Abstract
An ASTM method was used to determine the methyl content of linear polyethylene SRM 1475 by measuring the absorbance at 1378 cm−1 (7.25 nm) of methyl groups on compensated infrared spectra. The methyl content of this polymer was found to be 0.15 methyls per 100 carbon atoms. Considering the methyls to be polymer end groups, the number average molecular weight computed approximates that determined by gel permeation chromatography within the experimental error. These values indicate that the polymer is essentially free of branching.
Keywords: Infrared, linear polyethylene, methyl, methylene, number average molecular weight, spectra, spectrophotometry
1. Introduction
The total methyl group content in polyethylene may be used to estimate the amount of branching in the polymer. If, for example, it is assumed that all chain-ends are methyl groups, then the methyl-group content of the polymer may be combined with its number-average molecular weight, Mn, to yield an estimate of the average number of chain ends per molecule.
In the characterization of SRM 1475, the total methyl content was determined to obtain an estimate of the polymer’s linearity. Infrared spectrophotometry was used to determine the methyl content from the absorbance at about 1378 cm−1 due to methyl groups. This band is overlapped by bands at 1368 and 1352 cm−1, and therefore cannot be used directly to estimate methyl group content. However, it has been shown [1–5]1 that both the latter two bands and another band at 1304 cm−1 arise from methylene-groups in the amorphous or disordered regions only, while the 1378 cm−1 band arises from methyl groups in both amorphous and crystalline regions. If we assume [5] that the spectrum of solid polyethylene is a superposition of contributions from amorphous and crystalline regions, then the spectrum arising from methyl groups alone may be obtained as a difference spectrum between two films of the same polymer, but different degrees of crystallinity and appropriately different thicknesses.
In this paper, we report the results for total methyl content in SRM 1475 by the method described in American Society for Testing and Materials (ASTM) Designation: D 2238–68, “Standard Method of Test for Absorbance of Polyethylene Due to Methyl Groups at 1378 cm−1.”2 By this method, the absorbance is measured at the methyl frequency (1378 cm−1) on films which are largely crystalline while compensating for the amorphous methylene absorbance with thinner, less crystalline films. Graphically, the absorbance resulting only from methyl groups is interpolated to the point of zero methylene absorbance at 1304 cm−1. Each of the absorbanccs at 1378 and 1304 cm−1 is corrected for the effect produced by the difference in thickness and density of the crystalline and amorphous films. The resulting absorbance at 1378 cm−1 is then used to calculate the number of methyl groups per 100 carbon atoms. The conversion of absorbance to methyls per 100 carbon atoms is computed from the absorbance at 1378 cm−1 due to methyl groups in n-hexadecane (cetane).
2. Experimental Procedure
Films were prepared from SRM 1475 pellets taken from each container and blended as described in the first paper of this series [6]. In accordance with the ASTM procedure, several films ranging in thickness from 0.01 to 0.04 cm were molded in a hydraulic press heated to 168 °C. The molten polyethylene in the mold assembly was then quenched in an ice-water mixture. Films prepared in this manner are designated reference films. Four films about 0.05 cm thick were also molded from the SRM pellets by the above procedure except that these films were annealed so as to produce an increase in density of at least 0.02 g/cm3 greater than the density of the quenched reference films. The annealed films will later be referred to as samples I through IV. Difference spectra of each sample (high crystalline content) were obtained with three or four of the thinner reference films (low crystalline content). The spectra were recorded in the region from about 1430 to 1250 cm−1 using the prism optics of a double-beam, infrared spectrophotometer, (Perkin-Elmer Model 221).3 From these spectra, which range from partial to over-compensation of the absorption due to amorphous methylene groups, the absorbance at the methyl frequency, A1378, and that at the methylene frequency, A1304, were determined as directed by the ASTM method.
3. Results and Discussion
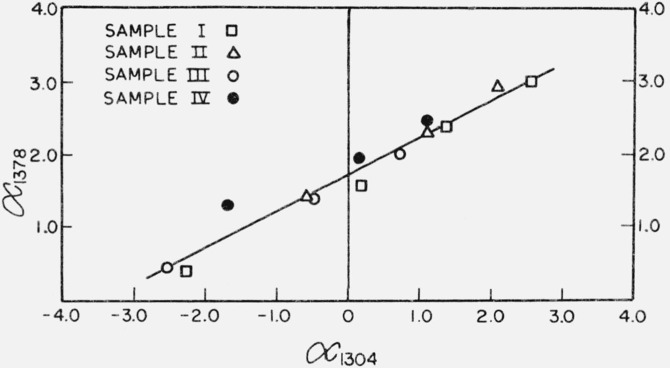
Effective absorptivities, α, at 1378 and 1304 cm−1 were calculated from the difference spectrum of each sample-reference pair by the relation α = A/(dsts − drtr), where A is absorbance and ds, ts, dr and tr are sample density, sample thickness, reference density, and reference thickness, respectively. The resulting pairs, (α1304, α1378), were plotted as shown in figure 1, together with the straight line determined by a linear least squares fit. The value of α1378, on the least squares line, corresponding to α1304 = 0 is the absorbtivity in cm2/g at 1378 cm−1 at which the methylene absorptivity at 1304 cm−1 is completely compensated. This quantity is designated K’ in table 1. The number of methyl groups per 100 carbon atoms is also shown in table 1. The methyl concentration is computed from the product of K’ and a calibration factor derived from measurements on cetane, as described in the ASTM procedure.
Figure 1. Ordinate: Absorptivity, α1378, cm2/g; Abscissa: Absorptivity, α1304, cm2/g.
The line shown in this graph represents a least squares fit of the absorptivity at 1378 cm−1 resulting from methyl groups against the absorptivity at 1304 cm−1 arising from amorphous methylene groups. The slope is equal to 0.51 ± 0.03 with the intercept at 1.74 ± 0.05 where the symbol ± stands for the standard deviation in these quantities inferred from the linear least-squares fit. The intercept represents the methyl absorption at perfect compensation for the methylene groups.
Table 1.
Results of methyl group determination from compensated spectra
| Absorptivity K’, cm2/g | Sample standard deviation of K’ | CH3 groups per 100 C atoms |
|---|---|---|
| 1.74 | 0.053 | 0.15 |
K’ in cm2/g is the reduced absorptivity of methyl groups, at com-plete compensations for the absorption due to methylene groups determined for SRM 1475.
High density (linear) polyethylene chains may be presumed to be terminated with methyl groups as demonstrated by Willbourn [4] in a study of chain branching in polyethylene. If we assume two methyl groups per chain, then our estimate of 0.15 methyl groups per 100 carbon atoms leads to an estimate of 18,700 for the number-average molecular weight, Mn. This value closely approximates the Mn (18,310) from the gel permeation chromatography measurement as shown in Paper X of this series [7]. This correlation and the density [8] suggests that the polymer, SRM 1475, is essentially free of branching.
Footnotes
Figures in brackets indicate the literature references at the end of this paper.
Available from American Society for Testing and Materials, 1916 Race Street, Philadelphia, Pennsylvania 19103.
Certain commercial equipment, instruments, or materials are identified in this paper in order to adequately specify the experimental procedure. In no case does such identification imply recommendation or endorsement by the National Bureau of Standards, nor does it imply that the material or equipment identified is necessarily the best available for the purpose.
4. References
- [1].Cross L. H., Richard R. B., and Willis H. A., Discussions Faraday Soc., No. 9, 235 (1950). [Google Scholar]
- [2].Nielson J. R. and Holland R. F., J. Mol. Spectr. 4, 488 (1960). [Google Scholar]
- [3].Okada T. and Mandelkern L., J. Polymer Sci. A–2 5, 239 (1967). [Google Scholar]
- [4].Willbourn A. H., J.Polymer Sci. 34, 569 (1959). [Google Scholar]
- [5].Krimm S., Liang C. Y., and Sutherland G. B. B. M., J. Chem. Phys. 25, 549 (1956). [Google Scholar]
- [6].Hoeve C. A. J., Wagner H. L., and Verdier P. H., J. Res. Nat. Bur. Stand. (U.S.), 76A (Phys. and Chem.), No. 2, 137–140 (Mar-Apr 1972). Paper I of this series. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ross G. S. and Frolen L. J., J. Res. Nat. Bur. Stand. (U.S.). 76A (Phys. and Chem.), No. 2 163–170 (Mar-Apr 1972). Paper X of this series. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Brown J. E., J. Res. Nat. Bur. Stand. (U.S.), 76A (Phys. and Chem.), No. 2, 143–144 (Mar-Apr 1972). Paper III of this series. [DOI] [PMC free article] [PubMed] [Google Scholar]