Abstract
In this work, mint essential oil (MEO) was added into gelatin films and antifungal activity was evaluated. Five concentrations of MEO (0, 0.06, 0.13, 0.25, 0.38, 0.50% (g/g gelatin)) were incorporated into gelatin solutions. The films were prepared by casting and characterized for their barrier properties, mechanical resistance, morphology, thermal and antifungal activity. The addition of oil into the solution slightly improved water vapor barrier, increased thickness and opacity, decreased transparency and modified thermal and mechanical properties of films. With addition of oil above 0.38%, the films were effective against the growth of Botrytis cinerea and Rhizopus stolonifer, indicating an inhibitory activity. Thus, gelatin-based edible films incorporated with MEO showed to be an effective way to inhibit microbial growth on the film surface.
Keywords: Edible film, Gelatin, Mint essential oil, Antifungal activity
Introduction
Recently, the interest in preserving food in a safe, natural and easy-to-prepare way has increased, as well as the worries to reduce the use of synthetic non-biodegradable polymers. Many materials can be used to produce biodegradable and/or edible films and coatings. Currently, bovine and fish proteins have been investigated as raw material for preparation of edible films as substitute for synthetic polymers due to their biocompatibility, biodegradability and good mechanical and barrier properties to gases, volatile compounds, oils and UV light (Kanmani and Rhim 2014; Tongnuanchan et al. 2016). However, these properties may change according to the coating solution formulation. Several authors report that bovine and porcine gelatin-based films show low deformation and high puncture force (Sobral et al. 2001). Gelatin-made edible coatings present low barrier to water vapor due to the hydrophilic nature of both gelatins and plasticizers used for producing the film (Limpisophon et al. 2010; Tongnuanchan et al. 2015). The use of active compounds in the preparation of edible coating films, such as plant essential oils with hydrophobic properties, can improve the barrier properties against water vapor. Different types of essential oils have been used in gelatin films such as bergamot, lemongrass, basil leaf and citronella essential oils (Ahmad et al. 2012; Arfat et al. 2014; Tongnuanchan et al. 2014, 2016). Mint essential oil (MEO) contains volatile components with flavonoids, organic acids and quinones. It has analgesic, anti-inflammatory and anti-spamodic properties and has been used for antimicrobial applications (Hussain et al. 2010). Kalemba and Kunicka (2003) and Guerra et al. (2015) reported the antimicrobial capacity of the coating of shrimp chitosan supplemented with Mentha piperita L. or Mentha × villosa Huds essencial oils against Aspergillus niger, Botrytis cinerea, Penicillium expansusm and Rhyzopus stolonifer, which were applied on the surface cherry tomato fruits. In addition, MEO can be used to improve the chemical and physical characteristics of the edible films (Guerra et al. 2015) thanks to its barrier property to hinder the development of fungi and avoid external and internal food contamination. This hydrophobic compound can also reduce water vapor permeability of films preventing food water loss during storage (Abbaszadeh et al. 2014; Guerra et al. 2015).
The present study aimed at production of gelatin-based edible films with different mint oil concentrations and characterization of their physical–chemical properties and effectiveness against B. cinerea and R. stolonifer.
Materials and methods
Materials
Bovine gelatin type B (Bloom 250, viscosity 34 mPa s, moisture 9%, ashes ≤ 2%, pH 5.8) was obtained from Gelnex (Itá, Santa Catarina, Brazil). Mint (Mentha arvensis) essential oil (MEO, approximately l-menthol = 40%, menthone = 24%, menthyl acetate = 4%, isomenthone = 10%) was acquired from Ferquima Indústria e Comercio Ltda (Vargem Grande, SP, Brazil), and glycerin (C3H8O3) and Tween 80 (C64H124O26) were supplied by Sigma Aldrich (St. Louis, Missouri, USA). The Rhizopus stolonifer (CBMAI 1551) strain was donated by Laboratory of Soil Microbiology, Federal University of Santa Catarina (Florianópolis, SC, Brazil) and the B. cinerea strain (CBMAI 863) was obtained from the Brazilian Collection of Environmental and Industrial Microorganisms—CBMAI/DRM, UNICAMP (Paulínia, SP, Brazil).
Preparation and formulation of coatings
Filmogenic solution formulations were adapted from Tongnuanchan et al. (2015) and were set as follows: gelatin 4% (m/v) was dissolved in distilled water under agitation for 20 min at 25 °C. Then, glycerol 0.13% (w/w gelatin) and Tween 80 0.06% (w/w gelatin) were added and the solution was incubated in a water bath at 80 °C for 10 min. Then, the solution was cooled to 25 °C, and mint essential oil was added in several concentrations: 0; 0.06; 0.13; 0.25; 0.38; 0.5% (w/w gelatin). The solutions were then homogenized using Ultra-Turrax T25 IKA-Werke (Steufen, Germany), at 14,500 rpm for 3 min. The air bubbles were removed under vacuum. The films were formed by pouring 20 g of the solution into 15 cm (ID) acrylic dishes, following drying at 25 °C for 24 h. The films were removed from the dishes, cut and stored under 58% RH at 25 °C for 48 h.
Thickness
Film thickness was determined using a digital micrometer (Mitutoyo, MDC-25P, Japan) with accuracy of 0.001 mm. The results were obtained by calculating the arithmetic mean of eight random measurements from the surface of each sample.
Opacity
Opacity was determined according to Hunterlab method using the colorimeter Ultra Scan Vis 1043 (Hunter Lab, Reston, USA) operating in the reflectance mode. The opacity values of each sample were calculated by software (Easy Match QC 3.83.00, EUA), as the relation between the opacity on the black standard and white standard in the CIELab* scale (Vanin et al. 2005; Sobral et al. 2005).
Transparency
Film samples with 1.0 cm × 4.0 cm were adapted on a glass cuvette with a 10 mm optical path. Transparency was measured by transmittance (%) at 500 nm using a spectrophotometer (SQ-2800 UV/VIS, UNICO, United Products & Instruments Inc., New Jersey, USA). Total transmittance (100% T) was determined using the same cuvette with no film attached (Tang et al. 2005).
Water vapor permeability (WVP)
WVP was determined by gravimetric analysis at 25 °C (Bertan et al. 2005) according to the method ASTM E96/E96M-5. Diffusion capsules filled with previously dried silica gel (0% RH) were covered with the film sample, sealed and stored in a chamber under 75% RH. The mass gain of each cell was determined by difference of weigh measured with an analytical balance (Shimadzu, AY220, Brasil) after 24 h of storage. Film permeability was calculated by Eq. 1:
| 1 |
where W is the mass gain (water) of the diffusion cell (g/h); L is the thickness of the film (m); A is the film area (m2); ps is the saturation pressure of water vapor (Pa) at the assay temperature. aw is water activity determined using a digital hygrometer (Aqualab Model -Series 3 TE, Decagon Devices, Inc., Pullman, USA) at 25 °C, and (aw1 − aw2) is the water activity difference between the external and internal environment, respectively.
Mechanical properties
The parameters of tensile strength (MPa) and strain (%) were determined using a universal texture analyzer with a 25 kg load cell, (TA-XT plus model, Stable Micro Systems, Surrey, England) according to ASTM D882-00 method (ASTM International 2003). Eight measurements were carried out for each sample (9.0 cm × 2.5 cm). The samples were fixed to a pair of grips on the TA-XT probe, with separation of 60 mm and test speed of 1 mm/s, pre- and post-test speeds 5 mm/s. Film thickness was measured before each analysis and taken at four random points using a digital micrometer (Mitutoyo, MDC-25P, Japan). The determinations were performed at 25 °C for 3 min, so that room conditions did not interfere with the results. From stress–strain curves the following parameters were calculated: (1) tensile strength was calculated as maximum stress, (2) strain, at break where the film is torn.
Morphology, chemical and thermal properties of films
Film morphology was analyzed by scanning electron microscopy (SEM, JEOL, JSM-6390LV, Japan). The film surface and cross section, fractured in liquid nitrogen, were analyzed with secondary electron sensor at an acceleration voltage of 15 kV and magnification of 1000× for the surface and 1500× for the cross section of the films. The films were previously cut into small rectangles of 1.0 cm by 0.5 cm and fixed in a metallic cylindrical support and covered by a thin layer of gold using a sputter coater (Bal-tec, SCD 0005, Japan).
Image analysis of the essential oil distribution on the film surface was performed using laser scanning confocal microscope model TCS SP5 (Leica Microsystems Inc. Wetzlar, Germany) captured with a 63× objective lens in oil immersion, laser set to green fluorescence mode, in a wavelength of 514 nm. The images were analyzed in Leica Application Suite software—LAS lite—TCS MP5 (Leica Microsystems inc. Wetzlar, Germany).
Prior to the formation of the films, the mint oil was stained with Nile Red (0.004 mg/10 mL of oil) until complete solubilization. Then, the films were produced following the method described earlier. For confocal microscope analysis the films were cut (diameter = 1.5 cm), placed onto a glass slide with a cover plate fixed to the sides with adhesive (Bertan et al. 2005). MEO droplet size was obtained with Leica Application Suite software—LAS lite—TCS MP5 (Leica Microsystems inc. Wetzlar, Germany) from mean droplet radius.
Interaction of the MEO in the matrix of films was assessed by infrared spectra of the films obtained using a FTIR (Agilent, model Carry 660, USA) spectrometer with a horizontal attenuated total reflectance (ZnSe) accessory (ATR). Samples were placed directly on the crystal and 20 scans were performed in the range of 4000–650 cm−1 and resolution of 4 cm−1 for each sample, as described by Tongnuanchan et al. (2015). The measurements of the samples were discounted by the background with air. Three replicates were performed for each film.
Thermal properties of the films were determined by Differential Scanning Calorimetry (DSC), using a TA-MDSC-2920 calorimeter (TA Instruments, New Castle, USA) with Refrigerated Cooling Accessory (RCS), operating with nitrogen gas at 150 mL/min. Calibration of the equipment was carried out with Indium (Tmelt = 156.6 °C), using helium as purge gas, with a constant flow rate of 25 mL/min. About 3 mg of sample were placed in a hermetically sealed aluminum capsules (20 μL) and analyzed using an empty capsule as a reference at a heating rate of 10 °C/min, in the temperature range of 0–180 °C. The same samples were analyzed in a second scan from 0 to 250 °C (10 °C/min) for eliminating the thermal history of the samples. The glass transition temperatures (Tg), melting points (Tmax) and enthalpies (ΔH) were determined only in the first heating scans, because the values obtained in the second scans were not relevant, since no changes were observed during heating. The analyses were performed in duplicates and the data were analyzed using the Universal Analysis 2.6 software (TA Instruments, New Castle, EUA) (Ma et al. 2012).
Contact angle and superficial free energy
Contact angles of films were measured by Tracker-S tensiometer equipment (Teclis Sarl, Longessaigne, France) following the method proposed by Rotta et al. (2009) with some modifications. Samples films (1 cm × 5 cm) were superimposed on a flat surface. One drop of 1 μL of the standard liquids (water, formamide and diiodomethane) was generated by a graduated microsyringe and applied separately on the surface of the films. The contact angle was calculated by the mean values of the left and right angles of the drop, from the digital image of the drop cross section. Measurements were performed for 30 s to avoid liquid absorption by the film at room temperature (23 °C). Four replicates were taken for each liquid in each film formulation. The surface free energy and its components (polar and dispersive) were calculated by the method of Owens and Wendt (1969) (Eqs. (2) and (3)):
| 2 |
| 3 |
where is the total free surface energy of the solid, is the surface free dispersive energy of the interface between liquid and solid (apolar), is the non-dispersive (polar) surface free energy of the liquid.
In Eq. (2), the surface free energy of the solid is described by using the angle θ, surface tension of the liquid γl and interfacial tension between solid and liquid γsl.
Antifungal assay
The antifungal activity of gelatin films with and without mint oil was evaluated in vitro against R. stolonifer and B. cinerea. A sample of 0.1 mL of the spore suspension (104 spores/mL) was spread on the surface of the PAD (Potato Agar Dextrose) medium. The films (diameter = 2.0 cm), pre-cut with a cylindrical blade, were positioned in the center of the plates, which were then incubated at 26 °C for 3 days for R. stolonifer and 26 °C for 5 days for B. cinerea. The antifungal activity of the films was determined by measuring the diameter of the zone of inhibition of fungal growth around the film with a Mitutoyo caliper (Kawasaki, Japan). The tests were performed in duplicate for each film (Perdones et al. 2014).
The antifungal activity of the oil was determined by the disk diffusion method described above according to Alexandre et al. (2016) with modifications. Sterile filter paper (diameter = 2.0 cm) impregnated with 5 μL of MEO was used. Disks impregnated with soybean oil were used as positive control and absolute ethanol as negative control. This analysis aimed to characterize the oil used for its antifungal activity against B. cinerea and R. stolonifer.
Statistical analysis
The results were evaluated by Analysis of variance (ANOVA) using the software Statistica 10 (Stafsoft Inc., USA). The means were compared by Tukey´s test. The level of significance adopted was p < 0.05.
Results and discussion
Thickness, transparency and opacity
Thickness, transparency and opacity of gelatin films incorporated with different concentrations of MEO are presented in Table 1. Films thickness increased with incorporation of oil into edible solution when compared to control films (without MEO), varying from 41.0 to 44.0 µm. However, no difference (p > 0.05) in thickness was observed between the lowest oil concentrations (0.06, 0.13 and 0.25%) and control films. Actually, the significant differences (p < 0.05) in comparison to control films were observed only in samples containing the highest oil concentrations (0.38 and 0.50%). The variation of film thickness can be related to the increase of solids content or due to peptide chains in gelatin being unable to form a compact film network in the presence of oil, as suggested by Ahmad et al. (2012) and Tongnuanchan et al. (2015), who reported similar results for fish gelatin films incorporated with oils at different concentrations.
Table 1.
Thickness, opacity, transparency, water vapor permeability (WVP), tensile strength, elongation and Young’s modulus of the gelatin films incorporated with mint essential oil (MEO) in different concentrations
| Formulations (% MEO) |
Thickness (µm) |
Opacity (%) |
Transparency (%) |
WVP (10−4 g mm/m2 Pa h) |
Tensile strength (MPa) |
Elongation (%) |
Young’s modulus (MPa) |
|---|---|---|---|---|---|---|---|
| Control (0) | 37.0 ± 4.0a | 11.0 ± 1.0a | 83.3 ± 1.1a | 1.55 ± 0.08a | 41.0 ± 2.0a | 13.3 ± 2.2ac | 1596 ± 60a |
| A1 (0.06) | 41.0 ± 1.3ab | 13.0 ± 1.0b | 82.0 ± 1.0ab | 1.12 ± 0.02b | 32.1 ± 3.0b | 18.1 ± 3.0b | 1118 ± 98bc |
| A2 (0.13) | 42.0 ± 3.3ab | 13.1 ± 1.0b | 81.2 ± 1.2ab | 1.11 ± 0.06b | 32.4 ± 2.0b | 17.0 ± 2.3ab | 1187 ± 106bc |
| A3 (0.25) | 43.0 ± 2.1ab | 14.0 ± 1.0b | 80.0 ± 1.0bc | 1.10 ± 0.07b | 32.0 ± 3.0b | 15.2 ± 2.0ab | 1258 ± 111b |
| A4 (0.38) | 43.0 ± 2.1b | 16.0 ± 1.0c | 77.4 ± 2.0cd | 1.46 ± 0.01a | 29.0 ± 2.1bc | 11.1 ± 2.0c | 1047 ± 82c |
| A5 (0.50) | 44.0 ± 2.0b | 18.0 ± 1.0c | 76.3 ± 1.3d | 1.51 ± 0.06a | 27.0 ± 3.0c | 10.0 ± 1.3c | 1048 ± 117c |
Equal superscript letters in the columns for the same parameter indicate that the samples do not differ significantly (p > 0.05)
On the other hand, the transparency of films containing MEO decreased when compared to the control film. This reduction (p < 0.05) was observed for samples containing 0.25% or higher concentrations of MEO in the formulation. Ahmad et al. (2012) reported that the incorporation of different concentrations of mandarin and citronella essential oils in gelatin films did not increase the transparency. Rauwendaal (2001), cited by Ahmad et al. (2012), reported that optical properties of materials can be significantly modified by the heterogeneity of material composition and can significantly modify its transparency. Pires et al. (2011) showed that the addition of thyme oil into gelatin films resulted in less transparent films. The authors pointed out, however, that films containing oil were clear enough to be used as films or food coating material. The transparency reduction was also reported by Tongnuanchan et al. (2014) and Tongnuanchan et al. (2015) in studies with fish-based gelatin incorporated with basil and citronella essential oils and palm oil, respectively.
Film optical properties are attributes that can influence the product aspect, commercial value and the adequacy for several applications (Ahmad et al. 2012). The opacity of films increased with the increase in oil concentration, ranging from 13.0 to 18.0%, consequently increasing the opacity of gelatin films. Several studies focused on gelatin films also described an increment in opacity in the presence of essential oils. Kavoosi et al. (2014) characterized gelatin films added with Zataria essential oil and observed an enhancement to opacity with the increase of oil concentration. The same was reported by Ma et al. (2012) who observed an increase in gelatin films opacity when different concentrations of essential oil were added, due to the color of the oil.
Water vapor permeability (WVP)
Water vapor permeabilities (WVP) of films incorporated with different concentrations of MEO are presented in Table 1. Gelatin films showed low barrier to water vapor due their hydrophilic characteristic (Dangaran et al. 2009). Significant reductions (p < 0.05) of WVP were obtained between oil concentrations of 0.06, 0.13 and 0.25% and the control film. However, WVP of samples containing 0.38 and 0.50% MEO did not differ (p > 0.05) from films without MEO. This behavior can be due to heterogeneous dispersion of MEO in the film, forming oil agglomerates, which were observed by scanning electron and confocal scanning laser microscopy (Fig. 1). At 0.06, 0.13 and 0.25% MEO, WVP was lower due to better oil droplet distribution within the polymeric matrix. Thus, microstructure of films, droplet size and lipid distribution in the film matrix are important characteristics to understand the water barrier properties of films (Ma et al. 2012). Pires et al. (2011) characterized fish-based gelatin films incorporated with 5–20% of essential oils of citronella, cilantro, tarragon and thyme. The authors showed the addition of essential oil in the films caused a reduction in water vapor permeability. Nevertheless, they also observed an increase in WVP for films containing higher amounts (10 and 15%) of essential oils. Ahmad et al. (2012) also described an increase in WVP for gelatin films added with mandarin oil. This behavior was attributed to the difference in the hygroscopic nature of the oils used what caused changes in the affinity of water molecules with the film polymeric network.
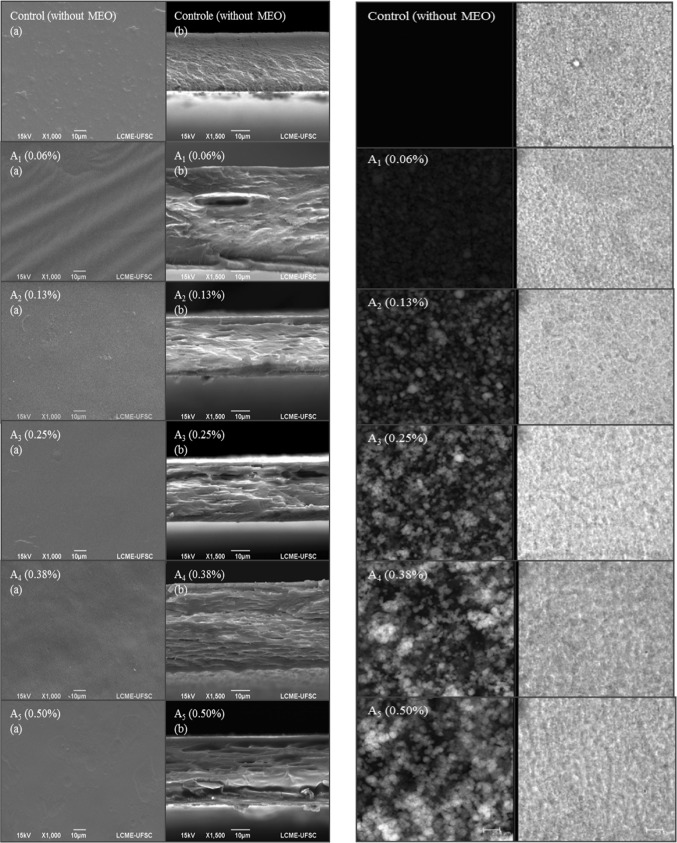
Fig. 1.
Scanning electron (left) and confocal scanning laser microscopy (right) micrographs of the gelatin films incorporated with mint essential oil (MEO) at different concentrations. Surface (a) and cross-section fracture (b). Size bars in confocal scanning laser microscopy represent 25 μm
Mechanical properties
Mechanical properties of gelatin film added of MEO are shown in Table 1. Tensile strength of films with oil decreased significantly (p < 0.05) from 32 to 27 MPa. Films containing lower oil concentration (0.06, 0.13%) showed a significant (p < 0.05) increment on elongation at break values. These results corroborate with Young’s modulus, which decreased significantly (p < 0.05) with increase of oil concentration, indicating that the films became less rigid than the control film, as Young’s modulus display the intrinsic rigidity of films (Kanmani and Rhim 2014).
The mechanical properties at higher amount of essential oil showed the further increase in MEO concentration resulted in more fragile films, less rigid and less resistant, consistent with the results of water vapor permeability (Table 1) and heterogeneous distribution of lipid in the matrix of these films, according to photomicrographs (Fig. 1). The results are in accordance with Ahmad et al. (2012) and Kavoosi et al. (2014), who reported that increasing the concentration of different essential oils in gelatin films caused reductions in tensile resistance, elongation and Young’s modulus.
Lower MEO concentrations (0.06, 0.13 and 0.25%) resulted in an increase of film flexibility as result of resistance to traction and Young´s modulus, with simultaneous increase in elongation, characterizing the plasticizer effect of MEO on film matrix at these concentrations.
Morphology, chemical and thermal properties of films
Film microstructure obtained by SEM is presented by the micrographs in Fig. 1 (left). Film without MEO showed a smooth surface, with presence of air microbubbles that probably were not removed from the solution by vacuum treatment. Cross-sections of film without MEO (Fig. 1b) show a more homogeneous, compact structure, slightly rough, and without aggregates, compared to the films added with MEO. Other studies (Ahmad et al. 2012; Tongnuanchan et al. 2014, 2015; Martucci et al. 2015) analyzing pure gelatin films also showed the same morphological characteristics of gelatin films found in the present study.
The films incorporated with MEO showed smoother and homogeneous surfaces, when compared to control, except the film containing 0.50% of oil, which showed coalescence regions. This is probably due to the non-homogeneous distribution of MEO within the film protein matrix. Samples with lower concentrations of MEO (0.06, 0.13 and 0.25%) showed structures less rough and more compact, suggesting a better incorporation MEO within the film matrix.
Confocal laser scanning microscopy was also used as tool to evaluate MEO dispersion in the film matrix. Increase in yellow fluorescence due to the presence of essential oil can be evidenced in Fig. 1 (right). Control film images (without MEO) do not show any yellow color. We did not observe any MEO drop aggregates in gelatin films with 0.06, 0.13 and 0.25% of oil, confirming results observed by SEM images. Droplet size of MEO was lower than 9 µm. For films containing 0.38 and 0.50% of MEO, aggregation in the matrix varied from 5 to 18 µm. These results are in accordance with SEM observations.
All the films presented FT-IR spectra with the main characteristic peaks of proteins. The peak amplitude varied with the concentration of MEO (Fig. 2). Bands at 1045 cm−1 were found in all film samples, probably due to the interaction of the protein (–CH2 of amino acid residue of gelatin molecules) and plasticizer (OH group of glycerol) (Bergo and Sobral 2007). The amplitude of these peaks increased with the incorporation of the different concentrations of MEO in the films, showing no effect of dilution of the glycerol by the MEO, as reported by Tongnuanchan et al. (2015).
Fig. 2.
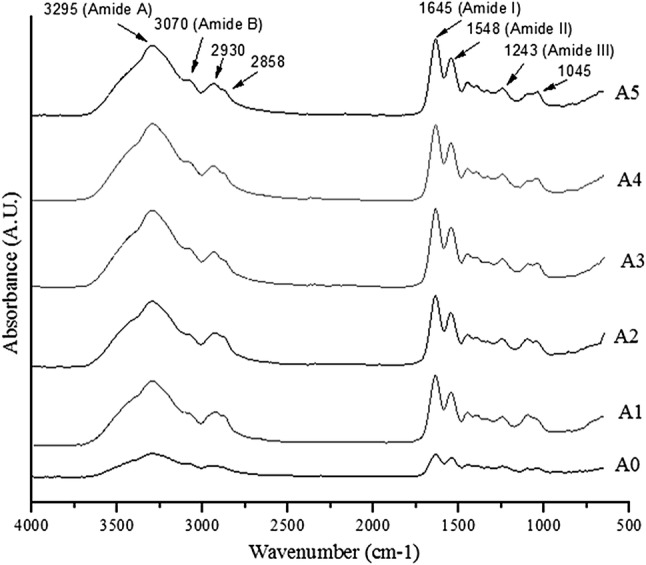
Infrared spectra of the gelatin films incorporated with mint essential oil (MEO) at different concentrations
The spectra of the films also showed bands in the amide region at 1645 cm−1 (Amide I), 1548 cm−1 (Amide II), 1243 cm−1 (Amide III), 3295 cm−1 (Amide A), 3070 cm−1 (Amide B) (Bergo and Sobral 2007; Ahmad et al. 2012; Tongnuanchan et al. 2015). The amplitudes of Amide I, II, and III were smaller for the control film compared to films incorporated with MEO, while Amide A and B bands were not shifted by the addition of MEO. Shift in amide bands and changes in amplitude of such bands are reported when adding palm oil and cinnamon oil in gelatin films (Tongnuanchan et al., 2015, Bahram et al., 2014) and attributed to the interaction of the protein with the oil compounds. The addition of MEO in the films probably caused less intermolecular interaction among the components of the formulations during the film drying process (Ahmad et al. 2012), correlating with the reduction in tensile strength values of the films added of oil, as shown later in this paper. Wu et al. (2015) also found similar Amide I, II and III spectra for fish gelatin films incorporated with cinnamon essential oil nanoliposomes, at wavenumber values of 1642 cm−1, 1552 cm−1 and 1237 cm−1, respectively, and observed that amplitudes of Amides I, II and III were higher for the film with oil nanoliposomes.
Bands at 2858 cm−1 and 2930 cm−1 were also observed in all samples of the films in the present study, with higher intensity and area in the films added with MEO. These bands can be attributed to the asymmetric and symmetric stretching vibrations of C–H aliphatic type in CH2 and CH3 groups, respectively. These bands showed greater intensity in the presence of hydrophobic components, such as terpenoids. Thus, films containing different concentrations of MEO indicated the presence of hydrocarbons that cause vibrations in these peaks, consequently altering the molecular organization and intermolecular interaction in the matrix of the film (Ahmad et al. 2012; Guerra et al. 2015; Tongnuanchan et al. 2016).
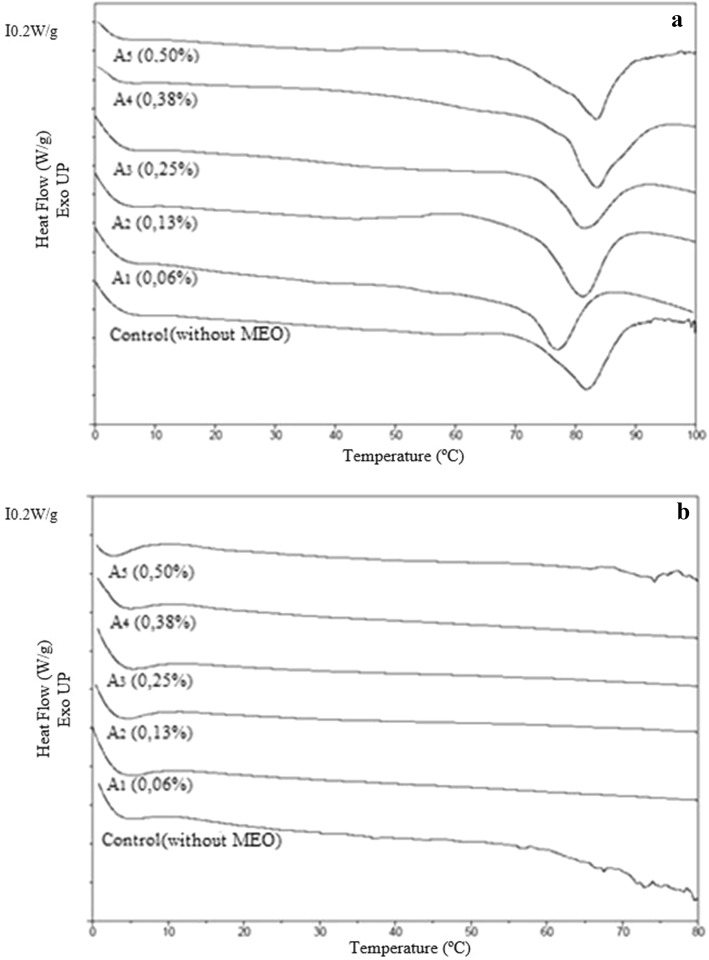
DSC thermograms of gelatin film samples, of the first and second heating steps, are illustrated in Fig. 3. The thermograms of all samples in the first heating exhibited glass transition temperatures (Tg), melting temperatures (Tmax) and enthalpies (ΔH), which are summarized in Table 2. In the second heating, no phase changes or any melting event was observed. The film with the highest concentration of MEO (0.50%) showed the highest Tg value (27.0 °C), while the lowest Tg (17.0 °C) was found for the sample with lower concentration of MEO (0.06%). There was no significant difference (p > 0.05) between the samples with MEO when compared to the control sample without MEO (22.02 °C). Tg is related to the displacement of polymer chain segments from amorphous materials. This transition is important and influences the properties of materials and potential applications, thus helping to explain the physical and chemical behavior of the material (Perdomo et al. 2009; Nagarajan et al. 2012; Tongnuanchan et al. 2016).
Fig. 3.
Thermograms of the first heating (a) and second heating (b) of the gelatin films incorporated with mint essential oil (MEO) at different concentrations
Table 2.
Glass transition temperature (Tg), melt temperature (Tmax), fusion enthalpy (ΔH), surface free energy, polar component and dispersive component of the gelatin films incorporated with mint essential oil (MEO) at different concentrations
| Formulations (% MEO) |
Tg (°C) |
Tmax (°C) |
ΔH (J/g) |
Surface free energy (mN m−1) |
Polar component (mN m−1) |
Dispersive component (mN m−1) |
|---|---|---|---|---|---|---|
| Control (0) | 22.3 ± 2.0ab | 82.0 ± 0.1ab | 14.4 ± 1.4a | 59.2 ± 0.3a | 17.0 ± 0.1a | 43.0 ± 0.1a |
| A1 (0.06%) | 17.0 ± 1.0a | 80.2 ± 2.0a | 14.0 ± 0.1a | 59.0 ± 0.3a | 13.3 ± 0.5b | 45.3 ± 0.2b |
| A2 (0.13%) | 22.0 ± 3.0ab | 81.0 ± 0.4ab | 15.0 ± 1.0ab | 58.0 ± 0.1b | 12.0 ± 0.1c | 46.1 ± 0.2c |
| A3 (0.25%) | 24.2 ± 1.0ab | 81.0 ± 0.4ab | 16.0 ± 2.0ab | 56.2 ± 0.3c | 11.0 ± 0.4d | 45.4 ± 0.2b |
| A4 (0.38%) | 22.0 ± 2.0ab | 83.4 ± 0.1b | 17.2 ± 0.3ab | 55.1 ± 0.2d | 11.0 ± 0.3d | 44.4 ± 0.2d |
| A5 (0.50%) | 27.0 ± 2.0b | 83.1 ± 0.3ab | 18.4 ± 0.4b | 57.0 ± 0.1c | 8.3 ± 0.2e | 48.3 ± 0.1e |
Equal superscript letters in the columns for the same parameter indicate that the samples do not differ significantly (p > 0.05)
The gelatin films added of different concentrations of MEO presented Tg close to the control film. This suggests that test concentration of MEO was too low to increase the chain mobility. The decrease of Tg could explain, in part, the reduction in the strength and stiffness values of the gelatin films incorporated with the MEO. Tongnuanchan et al. (2014) reported lower values of Tg for fish gelatin films incorporated with basil and citronella oils, compared to the control film, independently of the surfactant used. The authors explained that the Tg of gelatin films increases with increasing chain stiffness, as measured by attractive inter/intramolecular forces, and that the weaker structure of the film was developed when essential oils were incorporated, causing a decrease in mechanical strength of films. The authors further explained that better dispersion and greater stability of the oil droplets, regulated by the surfactants, could interfere in the inter/intramolecular interaction of the protein network.
The melting temperature of the control gelatin film was characterized by an endothermic peak at 82.0 °C, caused by the melting of the crystal structure of a triple helix of gelatin, as well as the range of other types of ordered molecular structures (Sobral and Habitante 2001; Ma et al. 2012; Staroszczyk et al. 2012). During the drying stage of gelatin films, they undergo a renaturation, changing into a more regular structure (Rahman et al. 2008; Tongnuanchan et al. 2016). The incorporation of the different concentrations of MEO in the matrix of the films resulted in a small increase in the melting temperature (Tmax) of the samples containing 0.38% and 0.50% of oil, showing no significant difference (p > 0.05) compared to the control sample. This transition is associated with the rupture of ordered or aggregate molecular structure (Tang et al. 2005; Tongnuanchan et al. 2016). This displacement of the Tmax may be associated with the lower water content of these samples (Guerrero and De La Caba 2010). Similar results for fish gelatin films with essential oils were reported by Ma et al. (2012) and Tongnuanchan et al. (2016).
The control film (without OEM) presents a lower fusion enthalpy (ΔH) of 14.4 J/g (p < 0.05) than the film with the highest concentration of MEO (0.50%) (ΔH = 18.4 J/g). For the other samples, the enthalpy values did not present a significant difference (p > 0.05) compared to the control film. The higher fusion enthalpy suggests better ordination among the components (gelatin, oil, water, plasticizer) in the structures of the films, which need to be overcome to achieve fusion. The opposite behavior was reported in the study of Tongnuanchan et al. (2016), who showed fish gelatin films incorporated with both citronella or basil oils required lower enthalpies to break the interactions among chains. They also explained the addition of essential oils in high amounts may increase the amorphous phase and decrease the ordered phase, thus enhancing the molecular mobility.
Contact angle and surface free energy
Figure 4 shows the contact angles of the standard liquids: water, formamide and diiodomethane, dispensed on the surfaces of the films added with different concentrations of MEO. The contact angle is an indirect measurement of hydrophilicity or hydrophobicity and wettability of films (Su et al. 2010). Usually, films that have an angle of contact with water greater than 65° are considered as hydrophobic surfaces (Vogler 1998).
Fig. 4.
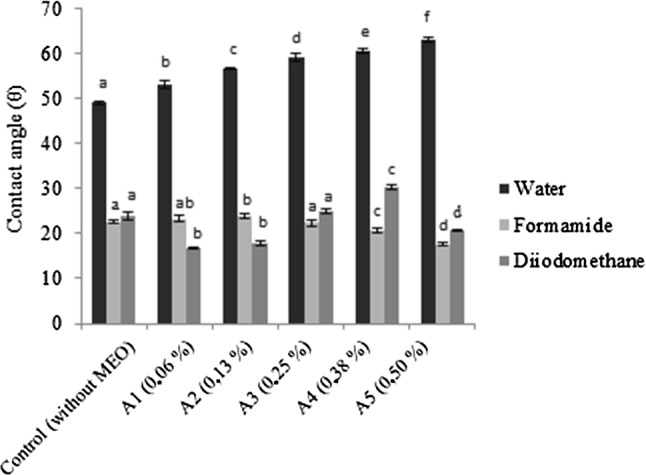
Contact angles of water, formamide and diiodomethane on the surface of gelatin films incorporated with mint essential oil (MEO) at different concentrations. Equal letters in the columns for the same parameter mean that the samples did not differ significantly (p > 0.05)
Compared with the control film, the addition of different concentrations of MEO has shown a significant effect (p < 0.05) on the water contact angle (polar). The contact angles of the water with the films varied from 49.0° to 63.1°, indicating an increase in the hydrophobic character of the films with the increase of the oil concentration incorporated into them. The lower contact angles were found using formamide (24.0°–17.4°) and diiodomethane (30.2°–17.0°). The former is a liquid of intermediate polarity between water and diiodomethane. The lowest contact angle of the water with the control film (without MEO) can be attributed to the water binding capacity with plasticizer (glycerol) and functional groups in gelatin (Ojagh et al. 2010). The hydrophobicity of the MEO was an important factor for the increase of contact angles with water, decrease in hydrophilicity, and thus, decrease in the WVP values, as observed previously (Sect. 3.2).
Table 2 shows the surface free energy and the polar and dispersive components for the films. The surface free energy values of the polar components of the films were lower than the values of the dispersive components. This may characterize the films as hydrophobic. The contact angles indicated that the water (polar component) was the liquid that presented less interaction with the films. When the film is more hydrophilic, the polar component of the surface free energy is higher, while the dispersive component (non-polar) of the surface free energy becomes lower (Rotta et al. 2009; Mihaly Cozmuta et al. 2015). In the present study, gelatin films added with MEO showed hydrophobic characteristics, since the increase in MEO concentrations in the formulations caused a decrease in the polar component from 17.0 to 8.3 mN m−1 and increased the dispersive component from 43.0 to 48.3 mN m−1. It is worth mentioning these results agree with the lower values of the water vapor permeabilities of the films incorporated with MEO.
Antifungal capacity
The pure MEO inhibited completely the growth of fungus during the period of 15 days, thus confirming antifungal activity against B. cinerea and R. stolonifer. When MEO was added in the film at various concentrations, antifungal activity against B. cinerea and R. stolonifer was observed at 0.38 and 0.50% MEO in films, with an inhibition halo of 2.0 cm in diameter (Table 3). For MEO concentrations lower than 0.38% no inhibition was observed. Despite the lack of researches about the use of MEO in edibles films or coatings in the literature, antifungal activity of the MEO could be attributed to menthol. According to Abbaszadeh et al. (2014), pure menthol effectively inhibited B. cinerea and Rhizopus oryzae PTCC 5174. Kazemi et al. (2012) showed the MEO has strong effectiveness against pathogenic fungus. Moleyar and Narasimham (1986) used essential oil components to inactivate fungal such as A. niger, R. stolonifer and Mucor spp. and showed the menthol was most inhibitory to R. stolonifer. In the MEO used in this work, menthol was the major component (41%), according to the technical report of the supplier.
Table 3.
Antifungal activity against B. cinerea and R. stolonifer, measured by the halo of inhibition technique, for gelatin films incorporated with mint essential oil (MEO) at different concentrations
| Formulations (% MEO) |
B. cinerea
(cm) |
R. stolonifer
(cm) |
|---|---|---|
| Control (0) | – | – |
| A1 (0.06%) | – | – |
| A2 (0.13%) | – | – |
| A3 (0.25%) | – | – |
| A4 (0.38%) | 2.0 ± 0.1a | 2.0 ± 0.1a |
| A5 (0.50%) | 2.0 ± 0.1a | 2.0 ± 0.1a |
Equal superscript letters in the columns for the same parameter indicate that the samples do not differ significantly (p > 0.05)
–No presence of inhibition halo
Conclusion
Gelatin films added with lower concentrations of mint essential oil (MEO) were prepared in this work. The addition of concentrations equal or higher than 0.38% of MEO in the filmogenic solution yielded gelatin films with effective antifungal activity against B. cinerea and R. stolonifer. The essential oil caused some changes in the physical–chemical characteristics of the films. The most relevant changes occurred in water vapor permeation (29% decrease in WVP at 0.25% MEO), as well as in tensile strength and Young’s module (up to 35% decrease in both parameters at 0.5% MEO). On the other hand, films with higher amounts of MEO were more effective against the fungi growth. Thus, the choice of the film formulation for posterior studies or application should considered a balance between physical–chemical and antifungal characteristics. Hence, based on our results, we can conclude the 0.38% of MEO is the most promising concentration for application in the preparation of gelatin films, which may later be used as a protective edible film on fruits and vegetables.
Acknowledgements
Authors acknowledge National Council of Research and Technological Development (CNPq) for the scholarship provided to L. Scartazzini, Gelnex (Itá, Santa Catarina, Brazil) for kindly supplying gelatin samples. Research Foundation of State Santa Catarina is acknowledged for financial support (FAPESC). Central Laboratory of Electron Microscopy (LCME/UFSC) is acknowledged for SEM and laser confocal microscopy analyses.
Funding
Research Foundation of State Santa Catarina for financial support (FAPESC)-Grant Number 17.289/2009-8
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abbaszadeh S, Sharifzadeh A, Shokri H, et al. Antifungal efficacy of thymol, carvacrol, eugenol and menthol as alternative agents to control the growth of food-relevant fungi. J Mycol Med. 2014;24:e51–e56. doi: 10.1016/j.mycmed.2014.01.063. [DOI] [PubMed] [Google Scholar]
- Ahmad M, Benjakul S, Prodpran T, Agustini TW. Physico-mechanical and antimicrobial properties of gelatin film from the skin of unicorn leatherjacket incorporated with essential oils. Food Hydrocoll. 2012;28:189–199. doi: 10.1016/j.foodhyd.2011.12.003. [DOI] [Google Scholar]
- Alexandre EMC, Lourenço RV, Bittante AMQB, et al. Gelatin-based films reinforced with montmorillonite and activated with nanoemulsion of ginger essential oil for food packaging applications. Food Packag Shelf Life. 2016;10:87–96. doi: 10.1016/j.fpsl.2016.10.004. [DOI] [Google Scholar]
- Arfat YA, Benjakul S, Prodpran T, et al. Properties and antimicrobial activity of fish protein isolate/fish skin gelatin film containing basil leaf essential oil and zinc oxide nanoparticles. Food Hydrocoll. 2014;41:265–273. doi: 10.1016/j.foodhyd.2014.04.023. [DOI] [Google Scholar]
- ASTM International Standard test method for tensile properties of plastics. ASTM Int. 2003;08:46–58. [Google Scholar]
- Bahram S, Rezaei M, Soltani M, et al. Whey protein concentrate edible film activated with cinnamon essential oil. J Food Process Preserv. 2014;38:1251–1258. doi: 10.1111/jfpp.12086. [DOI] [Google Scholar]
- Bergo P, Sobral PJA. Effects of plasticizer on physical properties of pigskin gelatin films. Food Hydrocoll. 2007;21:1285–1289. doi: 10.1016/j.foodhyd.2006.09.014. [DOI] [Google Scholar]
- Bertan LC, Tanada-Palmu PS, Siani AC, Grosso CRF. Effect of fatty acids and “Brazilian elemi” on composite films based on gelatin. Food Hydrocoll. 2005;19:73–82. doi: 10.1016/j.foodhyd.2004.04.017. [DOI] [Google Scholar]
- Dangaran K, Tomasula PM, Qi P. Structure and function of protein-based edible films and coatings. Int Edible Films Coat Food Appl. 2009 [Google Scholar]
- Guerra ICD, de Oliveira PDL, de Souza Pontes AL, et al. Coatings comprising chitosan and Mentha piperita L. or Mentha × villosa Huds essential oils to prevent common postharvest mold infections and maintain the quality of cherry tomato fruit. Int J Food Microbiol. 2015;214:168–178. doi: 10.1016/j.ijfoodmicro.2015.08.009. [DOI] [PubMed] [Google Scholar]
- Guerrero P, De La Caba K. Thermal and mechanical properties of soy protein films processed at different pH by compression. J Food Eng. 2010;100:261–269. doi: 10.1016/j.jfoodeng.2010.04.008. [DOI] [Google Scholar]
- Hussain AI, Anwar F, Nigam PS, et al. Seasonal variation in content, chemical composition and antimicrobial and cytotoxic activities of essential oils from four mentha species. J Sci Food Agric. 2010;90:1827–1836. doi: 10.1002/jsfa.4021. [DOI] [PubMed] [Google Scholar]
- Kalemba D, Kunicka A. Antibacterial and antifungal properties of essential oils. Curr Med Chem. 2003;10:813–829. doi: 10.2174/0929867033457719. [DOI] [PubMed] [Google Scholar]
- Kanmani P, Rhim JW. Physical, mechanical and antimicrobial properties of gelatin based active nanocomposite films containing AgNPs and nanoclay. Food Hydrocoll. 2014;35:644–652. doi: 10.1016/j.foodhyd.2013.08.011. [DOI] [Google Scholar]
- Kavoosi G, Rahmatollahi A, Mohammad Mahdi Dadfar S, Mohammadi Purfard A. Effects of essential oil on the water binding capacity, physico-mechanical properties, antioxidant and antibacterial activity of gelatin films. LWT Food Sci Technol. 2014;57:556–561. doi: 10.1016/j.lwt.2014.02.008. [DOI] [Google Scholar]
- Kazemi M, Rostami H, Shafiei S. Antibacterial and antifungal activity of some medicinal plants from Iran. J Plant Sci. 2012;7:55–66. doi: 10.3923/jps.2012.55.66. [DOI] [Google Scholar]
- Limpisophon K, Tanaka M, Osako K. Characterisation of gelatin-fatty acid emulsion films based on blue shark (Prionace glauca) skin gelatin. Food Chem. 2010;122:1095–1101. doi: 10.1016/j.foodchem.2010.03.090. [DOI] [Google Scholar]
- Ma W, Tang CH, Yin SW, et al. Characterization of gelatin-based edible films incorporated with olive oil. Food Res Int. 2012;49:572–579. doi: 10.1016/j.foodres.2012.07.037. [DOI] [Google Scholar]
- Martucci JF, Gende LB, Neira LM, Ruseckaite RA. Oregano and lavender essential oils as antioxidant and antimicrobial additives of biogenic gelatin films. Ind Crops Prod. 2015;71:205–213. doi: 10.1016/j.indcrop.2015.03.079. [DOI] [Google Scholar]
- Mihaly Cozmuta A, Turila A, Apjok R, et al. Preparation and characterization of improved gelatin films incorporating hemp and sage oils. Food Hydrocoll. 2015;49:144–155. doi: 10.1016/j.foodhyd.2015.03.022. [DOI] [Google Scholar]
- Moleyar V, Narasimham P. Antifungal activity of some essential oil components. Int Food Microbiol. 1986;3:331–336. doi: 10.1016/0740-0020(86)90017-1. [DOI] [PubMed] [Google Scholar]
- Nagarajan M, Benjakul S, Prodpran T, Songtipya P. Properties of film from splendid squid (Loligo formosana) skin gelatin with various extraction temperatures. Int J Biol Macromol. 2012;51:489–496. doi: 10.1016/j.ijbiomac.2012.06.017. [DOI] [PubMed] [Google Scholar]
- Ojagh SM, Rezaei M, Razavi SH, Hosseini SMH. Development and evaluation of a novel biodegradable film made from chitosan and cinnamon essential oil with low affinity toward water. Food Chem. 2010;122:161–166. doi: 10.1016/j.foodchem.2010.02.033. [DOI] [Google Scholar]
- Owens DK, Wendt RC. Estimation of the surface free energy of polymers. J Appl Polym Sci. 1969;13:1741–1747. doi: 10.1002/app.1969.070130815. [DOI] [Google Scholar]
- Perdomo J, Cova A, Sandoval AJ, et al. Glass transition temperatures and water sorption isotherms of cassava starch. Carbohydr Polym. 2009;76:305–313. doi: 10.1016/j.carbpol.2008.10.023. [DOI] [Google Scholar]
- Perdones Á, Vargas M, Atarés L, Chiralt A. Physical, antioxidant and antimicrobial properties of chitosan-cinnamon leaf oil films as affected by oleic acid. Food Hydrocoll. 2014;36:256–264. doi: 10.1016/j.foodhyd.2013.10.003. [DOI] [Google Scholar]
- Pires C, Ramos C, Teixeira G, et al. Characterization of biodegradable films prepared with hake proteins and thyme oil. J Food Eng. 2011;105:422–428. doi: 10.1016/j.jfoodeng.2011.02.036. [DOI] [Google Scholar]
- Rahman MS, Al-Saidi GS, Guizani N. Thermal characterisation of gelatin extracted from yellowfin tuna skin and commercial mammalian gelatin. Food Chem. 2008;108:472–481. doi: 10.1016/j.foodchem.2007.10.079. [DOI] [PubMed] [Google Scholar]
- Rauwendaal C. Polymer extrusion. 4. Cincinnati: Ohio Hanser Gardner Publications; 2001. [Google Scholar]
- Rotta J, Ozório RÁ, Kehrwald AM, et al. Parameters of color, transparency, water solubility, wettability and surface free energy of chitosan/hydroxypropylmethylcellulose (HPMC) films plasticized with sorbitol. Mater Sci Eng, C. 2009;29:619–623. doi: 10.1016/j.msec.2008.10.032. [DOI] [Google Scholar]
- Sobral PJA, Habitante AMQB. Phase transitions of pigskin gelatin. Food Hydrocoll. 2001;15:377–382. doi: 10.1016/S0268-005X(01)00060-1. [DOI] [Google Scholar]
- Sobral PJA, Menegalli FC, Hubinger MD, Roques MA. Mechanical, water vapor barrier and thermal properties of gelatin-based edible films. Food Hydrocoll. 2001;15:423–432. doi: 10.1016/S0268-005X(01)00061-3. [DOI] [Google Scholar]
- Sobral PJDA, Dos Santos JS, Garcia FT. Effect of protein and plasticizer concentrations in film forming solutions on physical properties of edible films based on muscle proteins of a Thai Tilapia. J Food Eng. 2005;70:93–100. doi: 10.1016/j.jfoodeng.2004.09.015. [DOI] [Google Scholar]
- Staroszczyk H, Pielichowska J, Sztuka K, et al. Molecular and structural characteristics of cod gelatin films modified with EDC and TGase. Food Chem. 2012;130:335–343. doi: 10.1016/j.foodchem.2011.07.047. [DOI] [Google Scholar]
- Su JF, Huang Z, Yuan XY, et al. Structure and properties of carboxymethyl cellulose/soy protein isolate blend edible films crosslinked by Maillard reactions. Carbohydr Polym. 2010;79:145–153. doi: 10.1016/j.carbpol.2009.07.035. [DOI] [Google Scholar]
- Tang CH, Jiang Y, Wen QB, Yang XQ. Effect of transglutaminase treatment on the properties of cast films of soy protein isolates. J Biotechnol. 2005;120:296–307. doi: 10.1016/j.jbiotec.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Tongnuanchan P, Benjakul S, Prodpran T. Structural, morphological and thermal behaviour characterisations of fish gelatin film incorporated with basil and citronella essential oils as affected by surfactants. Food Hydrocoll. 2014;41:33–43. doi: 10.1016/j.foodhyd.2014.03.015. [DOI] [Google Scholar]
- Tongnuanchan P, Benjakul S, Prodpran T, Nilsuwan K. Emulsion film based on fish skin gelatin and palm oil: physical, structural and thermal properties. Food Hydrocoll. 2015;48:248–259. doi: 10.1016/j.foodhyd.2015.02.025. [DOI] [Google Scholar]
- Tongnuanchan P, Benjakul S, Prodpran T, et al. Mechanical, thermal and heat sealing properties of fish skin gelatin film containing palm oil and basil essential oil with different surfactants. Food Hydrocoll. 2016;56:93–107. doi: 10.1016/j.foodhyd.2015.12.005. [DOI] [Google Scholar]
- Vanin FM, Sobral PJA, Menegalli FC, et al. Effects of plasticizers and their concentrations on thermal and functional properties of gelatin-based films. Food Hydrocoll. 2005;19:899–907. doi: 10.1016/j.foodhyd.2004.12.003. [DOI] [Google Scholar]
- Vogler EA. Structure and reactivity of water at biomaterial surfaces. Adv Colloid Interface Sci. 1998;74:69–117. doi: 10.1016/S0001-8686(97)00040-7. [DOI] [PubMed] [Google Scholar]
- Wu J, Liu H, Ge S, et al. The preparation, characterization, antimicrobial stability and invitro release evaluation of fish gelatin films incorporated with cinnamon essential oil nanoliposomes. Food Hydrocoll. 2015;43:427–435. doi: 10.1016/j.foodhyd.2014.06.017. [DOI] [Google Scholar]