Abstract
The National Bureau of Standards has issued a linear polyethylene standard reference material, SRM 1475. In this report a general description of the sample is given, and the characterization work described in the subsequent reports is outlined. Some pellet-to-pellet variability was found and estimated.
Keywords: Fractionation, linear polyethylene, molecular weight, molecular weight distribution, National Bureau of Standards, pellet variability, standard reference material
1. Purpose
The National Bureau of Standards has issued a linear polyethylene standard reference material, SRM 1475,** to fill the need for a well-characterized sample of this polymer. The sample may be used for the calibration of instruments for measuring molecular weight and molecular weight distribution, such as light scattering photometers and gel permeation chromatographs. It should also be useful in other areas of polymer research such as dilute solution studies, polymer rheology, and polymer crystal physics.
2. Properties Measured
The characterization results which are described in the succeeding papers in this series [1]1 include a determination of:
(1) Weight average molecular weight by light scattering,
(2) molecular weight distribution by gel permeation chromatography and, from this distribution, weight average, number average, and Z average molecular weights,
(3) limiting viscosity number in 1-chloronaphthalene, 1,2,4-trichlorobenzene, and decalin,
(4) melt flow rate by a melt index type apparatus,
(5) density by ASTM procedures.
No attempt was made to determine number average molecular weight of this whole polymer by osmometry since diffusion of even small amounts of low molecular weight species through the membrane makes such a determination meaningless. However, by calibration of the gel permeation chromatograph with fractions that were well characterized by light scattering and osmometry, it was possible to determine number average molecular weight as well as weight average and molecular weight distribution. With these data, SRM 1475 may be used conveniently for the precise calibration of other gel permeation chromatographs.
In the papers which follow, the various techniques are described. More detail is given in those cases where there has been some deviation from the usual procedure. The certificate for SRM 1475 is reproduced at the end of this paper.
3. Sample Description
The sample of linear polyethylene chosen for SRM 1475, an ALATHON 7050, was kindly donated by the E. I. duPont Company, Wilmington, Delaware.* The material is in the form of pellets, each weighing about 20 mg. An antioxidant, tetrakis [methylene-3-(3′,5′-di-t-butyl-4′-hydroxyphenyl)propionate] methane, known commonly as Irganox 1010 (Geigy Chemical Company), was added to the polymer at a concentration of 111 ppm by the manufacturer. The linearity of the polymer is demonstrated, as shown in part II [1], by infrared analysis. Ash content, determined by ashing a 10-gram portion of the sample at 750 °C, was found to be 0.002 percent.
The determination of volatile components, or at least those components soluble in xylene, was made as follows. Three grams of polyethylene and 7 grams of xylene were sealed in one container and 7 grams of xylene was sealed in another as a control. These containers were heated at 140 °C until the contents dissolved. They were cooled to precipitate the polyethylene, opened, and samples of liquid from each were analyzed by gas chromatography.
It is estimated that any xylene-soluble volatiles that amounted to as much as ½ percent of the polyethylene could be readily detected by this method. No evidence of such volatile material was found.
4. Sampling
The sample was received in forty 50-lb bags. To determine the conditions necessary for uniform sampling, polymer variability from pellet-to-pellet and bag-to-bag was determined. The bags were numbered at random from 1 to 40 and samples were taken from the top and bottom of each of bags 1 to 20. Several grams of each of these were mixed and samples drawn from this “pool” to make up blended samples for light scattering, limiting viscosity number, and gel permeation chromatography. These blends were made up of several hundred pellets dissolved in xylene at 140 °C and precipitated in ethanol at room temperature.
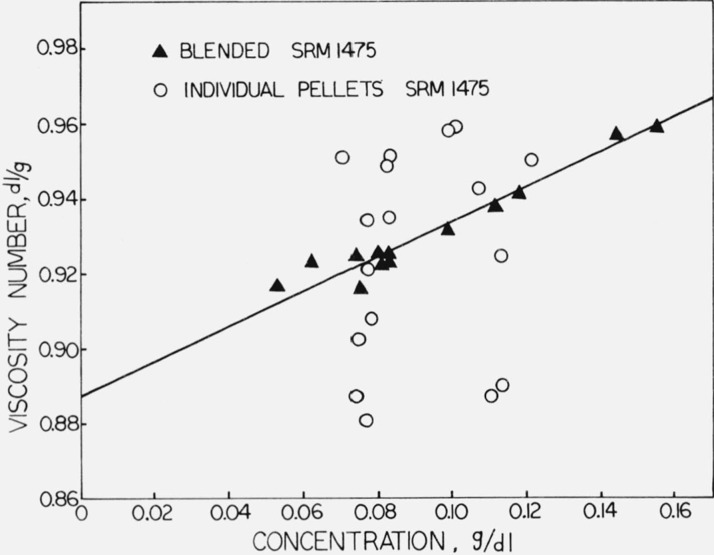
Pellet-to-pellet variability was determined by solution viscosity measurements [1d] in 1-chloronaphthalene at concentrations of from 0.06 to 0.12 g/dl at 130 °C on individual pellets and on samples of the blends. The viscosity number, (η − η0)/(η0c), in dl/g, where η is the viscosity of the solution, η0 is the viscosity of the solvent, and c is the concentration in g/dl, is plotted against concentration in figure 1. The viscosity numbers obtained for the blended samples were fitted by least squares to a linear function of concentration, yielding the straight line shown in the figure. The standard deviation in viscosity number obtained from the least-squares analysis for the blended samples was 0.0034 dl/g (0.38%). The root-mean-square deviation from the straight line of the viscosity numbers obtained for solutions made up from individual pellets was found to be 0.028 dl/g (3.1%). Thus the standard deviation of a single measurement is 0.38 percent and the standard deviation due to pellet-to-pellet variation is
Figure 1.
The viscosity number of a blended sample and of individual pellets of SRM 1475 plotted against concentration.
Since the coefficient of variation is 3 percent, it is recommended that all determinations be performed on samples containing at least 50 pellets or one gram of polymer (or material from a blend of one gram). This will reduce the expectation of the standard error due to pellet variability to less than 0.5 percent.
Details of the method of measurement of melt flow rate and the results obtained are discussed elsewhere [1c]. These measurements were employed to investigate variations in material taken from different regions of the entire material. Forty-two samples for melt flow rate determination were taken from 13 different regions in seven of the bags. The standard deviation for samples within a region was found to be 2.1 percent, based on 29 degrees of freedom. The standard deviation between regions was found to be 1.7 percent, based on 12 degrees of freedom. We conclude that variations from region to region are too small to be detected by this method.
Footnotes
Available through the Office of Standard Reference Materials, National Bureau of Standards, Washington, D.C. 20234.
Figures in brackets indicate the literature references at the end of this paper.
Certain commercial equipment, instruments, or materials are identified in this paper in order to adequately specify the experimental procedure. In no case does such identification imply recommendation or endorsement by the National Bureau of Standards, nor does it imply that the material or equipment identified is necessarily the best available for the purpose.
5. References
- [1].The Characterization of Linear Polyethylene 1475:; (a) Brown J. E., Paper II, Determination of Total Methyl Content by Infrared Spectrophotometry, J. Res. Nat. Bur. Stand. (U.S.), 76A (Phys. and Chem.), No. 2, 141–142 (Mar-Apr 1972). [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Brown J. E., Paper III, Density Determination, Ibid, 76A (Phys. and Chem.), No. 2, 143–144 (Mar-Apr 1972). [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Maurey J. R., Paper IV, Melt Flow Rate, Ibid, 76A (Phys. and Chem.), No. 2,145–146 (Mar-Apr 1972). [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Christensen R. G., Paper V, Solution Viscosity Measurements, Ibid, 76A (Phys. and Chem.), No. 2, 147–149 (Mar-Apr 1972). [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Christensen R. G., Paper VI, Preparation of Calibrating Fractions, Ibid, 76A (Phys. and Chem.), No. 2, 149–150 (Mar-Apr 1972). [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Wagner H. L., Paper VII, Differential Refractive Index of Polyethylene Solutions, Ibid, 76A (Phys. and Chem.), No. 2, 151–155 (Mar-Apr 1972). [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Frolen L. J., Ross G. S., Wims A. M., and Verdier P. H., Paper VIII, Light Scattering Studies on Polyethylenes in 1-Chloronaphthalene, Ibid, 76A (Phys. and Chem.), No. 2, 156–160 (Mar-Apr 1972). [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Brown J. E., and Verdier P. H., Paper IX, Number Average Molecular Weight of Fractions by Membrane Osmometry, Ibid, 76A (Phys. and Chem.), No. 2, 161–163 (Mar-Apr 1972). [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Ross G. S. and Frolen L. J., Paper X, Gel Permeation Chromatography, Ibid, 76A (Phys. and Chem.), No. 2, 163–170 (Mar-Apr 1972). [DOI] [PMC free article] [PubMed] [Google Scholar]