Abstract
Cutaneous squamous cell carcinomas (SCCs) and basal cell carcinomas (BCCs) have different clinical behaviors, despite both being keratinocyte carcinomas mainly caused by ultraviolet radiation. Whether these distinct features are associated with tumor-associated macrophages (TAMs) is largely unknown. The main goal of this study was to conduct a comprehensive analysis of density and polarization states of TAMs in SCCs versus BCCs. The role of lactic acid in TAM polarization in SCC versus BCC was examined. We found that SCCs have a higher density of CD68 + TAMs compared to BCCs. TAMs in SCCs express higher levels of TAM-associated markers (arginase-1, MMP9, CD40 and CD127) than those in BCCs. Interestingly, differential expression of TAM-associated markers between SCCs and BCCs was reproduced in human monocytic THP-1 cells stimulated with SCC- or BCC-conditioned media. Analysis of soluble factor(s) in these tumors further revealed that SCCs have a significantly higher concentration of lactic acid than BCCs, and lactic acid was sufficient to upregulate TAM markers. Our results demonstrate that TAMs in SCCs versus BCCs differ in density and polarization states, which can be determined by soluble factors including tumor-derived lactic acid. These differences in TAMs may contribute to the distinct clinical behaviors of SCCs versus BCCs. This work was supported by grants from the National Institutes of Health and the Doris Duke Charitable Foundation.
Research in context
Few studies have studied tumor-associated macrophages in the context of SCC versus BCC. It has been demonstrated that macrophages mobilize to the epidermis after being exposed to ultraviolet-B radiation and produce interleukin-10 (IL-10). It has also been shown that the production of IL-10 results in the evasion of T cell-mediated immunity in BCCs and SCCs. However, the relationship between TAMs and the clinical behaviors of SCCs and BCCs remains largely unclear. Our study shows that despite their similar origins, human cutaneous SCCs and BCCs are considerably different in their TAMs. To our knowledge, these results provide the first evidence of differential TAM density and polarization in SCCs versus BCCs, which may contribute to their characteristic clinical behaviors. Future studies are necessary to elucidate the mechanisms by which TAMs influence these cancers with the goal of developing therapies tailored to each type of malignancy.
Keywords: Cell differentiation, Immune response, Cancer research, Immunology, Tumor-associated macrophages, TAMs, Squamous cell carcinoma, SCC, Basal cell carcinoma, BCC, Macrophage polarization and density, Lactic acid
1. Introduction
Cutaneous squamous cell carcinomas (SCCs) and basal cell carcinomas (BCCs) are the most common skin cancers in the United States. Approximately, 5.4 million SCCs and BCCs are diagnosed annually and their incidence is rising [1] Both SCCs and BCCs are keratinocyte carcinomas (KCs) and exposure to ultraviolet (UV) radiation is the most important environmental factor involved in their initiation [2]. Despite the similarities in their origin and cause, SCCs and BCCs have distinct clinical features: SCCs are more aggressive and more likely to metastasize at late stages, while BCCs are more indolent and rarely metastasize [3]. Considerable effort has been devoted to elucidating the genetic events that drive the pathogenesis of cutaneous SCCs and BCCs. For example, UV-induced TP53 gene mutations are an early event in SCC development, while aberrant activation of the Hedgehog signaling pathway through acquired mutations in the PTCH1 and SMO genes is a pathognomonic feature of BCC development [4, 5]. It was proposed that the difference in cellular capacity for DNA repair, apoptosis, and proliferation plays an important role in the development of UV-induced SCCs and BCCs [6, 7]. Further, the importance of the immune system in skin cancer has been long recognized. UV radiation can induce more regulatory T cells than effector T cells in the skin, leading to an immunosuppressive microenvironment that is closely correlated with the development of cutaneous malignancies including KCs [8]. However, little is known about how tumor-infiltrating immune cells shape the clinical differences between SCCs and BCCs.
Numerous studies have demonstrated that macrophages are involved in all stages of carcinogenesis, including initiation, growth, invasion, and metastasis [9, 10]. While macrophages are generally characterized by two distinct polarization states, namely M1 and M2, most tumor-associated macrophages (TAMs) have an M2-like polarization state and their density positively correlates with a poor prognosis [9, 10, 11]. The published studies analyzing TAMs in cutaneous SCCs or BCCs are few: two studies showed that macrophages infiltrate into human epidermis upon exposure to UVB radiation and resultant TAMs, in both SCCs and BCCs, produce IL-10 leading to evasion of the local T cell-mediated immune response [12, 13]. Recently, TAMs in cutaneous SCCs were found to be a major source of vascular endothelial growth factor-C (VEGF-C), a critical lymphangiogenic factor [14]; while the number of TAMs in BCCs is directly correlated with depth of invasion, microvessel density, and induction of cyclooxygenase-2 [15]. Interestingly, significantly enhanced tumor growth has been reported in a BCC mouse model (induced in conditional Ptchflox/floxERT2+/− mice) when cutaneous macrophages were depleted using clodronate liposomes [16]. While the published studies have used SCC or BCC cell lines or mouse models, the role of TAMs in the distinct clinical behaviors of human SCCs and BCCs has yet to be determined.
To pursue these questions, we conducted a comprehensive comparison of TAMs in human cutaneous SCC and BCC. Our data revealed that SCCs have a higher density and polarization state of TAMs than BCCs and express higher levels of TAM-associated markers. Moreover, tumor-derived lactic acid was demonstrated to be one critical soluble factor involved in the functional polarization of TAMs in SCCs.
2. Materials and methods
2.1. Keratinocyte carcinomas
De-identified, discarded tissue was obtained consistent with the general requirements of the protocol approved by the Yale Human Investigations Committee (HIC# 200002048). Histology was evaluated by board-certified dermatopathologists to ensure that tumors were cutaneous SCCs and BCCs prior to being included in experiments.
2.2. Immunohistochemistry
SCC and BCC formalin-fixed paraffin-embedded samples were sectioned (5μm thickness) and the slides were stained with haematoxylin and eosin and anti-CD68 antibody. CD68 + macrophages were visualized at 40x magnification with OTIPHON Nikon microscope. Numbers of TAMs per 40x high power fields were counted with ImageJ by two independent researchers.
2.3. Preparation of single cell suspensions
The freshly excised tumor samples were mechanically dissociated and digested with 10 μg/ml LiberaseTM and 100 μg/ml DNase I in 20 ml PBS with calcium and magnesium at a 37 °C shaking incubator (180 r.p.m.) for 20–30 min. 200 μl 0·5 M EDTA was added to stop the enzymatic reaction. After filtrating through a 70 μm nylon cell strainer, cells were collected and washed with cold FACS buffer (0·5% FBS in PBS) and centrifuged (1,000 r.p.m.) at 4 °C. Red blood cells were lysed with ACK lysing buffer, then washed with FACS buffer. The samples were kept on ice throughout the rest of the staining process.
2.4. Flow cytometry
Human FcR Binding Inhibitor (eBioscience) was added to the samples (1:200) and incubated for 20 min on ice to block non-specific Fc-receptor binding. After, cells were washed with FACS buffer and stained with surface antibodies, which included anti-CD45 (HI30, eBioscience); anti-CD40 (5C3, BioLegend); and anti-CD127 (A019D5, BioLegend), at room temperature for 15 min in the dark. Samples were washed twice with FACS buffer. Intracellular staining with anti-CD68 (eBioY1/82A, eBioscience), anti-arginase-1 (R&D systems), and anti-MMP9 (R&D systems) was performed using the Fixation and Permeabilization Solution kit (BD Biosciences). Dead cells were excluded using 7-AAD staining. Samples were run on BD LSRII flow cytometer and data were analyzed using FACSDiva software and FlowJo.
2.5. Tumor-conditioned media and THP-1 stimulation
Fresh human cutaneous SCC and BCC specimens were dissociated with surgical scissors and razor blades. The minced samples were resuspended with complete RPMI 1640 media and centrifuged at 1,000 r.p.m. (4 °C) for 5 min. Cells were counted and equal number of live cells from each tumor was incubated with complete RPMI 1640 media in a humidified tissue culture incubator (37 °C) for 24 h. The tumor supernatant was carefully aspirated, centrifuged, and filtered with a sterile syringe filter (0.45 μm). The tumor-conditioned and control (RPMI 1640) media were stored at −80 °C for future experiments. For in vitro stimulation of THP-1 (human monocytic) cells, 1 × 106 THP-1 cells were incubated with 1 ml of tumor-conditioned or control media in sterile six-well Petri dishes for 48 h. Then, THP-1 cells were then harvested for flow cytometry as described above.
2.6. Determination of lactic acid concentration
Human cutaneous SCC or BCC samples were mechanically dissociated, enzymatically digested as above, and washed with cold PBS. Live cells were counted and lactic acid concentration was measured using the Lactate Colorimetric Assay Kit II (BioVision) following the manufacturer's instructions. 500,000 live cells in lactate assay buffer (50 μl/well) were added to a 96-well plate. 50 μl of reaction mix was added into each well and incubated for 30 min at room temperature. Absorbance was measured at 450 nm using a SpectraMax M5 microplate reader (Molecular Devices), then background absorbance was subtracted. A standard curve was made, and lactic acid concentration (mM) of each sample was calculated by applying the sample reading to the standard curve. The final lactic acid concentration was shown on a per-cell basis (nmol/cell).
2.7. THP-1 stimulation with lactic acid and quantitative PCR
1 × 106 THP-1 cells were incubated with complete RPMI media containing 0 mM (control) or 25 mM lactic acid in a 12-well plate for 24 h (37 °C). Total RNA was extracted from the stimulated cells and cDNA was synthesized with the SMARTer PCR cDNA Synthesis kit (Clontech). Quantitative PCR (qPCR) was performed using an Mx3000P Thermocycler (Stratagene) with 3 μl of cDNA plus 6 μl of PerfeCTa SYBR Green Supermix (Quanta BioSciences) and 3 μl (10 μM) of specific primers: ARG1: FP, 5′-GCAAGGTGGCAGAAGTCAAGA-3’; RP, 5′-TGGCATGGCCAGAGATGCTT-3’. MMP9: FP, 5′-GCCTCTGGAGGTTCGACGTG-3’; RP, 5′-GGGAACTCACGCGCCAGTAG-3’; CD40: FP, 5′-GCTGGCACTGTACGAGTGAGGC-3’; RP, 5′-CTTATTGGTTGGCTTCTTGGC-3’; IL7R: FP, 5′-CCGCCAGGAAAAGGATGAAAAC-3’; RP, 5′-GACTCCATTCACTCCAGAAGCCT-3’; ACTB: FP, 5′-GTCCACACCCGCCGCCAG-3’; RP, 5′-CGGGGGGCATCGTCG-3’. All samples were run in triplicate and fold change in gene expression was calculated using the reference sample (housekeeping gene ACTB).
2.8. Statistics
Statistical difference in values between two experimental groups was determined using the unpaired Student's t test (2-tailed distribution) and nonparametric Mann-Whitney test. Statistical differences in values between three experimental groups were determined by using one-way analysis of variance (ANOVA). P < 0.05 was considered statistically significant.
3. Results
3.1. Human cutaneous SCCs have a higher TAM density than BCCs
To characterize TAMs in SCCs and BCCs, we collected specimens of human cutaneous SCCs and BCCs and stained the slides of biopsy-proven SCCs (n = 5) and BCCs (n = 6) with CD68, a marker of human macrophages. Our results show that SCCs consistently had an approximately 6-fold higher density of CD68 + TAMs than BCCs (Fig. 1A). We also quantified the number of TAMs in SCCs and BCCs per high-powered field (Fig. 1B, P < 0.0001). To further confirm the difference in TAM-density, we analyzed CD45 + CD68 + TAMs in a second set of SCCs (n = 6) versus BCCs (n = 6) by flow cytometry. As shown in Fig. 1C, SCCs have a higher percentage of TAMs than BCCs. The difference was statistically significant (Supplementary Figure 1, P = 0.0005). Together, these data demonstrate that SCCs have a significantly greater density of TAMs than BCCs.
Fig. 1.
Human cutaneous SCCs have a greater TAM density than BCCs. A, Representative histologic images of SCC versus BCC comparison staining with anti-human CD68 antibody (40X). B, Number of TAMs per 40x high power fields were counted. ***: P < 0·001. SCC: n = 5; BCC: n = 6. C, CD45+CD68+ TAMs in human SCCs (n = 6) and BCCs (n = 6) analyzed by flow cytometry. Data are representative of at least four independent experiments.
3.2. TAMs in human cutaneous SCCs express higher levels of TAM-associated polarization markers than TAMs in BCCs
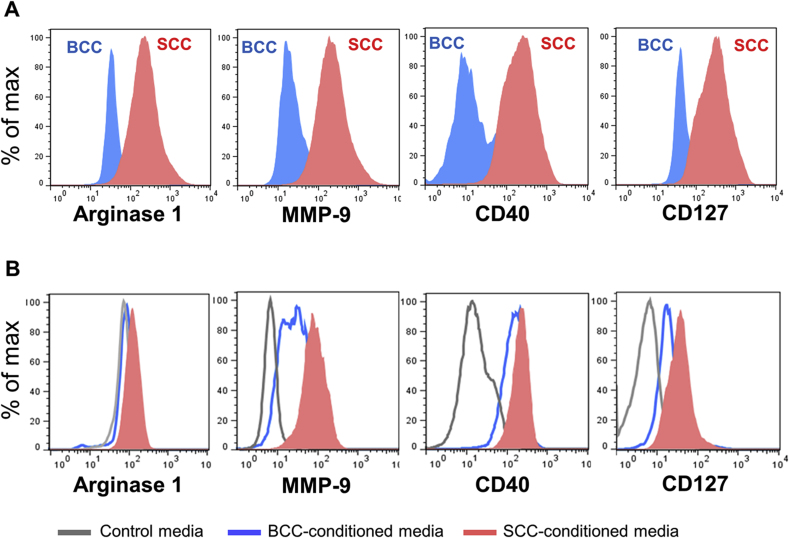
TAM-like polarization of macrophages has been associated with an increase in various expression of markers including arginase-1, MMP-9, CD127 and CD40 [9, 10, 11]. Considering the difference in clinical outcomes observed in SCCs versus BCCs, we hypothesized that TAMs in these skin cancers had different phenotypic characteristics. To test this, we analyzed the expression of TAM-associated markers on BCC (n = 9) and SCC (n = 13) TAMs. Compared to BCC TAMs, SCC TAMs expressed higher levels of the TAM-associated markers including arginase-1, MMP-9, CD40 and CD127 (Fig. 2A). Traditionally, arginase-1 and MMP9 are M2-associated markers while CD40 and CD127 are M1-associated markers. Therefore, our results support the observation that macrophages in human cutaneous SCCs exhibit both M1- and M2-associated markers [17].
Fig. 2.
TAMs in SCCs versus BCCs or THP-1 cells incubated with SCC- or BCC-conditioned media are different in their polarization states. A, Biopsy-proven human cutaneous SCCs (n = 13) and BCCs (n = 9) were dissociated by enzyme and the expression of arginase-1, MMP9, CD40, and CD127 on TAMs (CD45 + CD68+) were analyzed by flow cytometry. B: THP-1 cells were incubated with SCC- or BCC-conditioned media, or control RPMI media for 48 h. Cells were then collected for flow cytometry analysis of arginase-1, MMP9, CD40, and CD127. Data are representative of at least three independent experiments.
3.3. SCC- and BCC-conditioned media differentially drive polarization of human THP-1 cells
We and others have demonstrated that TAM polarization is profoundly affected by the tumor microenvironment [18, 19] Given that TAMs in human cutaneous SCCs and BCCs have different polarization states, we hypothesized that soluble factors in the tumor microenvironment lead to this difference. To test this, we generated SCC- and BCC-conditioned media to act as ex vivo models of the tumor microenvironments in SCCs and BCCs, respectively. THP-1 human monocytic cells were stimulated with SCC- versus BCC-conditioned media, and compared to control RPMI 1640 media. Their activation states were analyzed after 48 h. Compared to those cells stimulated with BCC-conditioned or control media, THP-1 cells stimulated with SCC-conditioned media showed an increase in TAM-like polarization, which was demonstrated by higher levels of arginase-1, MMP-9, CD40 and CD127 (Fig. 2B). The differences in MMP-9 and CD127 were statistically significant (Supplementary Figure 3, P = 0.03, P = 0.02, respectively). In THP-1 cells stimulated with SCC-conditioned media, arginase-1 trended upward compared to those stimulated with BCC-conditioned media (P = 0.15). These results suggest that soluble factor(s) present in the tumor microenvironment are able to partially drive TAM polarization through contact-independent, paracrine communication; however, cell-to-cell contact may play a more important role in TAM polarization.
3.4. Lactic acid is sufficient to polarize human macrophages to a TAM-like state
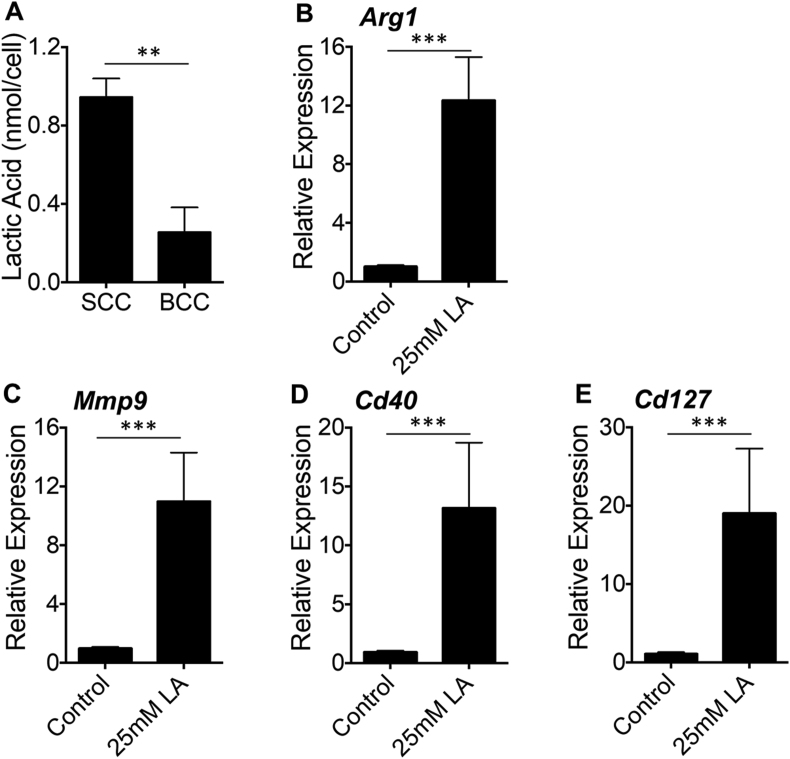
Using murine tumor models, we demonstrated that tumor-derived lactic acid induces TAM-like polarization in macrophages in a hypoxia-inducible factor 1α (HIF1α)-dependent manner [18, 20]. In this study, we asked whether human cutaneous SCCs and BCCs have different concentrations of lactic acid as a functional difference in their ability to polarize macrophages. Lactic acid concentrations in primary human SCCs and BCCs were measured and revealed that SCCs have an approximately four-fold higher concentration of lactic acid than BCCs (Fig. 3A). We next asked whether lactic acid could be a determinant of TAM polarization in SCCs and BCCs. To address this, we stimulated THP-1 cells with lactic acid. Compared to the control media (0 mM lactic acid), 25 mM lactic acid, a concentration observed in numerous cancers [18], significantly induced the expression of the TAM-associated markers arginase-1, MMP-9, CD40, and CD127 (Fig. 3B, C, D, E); this phenotypic change was consistent with what we observed when THP-1 cells were stimulated with SCC-conditioned media (Fig. 2B). Taken together, these data demonstrate that human cutaneous SCCs have higher level of lactic acid than BCCs and that lactic acid is sufficient to induce a heterogeneous polarization state of TAMs.
Fig. 3.
Human cutaneous SCCs have a greater concentration of lactic acid than BCCs and lactic acid is sufficient to induce arginase-1, MMP9, CD40, and CD127 in THP-1 cells. A, Biopsy-proven SCCs and BCCs were digested as described. 500,000 live cells were used to measure intracellular concentrations of lactic acid using Lactate Colorimetric Assay Kit II. Data were showed in nmol/cell (means ±S.E.M.). **: P < 0·01. SCCs: n = 5; BCCs: n = 5. B–F, Gene expression analysis (B, Arg1; C, Mmp9; D, Cd40; E, Cd127) on THP-1 cells stimulated with 0 mM (control) or 25 mM lactic acid was performed by qPCR and relative expressions were showed with means ±S.E.M. ***: P < 0·001. n = 9. Data are representative of at least three independent experiments.
4. Discussion
A majority of malignancies are primarily initiated by genetic alterations or mutations, but their subsequent development and behaviors requires support from the surrounding cells, such as TAMs [9, 10, 21]. In this study, we found that cutaneous SCCs have an approximately 6-fold higher TAM density than BCCs. Given that SCCs are clinically more aggressive than BCCs, this finding highlights the observation that a greater density of TAMs is associated with tumors with a worse prognosis. We do note that there exists a subset of immature myeloid dendritic cells (mDCs) that are CD68-positive and may impact the interpretation of the TAM densities [22, 23]; however, mDCs are found in both cutaneous SCCs and BCCs and we do not expect that the relative difference between the two tumor sets would be changed [24, 25]. Interestingly, TAMs in cutaneous SCCs not only express arginase-1 and MMP-9, two functional M2-associated markers, but also highly up-regulate the M1-associated markers CD40 and CD127. Indeed, macrophages with combinations of M1- and M2-associated markers within human skin cancers have been described by others [17, 26], indicating that macrophages have dynamic polarization states in response to different microenvironments within tumors. Acidic and/or hypoxic microenvironment induced by vigorous glycolysis of tumor cells has been demonstrated to promote M2-like macrophage polarization [19, 27]. Due to a higher growth rate, SCCs may have more areas with an acidic and/or hypoxic microenvironment than BCCs, leading to more TAM-like macrophages polarization. TAM-derived arginase-1 is a key enzyme that is involved in the production of polyamines, which can be in turn utilized by tumor cells for cellular proliferation and growth [28]. MMP-9 contributes to many aspects of tumor development including angiogenesis, local tissue invasion, and metastasis [29]. Therefore, the difference in levels of arginase-1 and MMP-9 produced by TAMs in SCCs and BCCs may be one important reason for the distinct clinical behavior of SCCs and BCCs.
Remarkably, the in vivo immunophenotype of TAMs in human cutaneous SCCs and BCCs was partially reproduced by in vitro stimulation of a human monocytic cell line (THP-1) with SCC- and BCC-conditioned media. This suggests that monocytes recruited to tumors are exposed to many soluble factor(s) in the tumor microenvironment, leading to TAM polarization through contact-independent, paracrine communication. Given the heterogeneity and complexity of the tumors, the source of such signaling factors could be derived from a variety of cells in the microenvironment, including tumor cells, Th1/Th2 cells, other immune cells and fibroblasts. Here we demonstrate that cutaneous SCCs have a higher concentration of lactic acid than BCCs and, in line with our findings shown in mouse tumor models [18], lactic acid stimulates THP-1 cells to upregulate TAM-associated molecules including arginase-1 and MMP-9 in a dose-dependent manner. Interestingly, we also observed a significant upregulation of more classical M1-like markers including CD40 and CD127. These data suggest that tumor-derived lactic acid is important in inducing a heterogeneous polarization state of TAMs within the tumor microenvironment. However, our study does not exclude other soluble factors that might also act through a paracrine manner to induce an intricate phenotype in TAMs that involves both M1- and M2-like activation.
Our findings demonstrate that TAMs in human cutaneous SCCs and BCCs have different densities and functional immunophenotypes, potentially explaining their distinct clinical behaviors. Soluble factor(s) including tumor-derived lactic acid might be the key in determining TAM activation state within the tumor microenvironment. Our data provides the first evidence, to our knowledge, of different TAM density and polarization states in human cutaneous SCCs versus BCCs. We believe a better understanding of TAM polarization in SCCs will provide novel insights for therapies targeting keratinocyte carcinomas.
Declarations
Author contribution statement
Xiaodong Jiang, Nika Cyrus: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Mike Wang, Diana Yanez, Richard Lacher, Anne Marie Rhebergen, Carolyn Brokowski, Anjela Galan: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Samuel Book: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Oscar Colegio: Conceived and designed the experiments; Wrote the paper.
Funding statement
This work was supported by grants from the National Institutes of Health (5K08CA172580), Doris Duke Charitable Foundation (15-007374), and NIH Medical Scientist Training Program (T32 GM007205). The funding sources were not involved in the design/conduct of the study; collection, analysis and interpretation of data; preparation, review or approval of the manuscript; nor in the decision to submit the manuscript for publication. Oscar Colegio had full access to the data and has final responsibility for the decision to submit for publication.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank Vincent Klump (Dermatopathology, Yale School of Medicine) for providing technical support with immunohistochemistry.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Rogers H.W. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. Population, 2012. JAMA Dermatol. 2015;151(10):1081–1086. doi: 10.1001/jamadermatol.2015.1187. [DOI] [PubMed] [Google Scholar]
- 2.Mancebo S.E., Wang S.Q. Skin cancer: role of ultraviolet radiation in carcinogenesis. Rev. Environ. Health. 2014;29(3):265–273. doi: 10.1515/reveh-2014-0041. [DOI] [PubMed] [Google Scholar]
- 3.Alam M., Ratner D. Cutaneous squamous-cell carcinoma. N. Engl. J. Med. 2001;344(13):975–983. doi: 10.1056/NEJM200103293441306. [DOI] [PubMed] [Google Scholar]
- 4.Ratushny V. From keratinocyte to cancer: the pathogenesis and modeling of cutaneous squamous cell carcinoma. J. Clin. Investig. 2012;122(2):464–472. doi: 10.1172/JCI57415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emmert S., Schon M.P., Haenssle H.A. Molecular biology of basal and squamous cell carcinomas. Adv. Exp. Med. Biol. 2014;810:234–252. doi: 10.1007/978-1-4939-0437-2_13. [DOI] [PubMed] [Google Scholar]
- 6.Feller L. Basal cell carcinoma, squamous cell carcinoma and melanoma of the head and face. Head Face Med. 2016;12:11. doi: 10.1186/s13005-016-0106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reichrath J., Rass K. Ultraviolet damage, DNA repair and vitamin D in nonmelanoma skin cancer and in malignant melanoma: an update. Adv. Exp. Med. Biol. 2014;810:208–233. doi: 10.1007/978-1-4939-0437-2_12. [DOI] [PubMed] [Google Scholar]
- 8.Yu S.H., Bordeaux J.S., Baron E.D. The immune system and skin cancer. Adv. Exp. Med. Biol. 2014;810:182–191. doi: 10.1007/978-1-4939-0437-2_10. [DOI] [PubMed] [Google Scholar]
- 9.Qian B.Z., Pollard J.W. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noy R., Pollard J.W. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41(1):49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sica A., Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Investig. 2012;122(3):787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang K. CD11b+ macrophages that infiltrate human epidermis after in vivo ultraviolet exposure potently produce IL-10 and represent the major secretory source of epidermal IL-10 protein. J. Immunol. 1994;153(11):5256–5264. [PubMed] [Google Scholar]
- 13.Kim J. IL-10 production in cutaneous basal and squamous cell carcinomas. A mechanism for evading the local T cell immune response. J. Immunol. 1995;155(4):2240–2247. [PubMed] [Google Scholar]
- 14.Moussai D. The human cutaneous squamous cell carcinoma microenvironment is characterized by increased lymphatic density and enhanced expression of macrophage-derived VEGF-C. J. Investig. Dermatol. 2011;131(1):229–236. doi: 10.1038/jid.2010.266. [DOI] [PubMed] [Google Scholar]
- 15.Tjiu J.W. Tumor-associated macrophage-induced invasion and angiogenesis of human basal cell carcinoma cells by cyclooxygenase-2 induction. J. Investig. Dermatol. 2009;129(4):1016–1025. doi: 10.1038/jid.2008.310. [DOI] [PubMed] [Google Scholar]
- 16.Konig S. Depletion of cutaneous macrophages and dendritic cells promotes growth of basal cell carcinoma in mice. PLoS One. 2014;9(4) doi: 10.1371/journal.pone.0093555. e93555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pettersen J.S. Tumor-associated macrophages in the cutaneous SCC microenvironment are heterogeneously activated. J. Investig. Dermatol. 2011;131(6):1322–1330. doi: 10.103/jid.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colegio O.R. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513(7519):559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruffell B., Affara N.I., Coussens L.M. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012;33(3):119–126. doi: 10.1016/j.it.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colegio O.R. Lactic acid polarizes macrophages to a tumor-promoting state. Oncoimmunology. 2016;5(3) doi: 10.1080/2162402X.2015.1014774. e1014774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim Y.Z., South A.P. Tumour-stroma crosstalk in the development of squamous cell carcinoma. Int. J. Biochem. Cell Biol. 2014;53:450–458. doi: 10.1016/j.biocel.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 22.Strobl H. Identification of CD68+lin- peripheral blood cells with dendritic precursor characteristics. J. Immunol. 1998;161(2):740–748. [PubMed] [Google Scholar]
- 23.Ziegler-Heitbrock L. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116(16):e74–80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 24.Bluth M.J. Myeloid dendritic cells from human cutaneous squamous cell carcinoma are poor stimulators of T-cell proliferation. J. Investig. Dermatol. 2009;129(10):2451–2462. doi: 10.1038/jid.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nestle F.O. Human sunlight-induced basal-cell-carcinoma-associated dendritic cells are deficient in T cell co-stimulatory molecules and are impaired as antigen-presenting cells. Am. J. Pathol. 1997;150(2):641–651. [PMC free article] [PubMed] [Google Scholar]
- 26.Biswas S.K., Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat. Immunol. 2010;11(10):889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 27.Kumar V., Gabrilovich D.I. Hypoxia-inducible factors in regulation of immune responses in tumour microenvironment. Immunology. 2014;143(4):512–519. doi: 10.1111/imm.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerner E.W., Meyskens F.L., Jr. Polyamines and cancer: old molecules, new understanding. Nat. Rev. Cancer. 2004;4(10):781–792. doi: 10.1038/nrc1454. [DOI] [PubMed] [Google Scholar]
- 29.Kerkela E., Saarialho-Kere U. Matrix metalloproteinases in tumor progression: focus on basal and squamous cell skin cancer. Exp. Dermatol. 2003;12(2):109–125. doi: 10.1034/j.1600-0625.2003.120201.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.