Abstract
Synthetic antioxidants can reduce postharvest losses, but consumers are concerned about chemical residues in the product. There is a growing interest in using natural compounds for the preservation of foods. In this study, the efficiency of juices or extracts obtained from three fruit species with high antioxidant activity as browning inhibitors was measured and then compared with that obtained from pomegranate by-product (PBP). The aim was to offer the most significant contributions concerning fresh-cut fruit preservation, using ‘Shahmive’ pear as the fruit model. Slices of pear were dipped in pomegranate juice (PJ) or extract (PE), kiwifruit juice (KJ) or extract (KE), grape juice (GJ) or extract (GE), PBP and chitosan before being stored at 4 °C. The total phenolic content of solutions ranged from 11 to 127.5 mg gallic acid/100 mL. Extracts of pomegranate peel and grape had the uppermost and the lowermost antioxidant activity (%DPPH), respectively. In comparison with other treatments including the control one, the minimum peroxidase (POD) activity, the maximum ascorbic acid content and the retention of firmness were seen in the fruit which was treated with kiwifruit extract. PJ + GJ treatment decreased POD and polyphenol oxidase activity and improved L* and a* values in the slices. The use of fruit juice or extracts had no adverse effect on the sensory quality of slices, with the exception of samples subjected to PJ or GJ + KJ treatments. Improving antioxidant capacity of fresh cut pear treated with KE and PJ + GJ would have a big advantage of preventing enzymatic browning.
Keywords: Antioxidants, Browning, Phenolic compounds, Postharvest disease
Introduction
By-products such as unused flesh, fruit pomace, pulps, seeds and peels, produced while processing the food, are inevitable. These secondary products, can pose serious disposal problems for the food industry. However, their outstanding features, both nutritional and technological, have so far made them encouraging sources of food processing. Gyawali and Ibrahim (2014) quite rightly noted to the various antimicrobial properties hidden in the enormous amount of organic acid, minerals, and phenolics that can be found in these byproducts remaining after the fruit and vegetables are processed. Organic acids play an important role in fresh fruits and their by-products by providing a sharp sour fresh taste (Muchuweti et al. 2005). The level of these organic acids which are the main cause of low pH values is relatively high in most fruits. These factors, namely a low pH, together with the high concentrations of organic acids are vital issues in preserving fruit. Beside organic acids, there are other known bioactive compounds including vitamins E and C, phenolic compounds, dietary fiber, and carotenoids in fruits which have been indicated to have antimicrobial effects, perform a chief function in protecting fruits against pathogens, lead to lysis, and penetrate the microorganisms’ cell membrane (Ayala-Zavala et al. 2011). Jan et al. (2013) attributed most of these effects to phenolic compounds and their free-radical scavenging activities and existing strong antioxidant.
Fresh-cut fruits are ready-to-eat products with increasing consumption growth. Peeling, cutting and other processing operations, however, can cause physiological effects including water loss, membrane deterioration, loss of acidity and speed up the plant tissues’ metabolic activities and enhance the fragility of fresh-cut fruit (Yousuf et al. 2018). Other biochemical and physiological changes related to the fresh-cut including browning enhancement on the cut surface. Browning is in fact the main reason why consumers do not like to buy fresh-cut fruits (Remorini et al. 2015). To hinder the growth of surface browning and control post-harvest diseases, a number of chemical treatments have so far been introduced. However, efforts have recently been made to find alternative ways as some postharvest fungal pathogens have become more resistant to the few approved fungicides; and the consumer demand for not only high quality but also harmless fruit and vegetables has lately increased (Gatto et al. 2011). Natural preserving agents are currently implemented to avoid fresh-cut’s tissue browning by preventing the activity of peroxidase (POD), phenylalanine ammonia lyase (PAL) and polyphenol oxidase (PPO), greatly engaged in the enzymatic browning of fresh-cut products (Supapvanich et al. 2012). To increase the shelf life of food products, researchers have recently focused largely on adding the natural antioxidants of vegetable or fruit extracts. For example Dave et al. (2017) used edible coatings containing soy protein isolate in combination with additives like hydroxypropyl methylcellulose and olive oil on ‘Babughosha’ pears. With the objective of utilizing agro-industrial by-products, Todisco et al. (2018) evaluated the effects of edible coatings containing disintegrated guava by-products on the nutritional properties of dried red guava. The results of Ciftci and Ozilgen (2019) showed that addition of black carrot juice delayed lipid oxidation, and improved the shelf life of the almond pastes. This area of research, however, is still in its infancy and to grow fresh-cut fruit that are safe and harmless and have a high nutritional value and sensory quality, more studies should be done.
The researchers of this study, taking all the mentioned issues into consideration, attempted to examine the antioxidant activity of pomegranate by-product (PBP). In addition to PBP, they selected three different fruit juice [pomegranate (PJ), kiwifruit (KJ) and grape (GJ)], which due to their phenolic and organic acid composition were of interest for the determination of antioxidant properties and for their contribution to the postharvest quality of fresh-cut fruits. According to Halvorsen et al. (2002), pomegranate, grape and kiwi had the highest antioxidant concentrations of all the fruits and vegetables analyzed. This study tried to offer the most significant contributions concerning fresh-cut fruit preservation, using ‘Shahmive’ pear as the fruit model. In parallel, their protective effect was compared with that of chitosan (as a natural fungistatic and preservative agent for the extended shelf-life of fruit products).
Materials and methods
Plant material
In September 2016, freshly harvested pears (Pyrus communis L. cv. Shahmive) were obtained from a commercial orchard in Khomeini Shahr (Isfahan, Iran) and used in this study. Flesh firmness was used to decide pear maturity and date of harvest. Fruits of uniform shape, colour, size and maturity stage without any damage or visual defects were immediately delivered to the laboratory and stored at 4 °C for 1 day prior to processing.
Antioxidant solutions
Natural juices
To prepare natural fruit juice, three different kinds of freshly harvested fruit (i.e. kiwifruit, grape, and pomegranate) with no observable external cuts or spoilage were purchased from the local market in Dorcheh, Isfahan. Initially, with clean running water, the fruits were washed. Next, using chlorinated water (0.3 w/v), the fruits were treated for 1 min. The fruits were then immersed in sterile distilled water for a minute and air-dried. The juicing processes are explained in the following: for kiwifruit, after washing and drying, the researcher cut the fruits in halves. Using a mixer, the endocarpus and seeds were triturated. Then, by means of cheesecloth, the solid residues from the liquid portion were separated. In the case of grape, the pedicels of black grape berries are bitter and hence must be separated before juice manufacture. The researcher added the berries without pedicle to a mixer. The seeds were removed by filtration. Then, glycerol (0.3% v/v) was incorporated into the fruit juices to act as a plasticizer (Supapvanich et al. 2012). For pomegranate, after washing and drying, the researcher separated the peel and arils and subsequently pressed them to get juice and seed. Juice and solid parts were separated by means of filtration.
Pomegranate by-product extract
To prepare the extract of pomegranate, the researcher used one of the previously tested experiments (Osorio et al. 2010). To do so, 150 g dried powder together with 750 mL of water were placed in a flask. For 12 h, at 60 °C, the mixture was refluxed. Next, for 15 min, the sample was filtered and centrifuged at 3500 rpm. Subsequently the extract with a concentration of 0.2 ppm was obtained. Having obtained a concentration of 0.3% (v/v), the researcher added the glycerol which acted as a plasticizer to the concentrate.
Fruit extracts
To obtain polyphenolic extracts of pomegranate (PE), kiwifruit (KE) and grape (GE), the researcher followed the procedure of Negi et al. (2003) with some modification. Briefly, 150 mL fruit juices with 750 mL water were placed in a flask. For 12 h, at 60 °C, the mixture was refluxed. The sample subsequently was filtered and centrifuged at 3500 rpm for 15 min. Next, the extract with a concentration of 0.2 ppm was obtained. Having obtained a concentration of 0.3% (v/v), the researcher added the glycerol which acted as a plasticizer to the concentrate.
Chitosan
Dissolving 1.5 g of chitosan (90% deacetylation and viscosity of 50–800) in 1% (w/v) aqueous acetic acid, the researcher prepared a 1.5% (w/v) chitosan coating solution. To be completely dissolved, the mixture was stirred at room temperature overnight. Then, glycerol [0.3% (v/v)] was added to the solution.
Pear processing and fresh-cut slice storage
To avoid surface contamination, at first, the researcher washed the pears with chlorinated water for 5 min (100 ppm active chlorine), rinsed them by tap water (5–13 °C) and then air-dried the sample for about 30 min. After peeling, based on the size of the sample, using a sharp stainless steel knife, each pear was sliced (approximately 6–9 pieces). Once the seed cavity was removed, to decrease the effect of browning and preserve the firmness of the sample, all samples of pear were immediately dipped into an aqueous solution containing 2% ascorbic acid, 0.75% N-acetyl-l-cysteine, and 1% calcium chloride for 15 min (Oms-Oliu et al. 2010). The researcher then immersed the samples in different solutions or distilled water (control) according to the design of the experiment at 4 °C for 2 min and then dried them at the same temperature for 10 min. For every treatment, fresh cut pears were packed in four individual polyethylene tray, containing 10 pieces of the fresh-cut pear in each tray and kept them at 4 ± 1 °C and 95% RH for 7 days.
Estimation of antioxidants and antioxidant activity of juices and extracts
The content of total flavonoid (FLV) was determined using aluminium trichloride method, according to Wang et al. (2014). To calculate the FLV concentration, a calibration curve (rutin as standard) was applied and expressed the output as mg/100 mL. To measure the total ascorbic acid (AsA) content, colorimetric method was used (see Kampfenkel et al. 1995). In order to form a standard curve, commercial l-Ascorbic acid was used. Spectrophotometry was applied to define the carotenoid (CAR) content; making use of the formula, the researcher calculated the concentration of the sample from the absorbance of extract at 663, 648 and 470 nm (Gunes et al. 2007). Anthocyanin (ANT) was also extracted according to methanol containing HCl method (Alexieva et al. 2001). Using Folin–Ciocalteu method with slight modifications, the amount of total phenolic (TF) compounds was estimated. Measuring the absorbance of gallic acid standards, the researcher made a calibration curve and then quantified TF using the given curve and expressed the results in mg gallic acid/100 mL (Remorini et al. 2015). Based on Vinha et al. (2014), the anti-radical ability of the sample extracts was evaluated. However, minor modifications were made by decreasing the absorbance of the methanolic solution of DPPH. To do all spectrophotometric measurements, the researcher used Schimadzu UV–VIS AA 6300 spectrophotometer.
Quality evaluations of fresh cut pears
Ascorbic acid
A slightly modified version of the method used by Kampfenkel et al. (1995) was applied to measure the total AsA content. In 1 mL of cold 6% (w:v) trichloroacetic acid (TCA),the researcher homogenized 0.1 g of frozen ground pear. Then, at 4 °C, for about 15 min, the homogenate was centrifuged at 16,000g. Next, 200 μL of supernatant was mixed with 600 μL of 0.2 M phosphate buffer (pH 7.4), 200 μL ddH2O, 1 mL 10% TCA (w/v), 800 μL 42% H3PO4 (v/v), 800 μL 4% 2,2-bipyridyl (w/v), and 400 μL 3% FeCl3(w/v). After further incubation at 42 °C for 40 min, the researcher measured the absorbance at 525 nm. Using an AsA standard curve, the AsA content in seed samples was obtained.
Titratable acidity (TA) and total soluble solids content (TSS)
Using a blender, the researcher crushed three slices of pear from each repetition (Moulinex, Barcelona, Spain) and used the resulted juice to specify TSS and TA. Using a refractometer (Atago, Japan), at 20 °C, TSS was obtained and expressed that in °Brix. TA value was also calculated by titrating 10 mL of juice with 4 g L−1 NaOH to an 8.1 pH endpoint. TA was calculated as g of malic acid per 100 mL juice.
Firmness and weight loss
Making use a texture analyzer (TA-XT2i, Godalming, UK) equipped with a probe (5 mm in diameter and a penetrating depth of 10 mm), the researcher calculated the maximum puncture force on slices (midpoint between endocarp and skin). Firmness was expressed in Newton (N). Te weight loss at different storage times was also measured and calculated that using the equation below:
where N is the number of storage days. The results were reported as the percentage loss of initial weight.
Color measurement
The researcher directly measured the color [CIE L*(lightness) and a*(− green to + red)] in a total of 3 locations. To do so, ColorFlex colorimeter (Hunterlab, VA, USA) was used. The color was measured on both cut surfaces of each piece; to make sure that color readings were representative of each piece, the mean value was obtained. The results were reported as L* and a* values.
Peroxidase (POD) and polyphenol oxidase (PPO) activity determination
Slightly modified version of the method suggested by Liu et al. (2007) was used to extract POD and PPO. Consequently, 2 g frozen flesh was homogenized with 10 mL of ice-cold 50 mM sodium phosphate buffer (pH 7) having 0.2 g of polyvinyl polypyrrolidone (PVPP) and powdered at 4 °C. Centrifuging the homogenate was then done at 15,000×g for 30 min at 4 °C; for the enzyme assay, the supernatant was used.
The changes in the absorbance at 470 nm were the basis to assay POD activity (Chen et al. 2017). 2.8 mL of substrate solution and 50 μL of the enzyme extract were inserted in the reaction cuvette. The substrate solution included 20 mM guaiacol and 25 mM hydrogen peroxide that were dissolved in 50 mM sodium phosphate buffer (pH 7.0).
To determine PPO activity, every 3 mL reaction mix included 100 μL enzyme extract, 0.1 M catechol, and 0.1 M sodium phosphate buffer (pH 6.8). At 420 nm, an increase was observed in the absorbance (Hong et al. 2013).
Sensory analysis
Sliced pears were sensory evaluated using a hedonic scale of nine points. Number 9, 7, 5, 3, and 1 indicated excellent (just sliced), very good, good, (limit of marketability), fair (limit of usability) and poor (inedible), respectively. Fruit were evaluated using twenty untrained judges. Panelists assessed the combination of pear slices covered with natural juice or extracts. Color, aroma, texture, flavor, and overall acceptability were evaluated (Xu et al. 2015).
Statistical analysis
A complete randomized design was used in this study. Inferential statistics including ANOVA and LSD (95% confidence interval) was used to analyze the effect of antioxidant solutions (treatment) and the storage time. All statistical analysis was defined to be significant if p < 0.05.
Results and discussion
Natural antioxidant composition
Table 1 summarizes the contents of CAR, ANT, TF, FLV, AsA and anti-radical ability of sample extracts and juices. Based on the results of the study, all juices under investigation showed noticeable amounts of phenolic compounds. The highest TF was observed in PBP’s extract. PJ had the highest ANT. The highest total CAR was seen in GJ and PJ. The highest FLV content was recorded in KJ. The FLV and AsA contents of GJ were higher than those of juices prepared from other fruits. In general, prepared extracts had lower content of phenolic compound than juices. The extract of PBP was characterized by the presence of phenolic compounds and anti-radical ability (DPPH) and the absence of AsA and ANT. In comparison to the contents of grape juice extracts, DPPH radical-scavenging activity and TP content of KE and PE were almost twofold higher. Based on these data, differences in the TFs of the fruit extracts under investigation were significant. Under the test conditions, natural juices and extracts with higher total phenolics contents, namely PJ and PBP, had the uppermost antioxidant activity (%DPPH), in comparison with other treatments including the control one. As a variety of phenolic compounds and their derivatives are present in different plant species, the researchers expected variations in the total amount of phenolics in the tested fruit juices. The level of these compounds in the peels or the non-edible parts of fruits was significantly higher than that in the edible sections (aril juice). These findings were in line with the results of Orgil et al. (2014).
Table 1.
The total ascorbic acid, flavonoid, anthocyanin, carotenoid and phenolic content and related total antioxidant capacity determined as %inhibition of DPPH of used natural juices and extracts
| DPPH (%) | Ascorbic acid (mg/mL) | Flavonoid (mg RUT/100 mL) | Anthocyanin (μg/100 mL) | Carotenoid (μg 100/mL) | Phenolics (mg GAE/100 mL) | |
|---|---|---|---|---|---|---|
| Pomegranate juice (PJ) | 84.9a | 0.11de | 63.40ab | 177.50a | 91.50a | 108.36b |
| Kiwifruit juice (KJ) | 40.65c | 0.210b | 74.65a | 52.00d | 36.25cd | 53.82d |
| Grape juice (GJ) | 35.38c | 0.12d | 16.27d | 9.00f | 99.00a | 30.66e |
| PJ + KJ (1:1) | 71.55b | 0.24a | 72.55a | 104.50b | 52.25bc | 77.95c |
| PJ + GJ (1:1) | 63.67b | 0.23a | 40.21c | 76.00c | 72.00ab | 67.46cd |
| GJ + KJ (1:1) | 43.70c | 0.17c | 43.74c | 30.50e | 41.50c | 22.87e |
| Pomegranate extract | 20.34d | 0.09ef | 11.52d | 1.50fg | 7.10e | 33.15e |
| Grape extract | 10.17d | 0.09ef | 10.85d | 00.00g | 7.25e | 10.99f |
| Kiwifruit extract | 19.84d | 0.09f | 10.17d | 00.00g | 9.75de | 21.32ef |
| Pomegranate by-product extract | 93.61a | 0.03g | 54.99bc | 4.50fg | 57.75bc | 127.51a |
Data followed by the same letters are not significantly different at 5% level of probability using least significant differences test
Characterization of pear cuts
Ascorbic acid content
Table 2 shows the AsA content of the ‘Shahmive’ pear tissues. These data were collected immediately after treatment, and 3 and 7 days of cold storage. Initially, AsA concentration was approximately 0.053 mg/g fruit. As was expected, due to AsA content of the treatments, AsA concentration of all samples significantly increased in throughout 3 days at 4 °C. Nonetheless, this increment was not seen at 4 °C after 7 days, in pear slices. The AsA content of all fruit slices except PJ + KJ and KE treated slices dropped to endogenous control levels. As a general antioxidant and the main nonprotein reductant for redox homeostasis in plants, ascorbic acid performs a significant role. In general, PJ, PJ + KJ or KE treated slices contained higher contents of AsA than other treatments or control. This result can be attributed to the high antioxidant activity of these juices or extracts. Vitamin C decrease over time looks to be mainly because of oxidation. Thus, antioxidant treatment can inhibit the progress of such deterioration, thereby resulting in a fresh-cut product having a final AsA content as high as fresh fruits (González-Aguilar et al. 2008). Once chitosan film was applied, vitamin C deterioration could not be avoided. Recently, researchers have found a new tocopherol analogue (tocomonoenol) in the kiwifruit. The remarkable role of tocomonoenol in the total antioxidant activity of kiwifruits has been studied (Sârbu et al. 2012). Although not measured in our study, it is likely that the tocomonoenol well interacts with hydrophilic compounds (namely vitamin C and phenolic compounds) present in the fruit juice, resulting in an increase in its antioxidant activity.
Table 2.
Changes in ascorbic acid, soluble solid content, titrable acidity and firmness of fresh-cut pears of twelve treatments during 7 days at 4 °C
| Time (days) | Pomegranate juice (PJ) | Kiwifruit juice (KJ) | Grape juice (GJ) | PJ + KJ (1:1) | PJ + GJ (1:1) | GJ + KJ (1:1) | Pomegranate extract | Kiwifruit extract | Grape extract | Pomegranate by-product extract | Chitosan | Control |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ascorbic acid (mg/g FW) | ||||||||||||
| 0 | 0.053fgh | 0.053fgh | 0.053fgh | 0.053fgh | 0.053fgh | 0.053fgh | 0.053fgh | 0.053fgh | 0.053fgh | 0.053fgh | 0.053fgh | 0.053fgh |
| 3 | 0.208a | 0.192a | 0.145bc | 0.194a | 0.106d | 0.135c | 0.16bc | 0.149bc | 0.106d | 0.163b | 0.083def | 0.136bc |
| 7 | 0.058fgh | 0.06efgh | 0.05gh | 0.088de | 0.061e–h | 0.069efg | 0.04gh | 0.105d | 0.053fgh | 0.053fgh | 0.056fgh | 0.037h |
| Soluble solid (°Brix) | ||||||||||||
| 0 | 14.83a | 14.83a | 14.83a | 14.83a | 14.83a | 14.83a | 14.83a | 14.83a | 14.83a | 14.83a | 14.83a | 14.83a |
| 3 | 11.6g–j | 11.15h–l | 12.2e–h | 12.33efg | 10.83j–m | 13.86abc | 11.03i–l | 11.26h–l | 10.53j–m | 11.86f–i | 11.5g–k | 11.03i–l |
| 7 | 12.5d–g | 14.76a | 12.96c–f | 15.03a | 12.23e–h | 13.53bcd | 11.76ghi | 14.43ab | 12.5d–g | 11.73ghi | 11.83ghi | 12.13e–i |
| Titrable acidity (g malic acid/100 mL) | ||||||||||||
| 0 | 0.167a–e | 0.167a–e | 0.167a–e | 0.167a–e | 0.167a–e | 0.167a–e | 0.167a–e | 0.167a–e | 0.167a–e | 0.167a–e | 0.167a–e | 0.167a–e |
| 3 | 0.158b–g | 0.104j | 0.151c–h | 0.147c–i | 0.117ij | 0.151c–h | 0.144d–i | 0.138e–i | 0.125hij | 0.152b–h | 0.167a–e | 0.144d–i |
| 7 | 0.159b–g | 0.152b–h | 0.174a–d | 0.163a–f | 0.184ab | 0.127g–j | 0.138e–i | 0.193a | 0.178abc | 0.164a–f | 0.135e–i | 0.134f–i |
| Firmness (N) | ||||||||||||
| 0 | 7.93bc | 7.93bc | 7.93bc | 7.93bc | 7.93bc | 7.93bc | 7.93bc | 7.93bc | 7.93bc | 7.93bc | 7.93bc | 7.93bc |
| 3 | 7.90c | 7.75cd | 7.90bc | 7.38g | 7.68cde | 7.63def | 7.44efg | 8.20a | 7.62d–g | 7.43fg | 7.75cd | 7.79cd |
| 7 | 7.92bc | 7.68cde | 7.80cd | 7.75cd | 7.10h | 6.87i | 7.75cd | 8.10ab | 7.85cd | 7.11h | 8.10ab | 7.67cde |
Data followed by the same letters are not significantly different at 5% level of probability using least significant differences test
Total soluble solids and titrable acidity
TSS values (Table 2) fluctuated from 0 to 7 days, and decreased in the later storage time. The control samples and PJ + GJ, PE and GE treated slices had significantly lower TSS value than other samples and GJ + KJ sample showed different trends during the first 3 days. TSS was significantly more in KJ, PJ + KJ or KE treatments than in pomegranate-coated (PJ, PE, PBP) or grape-coated (GJ, GE, PJ + GJ) fruits once the experiment came to an end. As can be seen in Table 2, within the first 3 days, TA was gradually declined and lost in all samples. On 7th day, the TA values of GJ, PJ + GJ, GE or KE-treated fresh-cut pears was greatly higher than the values of untreated samples (Table 2). Moreover, TA values of pears examined instantly after treatment and those of PJ, GJ, PJ + KJ, PE, PBP and chitosan-treated pears were comparable. According to Salinas-Roca et al. (2016), in a number of treatments, when the researchers dip the fruit pieces into the given solution, sugar lixiviation may decrease the TSS parameter in fresh-cut fruits. On 7th day, TSS content of various fresh-cut pears increased, except the samples subjected to the GJ + KJ or PBP treatments. To explain the results, we can refer to the post-harvest preserving condition and continuation of acid metabolism as a result of fruit ripening and senescence, by transforming starch and acids to sugars to be used in metabolism. These results revealed that, in comparison with other coated fruits, glucose metabolism rapidly progressed in control, PE, PBP or chitosan treated fruits, as reported by Hashemi et al. (2017). The low respiration of pear slices can be the reason of the high content of TA as organic acids are, with sugars, respiratory substrates (Silveira et al. 2013). Similar result was reported in the previous studies, in which TA value was mostly constant in fresh-cut pears during the storage time and coating generally helped to retain the TA of fruit similarly to that provided by the controlled atmosphere preventing gas exchange (Xu et al. 2015). Barrier properties of the edible coatings can decrease the surface permeability of fruits to O2 and CO2, result in enhancement of the CO2 concentration of fruit tissues and the O2 concentration reduction, which, in turn, prolongs the shelf life of fruits (Vargas-Torres et al. 2017). Antioxidant coatings that resulted in higher TA, were less permeable to O2 and CO2 than others. Thus the application of such coating could retard the ripening process in pears.
Firmness and weight loss
Untreated fruit did not significantly lose their firmness during seven days of shelf-time. Slices from the control were significantly firmer after 7 days at 4 °C than GJ + KJ coated slices while the samples subjected to KE treatment had the highest firmness throughout the whole storage time (Table 2). Table 3 shows that all samples experienced a gradual loss of weight during the storage. Different antioxidants did not affect pear slice weight loss just after the processing (day 3). After 7 days, the weight loss of all samples largely increased and the PJ + KJ, PJ + GJ or GJ + KJ coated treated fruit and the control group compared to the other treatments lost significantly more weight. On 7th day, pears in the control group incurred 8.81% weight loss while 3.44% loss of weight was observed in the fruit coated with PE. Similarly, treating with PJ could well preserve the appearance of pear slices. With respect to quality parameters, TSS and firmness decreased and weight loss increased for all control and treated pears after storing for 7 days at 4 °C although we found that pears treated with PJ or KE had the highest quality, which indicated a lower metabolism (lowering production of ethylene and rate of respiration). It must be noted that each treatment had diverse pathways to increase quality. The content of TSS in the fruit treated with kiwifruit-based antioxidant (KJ, KE or PJ + KJ) was the highest whereas the weight loss of the pomegranate treatments (PJ, PE or PBP) was the least. After processing, treated pear slices, except those subjected to PJ + GJ, GJ + KJ and PBP treatments, were firmer than untreated pear pieces. The pectin gradual worsening of the cell wall and the starch hydrolysis to sugar usually lead to fruit softening (Vargas-Torres et al. 2017). Salinas-Roca et al. (2016) stated that the firmness loss of the minimally processed mango coated with alginate decreased in their study. Likewise, other researchers (e.g. Ghidelli et al. 2014) found similar findings in other fruits and vegetables like strawberry fruits coated with lemon extract and fresh-cut eggplant coated with soy protein–cysteine based edible coating.
Table 3.
Changes in weight loss (%) of fresh-cut pears of twelve treatments during 7 days at 4 °C
| Treatment | Time (days) | |
|---|---|---|
| 3 | 7 | |
| Pomegranate juice (PJ) | 1.09h | 3.55g |
| Kiwifruit juice (KJ) | 1.13h | 7.03cd |
| Grape juice (GJ) | 1.66h | 5.56f |
| PJ + KJ (1:1) | 1.96h | 8.00bc |
| PJ + GJ (1:1) | 1.60h | 8.00bc |
| GJ + KJ (1:1) | 1.16h | 9.44a |
| Pomegranate extract | 1.29h | 3.44g |
| Kiwifruit extract | 1.69h | 5.59f |
| Grape extract | 1.78h | 7.03cd |
| Pomegranate by-product extract | 1.05h | 5.29f |
| Chitosan | 1.36h | 5.84ef |
| Control | 1.32h | 8.81ab |
Data followed by the same letters are not significantly different at 5% level of probability using least significant differences test
Colour
The browning potential of the control and treated groups is shown in Table 4. Measured by L* value changes throughout storage at 4 °C, pear slices treated with PJ, PJ + KJ or GJ + KJ significantly revealed cut surface discoloration. No significant differences were seen between the treated and untreated samples except pears subjected to the PJ, PJ + KJ or GJ + KJ treatment once the experiment came to an end. Concerning a* values, compared to all other treatments, pear slices treated with PJ, PJ + KG and GJ + KJ received significantly higher a* values immediately after cutting (day 3). These results were in line with the corresponding L* values. However, while a* values for the control and some coated samples gradually rose, a* values for KJ, PJ + GJ, GE, PBP and chitosan treated samples were rather constant to the end of storing. Only a* values of PJ treated pears were positive indicating that the browning effect was progressing while the values of PJ + GJ, GE and chitosan coated slices slightly increased reflecting their yellow-greenish colour. Based on these results, researchers can well hinder the enzymatic browning of fresh cut pears using KJ, PJ + GJ, GJ and chitosan treatments. Using pineapple extract dip, Supapvanich et al. (2012) could successfully stopped the browning of fresh cut rose apple fruit. Chaisakdanugull et al. (2007) indicated that the browning of banana pulp could be slowed down using pineapple juice dip due to hindering PPO activity. Similarly, natural antioxidant preservative may preserve natural pigments of pear including carotenoids which are confined in the cells, thus avoiding the oxidation during storing time (Salinas-Roca et al. 2016). The positive value of a* and a decrease of L* value in PJ treated slices may be due to the deterioration or polymerization of anthocyanins at high temperatures indicating a fading of the typical red color of PJ. Consequently, the juices’ color looked browner.
Table 4.
Color changes of L* and a* from fresh-cut pears of twelve treatments during 7 days at 4 °C
| Time (days) | Pomegranate juice (PJ) | Kiwifruit juice (KJ) | Grape juice (GJ) | PJ + KJ (1:1) | PJ + GJ (1:1) | GJ + KJ (1:1) | Pomegranate extract | Kiwifruit extract | Grape extract | Pomegranate by-product extract | Chitosan | Control |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L* (lightness) | ||||||||||||
| 0 | 85ab | 85ab | 85ab | 85ab | 85ab | 85ab | 85ab | 85ab | 85ab | 85ab | 85ab | 85ab |
| 3 | 78de | 82a–e | 86ab | 80b–e | 86a | 79cde | 85ab | 84abc | 84abc | 84abc | 82a–d | 83a–d |
| 7 | 72f | 83a–d | 83a–d | 76ef | 83a–d | 72f | 80b–e | 80b–e | 81a–e | 81a–e | 83a–d | 81a–e |
| a*(− green to +red) | ||||||||||||
| 0 | − 11hi | − 11hi | − 11hi | − 11hi | − 11hi | − 11hi | − 11hi | − 11hi | − 11hi | − 11hi | − 11hi | − 11hi |
| 3 | 2b | − 9fgh | − 9fgh | − 6de | − 13i | − 5d | − 9fgh | − 9fgh | − 12i | − 10ghi | − 10ghi | − 13i |
| 7 | 5a | − 9fgh | − 6def | − 5de | − 10ghi | 00c | − 6def | − 8efg | − 11hi | − 9fgh | − 12i | − 5d |
Data followed by the same letters are not significantly different at 5% level of probability using least significant differences test
Peroxidase and polyphenol oxidase activity
POD activity was not significantly affected by various treatments during the first 3 days (Table 5). Nonetheless, the control samples had significantly higher POD activity than other samples after 7 days at 4 °C. Again, antioxidant coating generally helped to retain POD activity of fruit similar to that measured in raw materials. POD activity of the control was similar to that of the fruits coated with chitosan whereas the pears coated with PJ + KJ, PJ + GJ or KE had the lowest POD activity. The PPO activity of the fruit subjected to different treatments is reported in Table 5. Compared with fresh sliced fruit (8.2 U g FW−1 min−1), PPO activity of PE or PBP coated or control fruits significantly increased at the beginning of the storage. Concerning the untreated fresh-cut pear, when the experiment came to an end, the PPO activity reached 123.2% of the initial value. PJ + GJ, KE or chitosan treatments greatly prevented the rise of PPO activity; the maximal values obtained were only 39.0, 42.7 and 25.6% of that obtained in control groups (day 0), respectively.
Table 5.
Changes in peroxidase and polyphenol oxidase activity of fresh-cut pears during storage at 4 °C
| Time (days) | Pomegranate juice (PJ) | Kiwifruit juice (KJ) | Grape juice (GJ) | PJ + KJ (1:1) | PJ + GJ (1:1) | GJ + KJ (1:1) | Pomegranate extract | Kiwifruit extract | Grape extract | Pomegranate by-product extract | Chitosan | Control |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peroxidase activity (U g FW−1 min−1) | ||||||||||||
| 0 | 22fgh | 22fgh | 22fgh | 22fgh | 22fgh | 22fgh | 22fgh | 22fgh | 22fgh | 22fgh | 22fgh | 22fgh |
| 3 | 16f–i | 5hi | 8hi | 18f–i | 12ghi | 34ef | 16f–i | 12ghi | 18f–i | 20f–i | 12ghi | 10hi |
| 7 | 64cd | 48de | 80bc | 28fgh | 32efg | 98ab | 60d | 24fgh | 64cd | 94ab | 92ab | 105a |
| Polyphenol oxidase activity (U g FW−1 min−1) | ||||||||||||
| 0 | 8.2gh | 8.2gh | 8.2gh | 8.2gh | 8.2gh | 8.2gh | 8.2gh | 8.2gh | 8.2gh | 8.2gh | 8.2gh | 8.2gh |
| 3 | 10.8d–h | 10.0e–h | 11.7c–h | 6.7h | 6.5h | 13.0a–g | 15.2a–e | 8.9fgh | 13.0a–g | 16.9abc | 13.3a–g | 14.6a–f |
| 7 | 16.5abc | 14.7a–e | 18.3ab | 14.6a–f | 11.4c–h | 18.6a | 14.7a–e | 11.7c–h | 12.8b–g | 14.8a–e | 10.3d–h | 18.3ab |
Data followed by the same letters are not significantly different at 5% level of probability using least significant differences test
In this research, POD activity was lower in treated fruit than in controls suggestive of lesser oxidative stress during this period. Our findings were in line with the results of Chauhan et al. (2011) noting that coated apples displayed decreased POD activity. Investigating apples treated with shellac and aloe-gel-based surface coatings, they found that an increase in the respiration, as a result of cutting operation, could possibly induce these enzymes. In response to the stress created by the wounding process, in the form of cutting, PPO is activated. PPO catalyzes the oxidation of phenols to o-quinones, involved in the formation of polymeric dark-colored pigment deposits. During storage, samples registered decreased in L* value that is associated with the higher susceptibility to enzymatic browning (higher PPO levels) than others. Compared to the control, the low PPO activity suggested that the application of natural preservatives could effectively prolong the shelf life and preserve the quality of fresh-cut pear pieces during storage. Investigating chitosan, enriched with rosemary extract, Xiao et al. (2010) also found similar findings indicating that PPO activity of the fruit decreased and the browning reactions of the minimally processed pear were inhibited.
Sensory evaluation
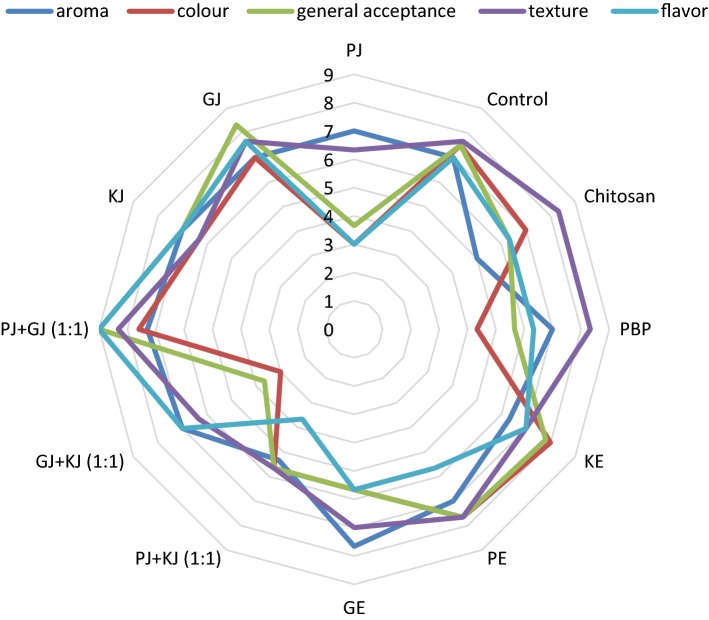
After seven days of storage, better results were obtained for flavor for PJ + GJ, GJ and KE-coated pears (Fig. 1). The maximum overall acceptability value was reached by fresh-cut pear in PJ + GJ followed by GJ and KE, associated with the above-mentioned point regarding flavor. It should be pointed out that although lower scores were obtained for aroma in chitosan-coated pears, judges did not describe that as the presence of an off-flavor. Concerning color pear cuts, GJ + KJ and PJ-treated were the lowest-rated samples while KE and PJ + GJ antioxidants possibly developed color properties of fresh cut fruits during the storage. Texture is the main sensory quality attribute in fresh-cut fruits. According to panelists, all pear cuts (control and coated) enjoyed good texture and aroma after eight days of storing (> 5 on a scale of 1 [definitely dislike] to 9 [definitely like]). Antioxidant treatments were effective in preventing fungal infection of pear slices. It has been indicated that using natural juice or extracts as preservative agents greatly influence the sensorial characteristics and consequently acceptability by consumers, which should be the focus of attention (Hashemi et al. 2017). Among the articles assessing the effect of fruit juices on the sensory quality of fresh-cut products, Silveira et al. (2013) compared untreated and pineapple, grape or apple juice treated fruit and concluded that fresh-cut melon in pineapple juice reached the uppermost overall quality. The effect of PJ + GJ, GJ and KE treatment on maintaining the quality of pear slices may be because of their influence on the prevention of O2 diffusion and in turn, TA reduction and weight loss. The quality properties of pears might be related to their ability to reduce oxidative stress (low POD activity).
Fig. 1.
Sensory characteristics of fresh-cut pears of different treatments stored for 7 days at 4 °C. Values represent the means of the replicates (n = 4). Control: untreated pear slices; PJ, GJ and KJ: pear samples coated with pomegranate, grape and kiwifruit juice, respectively; PE, GE and KE: pear samples dipped into extract solution of pomegranate, grape and kiwifruit, respectively; PBP: pear samples treated by pomegranate by-product extract; Chitosan: pear samples treated by chitosan (1.5%)
Conclusion
By investigating three different fruit juice and extract, we concluded that it is possible to substitute the synthetic compounds with preservatives safer for man and environment. We found that compared with the control, those fruits coated with KE and PJ + GJ had the highest TA and sensory perception after 7 days at 4 °C. Other treatments such as PJ + KJ and GJ were also effective in maintaining the acidity and sensory perception. KE treated slices exhibited higher AsA concentration, TSS content and firmness and lower POD and PPO activity than others. PJ + GJ treatment decreased POD and PPO activity and improved L* and a* values in the slices. Therefore, water extract of kiwifruit and combination of pomegranate and grape juices can be regarded as a safe alternative way to prolong the postharvest shelf life of fresh cut pears. Juices and extracts from different fruits exhibited the incidence and growth of fungi on the surface of the pear slices. The results obtained in this research could be advantageously used by industry as a request of consumer demand for pesticide-free food. Even though antioxidant features of the extracted phenolics in PBP were notable (96% inhibition of DPPH), this coating formula can not be considered as a good preservative because of the low quality attributes of the treated pears. The feasibility of PJ or GJ + KJ as new edible antioxidant coatings was not totally ensured because of the low sensory scores that were near the limit of rejection. More studies, however, should be done to understand how microorganisms grow and what physiological mechanisms may help to preserve the quality in these treatments. In the present study, a simple direct relationship between the antioxidant content of juices or extracts and the related activities to postharvest quality was not found.
Acknowledgements
The authors thank Dr. A.A. Ramin (Isfahan University of Technology) for his appreciated assistance during this study. The experiments done in this study were financially supported by the Isfahan University of Technology.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alexieva V, Sergiev I, Mapelli S, Karanov E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001;24:1337–1344. doi: 10.1046/j.1365-3040.2001.00778.x. [DOI] [Google Scholar]
- Ayala-Zavala JF, Vega-Vega V, Rosas-Domínguez C, Palafox-Carlos H, Villa-Rodriguez JA, Wasim Siddiqui Md, Dávila-Avina JE, González-Aguilar GA. Agro-industrial potential of exotic fruit byproducts as a source of food additives. Food Res Int. 2011;44:1866–1874. doi: 10.1016/j.foodres.2011.02.021. [DOI] [Google Scholar]
- Chaisakdanugull C, Theerakulkait C, Wrolstad RD. Pineapple juice and its fractions in enzymatic browning inhibition of banana [Musa (AAA group) Gros Michel] J Agric Food Chem. 2007;55:4252–4257. doi: 10.1021/jf0705724. [DOI] [PubMed] [Google Scholar]
- Chauhan OP, Raju PS, Singh A, Bawa AS. Shellac and aloe-gel-based surface coatings for maintaining keeping quality of apple slices. Food Chem. 2011;126:961–966. doi: 10.1016/j.foodchem.2010.11.095. [DOI] [Google Scholar]
- Chen X, Ren L, Li M, Qian J, Fan J, Du B. Effects of clove essential oil and eugenol on quality and browning control of fresh-cut lettuce. Food Chem. 2017;214:432–439. doi: 10.1016/j.foodchem.2016.07.101. [DOI] [PubMed] [Google Scholar]
- Ciftci D, Ozilgen S. Evaluation of kinetic parameters in prevention of quality loss in stored almond pastes with added natural antioxidant. J Food Sci Technol. 2019;56:483–490. doi: 10.1007/s13197-018-3510-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave RK, Ramana Rao TV, Nandane AS. Improvement of post-harvest quality of pear fruit with optimized composite edible coating formulations. J Food Sci Technol. 2017;54:3917–3927. doi: 10.1007/s13197-017-2850-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto MA, Ippolito A, Linsalata V, Cascarano NA, Nigro F, Vanadia S, Di Venere D. Activity of extracts from wild edible herbs against postharvest fungal diseases of fruit and vegetables. Postharvest Biol Technol. 2011;61:72–82. doi: 10.1016/j.postharvbio.2011.02.005. [DOI] [Google Scholar]
- Ghidelli C, Mateos M, Rojas-Argudo C, Pérez-Gago MB. Extending the shelf life of fresh-cut eggplant with a soy protein–cysteine based edible coating and modified atmosphere packaging. Postharvest Biol Technol. 2014;95:81–87. doi: 10.1016/j.postharvbio.2014.04.007. [DOI] [Google Scholar]
- González-Aguilar GA, Celis J, Sotelo-Mundo RR, de la Rosa LA, Rodrigo-Garcia J, Alvarez-Parrilla E. Physiological and biochemical changes of different fresh-cut mango cultivars stored at 5 °C. Int J Food Sci Technol. 2008;43:91–101. doi: 10.1111/j.1365-2621.2006.01394.x. [DOI] [Google Scholar]
- Gunes A, Inal A, Alpaslan M, Eraslan F, Guneri Bagci E, Cicek N. Salicylic acid induced changes on some physiological parameters symptomatic for oxidative stress and mineral nutrition in maize (Zea mays L.) grown under salinity. J Plant Physiol. 2007;164:728–736. doi: 10.1016/j.jplph.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Gyawali R, Ibrahim SA. Natural products as antimicrobial agents. Food Control. 2014;46:412–429. doi: 10.1016/j.foodcont.2014.05.047. [DOI] [Google Scholar]
- Halvorsen BL, Holte K, Myhrstad MCW, Barikmo I, Hvattum E, Remberg SF, Wold A-B, Haffner K, Baugerød H, Andersen LF, Moskaug JØ, Jacobs DR, Blomhoff R. A systematic screening of total antioxidants in dietary plants. J Nutr. 2002;132:461–471. doi: 10.1093/jn/132.3.461. [DOI] [PubMed] [Google Scholar]
- Hashemi SMB, Mousavi Khaneghah A, Ghaderi Ghahfarrokhi M, Eş I. Basil-seed gum containing Origanum vulgare subsp. viride essential oil as edible coating for fresh cut apricots. Postharvest Biol Technol. 2017;125:26–34. doi: 10.1016/j.postharvbio.2016.11.003. [DOI] [Google Scholar]
- Hong K, Xu H, Wang J, Zhang L, Hu H, Jia Z, Gu H, He Q, Gong D. Quality changes and internal browning developments of summer pineapple fruit during storage at different temperatures. Sci Hortic. 2013;151:68–74. doi: 10.1016/j.scienta.2012.12.016. [DOI] [Google Scholar]
- Jan S, Khan MR, Rashid U, Bokhari J. Assessment of antioxidant potential, total phenolics and flavonoids of different solvent fractions of Monotheca buxifolia fruit. Osong Public Health Res Perspect. 2013;4:246–254. doi: 10.1016/j.phrp.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampfenkel K, Van Montagu M, Inzé D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal Biochem. 1995;225:165–167. doi: 10.1006/abio.1995.1127. [DOI] [PubMed] [Google Scholar]
- Liu J, Tian S, Meng X, Xu Y. Effects of chitosan on control of postharvest diseases and physiological responses of tomato fruit. Postharvest Biol Technol. 2007;44:300–306. doi: 10.1016/j.postharvbio.2006.12.019. [DOI] [Google Scholar]
- Muchuweti M, Zenda G, Ndhlala AR, Kasiyamhuru A. Sugars, organic acid and phenolic compounds of Ziziphus mauritiana Fruit. Eur Food Res Technol. 2005;221:570–574. doi: 10.1007/s00217-005-1204-6. [DOI] [Google Scholar]
- Negi PS, Jayaprakasha GK, Jena BS. Antioxidant and antibacterial activities of Punica granatum peel extracts. J Food Sci. 2003;68:1473–1477. doi: 10.1111/j.1365-2621.2003.tb09669.x. [DOI] [Google Scholar]
- Oms-Oliu G, Rojas-Graü MA, González LA, Varela P, Soliva-Fortuny R, Hernando Hernando MI, Munuera IP, Fiszman S, Martín-Belloso O. Recent approaches using chemical treatments to preserve quality of fresh-cut fruit: a review. Postharvest Biol Technol. 2010;57:139–148. doi: 10.1016/j.postharvbio.2010.04.001. [DOI] [Google Scholar]
- Orgil O, Schwartz E, Baruch L, Matityahu I, Mahajna J, Amir R. The antioxidative and anti-proliferative potential of non-edible organs of the pomegranate fruit and tree. LWT Food Sci Technol. 2014;58:571–577. doi: 10.1016/j.lwt.2014.03.030. [DOI] [Google Scholar]
- Osorio E, Flores M, Hernández D, Ventura J, Rodríguez R, Aguilar CN. Biological efficiency of polyphenolic extracts from pecan nuts shell (Carya Illinoensis), pomegranate husk (Punica granatum) and creosote bush leaves (Larrea tridentata Cov.) against plant pathogenic fungi. Ind Crop Prod. 2010;31:153–157. doi: 10.1016/j.indcrop.2009.09.017. [DOI] [Google Scholar]
- Remorini D, Landi M, Tardelli F, Lugani A, Massai R, Graziani G, Fogliano V, Guidi L. Effect of chlorine dioxide and ascorbic acid on enzymatic browning and shelf life of fresh-cut red delicious and granny smith apples. J Food Proc Preserve. 2015;39:2925–2934. doi: 10.1111/jfpp.12544. [DOI] [Google Scholar]
- Salinas-Roca B, Soliva-Fortuny R, Welti-Chanes J, Martín-Belloso O. Combined effect of pulsed light, edible coating and malic acid dipping to improve fresh-cut mango safety and quality. Food Control. 2016;66:190–197. doi: 10.1016/j.foodcont.2016.02.005. [DOI] [Google Scholar]
- Sârbu C, Naşcu-Briciu RD, Kot-Wasik A, Gorinstein S, Wasik A, Namieśnik J. Classification and fingerprinting of kiwi and pomelo fruits by multivariate analysis of chromatographic and spectroscopic data. Food Chem. 2012;130:994–1002. doi: 10.1016/j.foodchem.2011.07.120. [DOI] [Google Scholar]
- Silveira AC, Aguayo E, Artés F. Shelf-life and quality attributes in fresh-cut Galia melon combined with fruit juices. Food Sci Technol. 2013;50:343–348. [Google Scholar]
- Supapvanich S, Prathaan P, Tepsorn R. Browning inhibition in fresh-cut rose apple fruit cv. Taaptimjaan using konjac glucomannan coating incorporated with pineapple fruit extract. Postharvest Biol Technol. 2012;73:46–49. doi: 10.1016/j.postharvbio.2012.05.013. [DOI] [Google Scholar]
- Todisco KM, Janzantti NS, Santos AB, Galli FS, Mauro MA. Effects of temperature and pectin edible coatings with guava by-products on the drying kinetics and quality of dried red guava. J Food Sci Technol. 2018;55:4735–4746. doi: 10.1007/s13197-018-3369-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Torres A, Becerra-Loza AS, Sayago-Ayerdi SG, Palma-Rodríguez HM, de Lourdes García-Magãna M, Montalvo-González E. Combined effect of the application of 1-MCP and different edible coatings on the fruit quality of jackfruit bulbs (Artocarpus heterophyllus Lam) during cold storage. Sci Hortic. 2017;214:221–227. doi: 10.1016/j.scienta.2016.11.045. [DOI] [Google Scholar]
- Vinha AF, Alves RC, Barreira SVP, Castro A, Costa ASG, Beatriz M, Oliveira PP. Effect of peel and seed removal on the nutritional value and antioxidant activity of tomato (Lycopersicon esculentum L.) fruits. Food Sci Technol. 2014;55:197–202. [Google Scholar]
- Wang Y, Xie X, Long LE. The effect of postharvest calcium application in hydro-cooling water on tissue calcium content, biochemical changes, and quality attributes of sweet cherry fruit. Food Chem. 2014;160:22–30. doi: 10.1016/j.foodchem.2014.03.073. [DOI] [PubMed] [Google Scholar]
- Xiao C, Zhu L, Luo W, Song X, Deng Y. Combined action of pure oxygen pretreatment and chitosan coating incorporated with rosemary extracts on the quality of fresh-cut pears. Food Chem. 2010;121:1003–1009. doi: 10.1016/j.foodchem.2010.01.038. [DOI] [Google Scholar]
- Xu M, Liu H, Huang M, Zhou D, Cao Q, Ma K. Effects of high pressure nitrogen treatments on the quality of fresh-cut pears at cold storage. Innov Food Sci Emerg Technol. 2015;32:56–63. doi: 10.1016/j.ifset.2015.09.006. [DOI] [Google Scholar]
- Yousuf B, Qadri OS, Srivastava AK. Recent developments in shelf-life extension of fresh-cut fruits and vegetables by application of different edible coatings: A review. LWT Food Sci Technol. 2018;89:198–209. doi: 10.1016/j.lwt.2017.10.051. [DOI] [Google Scholar]