used for both total phenol content (TPC) and HPLC–ESI–MS/MS analyses according to procedures reported elsewhere (Bodoira et al. 2017).
HPLC–ESI–MS/MS analysis
The composition of phenolic extracts was analyzed by means of HPLC–ESI–MS/MS using an Agilent 1200 HPLC Series system (Agilent Technologies, Santa Clara, USA). The chromatographic separations were achieved on a Kromasil (Bohus, Sweden) reversed-phase C18 column (5 µm, 250 mm × 4.60 mm i.d.) under analytical conditions used previously (Bodoira et al. 2017). The MS detector was programmed to perform a MS/MS scan of the most abundant ions, using collision energy of 13.0 eV. The identification of phenolic compounds was based on their retention times (Rt), elution order, and comparison of UV–Vis spectra and mass spectrometry data reported in the literature (Tanahashi et al. 1997; Di Donna et al. 2007; Kanakis et al. 2013; Klen et al. 2015; Michel et al. 2015). The Compass version 3.1 software and DataAnalysis version 4.1 software were used for data acquisition and processing, respectively.
Results and discussion
Proximate analysis
Olive seed proximate composition is summarized in Table 1. Seed oil content accounted for about 30% of the seed dry matter content. There is a lack of robust information on olive seed oil content. García-Inza et al. (2016) report seed oil concentrations around 35% in cv. Arauco from Argentine, but Moghaddam et al. (2012) have informed oil contents from 5.6 to 9.8% in kernels from several European cultivars. Compared with the oil content of common edible oil-bearing seeds, the oil concentration found in olive seeds results higher than those found in soybean and sunflower which range between 18–22 and 20–32%, respectively, but lower than peanut (40–50%) and rapeseed (40–60%) (Gunstone and Harwood 2007).
Table 1.
Proximate composition (%, DWB) of olive seeds
| Lipids | 30.4 ± 0.77 |
| Protein | 17.2 ± 0.35 |
| Total fibre | 47.6 ± 0.95 |
| Insoluble fibre | 32.7 ± 0.56 |
| Ash | 2.67 ± 0.21 |
| Carbohydrates | 2.13 ± 0.25 |
Mean values ± SD (n = 3)
The total protein content (17.2% DWB) of olive seeds compares well with those reported in some cereal grains, for example rice (average protein content 10.1% DWB), corn (10.3%), sorghum (12.3%) and wheat (13.4%) (Primo Yúfera 1998). Compared with oil-bearing seeds, it results lower than whole-seed meals from cultivated rapeseed and sunflower, which ranges between 23–24% and 26–34%, respectively (Park et al. 1997; Wanasundara 2011).
Olive seeds contained an unusually high quantity (47.6% DWB) of total fibre consisting of approximately equal amounts of both soluble and insoluble fractions. Such a value is higher than those found in chia (Salvia hispanica L.) seeds, which are known to be one of the best sources (39–42%) of dietary fibre (Reyes-Caudillo et al. 2008).
Oil chemical analyses
Identified FA in olive seed oils were palmitic (hexadecanoic), palmitoleic (cis-9-hexadecenoic), margaric (heptadecanoic), cis-10-heptadecenoic, stearic (octadecanoic), oleic (cis-9-octadecenoic), linoleic (cis-9-cis-12-octadecadienoic), linolenic (cis-9-cis-12-cis-15-octadecatrienoic), arachidic (eicosanoic), gondoic (cis-11-eicosenoic) and behenic (docosanoic) acids. Palmitic, oleic and linoleic acids were measured as major FA (Table 2). trans-FA were not detected. This qualitative pattern shows resemblance with that found in olive pulp oils (Moghaddam et al. 2012). Both seed and pulp tissues contain identical FA species but with some differences in concentrations, especially regarding unsaturated FA levels. In general, olive pulp oils are somewhat richer in individual monounsaturated FA (oleic and palmitoleic acids) and lower in linoleic acid contents than oils obtained from the seeds. On the other hand, as compared with FA profiles of oil-bearing seeds, the olive seed oil is found to be markedly higher in oleic acid content (≅ 62%) than soybean (average 23%), traditional sunflower (20–30%) and peanut (40–55%), and similar to canola (low-erucic acid rapeseed) oil (average 61%) (Gunstone and Harwood 2007).
Table 2.
Fatty acid, tocopherol and sterol compositions of olive seed oil
| Fatty acids | (%) |
|---|---|
| C16:0 (palmitic) | 8.82 ± 0.18 |
| C16:1 (palmitoleic) | 0.32 ± 0.06 |
| C17:0 (margaric) | 0.12 ± 0.01 |
| C17:1 (heptadecenoic) | 0.09 ± 0.01 |
| C18:0 (estearic) | 2.47 ± 0.12 |
| C18:1 (oleic) | 61.83 ± 0.78 |
| C18:2 (linoleic) | 24.24 ± 0.39 |
| C18:3 (linolenic) | 0.39 ± 0.08 |
| C20:0 (arachidic) | 0.53 ± 0.10 |
| C20:1 (gondoic) | 0.54 ± 0.09 |
| C22:0 (behenic) | 0.40 ± 0.08 |
| ΣSFAa | 12.34 ± 0.51 |
| ΣMUFAb | 62.78 ± 0.75 |
| ΣPUFAc | 24.63 ± 0.42 |
| Tocopherols | (mg/kg oil) |
|---|---|
| α-Tocopherol | 401.0 ± 7.21 |
| β-Tocopherol | 9.05 ± 0.91 |
| γ-Tocopherol | 39.0 ± 2.50 |
| δ-Tocopherol | 9.90 ± 1.61 |
| Sterols | (mg/kg oil) |
|---|---|
| Campesterol | 72.7 ± 3.56 |
| Stigmasterol | 53.9 ± 15.35 |
| β-Sitosterol | 1674.9 ± 144.5 |
| Lanosterol | 10.5 ± 3.20 |
| Cycloartenol | 109.7 ± 13.9 |
| Citrostadienol | 17.2 ± 3.40 |
| 24-Methylenecycloartanol | 365.3 ± 68.3 |
Mean values ± SD (n = 3)
aSaturated fatty acids
bMonounsaturated fatty acids
cPolyunsaturated fatty acids
Up to the moment, there seems to be no published reports on individual tocopherol concentration of olive seed oils. The study by Moghaddam et al. (2012) informs total tocopherol contents (TTC) between 15 and 39 mg/kg in kernel oils from several olive cultivars. In the present study, the average TTC was found to be 459 mg/kg oil (Table 2). This seed oil fraction was largely composed of α-tocopherol (approximately 87% of the seed oil TTC) and minor contents of γ-, β-, and δ-tocopherol. The α-tocopherol content we found appears to be intermediate in relation to those obtained from olive pulp oils which show important quantitative variations; Del Caro et al. (2006) and Ranalli et al. (2012) report average α-tocopherol contents of 230 and 179 mg/kg, respectively, in oils obtained from de-stoned olive fruits of Italian cultivars, but Katsoyannos et al. (2015) have found 382–562 mg/kg in pulp oils from Greek varieties.
Squalene, the biosynthetic precursor of both sterols and non-steroidal triterpenoids, is part of the wide array of olive compounds with nutritional and health properties. Among vegetable oils, VOO is possibly the richest source of this compound (700–1200 mg/kg). To our knowledge, there are no published data about squalene content in olive seed oils. In the present study, the average concentration was found to be 194 mg/kg oil (Table 2). This value is within the range reported for some crude edible vegetable oils (138–262 mg/kg) (Nergiz and Çelikkale 2011).
The three main classes of sterol compounds—i.e., 4-demethylsterols, 4-monomethylsterols and 4,4′-dimethylsterols (also named triterpene alcohols), all of which are usually present in the olive fruit (Ranalli et al. 2002)—were detected in the olive seed oil (Table 2). Campesterol, stigmasterol and β-sitosterol were the major sterol compounds identified from the 4-demethylsterol fraction. Their concentrations (72.7, 53.9 and 1674.9 mg/kg, respectively) were in the ranges reported for these compounds (26–88, 4–67 and 670–2035 mg/kg) in VOOs from several cultivars and geographical origins (Kyçyk et al. 2016). Similarly to these latter oils, the 4-demethylsterol fraction from the olive seed oil was largely composed (about 90%) of β-sitosterol. The 4-demethylsterols namely cholesterol, brassicasterol, 24-methylenecholesterol, chlerosterol, stigmastanol, Δ5-avenasterol, Δ7-stigmastenol and Δ7-avenasterol were quantified as minor components (below 10 mg/kg oil). The methylsterol fraction included citrostadienol (4-monomethylsterol) and three 4,4′-dimethylsterols (lanosterol, cicloartenol and 24-methylenecycloartanol). In addition, β-amyrin (a non-steroidal triterpenoid) was found to elute with this latter fraction. The compositional pattern described above matches in general with that reported by Ranalli et al. (2002) from pulp and whole olive oils of several Italian cultivars. However and interestingly, β-amyrin—which is known to have important biological functions—was present in olive seed oils at much higher quantities (921 mg/kg oil, in average) than those observed in pulp or whole olive oils (Ranalli et al. 2002).
From a technological standpoint, the Rancimat method is a useful analytical test in order to measure the oxidative stability of edible oils. The average OSI value of olive seed oil analyzed here was found to be 1.35 h. This value is markedly lower as compared with those obtained from VOOs (for example 11.6 and 34 hs for ‘Arbequina’ and ‘Picual’ olive oils, respectively, oxidized under the same conditions we used, Mateos et al. 2006). The oxidative stability of vegetable oils is strongly related to their FA composition and also to the presence of minor endogenous antioxidant substances, mainly tocopherols and other phenolic compounds. As mentioned previously, VOOs (obtained either from pulp or whole fruits) and olive seed oil show certain similarities in FA and tocopherol profiles (oleic acid higher than 60%, linoleic acid 10–25%, α-tocopherol as the major tocopherol isoform, 200–500 mg/kg oil). On the contrary, the content of phenolic compounds other than tocopherols could be markedly different. Although olive seeds contained phenolics at relatively high amounts (this result is discussed below), they were found at low concentrations (54.7 mg/kg) in the corresponding oils. Looking at the structures of phenolic compounds identified in the seeds (Fig. 1) it can be seen that most of them are glycosylated and a high number of OH groups are present. These features suggest rather polar and hydrophilic forms which could be poorly soluble in lipids, and therefore would not be extracted with the oil. Hence, the differences in oxidative stability between olive oils obtained from fruits or seeds are likely to be due in part to the high amount of polyphenols generally present in the former, but barely present in the latter.
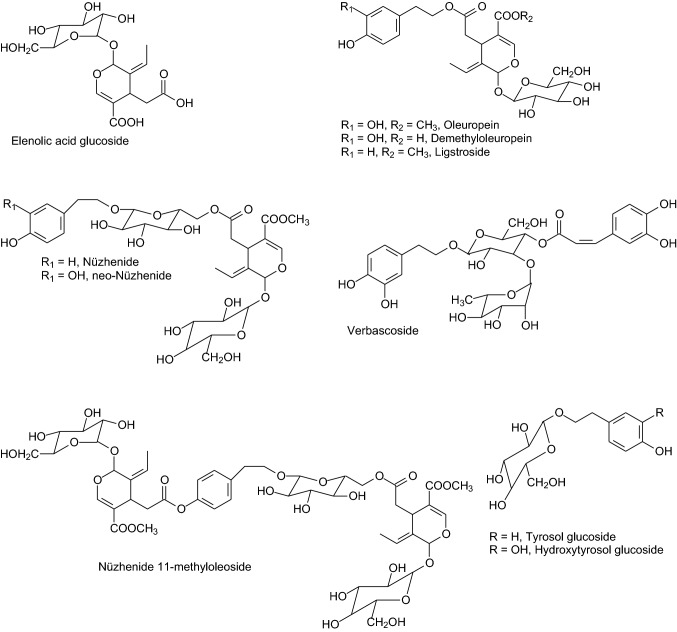
Fig. 1.
Chemical structures of the main phenolic (elenolic acid is not strictly a phenolic compound but is a key biosynthetic precursor of secoiridoid compounds present in olive) compounds found in olive seeds
Amino acid analysis
Up to the moment, there are no published studies on AA composition of olive seeds. A total of nineteen compounds were identified (Table 3). The total percentage of EAA, which is considered an indicator of protein quality, was about 46%. This percentage is substantially higher than those found in whole-wheat flour (about 27%) (Primo Yúfera 1998), which is one of the most widespread source of vegetable protein. On the other hand, the ratio of EAA to non-EAA in olive seed proteins (0.86) was markedly higher than the value recommended by FAO (1985) for human adults (0.38).
Table 3.
Amino acid (AA) composition from olive seeds and essential-AA requirements
| AA | Concentration (mg/g protein) | FAO (mg/g protein)b | AA scoree | Wheat (mg/g protein)f | |
|---|---|---|---|---|---|
| Child | Adult | ||||
| Asp + Asn | 88.17 ± 0.39 | ||||
| Glu + Gln | 170.45 ± 2.24 | ||||
| Ser | 44.55 ± 0.18 | ||||
| Hisa | 22.44 ± 0.28 | 19 | 15 | 1.18 | 41 |
| Gly | 45.47 ± 0.82 | ||||
| Thra | 36.75 ± 0.99 | 34 | 11 | 1.08 | 24 |
| Arg | 72.78 ± 0.86 | ||||
| Ala | 47.25 ± 0.28 | ||||
| Pro | 18.53 ± 0.33 | ||||
| Tyr | 33.72 ± 1.96 | 17 | |||
| Vala | 162.58 ± 7.22 | 35 | 15 | 4.64 | 42 |
| Meta | 18.84 ± 1.57 | 27c | 20c | 1.08 | 27 (Met + Cys) |
| Cys | 10.45 ± 0.59 | ||||
| Ilea | 43.83 ± 3.06 | 28 | 15 | 1.56 | 29 |
| Leua | 69.66 ± 8.01 | 66 | 21 | 1.05 | 51 |
| Phea | 53.41 ± 6.20 | 63d | 21d | 0.84 | 26 |
| Lysa | 46.63 ± 3.52 | 58 | 18 | 0.80 | 37 |
| % EAA | 46.5 | ||||
According to the EAA score (calculated on the basis of requirement values for children) olive seed protein was deficient in phenylalanine and lysine. These AA, however, were not limiting for adults. The other EAA were present in adequate amounts for both children and adults. Interestingly, olive seeds were found to be an unusually rich source of valine (162.58 mg/g protein); the value recorded was approximately four times higher than the value reported for wheat flour (Primo Yúfera 1998), and about twice as high as the values found in both casein and egg white protein (Friedman 1996) which are recognized as high quality protein sources.
With respect to non-EAA, the most abundant were those from the two pairs namely glutamic acid + glutamine (170.45 mg/g protein) and aspartic acid + asparagine (88.18 mg/g protein). These biosynthetically related compounds (glutamine and asparagine are derived forms of glutamic acid and aspartic acid, respectively) are relatively abundant in several seed proteins, including legumes and cereals (Primo Yúfera 1998). Interestingly, arginine (72.78 mg/g protein)—a semi-essential amino acid involved in many biological functions (Appleton 2002)—was present at much higher quantities than those observed in several cereal grains (Primo Yúfera 1998).
Mineral composition
To our knowledge, this is the first report on the concentration of individual minerals in olive seeds (Table 4). The values found showed potassium as the most abundant mineral, followed by sodium, calcium and magnesium. As compared with the mineral element content of whole olive fruits (Fernandez-Hernandez et al. 2010) or fruit pulp (Nergiz and Engez 2000), olive seeds are somewhat higher in these latter three elements but markedly lower in potassium concentration. Phosphorus content in olive seeds (≅ 745 mg/kg) was within the range observed in whole fruits from some Turkish olive varieties (605–1187 mg/kg) (Tanilgan et al. 2007).
Table 4.
Mineral profile (mg/kg, DWB) of olive seeds
| Macro-elements | |
| K | 5579.0 ± 93.3 |
| Na | 2758.2 ± 42.7 |
| Ca | 2615.4 ± 34.3 |
| Mg | 1878.5 ± 27.5 |
| P | 745.5 ± 44.5 |
| Micro-elements | |
| Fe | 12.8 ± 0.16 |
| Zn | 45.6 ± 0.64 |
| Mn | 31.5 ± 0.49 |
| B | 25.0 ± 0.63 |
| Cu | 23.4 ± 0.18 |
| Ba | 13.4 ± 0.31 |
| Cr | 6.3 ± 0.21 |
| Ni | 3.9 ± 0.20 |
| Bi | < 0.1 |
| Co | < 0.1 |
Mean values ± SD (n = 3)
An interesting feature is the large number of micro-elements found in olive seeds. These included iron, zinc, manganese, boron, copper, barium, chromium, nickel, bismuth and cobalt, in a decreasing order of concentration. Fe, Zn, Mn and Cu are essential nutrients for humans; they are constituents of many enzyme systems and play important functions in several physiological processes. The concentration of Fe was found to be lower whereas those of Zn, Mn and Cu were higher than those found by Fernandez-Hernandez et al. (2010) in whole olive fruits. In general, B, Ba, Cr, Ni, Bi and Co have not been quantified in olives; however, the study by Nergiz and Engez (2000) reports Ba, Cr and Co in fruits from Turkish olive cultivars.
On the other hand, the presence of vanadium, arsenic, cadmium and lead—which are associated to different degrees and types of toxicity in humans—was checked but no values were registered under the analytical detection conditions we used. It is important to remark these latter results because some heavy metals, especially lead and cadmium, are well known as potent toxic elements, and worldwide regulations set maximum limits in various foods.
Phenolic compounds
Studies concerning synthesis and accumulation of phenolic compounds in olive have focused on the fruit pulp. Other tissues, such as pits and seeds have received minor attention. The concentration of total phenolic compounds (TPC) in olive fruits varies widely; Bodoira et al. (2016) inform concentrations as high as 100 mg/g (whole fruits, DWB) in cv. Manzanilla. As compared to pulp, TPC of olive seeds analyzed here is markedly lower (2.79 mg/g). However, it is comparable to values reported in some edible seeds or nuts, such as hazelnut (2.9–8.3), almond (0.4–4.1), cashew (1.3–2.7), peanut (0.1–4.2), and macadamia (0.4–1.5) (Chang et al. 2016).
A total of 24 phenolic compounds were detected in the examined olive seeds (Table 5). The structural analysis of the most abundant compounds leads to their allocation into the group of secoiridoid compounds (elenolic acid derivatives) including oleuropein and structurally related substances (demethyloleuropein and ligstroside), and nüzhenide derivatives. In general, these compositional data agree with those obtained from olive pulp (Servili et al. 1999) and whole pits (Ben Mansour et al. 2015). Several of the identified compounds were present as glycosides in contrast with data from Alu’datt et al. (2011) who found the predominant phenolic compounds in olive seeds in free form.
Table 5.
Phenolic compound profile of olive seeds
| Compound | Rt (min) | Formula | [M–H]− measured (m/z) | Main fragments MS2 [M–H]− (m/z) | Relative abundance (%) |
|---|---|---|---|---|---|
| Caffeic acid derivative | 6.8 | C18H18O9 | 377.0883 | 341.1071–191.0582–179.0358 | ++ |
| Hydroxytyrosol glucoside | 11.2 | C14H20O8 | 315.1140 | naa | +++ |
| Elenolic acid glucoside | (i) 12.1 | C17H24O11 | 403.1356 | 223.0633–371.0979 | (i) ++++ |
| (ii) ++++ | |||||
| (iii) ++++ | |||||
| (ii) 13.3 | |||||
| (iii) 13.6 | |||||
| Epigallocatequin | 13.2 | C15H14O7 | 305.0782 | 125.1182 | ++ |
| Neo-Nüzhenide isomers | (i) 13.8 | C31H42O18 | 701.2298 | 523.1820–315.1123–403.1233 | (i) +++ |
| (ii) +++ | |||||
| (ii) 14.2 | |||||
| Verbascoside | 14.0 | C29H36O15 | 623.2096 | 461.1647 | ++ |
| Nüzhenide isomers | (i) 14.1 | C31H42O17 | 685.2338 | 523.1830–299.1190 | (i) ++++ |
| (ii) ++++ | |||||
| (ii) 15.8 | |||||
| Oleuropein aglycon | 15.3 | C16H26O10 | 377.1215 | 195.0666 | +++ |
| Methoxynüzhenide | 16.6 | C32H44O18 | 715.2455 | 523.1736–553.1927–329.1241 | ++ |
| Tyrosol glucoside | 18.1 | C14H20O7 | 299.1180 | na | +++ |
| Nüzhenide 11-methyloleoside isomers | (i) 18.8 | C48H64O27 | 1071.3551 | 685.2397–771.2381–523.1873–403.1231 | (i) + |
| (ii) ++ | |||||
| (iii) ++ | |||||
| (iv) + | |||||
| (ii) 21.3 | |||||
| (iii) 23.2 | |||||
| (iv) 23.7 | |||||
| Apigenin rutinoside | 19.2 | C27H30O14 | 577.1579 | na | + |
| Oleuropein | 19.8 | C25H32O13 | 539.1851 | 377.1215–307.0838–275.0949 | ++++ |
| Ligstroside | 21.9 | C25H32O12 | 523.1841 | 291.0896–361.1304 | +++ |
| Demethyloleuropein | 23.5 | C24H30O13 | 525.1982 | 405.13–291.09 | ++++ |
| Jaspolyanoside | 24.1 | C42H54O22 | 909.3192 | 523.1843–361.1289–291.0881 | ++ |
| Luteolin | 24.3 | C15H10O6 | 285.0402 | na | ++ |
Compounds are listed on the basis of increasing retention times (Rt)
Relative abundance (% precursor ion intensity) of the metabolites identified is indicated by + (0–5%), ++ (5–20%), +++ (20–50%) and ++++ (50–100%)
ana not available
Three peaks showing identical mass spectra data ([M–H]− signal at m/z 403.13, presence of major product ions at m/z 223.06 and m/z 371.09) were detected at 12.1, 13.3 and 13.6 Rt. These data match exactly with those from different elenolic acid glucoside isomers (Klen et al. 2015); the fragment at m/z 223.06 is due to the elimination of hexose, whereas that at m/z 371.09 is attributed to neutral loss of the methyl group from the elenolic acid moiety. The glycosylated elenolic acid, also named oleoside 11-methyl ester, is common in the olive fruit since it represents a key biosynthetic precursor in the metabolic pathway leading to the biosynthesis of oleuropein, ligstroside and other secoiridoids.
Oleuropein, one of the most representative polyphenolic compound in the olive fruit, was unambiguously identified by its typical [M–H]− signal at m/z 539.18 and by the appearance of its classical protonated aglycone ion at m/z 377.12 (Kanakis et al. 2013; Michel et al. 2015). The peak at 15.3 Rt showed this latter as parent [M–H]− ion, and a major daughter ion at m/z 195.06 that could correspond to the hydroxytyrosol acetyl ester formed through various cleavages of the iridoid part (Kanakis et al. 2013). So, such a peak was identified as oleuropein aglycone (the dialdehydic form of decarboxymethyl elenolic acid linked to hydroxytyrosol) which is formed by the cleavage of the glycosidic bond due to β-glycosidase activity. The identity of demethyloleuropein was based on its characteristic [M–H]− signal at m/z 525.19, which is 14 mass units lower than that of oleuropein. Oleuropein and demethyloleuropein have been previously found in all of the constitutive parts of the olive fruit such as peel, pulp and seed (Klen et al. 2015; Bodoira et al. 2016).
Ligstroside was identified on the basis of molecular weight and mass spectra data ([M–H]− signal at m/z 523.18, presence of major product ions at m/z 361.13—attributable to the loss of the glucose moiety—and m/z 291.08—probably derived from the C4H6O fragment loss from m/z 361.13) as described by Kanakis et al. (2013) and Michel et al. (2015). Ligstroside is a common phenolic component in different olive tissues (leaf, fruit pulp, stone) and olive oil (Klen et al. 2015; Michel et al. 2015), but it has been rarely reported in olive seeds (Michel et al. 2015).
Interestingly, a peak showing a parent ion [M–H]− signal at m/z 909.3192 was found. On the basis of its exact molecular mass and the presence of a major daughter ion at m/z 523.1843—which could be attributed to deprotonated ligstroside—it could be tentatively identified as jaspolyanoside (Tanahashi et al. 1997). This compound has been earlier reported as a minor component in olive wood (Pérez-Bonilla et al. 2011) but, at to the moment, it has not been found in the fruit. Jaspolyanoside is a relatively common compound in Jasminum (Tanahashi et al. 1997) and it was found to be structurally closed to jaspolyoside which has a ligstroside based structure.
Nüzhenide and derivatives are considered major components of olive seeds (Servili et al. 1999). Four nüzhenide-related chemical species, each showing various isomers, were detected in seed extracts analyzed here. The characteristic molecular ion at m/z 685.23 and the presence of major daughter ions at m/z 523.18 (corresponding to the nüzhenide aglycone) and m/z 299.11 (formed by the loss of the 11-methyloleoside unit) led to the unambiguous assignments of peaks at 14.1 and 15.8 Rt as nüzhenide isomers.
Two compounds with typical [M–H]− signal at m/z 701.22 were also detected at 13.8 and 14.2 Rt. Such a molecular mass is 16 mass units heavier than nüzhenide, which could be due to the replacement of tyrosol by hydroxytyrosol. In addition, the presence of a shared daughter ion (m/z 523.18) suggests those two compounds structurally similar to nüzhenide. Hence, they were assigned to neo-nüzhenide isomers. Neo-nüzhenide was previously detected as a micro-component in the olive fruit flesh (Di Donna et al. 2007).
The peak at 16.6 Rt ([M–H]− signal at m/z 715.24) is 30 mass units heavier than nüzhenide (probably due to a methoxy group attached to the tyrosol moiety), and shares with this latter compound a daughter ion at m/z 523.18, originated as indicated previously. Other daughter ions at m/z 553.19 and m/z 329.12 are possibly formed by neutral loss of glucose, followed by the consecutive loss of methoxytyrosol glucose. Hence, the identity of such a peak was tentatively assigned as methoxynüzhenide. This compound has been previously detected in olive stones (Klen et al. 2015); it has been suggested that its presence in other parts of the fruit could be a result of transfer from the stone.
Four peaks showing identical and characteristic [M–H]− signal at m/z 1071.35 were found at 18.8, 21.3, 23.2 and 23.7 Rt. Their molecular mass results 386 mass units heavier than that of nüzhenide, probably due to an addition of one unit of 11-methyl oleoside to the latter molecule. Likewise, the presence of major daughter ions at both m/z 685.23 and 523.18 (originated as indicated previously), and at m/z 403.12 (corresponding to the 11-methyloleoside fragment) confirms the identity as nüzhenide 11-methyloleoside isomers (Klen et al. 2015; Michel et al. 2015).
The identity of the peak at 6.8 Rt ([M–H]− signal at m/z 377.08) remains pending. Based on the presence of major product ions at m/z 179.03 (deprotonated caffeic acid) and m/z 341.10 (deprotonated caffeoyl glucose or dicaffeic acid) it could be preliminarily assigned as caffeic acid derivative.
The presence of verbascoside, a heterosidic ester of caffeic acid and hydroxytyrosol, was confirmed by its typical molecular ion at m/z 623.20 (Kanakis et al. 2013), and formation of a major product ion at m/z 461.16 that is attributed to the loss of a caffeoyl moiety. Verbascoside is an abundant compound in olive pulp tissues (Dermeche et al. 2013; Bodoira et al. 2016) but it is relatively uncommon in the seed (Servili et al. 1999).
The phenyl alcohols namely hydroxytyrosol and tyrosol were found as glycosylated derivatives. The spectra generated gave characteristic [M–H]− signals at m/z 315.11 and 299.11, respectively, as described previously (Kanakis et al. 2013). These compounds and their corresponding aglycones—which are precursors in oleuropein and ligstroside biosynthesis—are commonly found as minor constituents in all parts of the olive fruit (Klen et al. 2015; Michel et al. 2015).
Flavonoid-type compounds included the well-known flavones luteolin and apigenin—this latter as glycosylated derivative–, and the flavanol namely epigallocatequin (EGC). The identity of this latter compound, which is uncommon in olive fruits, was assigned on the basis of its exact molecular mass (parent ion [M–H]− signal at m/z 305.0782), and the presence of a major product ion at m/z 125.11 corresponding to the 1,2,3-trihydroxybenzene deprotonated fragment.
Conclusion
Overall, olive seeds are a good source of macronutrients. Oil content (≅ 30% seed dry weight) and composition compare favourably with those from many common oil-bearing seeds. Olive seed oil provides a complete and well-balanced fatty acid composition (high content of MUFA, ≅ 63%; moderate amounts of PUFA, ≅ 25%; low content of saturated fatty acids, ≅ 12%), and a myriad of bioactive phytochemicals, viz. tocopherols (≅ 460 mg/kg oil), squalene (≅ 194 mg/kg), steroidal and non-steroidal triterpenoids. Olive seeds are also an outstanding source of dietary fibre (≅ 47% seed dry weight) as compared to most known edible seeds. With the exception of lysine, olive seed proteins (≅ 17% seed dry weight) are a rich source of essential amino acids (about 46% of the total AA content) particularly valine, and arginine which is considered a semi-essential AA. The mineral composition shows important amounts of essential macro-elements (K, Ca, Mg, Na, P) and micro-elements (Zn, Mn, Cu) comparable to those found in many edible nuts and seeds. Phenolic compounds are present at relatively high quantities (≅ 2.8 mg/g seed); the most abundant belong to the group of secoiridoid compounds (elenolic acid derivatives) including oleuropein and structurally related substances (demethyloleuropein and ligstroside), and nüzhenide derivatives. Keeping in mind the overall chemical composition, olive seeds constitute a valuable source of nutritional and functional components, with potential value for edible oil production, proteins and meal serving as feed supplements.
Acknowledgements
This research was financed with funds from FEDER (Projects RTC-2016-4824-2, RTC-2017-6654-2, BFU2016-77243-P), and Grants from Programa de Cooperación Bilateral CONICET-CSIC and SeCyT-UNC.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alaiz M, Navarro JL, Girón J, Vioque E. Amino acid analysis by high-performance liquid chromatography after derivatization with diethyl ethoxymethylenemalonate. J Chrom A. 1992;591:181–186. doi: 10.1016/0021-9673(92)80236-N. [DOI] [PubMed] [Google Scholar]
- Alché JD, Jiménez-López JC, Wang W, Castro-López AJ, Rodríguez-García MI. Biochemical characterization and cellular localization of 11S type storage proteins in olive (Olea europaea L.) seeds. J Agric Food Chem. 2006;54:5562–5570. doi: 10.1021/jf060203s. [DOI] [PubMed] [Google Scholar]
- Alu’datt MH, Alli I, Ereifej K, Alhamad MN, Alsaad A, Rababeh T. Optimisation and characterisation of various extraction conditions of phenolic compounds and antioxidant activity in olive seeds. Nat Prod Res. 2011;25:876–889. doi: 10.1080/14786419.2010.489048. [DOI] [PubMed] [Google Scholar]
- AOCS . Official methods and recommended practices of the american oil chemists’ society. Champaign: AOCS Press; 2009. [Google Scholar]
- Appleton J. Arginine: clinical potential of a semi-essential amino acid. Altern Med Rev. 2002;7:512–522. [PubMed] [Google Scholar]
- Belitz HD, Grosch W, Schieberle P. Food chemistry. In: Belitz HD, Grosch W, Schieberle P, editors. Food chemistry. Berlin: Springer; 2009. [Google Scholar]
- Ben Mansour A, Porter EA, Kite GC, Simmonds MS, Abdelhedi R, Bouaziz M. Phenolic profile characterization of Chemlali olive stones by liquid chromatography-ion trap mass spectrometry. J Agric Food Chem. 2015;63:1990–1995. doi: 10.1021/acs.jafc.5b00353. [DOI] [PubMed] [Google Scholar]
- Bodoira R, Torres M, Pierantozzi P, Aguate F, Taticchi A, Servili M, Maestri D. Dynamics of fatty acids, tocopherols and phenolic compounds biogenesis during olive (Olea europaea L.) fruit ontogeny. J Am Oil Chem Soc. 2016;93:1289–1299. doi: 10.1007/s11746-016-2877-7. [DOI] [Google Scholar]
- Bodoira R, Rossi Y, Montenegro M, Maestri D, Velez A. Extraction of antioxidant polyphenolic compounds from peanut skin using water–ethanol at high pressure and temperature conditions. J Super Fluids. 2017;128:57–65. doi: 10.1016/j.supflu.2017.05.011. [DOI] [Google Scholar]
- Chang SK, Alasalvar C, Bolling BW, Shahidi F. Nuts and their co-products: the impact of processing (roasting) on phenolics, bioavailability, and health benefits—a comprehensive review. J Funct Foods. 2016;26:88–122. doi: 10.1016/j.jff.2016.06.029. [DOI] [Google Scholar]
- Del Caro A, Vacca V, Poiana M, Fenu P, Piga A. Influence of technology, storage and exposure on components of extra virgin olive oil (Bosana cv) from whole and de-stoned fruits. Food Chem. 2006;98:311–316. doi: 10.1016/j.foodchem.2005.05.075. [DOI] [Google Scholar]
- Dermeche S, Nadour M, Larroche C, Moulti-Mati F, Michaud P. Olive mill wastes: biochemical characterizations and valorization strategies. Process Biochem. 2013;48:1532–1552. doi: 10.1016/j.procbio.2013.07.010. [DOI] [Google Scholar]
- Di Donna L, Mazzotti F, Napoli A, Salermo R, Sajjad A, Sindona G. Secondary metabolism of olive secoiridoids. New microcomponents detected in drupes by electronspray ionization and high-resolution tandem mass spectrometry. Rapid Commun Mass Spectrom. 2007;21:273–278. doi: 10.1002/rcm.2830. [DOI] [PubMed] [Google Scholar]
- FAO/WHO/UNU (1985) Energy and protein requirements. Report of the joint FAO/WHO/UNU expert consultation. Technical report series no. 724. FAO. WHO and the United Nations University, Geneva
- Fernandez-Hernandez A, Mateos R, García-Mesa JA, Beltrán G, Fernandez-Escobar R. Determination of mineral elements in fresh olive fruits by flame atomic spectrometry. Span J Agric Res. 2010;8:1183–1190. doi: 10.5424/sjar/2010084-1206. [DOI] [Google Scholar]
- Friedman M. Nutritional value of proteins from different food sources. A review. J Agric Food Chem. 1996;44:6–29. doi: 10.1021/jf9400167. [DOI] [Google Scholar]
- García-Inza GP, Castro DN, Hall AJ, Rousseaux MC. Opposite oleic acid responses to temperature in oils from the seed and mesocarp of the olive fruit. Eur J Agron. 2016;76:138–147. doi: 10.1016/j.eja.2016.03.003. [DOI] [Google Scholar]
- Griboff J, Wunderlin DA, Monferran MV. Metals, As and Se determination by inductively coupled plasma–mass spectrometry (ICP–MS) in edible fish collected from three eutrophic reservoirs. Their consumption represents a risk for human health? Microchem J. 2017;130:236–244. doi: 10.1016/j.microc.2016.09.013. [DOI] [Google Scholar]
- Gunstone FD, Harwood JL. Occurrence and characteristics of oils and fats. In: Gunstone FD, Harwood JL, Dijkstra AJ, editors. The lipid handbook. Boca Raton: CRC Press; 2007. pp. 37–141. [Google Scholar]
- Kanakis P, Termentzi A, Michel T, Gikas E, Halabalaki M, Skaltsounis AL. From olive drupes to olive oil. An HPLC-Orbitrap-based qualitative and quantitative exploration of olive key metabolites. Planta Med. 2013;79:1576–1587. doi: 10.1055/s-0033-1350823. [DOI] [PubMed] [Google Scholar]
- Katsoyannos E, Batrinou A, Chatzilazarou A, Bratakos SM, Stamatopoulos K, Sinanoglou VJ. Quality parameters of olive oil from stoned and non-stoned Koroneiki and Megaritiki Greek olive varieties at different maturity levels. Grasas Aceites. 2015;66:1–10. [Google Scholar]
- Klen TJ, Wondra AG, Vrhovsek U, Vodopivec BM. Phenolic profiling of olives and olive oil process-derived matrices using UPLC–DAD–ESI–QTOF–HRMS analysis. J Agric Food Chem. 2015;63:3859–3872. doi: 10.1021/jf506345q. [DOI] [PubMed] [Google Scholar]
- Kyçyk O, Aguilera MP, Gaforio JJ, Jiménez A, Beltrán G. Sterol composition of virgin olive oil of forty-three olive cultivars from the World Collection Olive Germplasm Bank of Cordoba. J Sci Food Agric. 2016;96:4143–4150. doi: 10.1002/jsfa.7616. [DOI] [PubMed] [Google Scholar]
- Maestri D, Martínez M, Bodoira R, Rossi Y, Oviedo A, Pierantozzi P, Torres M. Variability in almond oil chemical traits from traditional cultivars and native genetic resources from Argentina. Food Chem. 2015;170:55–61. doi: 10.1016/j.foodchem.2014.08.073. [DOI] [PubMed] [Google Scholar]
- Martínez M, Mattea M, Maestri D. Varietal and crop year effects on lipid composition of walnut (Juglans regia L.) genotypes. J Am Oil Chem Soc. 2006;83:791–796. doi: 10.1007/s11746-006-5016-z. [DOI] [Google Scholar]
- Mateos R, Uceda M, Aguilera MP, Escuderos ME, Beltrán Maza G. Relationship of Rancimat method values at varying temperatures for virgin olive oils. Eur Food Res Technol. 2006;223:246–252. doi: 10.1007/s00217-005-0185-9. [DOI] [Google Scholar]
- Michel T, Khlif I, Kanakis P, Termentzi P, Allouche N, Halabalaki M, Skaltsouni A. UHPLC–DAD–FLD and UHPLC–HRMS/MS based metabolic profiling and characterization of different Olea europaea organs of Koroneiki and Chetoui varieties. Phytochem Lett. 2015;11:424–439. doi: 10.1016/j.phytol.2014.12.020. [DOI] [Google Scholar]
- Moghaddam G, Vander Heyden Y, Rabiei Z, Sadeghi N, Oveisi MR, Jannat B, Shokoufeh Hassani S, Behzad M, Hajimahmoodi M. Characterization of different olive pulp and kernel oils. J Food Comp Anal. 2012;28:54–60. doi: 10.1016/j.jfca.2012.06.008. [DOI] [Google Scholar]
- Nergiz C, Çelikkale D. The effect of consecutive steps of refining on squalene content of vegetable oils. J Food Sci Technol. 2011;48:382–385. doi: 10.1007/s13197-010-0190-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nergiz C, Engez Y. Compositional variation of olive fruit during ripening. Food Chem. 2000;69:55–59. doi: 10.1016/S0308-8146(99)00238-1. [DOI] [Google Scholar]
- Park CS, Marx GD, Moon YS, Wiesenborn D, Chang KC, Hofman VL. Alternative uses of sunflower. In: Schneiter AA, editor. Sunflower technology and production. Madison: Wiley; 1997. pp. 765–808. [Google Scholar]
- Pérez-Bonilla M, Salido S, van Beek TA, de Waar P, Linares-Palomino PJ, Sánchez A, Altarejos J. Isolation of antioxidative secoiridoids from olive wood (Olea europaea L.) guided by on-line HPLC–DAD–radical scavenging detection. Food Chem. 2011;124:36–41. doi: 10.1016/j.foodchem.2010.05.099. [DOI] [Google Scholar]
- Primo Yúfera E. Química de los Alimentos. Madrid: Editorial Alhambra; 1998. [Google Scholar]
- Ranalli A, Pollastri L, Contento S, Di Loreto G, Iannucci E, Lucera L, Russi F. Sterol and alcohol components of seed, pulp and whole olive fruit oils. Their use to characterise olive fruit variety by multivariates. J Agric Food Chem. 2002;50:3775–3779. doi: 10.1021/jf011506j. [DOI] [PubMed] [Google Scholar]
- Ranalli F, Ranalli A, Contento S, Casanovas M, Antonucci M, Di Simone G. Concentrations of bioactives and functional factors in destoned virgin olive oil: the case study of the oil from olivastra di Seggiano cultivar. J Pharm Nutr Sci. 2012;2:83–93. [Google Scholar]
- Reyes-Caudillo E, Tecante A, Valdivia-López MA. Dietary fibre content and antioxidant activity of phenolic compounds present in Mexican chia (Salvia hispanica L.) seeds. Food Chem. 2008;107:656–663. doi: 10.1016/j.foodchem.2007.08.062. [DOI] [Google Scholar]
- Rodríguez G, Lama A, Rodríguez R, Jiménez A, Guillén R, Fernández-Bolaños J. Olive stone an attractive source of bioactive and valuable compounds. Bioresour Technol. 2008;99:5261–5269. doi: 10.1016/j.biortech.2007.11.027. [DOI] [PubMed] [Google Scholar]
- Sanchez-Rodriguez E, Lima-Cabello E, Biel-Glesson S, Fernandez-Navarro JR, Calleja MA, Roca M, Espejo-Calvo JA, Gil-Extremera B, Soria-Florido M, de la Torre R, Fito M, Covas M, Alche J, Martinez de Victoria E, Gil A, Mesa MD. Effects of virgin olive oils differing in their bioactive compound contents on metabolic syndrome and endothelial functional risk biomarkers in healthy adults: a randomized double-blind controlled trial. Nutrients. 2018;10:1–17. doi: 10.3390/nu10050626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servili M, Baldioli M, Selvaggini R, Macchioni A, Montedoro G. Phenolic compounds of olive fruit: one- and two-dimensional nuclear magnetic resonance characterization of nüzhenide and its distribution in the constitutive parts of fruit. J Agric Food Chem. 1999;47:12–18. doi: 10.1021/jf9806210. [DOI] [PubMed] [Google Scholar]
- Tanahashi T, Takenaka Y, Akimoto M, Okuda A, Kusunoki Y, Suekawa C, Nagakura N. Six secoiridoid glucosides from Jasminum polyanthum. Chem Pharm Bull. 1997;45:367–372. doi: 10.1248/cpb.45.367. [DOI] [Google Scholar]
- Tanilgan K, Özcan M, Ünver A. Physical and chemical characteristics of five Turkish olive (Olea europea L.) varieties and their oils. Grasas Aceites. 2007;58:142–147. [Google Scholar]
- Varadharaj S, Kelly OJ, Khayat RN, Kumar PS, Ahmed N, Zweier JL. Role of dietary antioxidants in the preservation of vascular function and the modulation of health and disease. Front Cardiovasc Med. 2017;4:1–11. doi: 10.3389/fcvm.2017.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanasundara JPD. Proteins of Brassicaceae oilseeds and their potential as a plant protein source. Crit Rev Food Sci Nutr. 2011;51:635–677. doi: 10.1080/10408391003749942. [DOI] [PubMed] [Google Scholar]
- Zafra A, M’rani-Alaoui M, Lima E, Jiménez-López JC, Alché JD. Histological features of the olive seed and presence of 7S-type seed storage proteins as hallmarks of the olive fruit development. Front Plant Sci. 2018;9:1–15. doi: 10.3389/fpls.2018.01481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zienkiewicz A, Zienkiewicz K, Rejón JD, Alché JD, Castro AJ, Rodríguez-García MI. Olive seed protein bodies store degrading enzymes involved in mobilization of oil bodies. J Exp Bot. 2014;65:103–115. doi: 10.1093/jxb/ert355. [DOI] [PMC free article] [PubMed] [Google Scholar]