Abstract
Valorization of onion peel waste, considered to be a rich source of polyphenolic compounds, by employing green extraction techniques is the need of the hour. The aim of the present study was to optimize the microwave-assisted extraction of bioactive phenolic compounds from onion peel wastes employing ChCl:Urea:H2O deep eutectic solvent. Microwave power (100–200–300 W), time (5–15–25 min) and liquid to solid ratio (40:1–50:1–60:1) were studied as the major parameters affecting the extraction efficiency. A Box–Behnken design was adopted including 17 experiments with five centre points. The optimum conditions determined were 100 W microwave power, 15.03 min irradiation time and 54.97 mL g−1 liquid to solid ratio. Under the MAE optimized conditions, the recovery of TPC and FRAP were 80.45 (mg GAE g−1 dw) and 636.18 (µmol AAE g−1 dw), respectively. Morphology of onion peels before and after DES extraction were also studied to gain an insight in the effect of microwave irradiations on the biomass.
Electronic supplementary material
The online version of this article (10.1007/s13197-019-03891-7) contains supplementary material, which is available to authorized users.
Keywords: Onion peel, Deep eutectic solvents, Microwave-assisted deep eutectic solvent extraction, Total phenolic content, Antioxidant activities, Response surface methodology
Introduction
Polyphenols are an integral part of large portion of plant metabolites and play an important role in human and animal diets. Their main dietary sources are fruits, vegetables, cereals, chocolate, dry legumes and plant-derived beverages such as fruit juices, tea, coffee and red wine. Phenolics derived from food have immense antioxidant capacity and have potential in the treatment and prevention of diabetes, cancer, cardiovascular disease, asthma, etc. (Bravo 1998). The polyphenols market was valued around USD 757 million globally in 2015, mainly attributed to the increasing health awareness about consumption of polyphenolic compounds (Adebooye et al. 2018). Based on the present scenarios, there is an expectation of more boosts in the market up to 2024 owing to huge demand of polyphenol extracts in the food, beverages, pharmaceutical, and cosmetics industry (Adebooye et al. 2018). According to Grand View Research (2016), global natural antioxidants market is anticipated to reach USD 4.14 Billion by 2022 from USD 2.22 Billion in 2014 and with an 8.4% annual growth rate from 2014 to 2022. The current market scenarios suggest that the highest demand of polyphenols of around 40.3% of the global demand would be in Asia Pacific region (China, Japan and India) in 2024, followed by 27.8% in Europe. Polyphenols are generally used in the functional beverages, functional foods, juices, energy drinks, etc. In the terms of share of polyphenols in industrial utilization, these were used in functional beverages segment (44%), followed by functional foods (33%) (Adebooye et al. 2018).
Fruits and vegetables contain large amount of polyphenols. Onion is one of the vegetables which contain abundant flavonoids in its outer skins (Benitez et al. 2013). Quercetin, kaempferol, myricetin and isorhamnetin derivaties are the main flavonoids present in the onion peels (Pal and Jadeja 2019). India is the second largest producer of onion after China. 74.25 million tons of onion was produced in the world in 2016 (FAOSTAT 2016) while the production in India stood at 20.99 million tons (Department of Agriculture, Cooperation & Farmers Welfare (Horticulture Statistics Division): Agricultural Statistics at a Glance 2016). Waste part of onion in the form of peels are generated around 300–500 kg per day in India (Das and Mandal 2015). Very huge amount of this waste are generated in the worldwide. 6 MT of onion solid waste (OSW) was produced by European countries (EUROSTAT 2014). The onion wastes are generated in the different form of such as top and bottom of the bulbs, outer fleshy scales and roots etc. throughout industrial peeling (Benitez et al. 2013).
In the recent years, many investigators have reported that the onion peel is a good source of phenolic antioxidants (Das and Mandal 2015; Katsampa et al. 2015; Mouratoglou et al. 2016). So, it benefits our society to extract phenolic compounds from onion peels because it reduces the waste in the environment and these phenolic compounds can be used as natural antioxidants. Industries are suffering from this kind of waste disposal problems. These wastes are harmful for the environment as their dumping in the open space after industrial processing or domestic uses results in bad odor generation. Therefore, it is important to develop novel extraction methods to recover valuable bioactive compounds from onion peels. Onion peels have various pharmacological properties such as anti-carcinogenic activity, anti-mutagenic activity, anti-microbial activity and antioxidant activity etc. (Sharma et al. 2016; Pal and Jadeja 2019). Onion peels contain large amount of flavonoids which is used in preparing anti-AIDS drugs by inhibiting the function of viral protein (Yamazaki et al. 2014).
Of late, deep eutectic solvents (DESs) are emerging as a new type of green solvents rather than organic solvents and ionic liquids (ILs). Currently, DESs are widely used by researchers in place of organic solvents in many chemical processes. DESs are prepared by mixing a hydrogen bond acceptor with a hydrogen bond donor (Abbott et al. 2003; Zhang et al. 2012). Abbott et al. (2003) introduced for the first time a DES made up of urea as HBD and choline chloride as HBA. Compared with organic solvents or ILs, in the recovery of polyphenolic compounds from biomass waste, green solvents like deep eutectic solvents are used as suitable extraction medium because it possess relatively low cost, non-toxicity, biodegradability and easy preparation (Reinhardt et al. 2008; Yu et al. 2008). Therefore, DESs are widely being employed as solvents to extract bioactive compounds from solid plant matrices (Bi et al. 2013; Dai et al. 2013; Nam et al. 2015).
The conventional techniques have been used in the past decades, but these require longer extraction time thus running a severe risk of thermal degradation for most of the phyto-constituents. These are the major drawbacks of traditional technique. So, there is an increasing growth of new extraction technique with reduced solvent consumption, shortened extraction time and increased pollution prevention concern. In the present investigation, one of the novel extraction methods microwave assisted extraction (MAE) was employed for extraction purpose using onion peels as raw materials. The merits of using MAE include easy handling, automated techniques and shorter extraction time (Cui et al. 2015; Cvjetko Bubalo et al. 2016).
DES are known for their favorable properties like non-toxicity, low flammability, negligible vapor pressure, reduced waste generation and being environmental friendly; whereas use of microwaves can enhance the extraction efficiency with reductions in time as well as solvent consumption compared to that of conventional methods. Thus, Microwave assisted deep eutectic solvent extraction (MADESE) has a great potential for the extraction of phenolic compounds from biomass waste. MADESE has risen rapidly in the last decade, and for most applications it has proven to be effective in all aspects compared to traditional extraction techniques.
In the present study, MADESE was used to extract the major active compounds from onion peels instead of the organic solvents. Deep eutectic solvent consisted of mixture of choline chloride: urea: water in the molar proportion of 1:2:4. Optimized conditions for total phenolic content and antioxidant activity as measured by the FRAP were investigated by BBD with RSM. BBD has been widely applied to optimize the extraction of bioactive compounds from natural sources (Maran et al. 2013; Cui et al. 2015; Das and Mandal 2015; Fattahi and Rahimi 2016; Shirzad et al. 2017). The Box–Behnken is an excellent design for response surface methodology because it permits: (1) evaluation of the parameters of the quadratic model; (2) structure of chronological designs; and (3) recognition of lack of fit of the model. It was established that the efficiency of BBD was more than the central composite design and much greater than that of three-level full factorial designs (Ferreira et al. 2007).
To the best of our knowledge, this type of an approach has not been reported elsewhere. Microwave power, time and liquid to solid ratio are three main operating parameters which have profound effect on TPC and FRAP as investigated by BBD test. Therefore, the main objective of this study was to optimize the extraction parameters for recovery of antioxidant phenolic compounds from onion peels by employing Box–Behnken design (BBD) considering total phenolic content (TPC) and ferric reducing antioxidant power (FRAP) as responses.
Materials and methods
Chemicals and reagents
Analytical standard quercetin (≥ 95.0%), kaempferol (≥ 97.0%), rutin (≥ 97.0%) and myricetin (≥ 96.0%) were obtained from Sigma-Aldrich, Germany. Ferric chloride (FeCl3) (anhydrous) and ascorbic acid were from Rankem Laboratory, Thane, Maharashtra. Methanol was from Okhla Industries, New Delhi. Folin Ciocalteu’s reagent, gallic acid, sodium carbonate (Na2CO3) and 2,4,6-Trippyridyl-s-triazine (TPTZ) were supplied by Sisco Research Laboratories Pvt. Ltd. Taloja, Maharashtra. Ethyl acetate, hydrochloric acid, urea and choline chloride were purchased from Finar Ltd., Ahmedabad. Millipore ultrapure water, HPLC-grade acetonitrile and formic acid (Sigma-Aldrich, Germany) were used for HPLC analysis.
Onion peels collection and pretreatment
Only red-colored onion peels were selected for the extraction of phenolic compounds. The collected peels were dried in an oven at 60 °C for 10 h until the wetness was reduced from the peels (Lee et al. 2014). A high-speed mixer (Blender 7012S; Waring, Torrington, CT, USA) was used to grind onion peels and screened with B.S.S sieve (Kumar Test sieves, Mumbai). In this study, 500 µm peel powder was randomly selected in triplicate and carried out extraction process and rest of the peel powder was kept in a desiccator until used for later extraction operations.
Selection of deep eutectic solvent system
The DES was prepared by employing heating and stirring method. DESs were prepared in accordance with previously reported methodologies (Dai et al. 2015; Mouratoglou et al. 2016). In this study, four types of DES systems were screened for an effective solvent system for recovering phenolic compounds from onion peels (Pal and Jadeja 2019). The present investigation reveals the use of eutectic mixtures composed of choline chloride with four different HBDs like sucrose (4:1), urea (1:2), oxalic acid (1:1) and sorbitol (3:1) as extractants. ChCl:Urea DES system showed the best results for total phenolic content from onion peels while ChCl: Sorbitol DES system showed relatively lower extraction efficiency. The ChCl:U system gave the highest TPC at 1:2 (mol/mol). Therefore, ChCl:U with a molar ratio of 1:2 (mol/mol) was selected for further experiments. Choline chloride (HBA) and urea (HBD) were mixed and heated in a beaker at 70 °C, 600 rpm for 60 min resulting in a transparent viscous liquid which was subsequently used as extraction solvent.
Single factor analyses of different factors, viz., liquid to solid ratio (10:1–60:1 mL g−1), time (60–150 min), temperature (50–90 °C) and molar ratio of ChCl:U:H2O (1:1:4–1:3:4 mol/mol/mol) were performed to find the most significant extraction variables (Pal and Jadeja 2019). Out of these, four parameters (liquid to solid ratio, time, temperature and molar ratio) were found to have greater influence on the extraction efficiency and the same were chosen for further MADESE experiments.
Microwave assisted deep eutectic solvent extraction (MADESE)
Phenolic compounds were extracted from onion peels using modified Samsung microwave oven (Samsung, Seremban, Malaysia) (Thakker et al. 2016; Pal and Jadeja 2019). The microwave power ranges from 100 to 850 W. The experiments were performed with microwave power (100, 200, 300 W), irradiation time (5, 15, 25 min) and liquid to solid ratio of (40:1, 50:1, 60:1 mL g−1) using deep eutectic solvent (ChCl:Urea). Appropriate quantity of onion peel was placed into a round bottom flask (100 mL) with DES solvent which was then placed in the microwave cavity and subjected to fixed parameters as mentioned by BBD. The resulting extracts were filtered using Whatman paper No. 1 and then used for subsequent analyses.
Conventional extractions
Heating–stirring extraction (HSE) was performed by adding 1 g peel powder to 55 mL ChCl:Urea eutectic mixture stirred at 500 rpm for 180 min. For comparative analysis, Soxhlet extraction was also implemented to recover the phenolic compounds from onion peels with the help of 70% aqueous methanol for 360 min. In both the cases, the slurry was filtered through filter paper (Whatman No. 1) followed by analysis of the extracts to evaluate the total phenolic content (TPC) using the Folin-Ciocalteu method.
Measurement of total phenolic content (TPC)
The method of total phenolic content of onion peels from deep eutectic solvents extracts using Folin Ciocalteu reagent (Blidi et al. 2015). The content of total phenolics in each extract was determined using calibration curve (Y = 0.0005x; R2 = 0.993) prepared for gallic acid and the results were expressed as mg GAE g−1 dw through the calibration curve of gallic acid (25–500 mg L−1). UV-spectrophotometer (HACH DR-6000) was used and the absorbance was recorded at 760 nm.
Ferric reducing antioxidant power (FRAP) assay
Blidi et al. (2015) developed methodology to determine FRAP. Calibration curve was prepared of ascorbic acid (30–220 µmol). The FRAP of the extracts was determined from the standard curve (Y = 0.001x; R2 = 0.995) and the results were represented in the form of µmol AAE per g−1 dw. Each extract (0.05 mL) was diluted with 0.05 mL of 4 mM FeCl3 buffer which was kept in a water bath at 40 °C for 30 min. After this, 0.9 mL of 1 mM (0.05 M HCl) TPTZ solution was added in each extract. After10 min, the absorbance of each extract was recorded at 620 nm using UV-spectrophotometer (HACH DR-6000).
Recovery of phenolic compounds by liquid–liquid extraction
Ethyl acetate as an aprotic solvent plays an important role to separate phenolic compounds from DES extracts (Liu et al. 2016). The ratio of DES extract and ethyl acetate was maintained at 1:3 (v/v) in separating funnel. Two layers were formed, with ethyl acetate portion settling on the top which contains maximum amount of phenolic compounds. This was filtered through filter paper using sodium sulfate anhydrous (Na2SO4) to absorb water. The remaining solvent was evaporated using rotary evaporator (Heidolph, Germany). Crude sample was dissolved with 25 mL of methanol. 0.5 mL of this dilute sample was added to 0.5 mL pure methanol. This sample was centrifuged at 6000 rpm at 28 °C for 10 min and filtered through 0.45 µm membrane filter before HPLC analysis.
Quantification of flavonoids in onion peel extracts by HPLC
The identification and quantification of flavonoids mainly quercetin, kaempferol and myricetin in DES extracts was according to the procedure described by Makasana et al. (2017). HPLC system (Shimadzu Corporation, Kyoto, Japan) equipped with UV–Vis detector (model SPD-20A), a degasser (model DGU-20A3), a quaternary pump (model LC-20AD), a column oven (model CTO-10ASvp) and an autosampler (model SIL-20ACHT) connected to CBM-20 communication bus module system was employed. The separation was done on a C18 chromatographic column (Altima, 250 × 4.6 mm ID, 5 µm, Grace, UK) with column temperature at 40 °C. The mobile phase consisted of isocratic acetonitrile (45%) and 0.1% formic acid in water (55%) at a flow rate 1.0 mL min−1 for a total run time of 20 min and the peaks were detected at wavelength of 370 nm using UV absorbance.
Morphological characterization of onion peels by SEM
Scanning electron microscopy (SEM) (Hitachi Series 3400 N) was applied to study the morphological changes of the peel samples before and after MADESE treatment. The samples were prepared using double sided adhesive tape and also gold coated before imaging for comparing the micrographs of the samples.
RSM
In this study, three variables were considered as independent: microwave power (A), time (B) and liquid to solid ratio (C). In BBD design, five centre points are needed to investigate the effects of independent parameters on two (TPC and FRAP) dependent responses. Three codes (− 1, 0, 1) were transformed for independent variables of three levels. In all, 17 experiments with five centre points (as shown in Table 1) were performed.
Table 1.
Box–Behnken design with experimental and predicted values of TPC and FRAP
| Run | Factors | Experimental | Predicted (RSM) | ||||
|---|---|---|---|---|---|---|---|
| A | B | C | TPC | FRAP | TPC | FRAP | |
| 1 | 1 | 0 | − 1 | 67.66 | 545.38 | 67.88 | 539.49 |
| 2 | − 1 | 0 | − 1 | 56.57 | 598.49 | 57.49 | 603.70 |
| 3 | 0 | 1 | 1 | 53.59 | 505.58 | 53.09 | 502.91 |
| 4 | 0 | 0 | 0 | 52.58 | 746.02 | 53.77 | 742.93 |
| 5 | − 1 | 0 | 1 | 93.69 | 568.92 | 93.25 | 574.94 |
| 6 | 0 | 0 | 0 | 53.80 | 738.94 | 53.77 | 742.93 |
| 7 | 1 | − 1 | 0 | 45.86 | 476.99 | 45.24 | 480.74 |
| 8 | 1 | 0 | 1 | 86.80 | 542.51 | 86.11 | 537.16 |
| 9 | 1 | 1 | 0 | 55.44 | 488.34 | 56.53 | 495.82 |
| 10 | 0 | − 1 | 1 | 49.40 | 575.31 | 51.03 | 577.31 |
| 11 | − 1 | 1 | 0 | 38.74 | 535.56 | 39.78 | 532.74 |
| 12 | 0 | 0 | 0 | 53.12 | 755.27 | 53.77 | 742.93 |
| 13 | − 1 | − 1 | 0 | 60.26 | 554.23 | 58.74 | 545.81 |
| 14 | 0 | 1 | − 1 | 17.05 | 595.68 | 15.42 | 593.68 |
| 15 | 0 | − 1 | − 1 | 30.70 | 520.25 | 31.20 | 522.92 |
| 16 | 0 | 0 | 0 | 54.43 | 735.46 | 53.77 | 742.93 |
| 17 | 0 | 0 | 0 | 54.90 | 738.94 | 53.77 | 742.93 |
| Variables | Symbols | Coded levels | ||
|---|---|---|---|---|
| − 1 | 0 | 1 | ||
| Microwave power (W) | A | 100 | 200 | 300 |
| Time (min) | B | 5 | 15 | 25 |
| Liquid to solid ratio (mL g−1) | C | 40 | 50 | 60 |
RSM was performed using the Design Expert 10.0.8 software (Stat-Ease, Inc., Minneapolis, MN, USA) and quadratic polynomial Eq. (1) was obtained. Experiments were performed with different ranges of microwave power (A = 100, 200 and 300 W); time (B = 5, 15 and 25 min); and liquid to solids ratio (C = 40, 50, and 60 mL g−1).
| 1 |
where TPC and FRAP stands (Yn) for the predicted responses for X1–X3; b0 represents the constant coefficient; b1, b2, and b3 are the linear coefficients; b11, b22, and b33 show the quadratic coefficients; and b12, b13, and b23 are the cross-coefficients.
ANOVA method and maximum R2, adjusted R2 gave the accuracy of the estimated coefficient and also exhibited low p values for quadratic model exhibiting most appropriate model for the extraction of phenolic compounds from onion peels (Table 2). The model adequacy was checked by an F-test at 1 and 5% by representing coefficient R2.
Table 2.
ANOVA results
| Source | Sum of squares | df | Mean square | F value | p value Prob > F | |
|---|---|---|---|---|---|---|
| TPC | ||||||
| Model | 5099.63 | 9 | 566.63 | 249.61 | < 0.0001 | Significant |
| A-power | 5.28 | 1 | 5.28 | 2.33 | 0.1710 | |
| B-time | 28.89 | 1 | 28.89 | 12.73 | 0.0091 | |
| C-sample ratio | 1429.82 | 1 | 1429.82 | 629.87 | < 0.0001 | |
| AB | 233.30 | 1 | 233.30 | 102.77 | < 0.0001 | |
| AC | 78.34 | 1 | 78.34 | 34.51 | 0.0006 | |
| BC | 79.57 | 1 | 79.57 | 35.05 | 0.0006 | |
| A2 | 1225.57 | 1 | 1225.57 | 539.89 | < 0.0001 | |
| B2 | 1873.32 | 1 | 1873.32 | 825.25 | < 0.0001 | |
| C2 | 105.77 | 1 | 105.77 | 46.59 | 0.0002 | |
| Residual | 15.89 | 7 | 2.27 | |||
| Lack of fit | 12.34 | 3 | 4.11 | 4.63 | 0.0864 | Not significant |
| Pure error | 3.55 | 4 | 0.89 | |||
| R2 = 0.9969 | ||||||
| Adj R2 = 0.9929 | ||||||
| FRAP | ||||||
| Model | 1.588 × 105 | 9 | 17,646.70 | 225.75 | < 0.0001 | Significant |
| A-power | 5200.98 | 1 | 5200.98 | 66.53 | < 0.0001 | |
| B-time | 1.97 | 1 | 1.97 | 0.025 | 0.8783 | |
| C-sample ratio | 474.21 | 1 | 474.21 | 6.07 | 0.0433 | |
| AB | 202.14 | 1 | 202.14 | 2.59 | 0.1519 | |
| AC | 178.15 | 1 | 178.15 | 2.28 | 0.1749 | |
| BC | 5267.86 | 1 | 5267.86 | 67.39 | < 0.0001 | |
| A2 | 43,040.75 | 1 | 43,040.75 | 550.61 | < 0.0001 | |
| B2 | 62,549.33 | 1 | 62,549.33 | 800.17 | < 0.0001 | |
| C2 | 21,729.26 | 1 | 21,729.26 | 277.98 | < 0.0001 | |
| Residual | 547.19 | 7 | 78.17 | |||
| Lack of fit | 297.72 | 3 | 99.24 | 1.59 | 0.3242 | Not significant |
| Pure error | 249.46 | 4 | 62.37 | |||
| R2 = 0.9966 | ||||||
| Adj R2 = 0.9922 |
Statistical analysis
Response surface analysis was performed using the Design Expert 10.0.8 software (Stat-Ease, Inc., Minneapolis, MN, USA). The Chi square (χ2) test was employed to check the significant differences in the experimental and the predicted values. All the experimental results are the average of triplicate runs and are expressed as mean ± standard deviation (SD).
Results and discussion
A Box–Behnken design (BBD) approach was adopted for microwave assisted deep eutectic solvent extraction (MADESE) of phenolic compounds from onion peels. Table 1 show the detailed experimental design along with predicted values of TPC and FRAP.
Fitting the RSM models
Table 2 represents the ANOVA conclusion for the RSM models which indicate the p values of < 0.01 were considered to be significantly accurate for each of the two responses. In this study, the value of regression models R2 and R2-adj for TPC and FRAP were (R2 and R2-adj > 0.99 and 0.99) recommends the fitness of the RSM. Regarding lack of fit in the RSM model, p values for all two responses were greater than 0.05 (non significant) means that the model fits well and there is significant effect of parameter on output response (Puertolas et al. 2011). The values were TPC (0.0864), FRAP (0.3242). In addition, Table 2 illustrates that the coefficients of responses p values showed that in the linear terms of TPC was highly significant, effective variables were time and liquid to solids ratio (p < 0.01) while on FRAP highly significant variable was microwave power (p < 0.01). Interaction effects of the independent variables AB, AC and BC were highly significant in the case of TPC and BC was highly significant on FRAP. If the values greater than 0.1 represent the model terms are not significant. From the above discussions, it can be seen that the lower p value and higher R2 show the higher significant effect on the respective response variables.
Experimental data by RSM models for each of the responses are expressed based on the below equations:
| 2 |
| 3 |
Y indicates the predicting responses and A, B, and C represent microwave power, time and liquid to solid ratio, respectively.
Effects of process parameters on TPC and FRAP based on RSM
Total phenolic content (TPC)
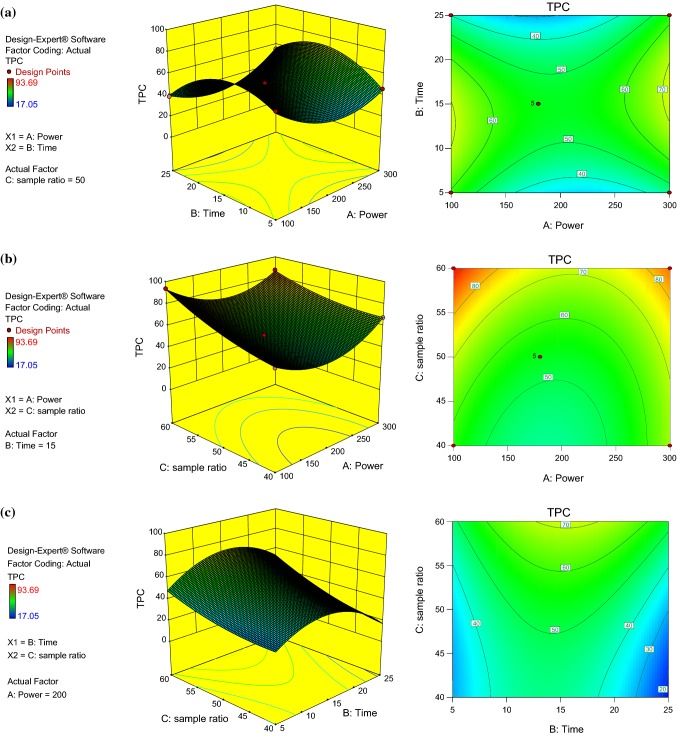
From the regression equations mentioned above, it can be seen that except A (microwave power) all model terms were highly significant for total polyphenolic content. 3D surface plots represent the interaction between variables A to C for TPC as shown in Fig. 1a–c.
Fig. 1.
Response surface plot showing the combined effect of microwave power and time (a), microwave power and sample ratio (b) and time and sample ratio (c) on the TPC
The interaction between microwave power (A) and time (B) are presented in Fig. 1a. Initially, the extraction efficiency increased by enhancing the time but after some time, it is clearly seen that there was continuous falling in the response with no effect on the extraction yield; this is probably due to the degradation of polyphenols and decrease in the polarity of solvent at higher microwave power (Maran et al. 2013). Polarity is one of the most important properties affecting the solubilizing capacity of DESs. The effective polarity of choline chloride combined with urea was found to be fairly dipolar. Urea has higher dipolarity with associated choline chloride in a 2:1 molar ratio because it contains –NH2 group and does not have any saturated carbons (Pandey et al. 2014). According to Fig. 1b, liquid to solid ratio (C) was another important independent parameter which was used for extraction of phenolic compounds from onion peels. Initially, the extraction efficiency was marginally significant in the range of liquid to solid ratio (40–50 mL g−1) but when liquid to solid ratio was increased (55–60 mL g−1) with respect to microwave power (100–120 W), high extraction efficiency was obtained.
When a higher liquid to solid ratio is used in the extraction operation, concentration gradient which is the driving force during mass transfer within the solid was higher between the liquid and solid, resulting in an increase in the diffusion rate. When the quantity of solvent compared to solid is not ample to obtain adequate transfer, various equilibria may take place, leading to a non negligible resistance to mass transfer. So, it is necessary to have a precise liquid solid ratio for better mixing leading to enhanced diffusion rate of the solute and subsequently improving the extraction yield (Cacace and Mazza 2003; Katsampa et al. 2015). The highest TPC 80–93.69 mg GAE g−1 dw was achieved in the range of liquid to solid ratio (55–60 mL g−1) and microwave power (100–150 W). There was a significant effect of time on TPC in the middle range (14–16 min) (Fig. 1c). Initially, no impact was observed on TPC in the time range (5–10 min) owing to improper mixing during this period. However, TPC reduced after 20 min as prolonged time might be due to degradation of polyphenolic compounds.
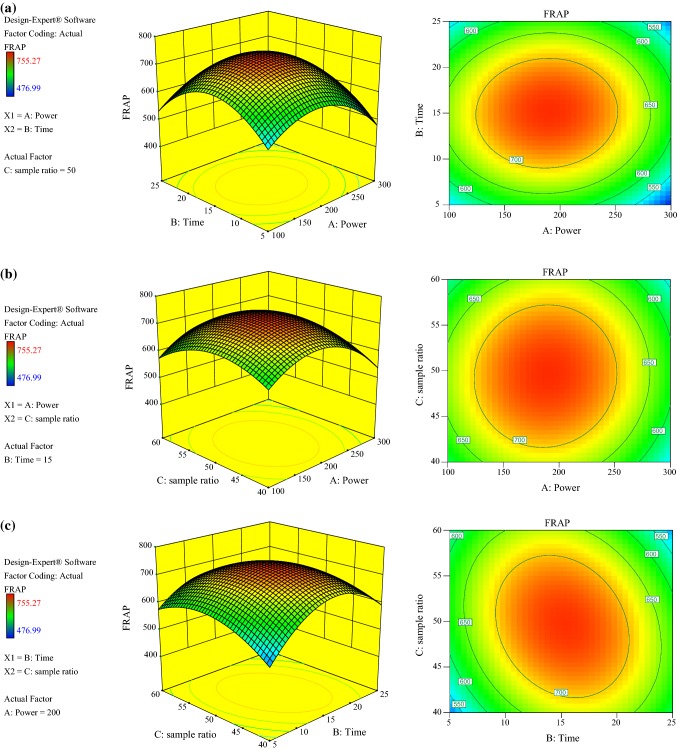
Ferric reducing antioxidant power (FRAP)
Figure 2a–c represent 3D surface plots of FRAP which explain the interaction between variables A to C. For reducing power, A, BC, A2, B2 and C2 were highly significant; C was significant while rest of the variables were not significant on the model terms.
Fig. 2.
Response surface plot showing the combined effect of microwave power and time (a), microwave power and sample ratio (b) and time and sample ratio (c) on the FRAP
From the Fig. 2a, it can be seen that the maximum reducing power was obtained in microwave power range 180–220 W and time range 14–16 min. By increasing microwave power, the diffusion coefficient increased which in turn raised the extraction rate and reduced the extraction time. As seen from the 3D surface plots, increase in microwave power beyond 250 W leads to a sharp decline in reducing power due to rise in temperature. This type of behavior is also observed in case of TPC. Hence, the reducing power is dependent on both TPC and the relative amounts of various polyphenols (Karakashov et al. 2015).
The extraction recovery rate was increased by enhancing the liquid to solid ratio but it was subsequently decreased when microwave power was increased up to 300 W. The higher reducing power was determined at middle power ranging between 180–220 W and liquid to solid ratio 50–55 mL g−1 (Fig. 2b). The cause for the stability of reducing power activity can be attributed to the formation of Maillard reaction products which posses antioxidant activity. This occurs due to heating which increases antioxidant activity in fruits and vegetables (Sharma et al. 2015). It was reported that, after heating there was an increase in reducing power due to alteration in phenolic compounds (Tsai and She 2006). Maximum FRAP was achieved between in 14–16 min (Fig. 2c) and similar situation was observed in the case of TPC. Under optimal conditions, the maximum reducing power activity was obtained 636.18 µmol AAE g−1 dw.
Simultaneous optimization of response values
Simultaneous optimization of two responses, namely, TPC and FRAP were carried out based on the desirability values at the optimal parameters viz; extraction time 15.03 min, microwave power 100 W and liquid to solid ratio was 54.96 mL g−1 are listed in supplementary information (SI) Fig. S1. In addition, under the optimum conditions, the corresponding desirability values were more than 0.76.
The corresponding values under the optimum conditions for TPC and antioxidant assay FRAP were 80.45 (mg GAE g−1 dw) and 636.18 (µmol AAE g−1 dw) respectively, which were very near to the experimental values performed under the optimized conditions. In order to validate these results, the experiments were performed at optimized conditions in duplicates and values were averaged. The results obtained were 80.09 (mg GAE g−1 dw) for TPC and 636.14 (µmol AAE g−1 dw) for FRAP.
Accuracy of RSM models
Chi square value (χ2) also known as “goodness of fit” statistics measures how well the observed distribution data are fitted with the expected distribution data (Fattahi and Rahimi 2016). If variables are independent, Eq. (4) can be used for calculation of Chi square value (χ2).
| 4 |
where Yexp and Ypred represents respectively, the experimental and the predicted real value of responses (TPC, FRAP) and n shows the number of sample points.
Generally, researchers have considered significance levels in the range of 0–1. In this study, all the two responses have 9 degrees of freedom and Chi square calculated values were 0.42 for TPC and 0.88 for FRAP, which clearly show that all the both response values are less than one (SI Fig. S2). High R2 values were observed between experimental data and predicted (RSM) data. Smaller Chi square calculated value point out the acceptable model fit for the predicted values with experimental values. Specially, optimized models of RSM can be employed for extractions of TPC from onion peels and in evaluating FRAP of the extracted polyphenolic compounds.
Polyphenolic profile by HPLC
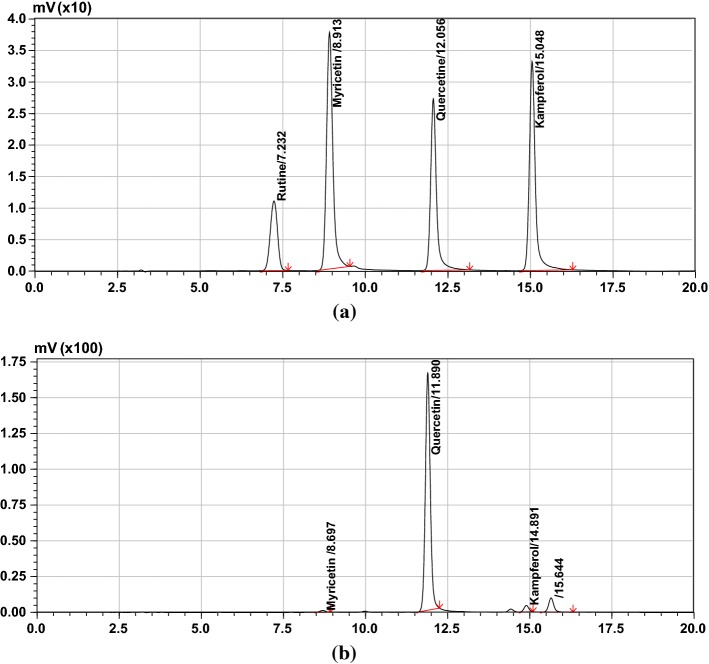
Main three flavonoids were quantified; quercetin (4.225375 mg g−1), kaempferol (0.091649 mg g−1) and myricetin (0.036879 mg g−1). Deep eutectic solvent exhibits very important properties such as solvation. That is robustly affected by hydrogen bonding. Protic solvents such as ethanol, methanol and water are miscible with DES, while aprotic solvents like hexane, ethyl acetate, toluene etc. are immiscible. Therefore, it is feasible to employ one of those aprotic solvents to be used in liquid–liquid extraction. EA was used to recover phenolic compounds from DES extract in this study. The mass transfer will take place among DES droplets and nearby aprotic solvents. Partition coefficient plays a key role in the separation of phenolic compounds between dispersed phase and aprotic solvents (Liu et al. 2016). Figure 3a, b show the HPLC chromatograms.
Fig. 3.
a HPLC chromatogram of commercial standard compounds and b HPLC chromatogram of onion peel extracts by MADESE
Scanning electron microscopy
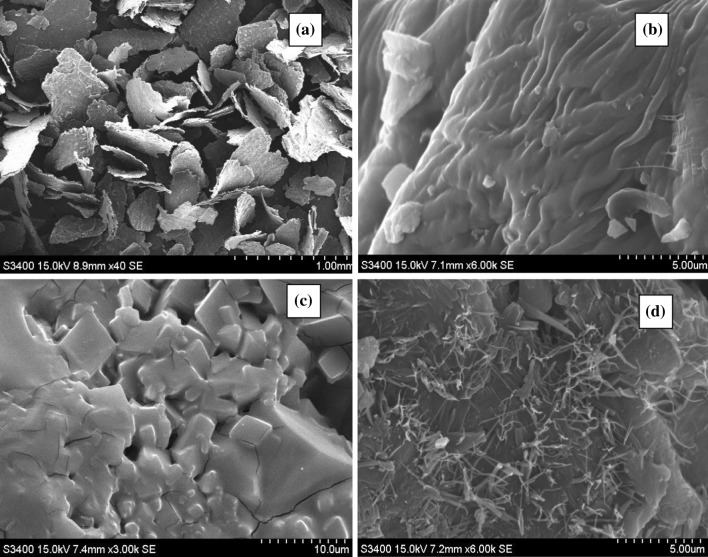
The morphological characterizations of raw as well as MADESE treated samples were carried out using a scanning electron microscope (Hitachi Series 3400 N). According to Fig. 4, noteworthy structural changes were shown on the peel surface before and after treatment with MADESE. Figure 4a shows the scanning electron micrograph of external surface of the raw samples. It can be seen that the raw sample is flaky in shape having a rough surface. The roughness of the raw materials surface is a valuable property because it increases the surface area.
Fig. 4.
SEM images of onion peel samples. a Raw materials, b magnified porous raw material, c MADESE based treated sample, d magnified MADESE based treated sample
Moreover, the magnified image Fig. 4b shows the presence of pores. Figure 4c shows the SEM image of the extracted sample with deep eutectic solvent by microwave assisted extraction. After extraction using MAE for 15 min, plant cells were not clear and appeared completely disrupted and collapsed in solvents. It can be seen that the surface of the sample is smooth and in rhombus shape. The diameter of this shape was ~ 0.5 mm which is in the range recommended for extraction operation. From Fig. 4d, it can be easily seen that in the process of MAE, pressure build-up within the glands could have exceeded their capacity for expansion and caused their rupture more speedily and completely. Therefore, higher extraction efficiency was achieved and the time utilized was less in the operation. MAE results represent that the material cells were effortlessly disrupted in DESs conditions because deep eutectic solvents break the cell wall with fiber dissolution (Gunny et al. 2015) and the bioactive compounds were extracted from the biomass waste. Swatloski et al. (2002) also demonstrated that cell rupture had a significant effect on extraction subsequently increasing efficiency.
Comparison of extraction methods
TPC obtained in case of MADESE, HSE and Soxhlet extraction were 80.09 (mg GAE g−1 dw), 63.27(mg GAE g−1 dw), 54.73 (mg GAE g−1 dw), respectively. The phenolic content of MADESE extract was superior to that of heating–stirring extract and that too achieved with a 12 fold reduction in extraction time. The enhanced extraction efficiency of MADESE was attributed to the mechanical effects of internal heating based on conduction and dielectric polarization caused by microwave irradiation, and the pressure built up within the cells. This leads to capable delivery of phenolic compounds to the plant material through the molecular interaction with the electromagnetic field (Proestos and Komaitis 2008). Therefore, MAE facilitated desorption and release of the TPC from plant matrix, thus accelerating the extraction process as well as increasing the extraction yield. The flavonoids in HSE extract were quercetin (3.287065 mg g −1), kaempferol (0.184662 mg g −1) and myricetin (0.045651 mg g −1) whereas Soxhlet extraction resulted in 6.185366 mg g−1 quercetin, 0.349224 mg g−1 kaempferol and 0.105077 mg g−1 myricetin. Some degradation of polyphenols was observed in case of MADESE extract owing to the high microwave energy. Nevertheless, the results are in agreement with those reported by Benitez et al. (2011)
Conclusion
The BBD approach was effectively useful to maximize the extraction of TPC and FRAP from onion peels. In addition, three independent variables microwave power (W), time (min) and liquid to solid ratio (mL g−1) were investigated by RSM using green solvent such as deep eutectic (ChCl:Urea:H2O) in the proportion molar ratio of 1:2:4. The results represents that the purpose of microwave for the duration of extraction and the process variables had a noteworthy outcome on the highest extraction of TPC and antioxidants from onion peels. Model summary data showed that the model was sufficient and accurate with the observed data. The ANOVA results representing the value of R2 for TPC, FRAP (R2 = 0.996, 0.996) showed the adequate fitness of the response surface methodology. To evaluate the interactive impact of the process parameters on the response variables these were used to the contour and response surface plots. Various models were fitted to the experimental data to develop a relevant model. The models were extremely significant (p < 0.01) for all the response variables. After optimizing for multi-response the optimum conditions were 100 W microwave power, 15.03 min irradiation time and 54.97 mL g−1 liquid to solid ratio. Under the MAE optimized conditions, the recovery of TPC, FRAP were 80.45 (mg GAE g−1 dw), 636.18 (µmol AAE g−1 dw) respectively, which were consistent to the experimental values performed under optimized conditions. According to SEM analysis, MADESE procedure confirmed that cell disruption also affected the extraction efficiency.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors gratefully acknowledge the support of Dr. N. A. Gajbhiye, Senior Scientist (Organic Chemistry), ICAR-Directorate of Medicinal & Aromatic Plants Research, Anand, Gujarat by providing necessary facilities for HPLC analysis of the samples and help rendered in analytical determinations.
Abbreviations
- AAE
Ascorbic acid equivalents
- BBD
Box–Behnken design
- ChCl:U
Choline chloride:urea
- DES
Deep eutectic solvent
- EA
Ethyl acetate
- FRAP
Ferric reducing antioxidant power
- GAE
Gallic acid equivalents
- HBA
Hydrogen bond acceptor
- HBD
Hydrogen bond donors
- HPLC
High performance liquid chromatography
- HSE
Heating–stirring extraction
- LLE
Liquid–liquid extraction
- MADESE
Microwave-assisted deep eutectic solvent extraction
- MAE
Microwave-assisted extraction
- PC
Phenolic compounds
- RSM
Response surface methodology
- SEM
Scanning electron microscopy
- TPC
Total phenolic content
- TPTZ
2,4,6-Trippyridyl-s-triazine
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chandra Bhushan T. Pal, Phone: +91-261-2201643, Email: ctp.svnit@gmail.com
Girirajsinh C. Jadeja, Phone: +91-261-2201643, Email: jgc@ched.svnit.ac.in, Email: gcjadeja@gmail.com
References
- Abbott AP, Capper G, Davies DL, Rasheed RK, Tambyrajah V. Novel solvent properties of choline chloride/urea mixtures. Chem Commun. 2003;1:70–71. doi: 10.1039/b210714g. [DOI] [PubMed] [Google Scholar]
- Adebooye OC, Alashi AM, Aluko RE. A brief review on emerging trends in global polyphenol research. J Food Biochem. 2018;10:10. [Google Scholar]
- Benitez V, Molla E, Martin-Cabrejas MA, Aguilera Y, Lopez-Andreu FJ, Cools K, Terry LA, Esteban RM. Characterization of industrial onion wastes (Allium cepa L.): dietary fibre and bioactive compounds. Plant Foods Hum Nutr. 2011;66:48–57. doi: 10.1007/s11130-011-0212-x. [DOI] [PubMed] [Google Scholar]
- Benitez FV, Molla E, Martin-Cabrejas MA, Aguilera Y, Lopez-Andreu FJ, Terry LA, Esteban RM. The impact of pasteurization and the sterilization on bioactive compounds of onion by-products. Food Bioprocess Technol. 2013;6:1979–1989. doi: 10.1007/s11947-012-0866-x. [DOI] [Google Scholar]
- Bi W, Tian M, Row KH. Evaluation of alcohol-based deep eutectic solvent in extraction and determination of flavonoids with response surface methodology optimization. J Chromatogr A. 2013;1285:22–30. doi: 10.1016/j.chroma.2013.02.041. [DOI] [PubMed] [Google Scholar]
- Blidi S, Bikaki M, Grigorakis S, Loupassaki S, Makris DP. A comparative evaluation of bio-solvents for the efficient extraction of polyphenolic phytochemicals: apple waste peels as a case study. Waste Biomass Valoriz. 2015;6:1125–1133. doi: 10.1007/s12649-015-9410-3. [DOI] [Google Scholar]
- Bravo L. Polyphenols: chemistry, dietary, sources, metabolism and nutritional significance. Nutr Rev. 1998;56(11):317–333. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- Cacace JE, Mazza G. Optimization of extraction of anthocyanins from black currants with aqueous ethanol. J Food Sci. 2003;68:240–248. doi: 10.1111/j.1365-2621.2003.tb14146.x. [DOI] [Google Scholar]
- Cui Q, Peng X, Yao X-H, Wei Z-F, Luo M, Wang W, Zhao C-J, Fu Y-J, Zu Y-G. Deep eutectic solvent-based microwave-assisted extraction of genistin, genistein and apigenin from pigeon pea roots. Sep Purif Technol. 2015;150:63–72. doi: 10.1016/j.seppur.2015.06.026. [DOI] [Google Scholar]
- Cvjetko Bubalo M, Curko N, Tomasevic M, Ganic KK, Redovnikovic IR. Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem. 2016;200:159–166. doi: 10.1016/j.foodchem.2016.01.040. [DOI] [PubMed] [Google Scholar]
- Dai Y, van Spronsen J, Witkamp G-J, Verpoorte R, Choi YH. Natural deep eutectic solvents as new potential media for green technology. Anal Chim Acta. 2013;766:61–68. doi: 10.1016/j.aca.2012.12.019. [DOI] [PubMed] [Google Scholar]
- Dai Y, Witkamp G-J, Verpoorte R, Choi YH. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015;187:14–19. doi: 10.1016/j.foodchem.2015.03.123. [DOI] [PubMed] [Google Scholar]
- Das S, Mandal SC. Effect of process parameters of microwave assisted extraction on natural product yield from onion peel. Int J Pharm Sci Rev Res. 2015;6(8):3260–3275. [Google Scholar]
- Department of Agriculture, Cooperation & Farmers Welfare (Horticulture Statistics Division): Agricultural Statistics at a Glance (2016) Government of India
- EUROSTAT (2014) http://ec.europa.eu/eurostat/statistics-explained/index.php/MainPage. Retrieved 30 Apr 2018
- FAOSTAT (2016) World onion area and production statistics reports. http://faostat.fao.org/. Accessed July–Aug 2018
- Fattahi M, Rahimi R. Optimization of extraction parameters of phenolic antioxidants from leaves of Capparis spinosa using response surface methodology. Food Anal Method. 2016;9:2321–2334. doi: 10.1007/s12161-016-0414-9. [DOI] [Google Scholar]
- Ferreira SLC, Bruns RE, Ferreira HS, Matos GD, David JM, Brandao GC, da Silva EGP, Portugal LA, dos Reis PS, Souza AS, dos Santos WNL. Box–Behnken design: an alternative for the optimization of analytical methods. Anal Chim Acta. 2007;597:179–186. doi: 10.1016/j.aca.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Grand View Research (2016) Natural antioxidants market analysis by product and segment forecasts to 2022. https://www.grandviewresearch.com/press-release/natural-antioxidants-market. Accessed 15 July–Aug 2018
- Gunny AAN, Arbain D, Nashef EM, Jamal P. Applicability evaluation of deep eutectic solvents-cellulase system for lignocellulose hydrolysis. Bioresour Technol. 2015;181:297–302. doi: 10.1016/j.biortech.2015.01.057. [DOI] [PubMed] [Google Scholar]
- Karakashov B, Grigorakis S, Loupassaki S, Makris DP. Optimisation of polyphenol extraction from Hypericumperforatum (St John’s Wort) using aqueous glycerol and response surface methodology. J Appl Res Med Aromat Plants. 2015;2:1–8. [Google Scholar]
- Katsampa P, Valsamedou E, Grigorokis S, Makris DP. A green ultrasound-assisted extraction process for the recovery of antioxidant polyphenols and pigments from onion solid wastes using Box–Behnken experimental design and kinetics. Ind Crops Prod. 2015;77:535–543. doi: 10.1016/j.indcrop.2015.09.039. [DOI] [Google Scholar]
- Lee KA, Kim KT, Kim HJ, Chung MS, Chang PS. Antioxidant activities of onion (Allium cepa L.) peel extracts produced by ethanol, hot water and subcritical water extraction. Food Sci Biotechnol. 2014;23(2):615–621. doi: 10.1007/s10068-014-0084-6. [DOI] [Google Scholar]
- Liu Y, Garzon J, Friesen JB, Zhang Y, McAlpine JB, Lankin DC, Chen SN, Pauli GF. Countercurrent assisted quantitative recovery of metabolites from plant-associated natural deep eutectic solvents. Fitoterapia. 2016;112:30–37. doi: 10.1016/j.fitote.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makasana J, Dholkiya B, Gajbhiye N, Raju S. Extractive determination of bioactive flavonoids from butterfly pea (Clitoria ternatea Linn.) Res Chem Intermed. 2017;43(2):783–799. doi: 10.1007/s11164-016-2664-y. [DOI] [Google Scholar]
- Maran JP, Manikandan S, Thirugnanasambandham K, Nivetha CV, Dinesh R. Box–Behnken design based statistical modeling for ultrasound-assisted extraction of corn silk polysaccharide. Carbohyd Polym. 2013;92:604–611. doi: 10.1016/j.carbpol.2012.09.020. [DOI] [PubMed] [Google Scholar]
- Mouratoglou E, Malliou V, Makris DP. Novel glycerol based natural eutectic mixtures and their efficiency in the ultrasound—assisted extraction of antioxidant polyphenols from agri-food solid wastes biomass. Waste Biomass Valoriz. 2016;7:1377–1387. doi: 10.1007/s12649-016-9539-8. [DOI] [Google Scholar]
- Nam MW, Zhao J, Lee MS, Jeong JH, Lee J. Enhanced extraction of bioactive natural products using tailor made deep eutectic solvents: application of flavonoid extraction from Flos sophorae. Green Chem. 2015;3:1718–1727. doi: 10.1039/C4GC01556H. [DOI] [Google Scholar]
- Pal CBT, Jadeja GC. Deep eutectic solvents-based extraction of polyphenolic antioxidants from onion (Allium cepa L.) peel. J Sci Food Agric. 2019;99(4):1969–1979. doi: 10.1002/jsfa.9395. [DOI] [PubMed] [Google Scholar]
- Pandey A, Rai R, Pal M, Pandey S. How polar are choline chloride-based deep eutectic solvents? Phys Chem Chem Phys. 2014;16:1559–1568. doi: 10.1039/C3CP53456A. [DOI] [PubMed] [Google Scholar]
- Proestos C, Komaitis M. Application of microwave-assisted extraction to the fast extraction of plant phenolic compounds. LWT Food Sci Technol. 2008;41:652–659. doi: 10.1016/j.lwt.2007.04.013. [DOI] [Google Scholar]
- Puertolas E, Saldana G, Alvarez I, Raso J. Experimental design approach for the evaluation of anthocyanin content of rose wines obtained by pulsed electric field. Influence of temperature and time of maceration. Food Chem. 2011;126:1482–1487. doi: 10.1016/j.foodchem.2010.11.164. [DOI] [Google Scholar]
- Reinhardt D, Iigen F, Kralish D, Konig B, Kreisel G. Evaluating the greenness of alternative reaction media. Green Chem. 2008;10:1170–1181. doi: 10.1039/b807379a. [DOI] [Google Scholar]
- Sharma K, Ko EY, Assefa AD, Ha S, Nile SH, Lee ET. Temperature-dependent studies on the total phenolics, flavonoids, antioxidant activities, and sugar content in six onion varieties. J Food Drug Anal. 2015;23:243–252. doi: 10.1016/j.jfda.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K, Mahato N, Nile SH, Lee ET, Lee YR. Economical and environmentally-friendly approaches for usage of onion (Allium cepa L) Food Funct. 2016;7:3354–3369. doi: 10.1039/C6FO00251J. [DOI] [PubMed] [Google Scholar]
- Shirzad H, Niknam V, Taheri M, Ebrahimzadeh H. Ultrasound-assisted extraction process of phenolic antioxidants from Olive leaves: a nutraceutical study using RSM and LC-ESI-DAD-MS. J Food Sci Technol. 2017;54(8):2361–2371. doi: 10.1007/s13197-017-2676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swatloski RP, Spear SK, Holbrey JD, Rogers RD. Dissolution of cellose with ionic liquids. J Am Chem Soc. 2002;124(18):4974–4975. doi: 10.1021/ja025790m. [DOI] [PubMed] [Google Scholar]
- Thakker MR, Parikh JK, Desai MA. Microwave assisted extraction of essential oil from the leaves of palmarosa: multi-response optimization and predictive modeling. Ind Crops Prod. 2016;86:311–319. doi: 10.1016/j.indcrop.2016.03.055. [DOI] [Google Scholar]
- Tsai PJ, She CH. Significance of phenol-protein interaction in modifying the antioxidant capacity of peas. J Agric Food Chem. 2006;54:8491–8494. doi: 10.1021/jf061475y. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Miyoshi N, Kawabata K, Yasuda M, Shimoi K. Quercetin-3-O glucuronide inhibits noradrenaline-promoted invasion of MDA-MB-231 human breast cancer cells by blocking β2-adrenergic signaling. Arch Biochem Biophys. 2014;557:18–27. doi: 10.1016/j.abb.2014.05.030. [DOI] [PubMed] [Google Scholar]
- Yu Y, Lu X, Zhou Q, Dong K, Yao H, Zhang S. Biodegradable naphthenic acid ionic liquids: synthesis, characterization, and quantitative structure-biodegradation relationship. Chem Eur J. 2008;14:11174–11182. doi: 10.1002/chem.200800620. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Vigier KDO, Royer S, Jerome F. Deep eutectic solvents: syntheses, properties and applications. Chem Soc Rev. 2012;41:7108–7146. doi: 10.1039/c2cs35178a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.