Abstract
Syringic acid (SA) is a phenolic acid and have been investigated for diverse pharmacological activities, but the safety and/or mechanism of toxicity is still lacking in the literature. Subacute toxicity studies will add value to its pharmacological profile and support its exploration as a future medicine. According to OECD TG 407 (OECD, 2008), rats were divided into 3 groups (n = 12). The dose of SA was decided by limit test. Treatment and satellite groups received SA (1000 mg/kg/day, p.o for 14 days), whereas an equal volume of vehicle was given to control groups. In order to access reversibility, satellite groups were kept for another 14 days post-treatment. The toxic signs, mortality and body weight changes were recorded. On day 15 and 29 the rats were anesthetized to collect blood for estimation of hematological and biochemical parameters and then sacrificed to collect internal body organ for weighing and histopathological studies. SA has no major adverse effect on the body weight, food intake, erythropoiesis, leucopoiesis and on internal body organs which was confirmed by evaluating various biochemical and hematological parameters, relative body organ weight and histopathological studies. Therefore, SA could be considered safe over limited period of time and this study may help researchers in establishing the doses for the longer-term subchronic studies. Further, subchronic and chronic toxicity studies are required to evaluate safety on long term use.
Keywords: Toxicology, Physiology, Subacute, Toxicity, Syringic acid, Polyphenol, Safety
1. Introduction
Polyphenols are the largest group of phytochemicals, secondary metabolites of plant produce to protect themselves from insects, mushrooms and diseases (Rong, 2010); classified as flavones, flavonols, phenolic acids, volatile phenols, anthocyanins, catechins and tannins (Khoddami et al., 2013). They have received tremendous attention among nutritionists, food scientists and consumers due to their roles in human health. Research in the recent years strongly supports their role in the prevention of the diseases, particularly cancer, cardiovascular diseases, diabetes and neurodegenerative disorders (Kanti et al., 2009).
Syringic acid (SA) is one of the examples of phenolic acid found in various dry fruits (olives, dates), spices, pumpkin, grapes, acai palm, honey, red wine and other plants (Srinivasulu et al., 2018). SA exhibits diverse pharmacological actions such as antidiabetic (Jayachandran, 2013), antibacterial (Aziz et al., 1998). It inhibits aldose reductase enzyme (Wei et al., 2012), showed antioxidant and antihypertensive (Kumar et al., 2010), hepatoprotective (Ramachandran and Raja, 2003) and neuroprotective activity (Tokmak et al., 2015). It has antiglycating properties (Abhishek and Abhijit, 2015), anti-steatotic and anti-inflammatory effect (Ju et al., 2016) and have improved diabetic cataract (Wei et al., 2012). Additionally, it has been proven to have anti-cancer property, protects the heart and brain/CNS, employed in the preparation of dental cement (Srinivasulu et al., 2018), attenuates clot formation and acute thromboembolism in mice (Choi and Kim, 2018).
SA is supposed to be administered to treat chronic disorders for a long period; therefore an evaluation of safety becomes essential. In addition, current scientific reports showed toxic effects of phytochemicals or plant extracts in experimental animals at high doses such as carcinogenic (capsaicin, chili powder, safrole), neurotoxic (Papaver somniferum, erytroxylum sp. and cannabis sativa), genotoxic (thymol and carvacro), teratogenic (pyrrolizidine alkaloid monster, heliotrine), cytotoxic (Allium sativum, Lagerstroemia speciosa, Calotropisprocera, Chenopodiummurale, Pulicariaorientalis, Tribulusterrestris, Withania somniferum, Blumealacera and safrole-2′, 3′-oxide), nephrotoxic (aristolochic acid, turmeric, Juniper berries, green tea-derived preparations, Teavigo, Polyphenon E), hepatotoxic (Cimicifugaracemosa, Herbalifeproducts), and gastrointestinal effects (myristicin, elemicin, omum/ajwain, red pepper, fennel, cardamom, dark pepper, cumin, coriander and ginger) (Guldiken et al., 2018). Brown (2017) documented case studies of twenty one herbs and twelve dietary supplements which posed a possible risk for liver injuries. In certain individuals, herbs with the high number of published reports (but not cases studies) were germander, black cohosh, kava extract, and green tea extract.
These reports are alarming and showed that there is a need of creating awareness, particularly amongst clinicians and patients for usage of safe phytochemical dose. At the same time, it compels researchers to evaluate safety of phytochemicals. Research in the toxicological domain (such as acute, subacute, subchronic and chronic studies) not only adds value to the ethanopharmacology of phytochemicals but also it will ensure the safe use and may avoid the occurrence of any untoward effects which were considered to be a major obstacle with the synthetic drugs. As per the OECD TG 407 (OECD, 2008), subacute toxicity studies are usually carried out after the availability of initial information on acute toxicity studies. It provides information on possible health hazards likely to arise from repeated exposure of drugs/chemical over a limited period of time (Hayes and Claire, 2007) and also, it helps in establishing-doses-for the longer-term subchronic studies (Eaton et al., 2018). In addition, subacute toxicology studies in rodents are considered to be an obligatory step to support the progression to clinical trials and the eventual marketing of drug molecules (Greaves, 2011).
However, to the best of our knowledge, no reports of subacute toxicity of SA are available in the literature. Hence, it was decided to conduct a subacute toxicity study. The objective was to find out safety aspects of SA on vital internal body organs (such as liver, brain, heart, kidney and sciatic nerve) in Wistar rats. This study will add value to its ethanopharmacological profile; provide preliminary data for researchers to conduct long-term toxicity studies (subchronic and chronic studies). Further, provide a platform to search ways of decreasing its toxic effects (if any).
2. Material and methods
2.1. Chemicals
Syringic acid (HSN-98020000) was purchased from TCI Chemicals (Tokyo, Japan) with 97% purity and sodium chloride solution (0.9 % w/v) was purchased from Aculife Healthcare Pvt. Ltd. (Gujrat, India) through local supplier. The SA was dissolved in sterile, non-pyrogenic sodium chloride solution (0.9 % w/v) on a daily basis in aseptic condition. World Health Organization has included sodium chloride solution (0.9 % w/v) in the List of Essential Medicines and considered it as an effective and safe medicine for the health system. All required biochemical kits were of analytical grade and purchased from the Lab-Care Diagnostics Pvt Ltd and Transasia Bio-Medicals Ltd, India.
2.2. Experimental animals
Healthy young adult male and female Wistar albino rats were obtained from the National Institute of Bioscience, Pune. The basic animal feed was provided by Nutrivet Life Sciences, Pune (57 % carbohydrate, 20 % protein, 4 % fat, 4.3 % fiber).
They were housed under standard environmental conditions of temperature at 22 ± 1 °C under a 12 h-light: 12 h-dark cycle (8.00 am–20.00 pm). Male and female rats were housed separately in sterile polypropylene cages. Fed with basic feed and purified water, allowed free access to drinking water and standard pelleted diet. All rats were allowed to adapt to the new environment for one week before the study.
2.3. Ethical approval
The experimental design of the present study was reviewed and approved with reference no. AIKTC/SOP/IAEC/2017/03 by the Institutional Animal Ethical Committee of AI's Kalsekar Technical Campus (AIKTC), Mumbai, Maharashtra, India (Reg.No.1771/PO/Re/S/14/CPCSEA) and animals were maintained as per the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), India.
2.4. Limit test/dose selection assay
As per OECD TG 425 (OECD,2008), initially a single dose of SA (2000 mg/kg b.w) was orally administered to 1 rat and then based on observation of mortality and morbidity, the SA (2000 mg/kg b.w) was administered to another 4 rats. All the rats were observed critically for any mortality and toxic signs for 14 days. The limit test was performed to determine the LD50 value and the dose for subacute toxicity study.
2.5. Subacute toxicity
According to OECD TG 407 (OECD, 2008), 36 rats (18 male and 18 female) were randomized into 3 groups having 12 rats each group (n = 12). The rats were marked to permit individual identification. Male and female rats were kept in separate cages and cages were numbered. Rats groups were as follows.
-
i.
Control group [1A (Male) and 1B (Female)] received a sodium chloride solution (0.9 % w/v) for 14 days.
-
ii.
Treatment group [2A (Male) and 2B (Female)] received SA (1000 mg/kg/day, p.o) for 14 days.
-
iii.
Satellite group [3A (Male) and 3B (Female)] received SA (1000 mg/kg/day, p.o for 14 days) and observed for another 14 days post treatment.
All the animals have been observed for mortality on daily basis and in-life clinical signs, body weight, food and water intake were recorded on weekly basis. The control group and treatment group animals were sacrificed on day 15 for required evaluations as per the experimental design.
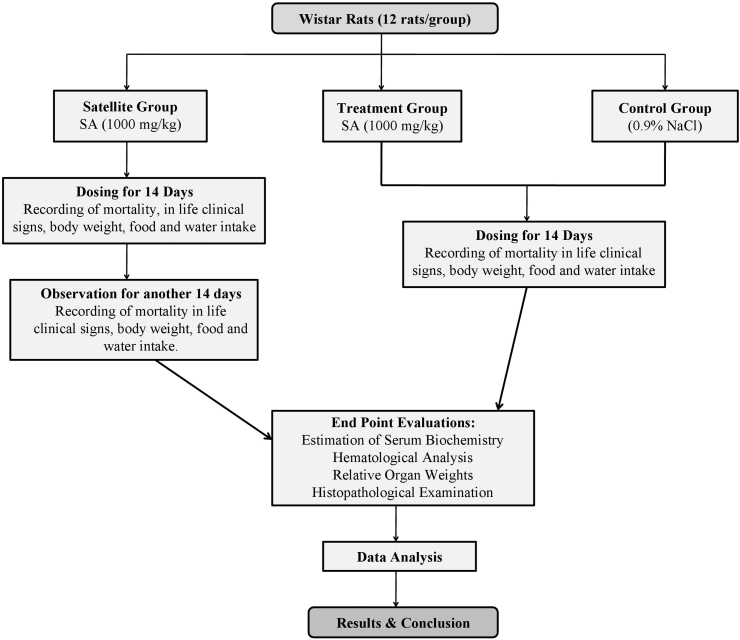
In order to access reversibility or recovery; satellite group was continued for another 14 days post-treatment. All the rats were observed on daily basis for mortality. In-life clinical signs, body weight, food and water intake were measured for additional day 21 and 28. On day 29 rats were sacrificed for endpoint evaluations (Thanabhorn et al., 2006; Witthawaskul et al., 2003; Sireeratawong et al., 2012; Eaton et al., 2018).The schematic illustration of experimental design is shown in Fig. 1.
Fig. 1.
The schematic illustration of experimental design.
2.6. In-life clinical observations
Animals were observed twice a day for mortality. Observations of clinical signs were recorded during handling and in open field on day 0,7,14 for animals of the control and treatment group. Animals of satellite group were observed on additional day 21 and 28. Clinical signs included changes in sensory organs (such as skin, eyes, nose), body secretion, autonomic activities (respiratory rate, pupil size, piloerections), response while handling, walking pattern, overall body language, muscle tremor and behavior, etc. (Wu et al., 2018).
2.7. Body weight and body weight gain
Animals were weighed twice prior to grouping. Body weights were recorded on days 1 (prior to dosing), 7, 14 and 15 (fasting body weight prior to sacrifice to calculate relative body organ weight) in treatment and control groups. Body weight measurements of animals of satellite group were continued on a weekly interval on day 21, 28 and 29 (prior to sacrifice) (Thanabhorn et al., 2006; Witthawaskul et al., 2003). At the end of the testing period, mean body weight gains were calculated for each group.
2.8. Food and water consumption
Animals were allowed ad libitum access to food and water throughout the study. Food and water consumption for animals of treatment and control groups were measured on Days 1 (prior to dosing), 7 and 14. Measurements were continued for animals of satellite group for day 21 and 28 (Kim et al., 2018; Thanabhorn et al., 2006). Animals were fasted overnight prior to termination of the study.
2.9. Hematological analysis
At the termination of the study, all surviving animals were anesthetized by isoflurane and blood samples were collected via the inferior vena cava in pre-calibrated tubes coated with ethylene diamine tetraacetic acid (EDTA) for hematological assessment which was conducted by Unique Biodiagnostics Enterprises (Mumbai, India). The parameters are as follows: Red blood cells count (RBC), hemoglobin concentration (HBG), platelet count (PLT), packed cell volume (PCV), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), total white blood cell (TWBC), mean corpuscular hemoglobin concentration (MCHC) and differential leukocyte count, which includes percentage of neutorophill (N%), eosinophill (E%), lymphocytes (L%) and monocytes (M%).
2.10. Serum biochemistry
The non-heparinized blood samples were collected for biochemical estimations. The coagulated blood samples were centrifuged at 2000 g to obtain serum. The serum was taken into new tubes and stored at -20 °C until analyzed. The serum biochemical parameters were estimated spectrophotometerically by using kits of analytical grade. Parameters include blood glucose (BG), blood urea nitrogen (BUN), creatnine (CR), total protein (TP), albumin (ALB), total billirubin (TB), direct billirubin (DB), Aspartate transaminase (AST), Alanine transaminase (ALT), alkaline phosphatase (ALP), calcium (Ca), sodium (Na), potassium (K), and chlorine (Cl), phosphate (P), lactate dehydrogenase (LDH), high density lipoprotein (HDL), triglyceride (TG) and total cholesterol (TC).
2.11. Gross necropsy and relative organ weight
At scheduled terminal sacrifice (Day 15 and 29), all surviving animals were anesthetized and dissected out. A gross necropsy examination was performed to identify the presence of lesions which included examinations of the external surface of the body, all orifices, skeletal systems and body cavities (such as cranial, thoracic, abdominal, and pelvic) (Kim et al., 2018). Immediately the lungs, liver, heart, spleen, kidneys, adrenal gland, testis/ovaries were excised, freed of fat, washed in cold saline, blotted with clean tissue paper, and observed for gross pathological changes. Then the organ weights were recorded in grams using a calibrated balance and relative body weights were calculated by taking ratio of these organ weights to body weight.
2.12. Histopathological examinations
Samples of the liver, heart, kidneys, pancreas, hippocampus, sciatic nerve were collected for histological studies. The tissues were fixed immediately in 10% formalin, dehydrated through a series of ethanol solutions, and embedded in paraffin. Thin sections of 4-5-μm-thickness were cut with the help of rotary microtome and were stained with hematoxylin and eosin for photomicroscopic observation. All histopathological changes were examined by a pathologist. The microscopic features of the organs of both treated and satellite groups were then compared with those of the control group. Slide preparation and histological assessment were carried out by Unique Biodiagnostic Enterprises (Mumbai, India).
2.13. Statistical analysis
Data analysis was performed using GraphPad Instat 3.0 version (GraphPad, San Diego, CA). Data were expressed as mean ± standard deviation (SD) and analyze using one-way analysis of variance (ANOVA), Dunnett's multiple range test was applied for post hoc analysis. A value of P < 0.05 was considered to be statistically significant.
3. Results
3.1. Limit test
A single dose of 2000 mg/kg/p.o of SA did not show any mortality and signs of toxic effects in all 5 rats. Therefore, it can be concluded that the LD50 value may be greater than 2000 mg/kg. Hence, 1000 mg/kg dose was considered to be safe and by referring the published literature and OECD TG 407 (OECD, 2008), this dose was selected to conduct a subacute toxicity study (Thanabhorn et al., 2006; Witthawaskul et al., 2003; Sireeratawong et al., 2012).
3.2. In-life clinical observations
All animals were observed daily for mortality. Observations for any clinical signs during handling and in the open field were recorded on a weekly basis till the termination of the study. No mortality was observed during and at the end of the study period in any of the animal groups. The experimental rats were observed critically during handling and in open field; no prominent clinical signs such as vocalization, lacrimation, salivation, irregular respiratory pattern, convulsion, tremors and no unusual behavior were observed in the animals of treatment group and satellite group as compared to control group. The details are summarized in Table S1 (Provided in supplementary material).
3.3. Body and relative organ weight
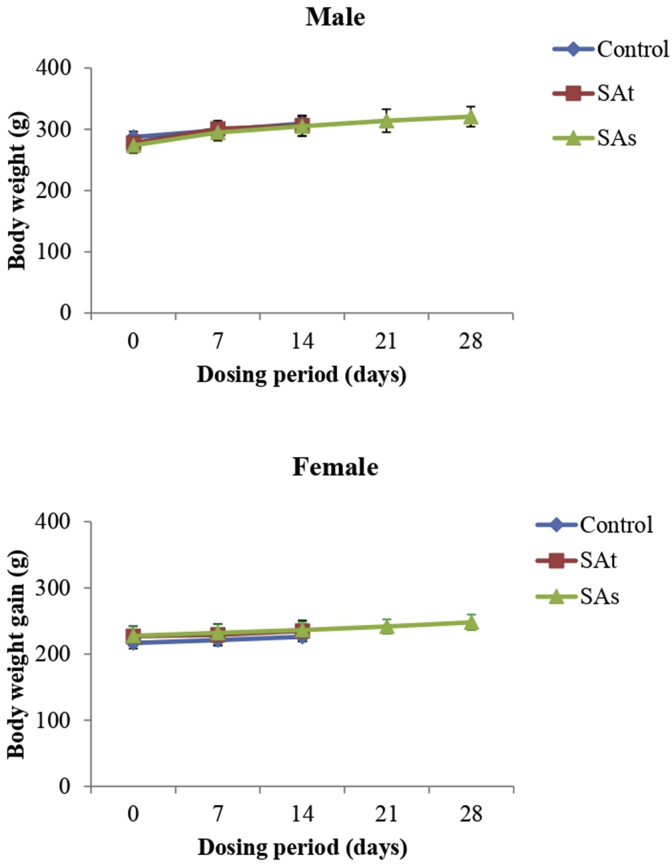
Changes in the body weights were summarized in Fig. 2. It was recorded on a weekly basis. Proper increase in body weights were found in animals of treatment and satellite group as compared to normal control animals.
Fig. 2.
Body weight gain of rats in subacute toxicity studies of the SA. Values are expressed as mean ± SD (n = 6), *p < 0.05, **p < 0.01 compared to control group with statistical analysis by one-way ANOVA followed by Dunnett's post-hoc test. SAt is a group of animals treated with syringic acid (1000 mg/kg) for 14 days and then sacrificed. SAs is a satellite group treated with syringic acid (1000 mg/kg) for 14 days, no treatment for another 14 days and then sacrificed.
3.4. Food and water consumption
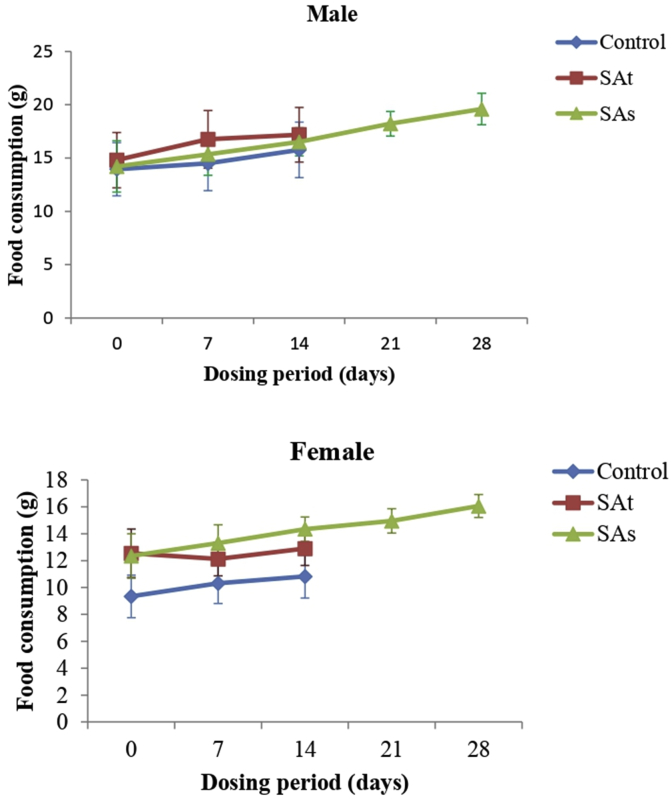
Food consumption for the treatment and satellite groups of male and female animals were compared with vehicle-treated control group. During the study, the proper increase in food consumption was observed in male animals of treatment and satellite groups. However, decreased food consumption on Day 7 in female treatment group was observed, followed by a proper increase on day 14 (Fig. 3), the female animals of satellite group showed a regular increase in food consumption. However, this initial decreased food consumption did not result in an overall decrease in body weights of female rats and hence are not interpreted to be toxicologically relevant. Further, oral administration of SA caused no major alteration in water consumption in the treatment and satellite groups compared to control group.
Fig. 3.
Food consumption of rats administered daily doses of SA. Values are expressed as mean ± SD (n = 6), *p < 0.05, **p < 0.01 compared to control group with statistical analysis by one-way ANOVA followed by Dunnett's post-hoc test. SAt is a group of animals treated with syringic acid (1000 mg/kg) for 14 days and then sacrificed. SAs is a satellite group treated with syringic acid (1000 mg/kg) for 14 days, no treatment for another 14 days and then sacrificed.
3.5. Hematological examination
The results of hematological examination were presented in Table 1.
Table 1.
Effect of SA on hematological parameters at the termination of study.
| Parameters | Groups (Male) |
Groups (Female) |
||||
|---|---|---|---|---|---|---|
| Control | SAt | SAs | Control | SAt | SAs | |
| Hb (gm%) | 13.00 ± 0.44 | 12.68 ± 0.95 | 15.33 ± 0.82** | 12.35 ± 0.80 | 12.10 ± 1.52 | 14.18 ± 0.82* |
| RBC (x 106/cmm) | 07.34 ± 0.23 | 07.32 ± 0.62 | 08.98 ± 0.37** | 06.89 ± 0.40 | 06.51 ± 0.76 | 07.97 ± 0.65* |
| WBC (x 103/cmm) | 11.88 ± 3.74 | 13.88 ± 1.88 | 11.51 ± 2.03 | 10.13 ± 2.91 | 10.63 ± 3.54 | 10.23 ± 2.94 |
| PLT(x 105/cmm) | 09.48 ± 1.20 | 08.47 ± 1.36 | 10.49 ± 1.22 | 08.87 ± 1.79 | 09.14 ± 1.90 | 09.11 ± 1.17 |
| PCV (%) | 33.86 ± 1.29 | 34.75 ± 2.40 | 49.33 ± 2.76** | 32.73 ± 1.90 | 32.40 ± 3.70 | 46.25 ± 2.43** |
| MCV(fl) | 46.06 ± 0.56 | 47.85 ± 1.40* | 54.56 ± 1.39** | 47.58 ± 0.75 | 49.85 ± 1.58 | 58.2 ± 2.31** |
| MCH (pg) | 17.61 ± 0.23 | 17.28 ± 0.26 | 17.00 ± 0.23** | 17.88 ± 0.33 | 18.50 ± 0.27* | 17.76 ± 0.54 |
| MCHC (gm/dl) | 38.33 ± 0.42 | 36.21 ± 0.84** | 30.95 ± 0.18** | 37.66 ± 0.75 | 37.26 ± 1.00 | 30.61 ± 0.34** |
| WBC (x 103/cmm) | 11.88 ± 3.74 | 13.88 ± 1.88 | 11.51 ± 2.03 | 10.13 ± 2.91 | 10.63 ± 3.54 | 10.23 ± 2.94 |
| N % | 44.66 ± 8.80 | 54.16 ± 4.02* | 64.66 ± 6.50** | 44.00 ± 17.29 | 46.33 ± 12.86 | 60.66 ± 4.54 |
| E % | 02.83 ± 2.85 | 07.16 ± 6.36 | 0.83 ± 0.75 | 06.00 ± 4.60 | 03.00 ± 3.22 | 1.16 ± 1.16* |
| L % | 51.00 ± 9.71 | 37.83 ± 9.80* | 34.00 ± 6.98** | 49.33 ± 21.22 | 49.50 ± 15.24 | 37.33 ± 4.59 |
| M % | 01.5 ± 1.76 | 0.83 ± 0.40 | 0.50 ± 0.54 | 0.66 ± 0.51 | 1.16 ± 0.75 | 0.83 ± 0.75 |
Values are expressed as mean ± SD (n = 6), *p < 0.05, **p < 0.01 compared to control group with statistical analysis by one-way ANOVA followed by Dunnett's post-hoc test.
SAt is a group of animals treated with syringic acid (1000 mg/kg) for 14 days and then sacrificed. SAs is a satellite group treated with syringic acid (1000 mg/kg) for 14 days, no treatment for another 14 days and then sacrificed.
The Male treatment group of rats showed no significant increase in the HGB, RBC, PCV, PLT, MCH except increased MCV (p < 0.05) and MCHC level (p < 0.01). In female treatment group no significant changes in hematological parameters were found except an increase in MCH (p < 0.05). All hematological parameters were found to be increased (p < 0.01) but within normal range in male and female satellite groups.
In male treatment group there was a similar WBC count when compared to a normal control group but, increase in neutrophills (p < 0.05) and decrease in lymphocyte count (p < 0.05) were observed with no recovery in satellite group. No significant change in WBC count was observed in female animals of treatment and satellite group, but a significant decrease in eosinophill count (p < 0.05) was observed in satellite group.
3.6. Blood biochemistry
The data of blood biochemistry represented in Table 2, biochemical analysis involved estimations such as BG, BUN, CR, TP, albumin, TB, DB, AST, ALT, ALP, electrolytes (Ca, K, Cl and P), LDH, lipid profile [HDL, TC and TG].
Table 2.
Effect of SA on biochemical estimations in Wistar rats.
| Parameters | Groups (Male) |
Groups (Female) |
||||
|---|---|---|---|---|---|---|
| Control | SAt | SAs | Control | SAt | SAs | |
| BG (mg/dl) | 89.26 ± 10.42 | 79.23 ± 11.60 | 91.92 ± 8.82 | 98.23 ± 13.75 | 91.73 ± 8.19 | 93.42 ± 10.09 |
| BUN (mg/dl) | 20.24 ± 1.94 | 19.70 + 1.88 | 21.12 ± 0.97 | 22.07 ± 11.67 | 23.34 ± 1.72 | 21.41 ± 1.26 |
| CR (mg/dl) | 0.51 ± 0.04 | 0.47 ± 0.07 | 0.50 ± 0.04 | 0.49 ± 0.10 | 0.52 ± 0.05 | 0.53 ± 0.06 |
| TP (g/dl) | 6.48 ± 0.76 | 6.66 ± 0.82 | 7.02 ± 0.38 | 7.37 ± 0.48 | 7.45 ± 0.34 | 7.30 ± 0.40 |
| ALB (g/dl) | 4.97 ± 0.23 | 4.83 ± 0.34 | 5.04 ± 0.43 | 4.99 ± 0.39 | 5.08 ± 0.28 | 5.09 ± 0.22 |
| TB (mg/dl) | 0.21 ± 0.03 | 0.23 ± 0.05 | 0.22 ± 0.03 | 0.24 ± 0.04 | 0.23 ± 0.03 | 0.22 ± 0.03 |
| DB (mg/dl) | 0.062 ± 0.01 | 0.04 ± 0.02 | 0.06 ± 0.027 | 0.081 ± 0.05 | 0.08 ± 0.06 | 0.102 ± 0.05 |
| AST (U/L) | 84.32 ± 7.32 | 87.02 ± 5.27 | 86.07 ± 4.40 | 73.08 ± 9.29 | 76.62 ± 6.33 | 71.37 ± 5.93 |
| ALT (U/L) | 32.81 ± 3.73 | 31.71 ± 3.73 | 31.42 ± 3.12 | 28.80 ± 3.94 | 31.71 ± 3.38 | 30.55 ± 3.50 |
| ALP (U/L) | 262.48 ± 6.69 | 246.16 ± 14.87 | 266.56 ± 32.91 | 152.77 ± 12.80 | 126.48 ± 10.70** | 151.86 ± 11.97 |
| Ca (mg/dl) | 11.21 ± 0.78 | 10.09 ± 1.19 | 10.43 ± 0.77 | 11.38 ± 1.02 | 11.01 ± 0.57 | 10.44 ± 0.66 |
| K (mEq/L) | 7.54 ± 1.89 | 8.00 ± 0.68 | 8.54 ± 1.27 | 8.75 ± 0.85 | 8.75 ± 1.00 | 7.87 ± 1.67 |
| Cl (mEq/L) | 103.76 ± 5.69 | 105.44 ± 12.77 | 112.2 ± 12.50 | 106.77 ± 7.27 | 108.87 ± 9.67 | 110.73 ± 10.70 |
| Na (mEq/L) | 86.03 ± 4.90 | 86.78 ± 27.84 | 81.00 ± 22.51 | 82.33 ± 15.46 | 64.33 ± 21.77 | 68.59 ± 9.74 |
| P (mg/dl) | 5.93 ± 1.87 | 5.83 ± 1.50 | 6.14 ± 1.60 | 6.11 ± 0.73 | 6.65 ± 1.52 | 6.68 ± 1.59 |
| LDH (U/L) | 361.44 ± 82.50 | 361.44 ± 82.50 | 356.93 ± 48.51 | 473.90 ± 117.43 | 365.68 ± 48.99 | 403.90 ± 40.26 |
| HDL (mg/dl) | 20.00 ± 3.53 | 21.25 ± 4.10 | 21.66 ± 5.79 | 21.66 ± 4.37 | 22.50 ± 5.47 | 24.58 ± 4.30 |
| TCl (mg/dl) | 81.44 ± 7.92 | 87.18 ± 5.73 | 83.41 ± 8.28 | 87.96 ± 4.14 | 88.45 ± 3.78 | 87.98 ± 6.15 |
| TG (mg/dl) | 110.66 ± 6.22 | 105.21 ± 5.73 | 108.08 ± 5.48 | 108.37 ± 8.08 | 114.26 + 7.74 | 110.65 ± 10.86 |
Values are expressed as mean ± SD (n = 6), *p < 0.05, **p < 0.01 compared to control group with statistical analysis by one-way ANOVA followed by Dunnett's post-hoc test.
SAt is a group of animals treated with syringic acid (1000 mg/kg) for 14 days and then sacrificed.
SAs is a satellite group treated with syringic acid (1000 mg/kg) for 14 days, no treatment for another 14 days and then sacrificed.
All biochemical parameters were found to be unaltered in male and female treatment and satellite groups when compared to the respective normal control group except ALP level (p < 0.01) in female treatment group was decreased significantly which was found recovered in satellite group.
3.7. Gross necropsy and relative organ weight
Macroscopic examination includes critical observation of body systems and/or organs such as gastrointestinal systems (stomach, duodenum, jejunum, ileum, cecum, colon and rectum), lungs, brain, pancreas, sciatic nerve, skeletal muscles, kidneys, adrenal gland, reproductive organs (testis or ovaries), heart, liver, spleen, all orifices and body cavities. Macroscopic findings showed no irregularities in gross anatomical features of organs of male and female treatment groups as compared to control group animals. Satellite group too showed no major alterations in structure of organs.
No significant decrease in relative body organ weights was observed in male treatment group but a significant increase in relative weight of heart and spleen was reported in respective satellite group. In female treatment group, a significant decrease in liver weight was observed and it was not recovered in satellite group. Other internal vital body organs had no major alteration in weight in female rats.
3.8. Histopathological findings
The 6 organs were used for histopathological findings in the current study. As shown in Fig. 4, no pathological changes were observed in treatment and satellite groups as compared to normal control animals.
Fig. 4.
Histological results of vital internal body organs after oral administration of SA. SAt is a group of animals treated with syringic acid (1000 mg/kg) for 14 days. SAs is a satellite group treated with syringic acid (1000 mg/kg) for 14 days, no treatment for another 14 days.
The detail analyses are as follows:
Hypothalamus: Sections revealed normal neuronal histomorphological features with intact supporting matrix.
Heart: Section of heart tissues showed normal features of cardiac muscle fibers, cardiac fibers were arranged in dense and compact fashion with intact length and normal cell striation and nuclei.
Kidney: Examination of kidney tissue sections showed normal glomeruli and renal tubules in the cortex and medulla.
Liver: The tissue sections showed intact hepatic parenchyma comprised of hepatocytes, portal triad and central vein. Hepatocytes were arranged in cords with intact nucleus and cellular borders.
Sciatic nerve: The tissue sections showed normal histomorphological features of nerve tissue with an intact length of nerve fiber and normal cellular detail.
Pancreas: Sections showed normal appearance of the islets of Langerhans.
No inflammatory or metabolic changes were observed in all histological sections of vital body organs.
4. Discussion
SA is a potent antioxidant and investigated for diverse pharmacological effects (Srinivasulu et al., 2018; Jayachandran, 2013; Aziz et al., 1998; Wei et al., 2012; Kumar et al., 2010; Ramachandran and Raja, 2003; Tokmak et al., 2015; Abhishek and Abhijit , 2015; Ju et al., 2016; Srinivasulu et al., 2018; Choi and Kim, 2018). To achieve optimum therapeutic benefits one has to take it for long duration and hence toxic or undesirable effects cannot be ignored. In the current literature no systematic information on toxicity is available. Therefore, it was decided to conduct a subacute toxicity study, the research finding will not only add value to its ethanopharmacological profile, but also provides information on possible health hazards likely to arise from repeated administration over a limited period of time (Hayes and Claire, 2007). In addition, the study will help to establish doses for the longer-term subchronic studies (Eaton et al., 2018). The study was planned (shown in Fig. 1) as per the OECD TG407 (OECD, 2008) and previous reported studies (Thanabhorn et al., 2006; Witthawaskul et al., 2003; Sireeratawong et al., 2012; Eaton et al., 2018). To select the dose of SA for subacute toxicity study; a limit test was performed by using a single dose (2000 mg/kg p.o) (OECD TG 425). Rats were observed critically for any toxic effect and mortality. No toxic symptoms and mortality were observed during and at the end of 14 days. Generally the toxic effect of drugs caused toxic signs and/or mortality. Therefore, this result showed that LD50 value of SA may be greater than 2000 mg/kg and 1000 mg/kg dose of SA may be safe for subacute toxicity study.
Toxic signs, food and water intake, if taken together, may indicate malaise or covert toxicity long before overt signs; if the animal does not feel well it will not eat sufficient food and/or drink the usual amount of water. Hence, body weight, food and water intake are the indicator of general health. Therefore, these parameters are crucial to assess the safety of the drug during the subacute toxicity study (Wang et al., 2017; Thanabhorn et al., 2006). Figs. 2 and 3 showed the effect of SA on body weight and food intake. Proper increase in body weight was reported along with normal increase in food and water intake compared with control group animals. In-life clinical observations were recorded during the study and the animals of treated and satellite groups didn't show any toxic symptoms, it could be concluded that oral administration of SA during subacute toxicity study period has no undesirable effect on the growth and physiological functions of rats.
Biochemical and hematological evaluations were amongst important parameters to assess safety of drug (Wang et al., 2017; Nyarko et al., 2005; Chung et al., 2015) therefore, they were estimated in current subacute toxicity study. Male treatment group of rats showed no significant increase in the HGB, RBC count, PCV, PLT count, MCH except MCV and MCHC with no recovery in the satellite group (Table 1). All hematological parameters were found to be increased in satellite group, but within the normal range which cannot be considered as a toxic effect (Kanu et al., 2016). This can be justified by taking into consideration, a condition of decrease in HBG, RBC count and other related parameters which showed anemic effect and adverse effect of SA on erythropoiesis. Total WBC count in male treatment group showed no increase as compared to normal control group, but increase in neutrophil and decrease in lymphocyte count was observed with no recovery in satellite group, this effect may justify antibacterial (Aziz et al., 1998) and anti-inflammatory effect (Ju et al., 2016) of SA. No significant change in WBC count was observed in female treatment and satellite group except eosinopenia in satellite group, but still it corroborate wide safety of SA (Adeneye et al., 2006). Hence, the results endorsed non toxicity of SA on physiology of red bone marrow.
Effect of SA on biochemical parameters were presented in Table 2. DB, AST, ALT and ALB levels are index of liver function (Mathew and Dushyant, 2014). No significant changes were reported in the treatment group and satellite group as compared to normal control group. In addition, histopathological reports showed no major alteration in the structural features of the liver. Hence, SA could not be considered toxic to the liver and this fact is supported by the non-significant change in BG and CR levels in the treated group as compared to normal control (Andy et al., 2010). ALP is a group of enzymes which catalyze the hydrolysis of phosphate esters generating organic radicals and inorganic phosphate (Tanuja et al., 2016). In the present study, low level of ALP in treated group with no recovery in the satellite group of female rats was reported, the low ALP level is an indicator of Wilson disease also known as hepatolenticular degeneration associated with pernicious anemia, hypophosphatasia and hypercupremia (Eve and Michael, 2008) but the same situation was not observed in male treatment group and not supported by histopathological studies of liver, non-significant change in serum ALT, AST, DB and phosphate level and no reduced RBC count. Therefore, it may not be considered as a severe toxic effect.
BUN, CR, TP and LDH are indicators of renal function (William, 1981; Bhargava et al., 1978). No statistically significant change was reported in the treatment and satellite groups of rats. These observations revealed that oral administration of SA may not affect the anatomical features of kidney, which is supported by the normal structural features found in histopathological studies and no significant change in relative kidney weight in the treatment and satellite group of both male and female rats. Therefore, administration of SA during the subacute study period might be safe for kidneys.
Electrolyte balance is an indicator of osmotic regulation by blood, kidney and cardiac function, digestion and intermediary metabolism (Simon, 2004; Rose and Post, 2001). Serum electrolytes (Na, K, Ca and P) levels were estimated at the end of the study period (Unuofin et al., 2018; Mei-Yin et al., 2018). No significant changes were observed in treatment and satellite groups as compared to control group. This observation showed that the SA is not affecting the digestive system, metabolism process, cardiac and renal functioning which is further supported by the non significant changes in renal, lipid parameters (TG, HDL and TC) and normal histological finding of heart and liver. Hence it could be concluded that SA may not have any adverse effect on electrolyte balance.
Observation of relative internal body organ weight is a crucial parameter to assess safety of drug (Wang et al., 2017; Thanabhorn et al., 2006). The relative organ weights were found unaltered in male treatment group, however an increased heart and spleen weight were observed in respective satellite group (Table 3). This may not be considered as a toxic effect and can be linked with body weight changes (Choi and Kim, 2018). In female treatment group, a significant decrease in relative liver weight was observed with no recovery in satellite group, but it is not consistent with observation of male groups, not in coordination with the results of histopathological study and estimation of liver enzymes. It may an adaptive change and non adverse change, hence may not be considered as a toxic effect (Hall et al., 2012). There were no major alterations in relative weight for other organs in both the male and female rats. Toxic effect of SA on internal vital organ was further verified with the help of histopathological studies which showed no abnormalities. Therefore, it could be concluded that SA might not have major toxic effects on internal vital body organs.
Table 3.
Effect of SA on relative body organ weight of Wistar rats.
| Organ weight (g % body weight) | ||||||
|---|---|---|---|---|---|---|
| Groups (Male) |
Groups (Female) |
|||||
| Organs | Control | SAt | SAs | Control | SAt | SAs |
| Lung | 0.47 + 0.01 | 0.43 + 0.01 | 0.45 + 0.10 | 0.49 + 0.001 | 0.51 + 0.02 | 0.51 + 0.02 |
| Heart | 0.37 + 0.01 | 0.36 + 0.02 | 0.40 + 0.02* | 0.31 + 0.02 | 0.28 + 0.02 | 0.29 + 0.03 |
| Liver | 3.71 + 0.04 | 3.64 + 0.16 | 3.76 + 0.06 | 2.92 + 0.11 | 2.70 + 0.09* | 2.62 + 0.03* |
| Spleen | 0.37 + 0.01 | 0.35 + 0.03 | 0.42 + 0.03** | 0.25 + 0.01 | 0.24 + 0.02 | 0.26 + 0.02 |
| Kidney | 0.47 + 0.01 | 0.46 + 0.01 | 0.48 + 0.005 | 0.46 + 0.07 | 0.41 + 0.02 | 0.48 + 0.09 |
| Testis/Ovary | 0.63 + 0.02 | 0.61 + 0.02 | 0.62 + 0.01 | 0.11 + 0.01 | 0.10 + 0.01 | 0.10 + 0.008 |
| Adrenal gland | 0.017 + 0.001 | 0.016 + 0.001 | 0.016 + 0.001 | 0.016 + 0.001 | 0.014 + 0.003 | 0.017 + 0.003 |
Values are expressed as mean ± SD (n = 6), *p < 0.05, **p < 0.01 compared to control group with statistical analysis by one-way ANOVA followed by Dunnett's post-hoc test.
SAt is a group of animals treated with syringic acid (1000 mg/kg) for 14 days and then sacrificed.
SAs is a satellite group treated with syringic acid (1000 mg/kg) for 14 days, no treatment for another 14 days and then sacrificed.
5. Conclusion
SA is a polyphenol with diverse pharmacological effects. Recent reports showed that safety is a major concern for phytochemicals too. No systematic study is available on subacute toxicity of SA. Therefore, it was decided to conduct a subacute study, which will be considered preliminary to subchronic and chronic studies. The results of physical, hematological and biochemical parameters during and at the end of the subacute toxicity study had shown no significant changes in SA treated rats. Thus the process of erythropoiesis, leukopoiesis and physiology of internal body organ were not disturbed. No structural abnormalities were observed in histopathological studies. Hence, SA could be considered safe over limited period of time and this study may help researcher in establishing doses for the longer-term subchronic studies. Further, subchronic and chronic toxicity studies are required to evaluate safety on long term use.
Declarations
Author contribution statement
Shital S Panchal, Anwar Baig Mirza: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by University of Mumbai under Minor Research Grant Scheme (Sanction letter No. APD/237/323 of 2018 dated 27th March 2018 of 2018. Proposal No: S-96, Research Project No. 183).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Author would like to acknowledge the infrastructural support provided by AI's Kalsekar Technical Campus and Institute of Pharmacy, Nirma University to complete the study.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Abhishek B., Abhijit D. Mechanism of antiglycating properties of syringic and chlorogenic acids in in vitro glycation system. Food Res. Int. 2015;3(77):540–548. [Google Scholar]
- Adeneye A.A., Ajagbonna O.P., Adeleke T.I., Bello S.O. Preliminary toxicity and phytochemical studies of the stem bark aqueous extract of Musanga cecropioides in rats. J. Ethnopharmacol. 2006;105:374–379. doi: 10.1016/j.jep.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Andy S., Andrew Y., Julia W. Renal dysfunction in chronic liver disease. Crit. Care. 2010;14(2):214. doi: 10.1186/cc8855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz N.H., Farag S.E., Mousa L.A., Abo-Zaid M.A. Comparative antibacterial and antifungal effects of some phenolic compounds. Microbios. 1998;93(374):43–54. [PubMed] [Google Scholar]
- Bhargava A.S., Khater A.R., Gunzel P. The correlation between lactate dehyrogenase activity in urine and serum and experimental renal damage in the rat. Toxicol. Lett. 1978;1(5-6) 319-232. [Google Scholar]
- Brown A.C. Liver toxicity related to herbs and dietary supplements: online table of case reports. Part 2 of 5 series. Food Chem. Toxicol. 2017;107:472–501. doi: 10.1016/j.fct.2016.07.001. [DOI] [PubMed] [Google Scholar]
- Choi J.H., Kim S. Mechanisms of attenuation of clot formation and acute thromboembolism by syringic acid in mice. J. Funct. Foods. 2018;43:112–122. [Google Scholar]
- Chung T.H., Myoung J.K., Seol H.M., Yu R.J., Jae S.H., Chunja N., Chong W.P., Sun H.L., Jae B.N., Chan S.P., Hee W.P., Jung M.L., Ho S.J., Sun H.P., Kyoung G.H., Young W.C., Hye Y.L., Jong K.K. Acute and 28-day subacute toxicity studies of hexane extracts of the roots of lithospermum erythrorhizon in sprague-dawley rats. Toxicol. Res. 2015;31(4):403–414. doi: 10.5487/TR.2015.31.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton D.L., Gallagher E.P., Vandivort T.C. General overview of toxicology. In: McQueen C., editor. Comprehensive Toxicology. Elsevier Science; USA: 2018. pp. 1–38. [Google Scholar]
- Eve A.R., Michael L.S. Diagnosis and treatment of wilson disease: an update. Hepatology. 2008;47(6):2089–2111. doi: 10.1002/hep.22261. [DOI] [PubMed] [Google Scholar]
- Greaves P. Academic Press; USA: 2011. Histopathology of Preclinical Toxicity Studies: Interpretation and Relevance in Drug Safety Evaluation; pp. 1–10. [Google Scholar]
- Guldiken B., Ozkan G., Catalkaya G., Ceylan F.D., Yalcinkaya I.E., Capanoglu E. Phytochemicals of herbs and spices: health versus toxicological effects. Food Chem. Toxicol. 2018;119:37–49. doi: 10.1016/j.fct.2018.05.050. [DOI] [PubMed] [Google Scholar]
- Hall A.P., Elcombe C.R., Foster J.R., Harada T., Kaufmann W., Knipple A., Kutttler K., Malarkey D.E., Maronpot R.R., Nishikawa A., Nolte T., Schulte A., Strauss V., York M.J. Liver hypertrophy: a review of adaptive (adverse and non-adverse) changes–conclusions from the 3rd international ESTP expert workshop. Toxicol. Pathol. 2012;40(7):971–994. doi: 10.1177/0192623312448935. [DOI] [PubMed] [Google Scholar]
- Hayes A.W., Claire L.K. Crc Press; New York: 2007. Principles and Methods of Toxicology; pp. 1223–1245. [Google Scholar]
- Jayachandran M. Syringic acid, a novel natural phenolic acid, normalizes hyperglycemia with special reference to glycoprotein components in experimental diabetic rats. J. Acute Dis. 2013:304–309. [Google Scholar]
- Ju R.H., Hae-In L., Ra-Yeong C., Mi-Ok S., Seo Kwon-I., Mi-Kyung L. Anti-steatotic and anti-inflammatory roles of syringic acid in high-fat diet-induced obese mice. Food Funct. 2016;7:689–697. doi: 10.1039/c5fo01329a. [DOI] [PubMed] [Google Scholar]
- Pandey Kanti B., Syed I.R. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell Longev. 2009;2(5):270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanu K.C., Ijioma S.N., Atiata O. Haematological, biochemical and antioxidant changes in Wistar rats exposed to dichlorvos based insecticide formulation used in southeast Nigeria. Toxics. 2016;4(4):28–29. doi: 10.3390/toxics4040028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoddami A., Wilkes M.A., Roberts T.H. Techniques for analysis of plant phenolic compounds. Molecules. 2013;18:2328–2375. doi: 10.3390/molecules18022328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Jaja-Chimedza A., Merrill D., Mendes O., Raskin I. A 14-day repeated-dose oral toxicological evaluation of an isothiocyanate- enriched hydro-alcoholic extract from Moringa oleifera Lam. seeds in rats. Toxicol. Rep. 2018;5:418–426. doi: 10.1016/j.toxrep.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Prahalathan P., Raja B. Syringic acid ameliorates (L)-NAME-induced hypertension by reducing oxidative stress. J. Basic Clin. Physiol. Pharmacol. 2010;21(4):369–385. doi: 10.1007/s00210-012-0802-7. https://link.springer.com/article/10.1007%2Fs00210-012-0802-7 [DOI] [PubMed] [Google Scholar]
- Mathew S., Dushyant S. Interpretation of abnormal liver function tests. Hosp. Med. Clin. 2014;3(1):e139–148. [Google Scholar]
- Mei-Yin C., Chih M.Y., Yi -T.L., Chao-Hsiang C. Dihydromyricetin-rich herbal mixture extracts as a potential prescription for treatment of metabolic syndrome in rats fed a high-fat diet and subacute toxicity assessment in rats. J. Tradit. Complement. Med. 2018;16(5):335–341. doi: 10.1016/j.jtcme.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyarko A.K., Okine L.K.N., Wedzib R.K., Addo P.A., Ofosuhene M. Subchronic toxicity studies of the antidiabetic herbal preparation ADD-199 in the rat: absence of organ toxicity and modulation of cytochrome P450. J. Ethnopharmacol. 2005;97:319–325. doi: 10.1016/j.jep.2004.11.021. [DOI] [PubMed] [Google Scholar]
- Ramachandran V., Raja B. Protective effects of syringic acid against acetaminophen-induced hepatic damage in albino rats. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2003;23(2):206–208. doi: 10.1515/jbcpp.2010.21.4.369. [DOI] [PubMed] [Google Scholar]
- Rong T. Chemistry and biochemistry of dietary polyphenols. Nutrients. 2010;2:1231–1246. doi: 10.3390/nu2121231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose B.D., Post T.W. Introduction to disorders of potassium balance. In: Rose B.D., Post T.W., editors. Clinical Physiology of Acid-Base and Electrolyte Disorders. McGraw-Hill; New York, NY: 2001. pp. 822–836. [Google Scholar]
- Simon A. Fluid, electrolytes and nutrition. Clin. Med. 2004;4:573–578. doi: 10.7861/clinmedicine.4-6-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sireeratawong S., Thamaree S., Ingkaninan K., Piyabhan P., Vannasiri S., Khonsung P., Parirat K., Tipaya S., Jaijoy K. Evaluation of acute and subacute oral toxicity of the ethanol extract from Antidesma Acidum Retz. Afr. J. Tradit., Complementary Altern. Med. 2012;9(4):465–469. doi: 10.4314/ajtcam.v9i4.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasulu C., Ramgopal M., Ramanjaneyulu G., Anuradha C.M., Suresh K. Syringic acid (SA) ‒ a review of its occurrence, biosynthesis, pharmacological and industrial importance. Biomed. Pharmacother. 2018;108:547–557. doi: 10.1016/j.biopha.2018.09.069. [DOI] [PubMed] [Google Scholar]
- Tanuja S., Ruchi, Ravish K., Vimal K., Anjali S. Evaluation of biochemical and histological effects on liver of Swiss albino mice due to acute oral toxicity of aqueous leaf extract of Phyllanthus niruri. IJPPR. 2016;8(1):85–90. [Google Scholar]
- Thanabhorn S., Jaijoy K., Thamaree S., Ingkaninan K., Panthong A. Acute and subacute toxicity study of the ethanol extract from Lonicera japonica Thunb. J. Ethnopharmacol. 2006;107:370–373. doi: 10.1016/j.jep.2006.03.023. [DOI] [PubMed] [Google Scholar]
- The Organisation of Economic Co-operation and Development (OECD) OECD Publishing; Paris: 2008. Test No. 407: Repeated Dose 28-day Oral Toxicity Study in Rodents, OECD Guidelines for the Testing of Chemicals, Section 4; pp. 1–13. [Google Scholar]
- The Organisation of Economic Co-operation and Development (OECD) OECD Publishing; Paris: 2008. Test No. 425: Acute Oral Toxicity-Up-and-Down Procedure, OECD Guidelines for the Testing of Chemicals, Section 4; pp. 4–5. [Google Scholar]
- Tokmak M., Yukse Y., Sehitoglu M.H., Guven M., Akman T., Aras A.B., Cosar M., Abbed K.M. The neuroprotective effect of syringic acid on spinal cord ischemia/reperfusion injury in rats. Inflammation. 2015;38(5):1969–1978. doi: 10.1007/s10753-015-0177-2. [DOI] [PubMed] [Google Scholar]
- Unuofin J.O., Otunola G.A., Afolayan A.J. Evaluation of acute and subacute toxicity of whole-plant aqueous extract of Vernonia mespilifolia Less. In Wistar rats. J. Integr. Med. 2018;16(5):335–341. doi: 10.1016/j.joim.2018.07.003. [DOI] [PubMed] [Google Scholar]
- Wang J., Sun F., Tang S., Zhang S., Lv P., Li J., Cao X. Safety assessment of vitacoxib: acute and 90-day sub-chronic oral toxicity studies. Regul. Toxicol. Pharmacol. 2017;86:49–58. doi: 10.1016/j.yrtph.2017.02.020. [DOI] [PubMed] [Google Scholar]
- Wei X., Chen D., Yi Y., Qi H., Gao X., Fang H., Gu Q., Wang L., Gu L. Syringic acid extracted from Herbadendrobii prevents diabetic cataract pathogenesis by inhibiting aldose reductase activity. Evid. Based Complement Alternat. Med. 2012:1–13. doi: 10.1155/2012/426537. 426537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- William M.K. Renal function test as indicator of kidney injury in subacute toxicity studies. Toxicol. Appl. Pharmacol. 1981;57(3):414–424. doi: 10.1016/0041-008x(81)90239-8. [DOI] [PubMed] [Google Scholar]
- Witthawaskul P., Panthong A., Kanjanapothi D., Taesothikul T., Lertprasertsuke N. Acute and subacute toxicities of the saponin mixture isolated from Schefflera leucantha Viguier. J. Ethnopharmacol. 2003;89(1):115–121. doi: 10.1016/s0378-8741(03)00273-3. [DOI] [PubMed] [Google Scholar]
- Wu Z., Ma Y., Zhao L., Cai S., Cheng G. Acute and subchronic toxicities of the ethanol and hot-water extracts from Chinese sumac (Rhuschinensis Mill.) fruits by oral administration in rats. Food Chem. Toxicol. 2018;119:14–23. doi: 10.1016/j.fct.2018.06.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.