Abstract
Background
Remote organ injury is one of the complications which are developed following ischemia-reperfusion induced acute kidney injury (AKI), dramatically increasing its mortality rate. The aim of the present study was to investigate the effect of piperine pretreatment on liver dysfunction following ischemia-reperfusion induced AKI.
Materials and methods
Acute kidney injury was induced by 30 min-bilateral renal ischemia followed by 24 h of reperfusion. To investigate liver damages, the levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP) enzymes were measured in plasma. In order to study oxidative stress, malondialdehyde (MDA) and ferric reducing antioxidant power (FRAP) levels were measured. Furthermore, the expression of intercellular adhesion molecule-1 (ICAM-1) mRNA along with infiltration of leukocytes in the liver tissue was measured for inflammation assessment. Histopathological damages were studied through measuring the extent of cellular fibrosis, sinusoidal dilatation, and vascular congestion in liver tissue.
Results
Following acute kidney injury, AST, ALT, and ALP levels in plasma, MDA level and ICAM-1 expression in the liver tissue, infiltration of leukocytes into the interstitium, and hepatic histopathologic damages increased significantly, while FRAP decreased. Pretreatment with piperine at 10 and 20 mg/kg body weight was able to improve these damages, such that some of them reached its value in the sham group, though piperine in the 20 mg/kg was more effective.
Conclusions
The results of this study suggest that ischemia-reperfusion induced AKI result in hepatic damages, and pretreatment with piperine can prevent development of these damages through its antioxidant and anti-inflammatory properties.
Keywords: Biochemistry, Plant biology, Physiology
1. Introduction
Acute kidney injury (AKI) is one of the major medical challenges for which no definite treatment has been found so far as the condition increases the duration of hospitalization and treatment costs [1]. Based on recent reports, throughout the world around 20% of adults and 33% of children suffer AKI during hospitalization [2]. The mortality rate caused by AKI is 11% and grows to 45–60% if it is accompanied by dysfunction of other organs, which is due to extra renal complications or AKI effects on remote organs [3].
Various factors can result in AKI, the most common being ischemia-reperfusion (I/R) [4]. The damages induced by renal I/R include inflammation, oxidative stress, and endothelial and epithelial cells injury [5]. Due to the organ crosstalk, kidney injury can cause damage to other organs including liver, heart, lungs, brain, digestive system, and bone marrow, known as remote organ injury [6, 7]. The precise mechanism of remote organ injury by renal I/R has still remained unknown. Nevertheless, potential pathways including activation of inflammation cascade and upregulation of systemic cytokines [8], transport of reactive oxygen species (ROS) to other organs, activation of proapoptotic pathways, and transport of leukocytes have been proposed [6]. It has been shown that glutathione or Malva sylvestris extract treatment, through anti-inflammatory and antioxidative effects, causes diminished liver injury caused by AKI [9, 10, 11]. Furthermore, it has been reported that melatonin, as a potent antioxidant, protects the liver against renal I/R induced damages [12].
Piperine (1-peperoylpiperidine) is the alkaloid present in black pepper (piper nigrum) and long pepper (piper longum) fruit [13]. It has been shown that piperine has various pharmacological properties including antioxidant [14] and anti-inflammation [15]. It can also protect and reduce damages in brain ischemia-reperfusion [16], and lead acetate induced nephrotoxicity in rats [17]. Mechanistically, it has been shown that piperine decreases intercellular adhesion molecule-1 (ICAM-1), tumor necrosis factor alpha (TNF-α), indcible nitric oxide synthase (iNOS), and nuclear factor-κB (NF-κB) expression [16, 18], and inhibits cytochrome P450 activity and endoplasmic reticulum stress [19]. In a previous study, we found that piperine gavage resulted in diminished renal inflammation, oxidative stress, tissue damage, and functional disturbances following 30 min of ischemia and 24 h of reperfusion (unpublished data). Nevertheless, so far the effect of piperine on the injuries caused by renal I/R in liver as a remote organ has not been examined. Therefore, the aim of the present study was to investigate the potential protective effects of piperine against liver damages induced by renal I/R in rats.
2. Materials and methods
2.1. Animals
This study was performed on 28 male Wister rats (200–250 g) prepared from the center for reproduction and breeding of laboratory animals, Kermanshah University of Medical Sciences. The animals were kept at 23 ± 2 °C with light/dark cycle of 12 h. Throughout the experiment, the studied animals had free access to standard food and water. During the study, attempts were made to minimize the pain and suffering for the animals. In the case of abnormal symptoms, they were excluded from the experiment and euthanized by deep anesthesia. During the study, the ethical principles of working with laboratory animals were observed, and all procedures were performed according to the European Economic Community Guidelines for the care and use of laboratory animals (EEC Directive of 1986; 86/609/EEC). The study procedure was also approved by the ethics committee of Kermanshah University of Medical Sciences (Approval number: IR.KUMS.REC.1396.452).
2.2. Experimental protocol
The studied animals were randomly divided into four groups (n = 7). The sham group received piperine solvent (20 μl Tween 80 + 980 μl normal saline) for 10 days orally. On the 10th day, 1 h after solvent gavage, they underwent sham surgery and the vessels of kidneys were not occluded. The second group (I/R) had a protocol similar to the sham group, but after the surgery, the artery and vein of both kidneys were occluded for 30 min. The third and fourth groups underwent a procedure similar to the I/R group, but they received piperine (Sigma, USA) at 10 (I/R + P10) or 20 (I/R + P20) mg/kg of body weight for 10 days, by gavage. These doses were chosen based on pilot studies conducted as well as previous similar studies [14, 16]. So that doses were chosen that have the best effect and the least side effects. After surgery, the rats were transferred to cages to pass the reperfusion period for 24 h during which they had free access to food and water. It needs to be mentioned that 30 min before inducing ischemia, 50 IU of heparin was injected to all animals intraperitoneally (ip). During the surgery period, the animal body temperature was controlled using a rectal thermometer and kept within the range of 37 °C ± 1 using a lamp and a heat plate. In order to induce anesthesia, sodium pentobarbital (Sigma, USA) was used (55 mg/kg, ip). After the 24 h of reperfusion, the animals underwent re-anesthesia, when a blood sample was taken from the abdominal aorta in order to measure AST, ALT, and ALP. Then, the liver was removed and one segment was immediately frozen in liquid nitrogen to investigate oxidative stress through measuring the levels of malondialdehyde (MDA) and ferric reducing antioxidant power (FRAP). Another segment of the liver was also kept in formaldehyde solution for histopathology study after hematoxylin-eosin (H&E) staining. Furthermore, expression level of ICAM-1 mRNA factor in the liver tissue was also measured by quantitative real-time PCR method. At the end of the experiments, the animals were euthanized by deep anesthesia [10, 11].
2.3. Measuring liver enzymes
In order to investigate hepatocellular leakage, the level of AST and ALT enzymes were measured in the plasma. Also, cholestatic induction was examined through measuring the level of ALP enzyme. Plasma concentrations of AST, ALT and ALP were determined by colorimetric method using commercially available kits (Man, Iran).
2.4. Assessing oxidative stress in the liver
Oxidative stress was assessed through measuring lipid peroxidation (MDA levels) as well as the total antioxidant activity (FRAP) of the liver tissue. Briefly, to measure the MDA level, first a tissue sample was homogenized in phosphate buffer saline (PBS). Next, acetic acid 20%, thiobarbituric acid 0.8%, sodium dodecyl sulfate 8.1%, and tissue extract were heated in water bath for 60 min at 95 °C. The resulting colored complex was extracted by adding 1-butanol, and its light absorption was measured using spectrophotometer at the wavelength of 532 nm. The obtained results were reported in terms of Nano mole per gram kidney weight (nmol/gKW).
In order to measure the level of FRAP, tripyridyl-s-triazine (TPTZ) was utilized. For this purpose, tissue extracts were added to TPTZ solution 10 mM, FeCl3 20 mM and acetate buffer 0.3 M, and the temperature was increased to 37 °C. The FRAP level was calculated by measuring the light absorption of the resulting complex at the wavelength of 593 nm. The obtained results were reported in terms of Micro mole per gram kidney weight (μmol/gKW) [20, 21].
2.5. Assessing inflammation in the liver tissue
To study inflammation, the expression level of ICAM-1 mRNA was measured through quantitative real-time PCR (qRT-PCR) through SYBR Green Kit in the liver tissue using beta-actin as housekeeping gene. Next, the relative expression of the gene was calculated using 2−ΔΔCT method. The sequence of forward primer for ICAM-1 was as 5′-GGGATGGTGAAGTCTGTCAA-3′ and reverse primer was as 5′- GGCGGTAATAGGTGTAAATGG-3′. For beta-actin, the forward primer sequence was 5′- TGCTATGTTGCCCTAGACTTC-3′ and reverse primer of 5′-GTTGGCATAGAGGTCTTTACGG-3′. Furthermore, the number of leukocytes infiltrated into interstitium was counted in 20 microscopic fields (the area of each field was 0.14 mm2), and the mean number was calculated per each square millimeter [10, 11].
2.6. Assessing liver histopathologic damages
In order to assess the histopathologic injuries of the liver, 5 μm thickness tissue slices were stained with H&E and studied using a light microscope. The liver tissue injuries for cellular fibrosis, sinusoidal dilatation, and vascular congestion were examined in 10 microscopic fields and graded. The level of each pathophysiological feature was graded according to the changes involved, scoring 0 with no changes, 1 with <20%, 2 with 20–40%, 3 with 40–60%, 4 with 60–80% and 5 with >80%. Then, the total histopathologic score was calculated, which was equal to the sum of all grades of different injuries, and analyzed statistically [11].
2.7. Statistical analysis
SPSS-23 software was utilized for statistical analysis, and the data have been represented as mean ± SEM. Comparison of the data related to hepatic enzymes and oxidative stress in different groups was performed using one-way ANOVA and Duncan's post hoc test. Also, the precise p-value was determined by LSD test. To analyze the total histopathologic scores, the nonparametric Kruskal-Wallis and Mann-Whitney tests were used. The significance level of the data was considered as P < 0.05.
3. Results
3.1. The effect of piperine on hepatic enzymes following renal I/R
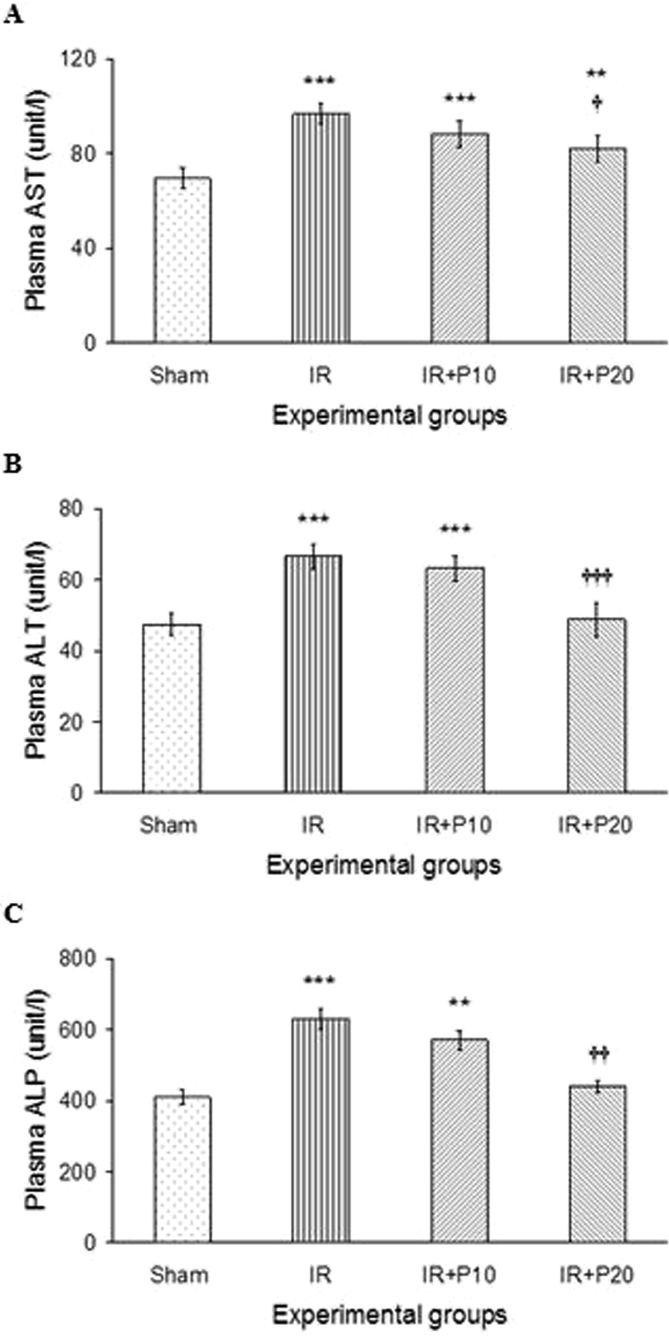
Fig. 1 demonstrates that 30 min of renal ischemia and 24 h of reperfusion resulted in elevated AST and ALT levels as compared to the sham group (p < 0.001 for both parameters). Piperine pretreatment at 10 mg/kg was not able to alter AST and ALT levels compared to I/R group, and their values were still significantly higher than the sham group. However, piperine at 20 mg/kg significantly reduced AST and ALT in I/R + P20 group in comparison to its value in I/R group (p < 0.05, p < 0.001, respectively), so that ALT level reached its values in the sham group.
Fig. 1.
Plasma AST (A), ALT (B) and ALP (C) levels in rats which underwent renal ischemia/reperfusion and pretreated with vehicle (I/R), or piperine at 10, or 20 mg/kg (I/R + P10 or I/R + P20 groups) compared to the sham group. Data is presented as mean ± SE (n = 7). **P < 0.01, ***P < 0.001 in comparison with the sham group. †P < 0.05, †††P < 0.001 in comparison with I/R group.
Furthermore, renal I/R resulted in a significant rise in ALP level (Fig. 1-C), where piperine at 20 mg/kg was able to reduce it to its value in the sham group.
3.2. The effect of piperine on liver oxidative stress induced by renal I/R
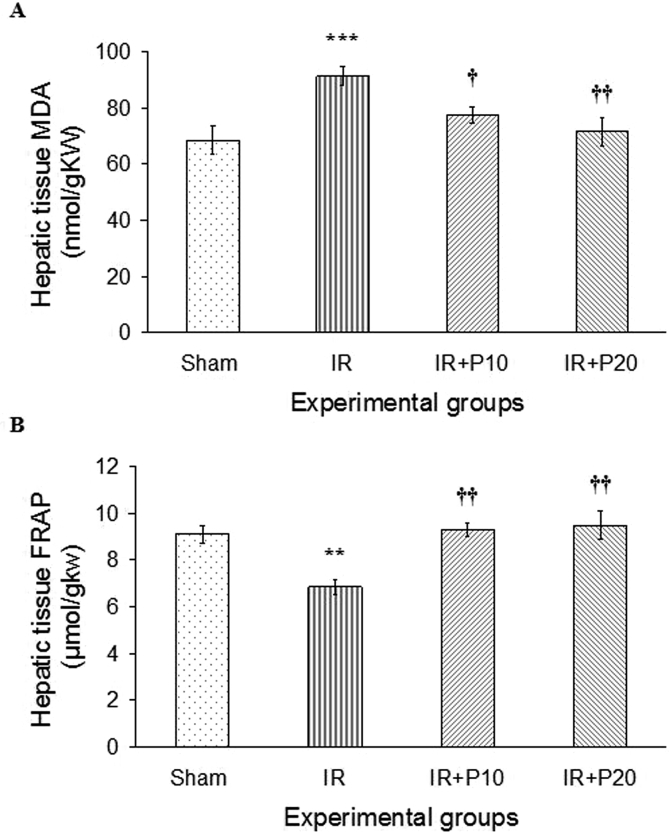
As shown in Fig. 2-A, the renal I/R led to elevated lipid peroxidation (MDA level) in the liver tissue compared to the sham group (p < 0.001). Piperine pretreatment with both studied doses was able to reduce MDA level in comparison to its value in I/R group, where the efficacy was higher at 20 mg/kg (p < 0.05 and p < 0.01, respectively). Further, renal I/R resulted in diminished total antioxidant capacity (FRAP) of the liver tissue in comparison to the sham group (p < 0.01). Piperine at both doses was able to enhance FRAP level in comparison to I/R group (p < 0.01), and raised it to the level of the sham group (Fig. 2-B).
Fig. 2.
A, Liver tissue lipid peroxidation level (MDA) and B, total antioxidant capacity (FRAP) in rats that underwent renal ischemia/reperfusion and pretreated with vehicle (I/R), or piperine at 10, or 20 mg/kg (I/R + P10 or I/R + P20 groups) compared to the sham group. Data is presented as mean ± SE (n = 7). **P < 0.01, ***P < 0.001 in comparison with the sham group. †P < 0.05, ††P < 0.01 in comparison with I/R group.
3.3. The effect of piperine on liver inflammation following renal I/R
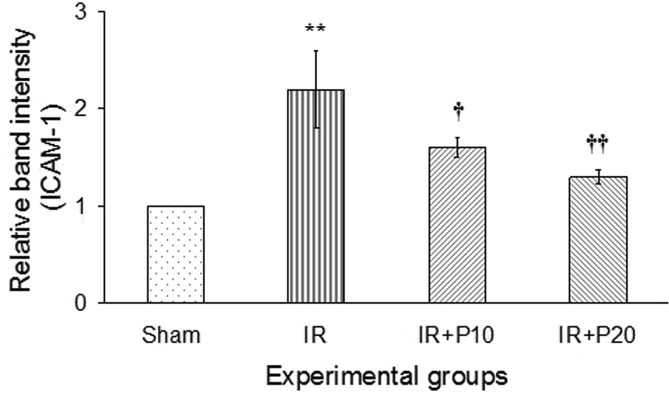
Following renal I/R, the expression level of ICAM-1 mRNA was increased in the liver tissue in comparison to its value in the sham group (p < 0.01). Following piperine gavage, the expression of ICAM-1 mRNA diminished in both groups of I/R + P10 and IR + P20 in comparison to the I/R group (p < 0.05 and p < 0.01, respectively), so that in both groups its expression level reached that of the sham group (Fig. 3). Also, Table 1 demonstrates that 30 min of ischemia and 24 h of reperfusion resulted in increased infiltration of leukocytes into the liver interstitium in comparison to the sham group. In this regard, administration of piperine at both doses of 10 and 20 mg/kg led to a significant decline in leukocytes infiltration compared to I/R group.
Fig. 3.
Representative mRNA fold change expression for intercellular adhesion molecule-1 (ICAM-1) in the liver tissue of the rats received vehicle (Sham), or piperine at 10, or 20 mg/kg (I/R + P10 or I/R + P20 groups) compared to the sham group. **P < 0.01, in comparison with the sham group. †P < 0.05, ††P < 0.01 in comparison with I/R group.
Table 1.
The effect of piperine on liver histopathologic injuries induced by AKI.
| Histopathologic damages | Experimental groups |
|||
|---|---|---|---|---|
| Sham | I/R | I/R + P10 | I/R + P20 | |
| Leukocytes infiltration | 0 | 3.4 | 0.7 | 0.4 |
| Cellular fibrosis | 0 | 4.6 | 1.6 | 0.7 |
| Sinusoidal dilatation | 1 | 5 | 1.8 | 1.2 |
| Vascular congestion | 0.9 | 5 | 2.1 | 1.1 |
| Total histopathologic score | 1.9 | 18*** | 6.2* †† | 3.4††† |
Histopathological scores in rats underwent sham surgery and received vehicle (Sham), ischemia/reperfusion and received vehicle (I/R), or piperine at 10 or 20 mg/kg (I/R + P10 and I/R + P20 groups).
*P < 0.05, ***P < 0.001, in comparison with sham group.
††P < 0.01, †††P < 0.001, in comparison with I/R group.
3.4. The effect of piperine on liver histopathologic injuries induced by renal I/R
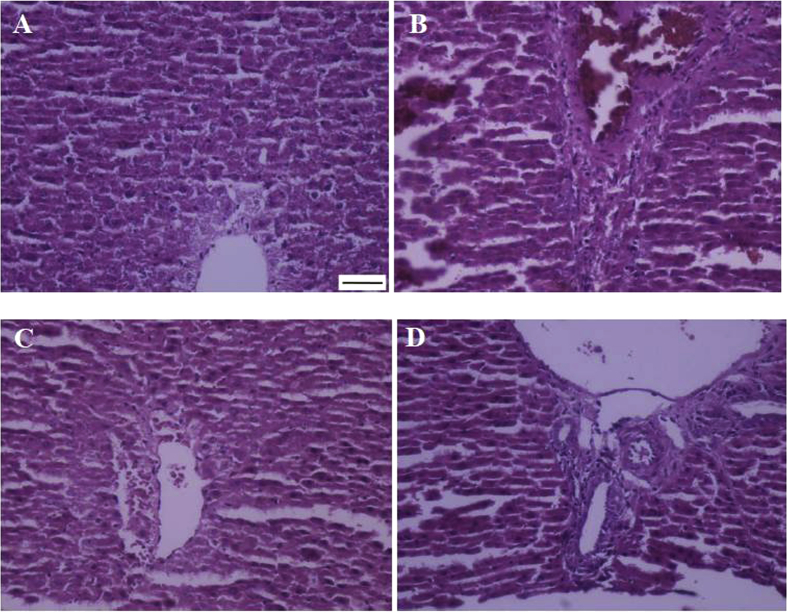
Fig. 4 and Table 1 show that the renal I/R has resulted in increased hepatic cellular fibrosis, sinusoidal dilatation, and vascular congestion (Fig. 4-A, B) so that the total histopathologic score has been significantly higher in I/R group as compared to the sham group (p < 0.001). Pretreatment with piperine in both I/R + P10 and I/R + P20 groups was able to reduce the grade of injuries in comparison to I/R group (p < 0.01 and p < 0.001, respectively). In this regard, the total histopathologic score in I/R + P20 group was not significantly different from the sham group (Fig. 4-C, D).
Fig. 4.
Representing histopathologic alterations in the liver of rats that underwent renal ischemia/reperfusion and pretreated with vehicle (B), or piperine at 10, or 20 mg/kg (C, D) compared to the sham group (A) (Haematoxylin-Eosin, 200x; scale bar: 100 μm).
4. Discussion
The mortality rate caused by AKI grows several times if it is accompanied by remote organ injury [3]. Therefore, the mechanism of damage development by AKI in remote organs should be understood more precisely, and proper therapeutic strategies should be designed for it accordingly. Potential side effects of treating with chemical anti-inflammatory drugs used for inflammatory diseases remain one of the problems which complicate the treatment process. Recently, herbal supplements have become more commonly used, due to their lower side effect profile, to protect and treat certain diseases. Piperine as the primary lipophilic component in black pepper and long pepper, has been reported to has anti-inflammatory and antioxidative properties [14, 15]. In this study, we found that gavage of piperine, through reducing inflammation and oxidative stress, resulted in improved histopathologic injuries and reduction of hepatic enzymes following their elevation by the renal I/R.
In the present study, we found that I/R induced AKI led to liver injury, which manifested itself as elevated levels of AST, ALT, and ALP as well as increased oxidative stress (MDA rise), diminished total antioxidant capacity (FRAP decline), increased expression of ICAM-1 mRNA adhesion factor, and increased infiltration of leukocytes into the interstitium, and eventually histopathologic injuries in the liver. Golab et al. demonstrated that AKI caused by I/R or bilateral nephrectomy results in elevated level of pro-inflammatory factors, activation of oxidative stress, reduction of antioxidant defense level, and increased expression of injury-promoting molecule SSAT [9]. Other studies have also suggested that following I/R, SSAT is induced, which plays a significant role in the pathophysiology of hepatic damage, so that the animals without SSAT would be protected against hepatic and renal injuries caused by I/R [22, 23].
Furthermore, renal I/R leads to production of TNF-α through damaging tubular epithelial cells and activating leukocytes, and TNF-α by inducing the expression of adhesion molecules including ICAM-1 in endothelial cells results in increased infiltration of leukocytes [24]. In the present study, we also found that the expression of adhesion molecule ICAM-1 in the liver increased following renal I/R, which had caused increased infiltration of leukocytes. It has also been proposed that AKI affects the liver, through developing metabolic acidosis, elevating the level of soluble mediators transferred from the kidney to the liver, induction of inflammation and oxidative stress, and accumulation of cytotoxic T-cells and neutrophils in the liver [25, 26, 27, 28]. Recently, Rabadi et al. reported that renal I/R leads to induction of peptidyl arginine deaminase 4 (PAD-4) in the liver, and liver damage in the mice lacking PAD-4 significantly declines following renal I/R, suggesting its role in the liver damage following AKI [29].
In this study, pretreatment with piperine led to diminished oxidative stress, expression of adhesion factor ICAM-1, histopathologic damages, level of hepatic enzymes, and enhanced total antioxidant capacity, suggesting its hepatoprotective effects against damages caused by renal I/R. In various studies, the antioxidant and anti-inflammatory effects of piperine have been examined. It has been reported that piperine supplement caused a significant decline in H2O2 production stimulated by puromycin [30], enhanced antioxidant capacity and reduction of lipid peroxidation [14], diminished oxidative stress of the endoplasmic reticulum [19] and reduction of MDA and elevation of superoxide dismutase and glutathione peroxidase [17]. Also, the results of other studies suggest that piperine causes diminished expression of NF-κB, TNF-α and ICAM-1 in different models of inflammation as well as ischemia-reperfusion [16]. Therefore, it can be inferred that piperine causes protection of liver against the damages caused by renal I/R through its anti-inflammatory and antioxidant properties. In line with this presumption, Golab et al. found that glutathione, as a well-known antioxidant, treatment leads to diminished damages caused by AKI in the liver [9]. Also, in other studies, the use of anti-inflammatory and antioxidant compounds resulted in reduced liver damages following AKI [10, 11].
Our limitation in this study was that we were not able to measure the level of pro-inflammatory factors, and in order to investigate the inflammation, only infiltration of leukocytes and the expression level of ICAM-1 were measured.
As the final conclusion, the results of the present study suggest that pretreatment with piperine can cause protection of the liver against the damages induced by renal I/R through its anti-inflammatory and antioxidative effects. Also, it is possible that part of this protective effect of piperine was mediated through reducing renal injuries following I/R, which requires further studies.
Declarations
Author contribution statement
Maryam Mohammadi: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Houshang Najafi: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Zeynab Mohamadi Yarijani: Performed the experiments.
Gholamhasan Vaezi, Vida Hojati: Conceived and designed the experiments.
Funding statement
This work was supported by research deputy of Kermanshah University of Medical Sciences, Kermanshah, Iran (grant no. 96496 to HN).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Rewa O., Bagshaw S.M. Acute kidney injury-epidemiology, outcomes and economics. Nat. Rev. Nephrol. 2014;10(4):193–207. doi: 10.1038/nrneph.2013.282. [DOI] [PubMed] [Google Scholar]
- 2.Susantitaphong P., Cruz D.N., Cerda J. World incidence of AKI: a meta-analysis. Clin. J. Am. Soc. Nephrol. 2013;8(9):1482–1493. doi: 10.2215/CJN.00710113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta R.L., Bouchard J., Soroko S.B. Sepsis as a cause and consequence of acute kidney injury: program to improve care in acute renal disease. Intensive Care Med. 2011;37(2):241–248. doi: 10.1007/s00134-010-2089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schiffl H., Fischer R. Clinical cause of presumed acute tubular necrosis requiring renal replacement therapy and outcome of critically ill patients: post hoc analysis of a prospective 7-year cohort study. Int. Urol. Nephrol. 2012;44(6):1779–1789. doi: 10.1007/s11255-011-9994-x. [DOI] [PubMed] [Google Scholar]
- 5.Doi K., Rabb H. Impact of acute kidney injury on distant organ function: recent findings and potential therapeutic targets. Kidney Int. 2016;89:555–564. doi: 10.1016/j.kint.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Grams M.E., Rabb H. The distant organ effects of acute kidney injury. Kidney Int. 2012;81(10):942–948. doi: 10.1038/ki.2011.241. [DOI] [PubMed] [Google Scholar]
- 7.Lee S.A., Cozzi M., Bush E.L., Rabb H. Distant organ dysfunction in acute kidney injury: a review. Am. J. Kidney Dis. 2018;72(6):846–856. doi: 10.1053/j.ajkd.2018.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoke T.S., Douglas I.S., Klein C.L. Acute renal failure after bilateral nephrectomy is associated with cytokine-mediated pulmonary injury. J. Am. Soc. Nephrol. 2007;18(1):155–164. doi: 10.1681/ASN.2006050494. [DOI] [PubMed] [Google Scholar]
- 9.Golab F., Kadkhodaee M., Zahmatkesh M. Ischemic and non-ischemic acute kidney injury cause hepatic damage. Kidney Int. 2009;75(8):783–792. doi: 10.1038/ki.2008.683. [DOI] [PubMed] [Google Scholar]
- 10.Mohamadi Yarijani Z., Godini A., Madani S.H., Najafi H. Reduction of cisplatin-induced renal and hepatic side effects in rat through antioxidative and anti-inflammatory properties of Malva sylvestris L. extract. Biomed. Pharmacother. 2018;106:1767–1774. doi: 10.1016/j.biopha.2018.07.115. [DOI] [PubMed] [Google Scholar]
- 11.Najafi H., Mohamadi Yarijani Z., Changizi-Ashtiyani S. Protective effect of Malva sylvestris L. extract in ischemia-reperfusion induced acute kidney and remote liver injury. PLoS One. 2017;12(11) doi: 10.1371/journal.pone.0188270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fadillioglu E., Kurcer Z., Parlakpinar H., Iraz M., Gursul C. Melatonin treatment against remote organ injury induced by renal ischemia reperfusion injury in diabetes mellitus. Arch Pharm. Res. 2008;31(6):705–712. doi: 10.1007/s12272-001-1216-3. [DOI] [PubMed] [Google Scholar]
- 13.Selvendiran K., Thirunavukkarasu C., Singh J.P., Padmavathi R., Sakthisekaran D. Chemopreventive effect of piperine on mitochondrial TCA cycle and phase-I and glutathione-metabolizing enzymes in benzo(a)pyrene induced lung carcinogenesis in Swiss albino mice. Mol. Cell. Biochem. 2005;271:101–106. doi: 10.1007/s11010-005-5615-2. [DOI] [PubMed] [Google Scholar]
- 14.Vijayakumar R.S., Surya D., Nalini N. Antioxidant efficacy of black pepper (Piper nigrum L.) and piperine in rats with high fat diet induced oxidative stress. Redox Rep. 2004;9:105–110. doi: 10.1179/135100004225004742. [DOI] [PubMed] [Google Scholar]
- 15.Wang-Sheng C., Jie A., Jian-Jun L., Lan H., Zeng-Bao X., Chang-Qing L. Piperine attenuates lipopolysaccharide (LPS)-induced inflammatory responses in BV2 microglia. Int. Immunopharmacol. 2017;42:44–48. doi: 10.1016/j.intimp.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Vaibhav K., Shrivastava P., Javed H. Piperine suppresses cerebral ischemia-reperfusion-induced inflammation through the repression of COX-2, NOS-2, and NF-κB in middle cerebral artery occlusion rat model. Mol. Cell. Biochem. 2012;367:73–84. doi: 10.1007/s11010-012-1321-z. [DOI] [PubMed] [Google Scholar]
- 17.Sudjarwo S.A., Eraiko K., Sudjarwo G.W., Koerniasari Protective effects of piperine on lead acetate induced-nephrotoxicity in rats. Iran J. Basic Med. Sci. 2017;20:227–231. doi: 10.22038/IJBMS.2017.9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu D., Wang Y., Chen Z. The protective effect of piperine on dextran sulfate sodium induced inflammatory bowel disease and its relation with pregnane X receptor activation. J. Ethnopharmacol. 2015;169:109–123. doi: 10.1016/j.jep.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Hammad A.S., Ravindran S., Khalil A., Munusamy S. Structure-activity relationship of piperine and its synthetic amide analogs for therapeutic potential to prevent experimentally induced ER stress in vitro. Cell Stress Chaperones. 2017;22:417–428. doi: 10.1007/s12192-017-0786-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Najafi H., Firouzifar M.R., Shafaat O., Changizi Ashtiyani S., Hosseini N. Protective effects of Tribulus Terrestris L extract against acute kidney injury induced by reperfusion injury in rats. Iran J. Kidney Dis. 2014;8(4):292–298. [PubMed] [Google Scholar]
- 21.Changizi-Ashtiyani S., Alizadeh M., Najafi H. Physalis alkekengi and Alhagi maurorum ameliorate the side effect of cisplatin-induced nephrotoxicity. Cancer Gene. Ther. 2016;23:235–240. doi: 10.1038/cgt.2016.24. [DOI] [PubMed] [Google Scholar]
- 22.Zahedi K., Lentsch A.B., Okaya T. Spermidine/spermine-N1-acetyltransferase ablation protects against liver and kidney ischemia-reperfusion injury in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296(4):G899–909. doi: 10.1152/ajpgi.90507.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Z., Zahedi K., Barone S. Overexpression of SSAT in kidney cells recapitulates various phenotypic aspects of kidney ischemia-reperfusion injury. J. Am. Soc. Nephrol. 2004;15(7):1844–1852. doi: 10.1097/01.asn.0000131525.77636.d5. [DOI] [PubMed] [Google Scholar]
- 24.Godet C., Goujon J.M., Petit I. Endotoxin tolerance enhances interleukin-10 renal expression and decreases ischemia-reperfusion renal injury in rats. Shock. 2006;25(4):384–388. doi: 10.1097/01.shk.0000209528.35743.54. [DOI] [PubMed] [Google Scholar]
- 25.Lane K., Dixon J.J., MacPhee I.A., Philips B.J. Renohepatic crosstalk: does acute kidney injury cause liver dysfunction? Nephrol. Dial. Transplant. 2013;28(7):1634–1647. doi: 10.1093/ndt/gft091. [DOI] [PubMed] [Google Scholar]
- 26.Miyazawa S., Watanabe H., Miyaji C., Hotta O., Abo T. Leukocyte accumulation and changes in extra-renal organs during renal ischemia reperfusion in mice. J. Lab. Clin. Med. 2002;139(5):269–278. doi: 10.1067/mlc.2002.122832. [DOI] [PubMed] [Google Scholar]
- 27.Park S.W., Chen S.W., Kim M. Cytokines induce small intestine and liver injury after renal ischemia or nephrectomy. Lab. Investig. 2011;91(1):63–84. doi: 10.1038/labinvest.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serteser M., Koken T., Kahraman A., Yilmaz K., Akbulut G., Dilek O.N. Changes in hepatic TNF-alpha levels, antioxidant status, and oxidation products after renal ischemia/reperfusion injury in mice. J. Surg. Res. 2002;107(2):234–240. doi: 10.1006/jsre.2002.6513. [DOI] [PubMed] [Google Scholar]
- 29.Rabadi M., Kim M., D'Agati V., Lee H.T. Peptidyl arginine deiminase-4-deficient mice are protected against kidney and liver injury after renal ischemia and reperfusion. Am. J. Physiol. Renal. Physiol. 2016;311(2):F437–449. doi: 10.1152/ajprenal.00254.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu H., Bigler S.A., Henegar J.R., Baliga R. Cytochrome P450 2B1 mediates oxidant injury in puromycin-induced nephrotic syndrome. Kidney Int. 2002;62:868–876. doi: 10.1046/j.1523-1755.2002.00515.x. [DOI] [PubMed] [Google Scholar]