Abstract
Nigella sativa seeds are traditionally reputed as possessing anti-diabetic properties. As a result, we aim to explore the mechanism of its anti-hyperglycemic activity. The present study uses various experimental designs including gastrointestinal (GI) motility, intestinal disaccharidase activity and inhibition of carbohydrate digestion and absorption in the gut. The animals used as type 2 diabetic models were induced with streptozotocin to make them as such. Oral glucose tolerance test was performed to confirm that the animals were indeed diabetic. The extract reduced postprandial glucose, suggesting it interfered with glucose absorption in the gut. It also improved glucose (2.5g/kg, b/w) tolerance in rats. Furthermore, treatment with N. sativa produced a significant improvement in GI motility, while reduced disaccharidase enzyme activity in fasted rats. The extract produced a similar effect within an acute oral sucrose (2.5g/kg, b/w) load assay. Following sucrose administration, a substantial amount of unabsorbed sucrose was found in six different parts of the GI tract. This indicates that N. sativa has the potentiality to liberate GI content and reduce or delay glucose absorption. A potential hypoglycemic activity of the extract found in insulin release assay, where the extract significantly improved insulin secretion from isolated rat islets. These concluded present findings give rise to the implication that N. sativa seeds are generating postprandial anti-hyperglycemic activity within type 2 diabetic animal models via reducing or delaying carbohydrate digestion and absorption in the gut as well as improving insulin secretion in response to the plasma glucose.
Keywords: Disaccharidase enzyme activity, GI motility, Glucose tolerance, Gut perfusion, Nigella sativa, Sucrose malabsorption
Introduction
Diabetes Mellitus (DM) is a chronic and complex metabolic group of disorders—its prevalence has increased rapidly on a global scale. Mortality rates for DM are estimated to reach a total of 2.9 million deaths by the year 2030. Increasingly, diabetes is cited as a significant global threat to public health [1,2], with 246 million individuals with this polygenic disorder around the world. Eighty percent reside in developing countries [3]. Diabetes is ranked seventh among the leading causes of mortality globally [4].
Type 2 DM is the most common and one of the life-threatening disease conditions among the current classified types. Type 2 DM is usually manifested via obesity and genetic disposition [5]. For the management of DM, the interruption of carbohydrate digestion and absorption is an active therapeutic approach of interest. The presence of aldohexose in the circulation over an extended period combined with the apprehended absorption technique, enables pancreatic β-cells of diabetic individuals to adjust their postprandial metabolic rate [6]. Moreover, the effectiveness of drug therapy is limited, and it shows a variety of complications and side effects [7].
Nigella sativa (N. sativa), also known as black seed, is a plant which belongs to the family Ranunculaceae and is native to Southern Europe, North Africa, and Southwest Asia [8]. Traditionally black seeds are used to treat a wide range of ailments including different airway disorders, chronic headaches, back pain, diabetes, paralysis, infections, inflammation, hypertension, and digestive tract-related problems. The black seed is administered in different kinds of preparations depending on the ailment. It also has topical uses to treat blisters, nasal abscesses, eczema, and swollen joints [9]. N. sativa components exhibit a remarkable array of biochemical, immunological, and pharmacological actions, including bronchodilatory [10], anti-inflammatory [11], antibacterial [12], hypoglycemic [13], and immunomodulatory effects [14]. Most of these properties have been attributed mainly to the quinone constituents of N. sativa like thymoquinone (TQ) (30–48%), thymohydroquinone and dithymoquinone (nigellone). Of the quinones, TQ is the most abundant active ingredient of the extracted volatile oils from the black seed [15]. In addition, TQ has been reviewed several times as an antioxidant, anti-inflammatory and anti-tumorigenic [16–18]. TQ has also been reported to reduce hippocampal neurodegeneration following chronic toluene exposure in rats [19] and protects the frontal cortex from similar toxin exposure [20]. Some other studies have reported too that TQs have potentiality to clear Aβ in AD model [21–23].
N. sativa has been reported for different anti-diabetic properties in various diabetic animal models, yet no mechanistic investigation has been carried out. Herein, we focused on exposing a more comprehensive mechanism of action of N. sativa within diabetic animal models. Previous studies with this extract claim that it may act by improving (or mimicking) insulin secretion or reducing the oxidative stress of β-cells [13,24]. We designed the present study to co-relate gastrointestinal (GI) absorption interference by this extract and observing its anti-diabetic effect as a result. The present study will provide an insight and give a thorough evaluation of the hypoglycemic effects of N. sativa in diabetic animal models and will examine the possible effects of N. sativa on intestinal glucose absorption and GI motility, as these are part of this plant’s anti-hyperglycemic efficacy.
Materials and methods
Plant collection and processing
The seeds of N. sativa were purchased from the commercial herbal medicine outlet in Uttara, Dhaka, Bangladesh. Seeds were collected in dried form as ordered and the extract was prepared following the procedure as previously described by Azad et al. [25]. Briefly, fully dried seeds were then ground to make a powder, and 500 g of the powdered material was soaked in 2.5 l of methanol inside a flat-bottomed glass container. The solution was kept for 1 week while being shaken continuously. The mixture after this was first filtered using fresh cotton and at the end it was filtered with filter paper (Whatman no. 1), and the obtained material was evaporated by a Rotary Evaporator (Bibby RE-200, Sterilin Ltd., U.K.) at 5–6 rpm at 57°C. Finally, a gummy, semi-solid crude extract was obtained and stored at 4°C until required in the study.
Determination of glucose-adsorption capacity
This assay was conducted as described by Ou et al. [26]. In brief, the glucose-adsorption capacity (mmol/l) was measured by mixing 1 g of either insoluble plant powder or carboxymethyl cellulose, with 100 ml of glucose solution. The mixture was incubated at 37°C for 6 h. Afterward, the mixture was centrifuged at 3500 rpm for 15 min. Glucose concentration in the supernatant was assayed using GOD-PAP method as previously described [27].
Experimental animal models
Long–Evans rats (both male and female), weighing 150–200 g were collected from icddr, b, and acclimatized and bred within the animal house of the Department of Pharmacy, East West University, Dhaka, Bangladesh. Animals were kept at an ambient temperature of 22 ± 5°C and at 50–70% humidity. A 12-h day-night cycle was maintained to avoid fluctuations of the circadian rhythm within the rats and the rats were kept in translucent plastic cages with wood shavings provided as bedding. The cages were replaced with bedding before fasted rat testing, to prevent and lessen coprophagy. Rats were provided with ad libitum diet pellets (a nutrient composition of 38.5% fiber, 36.2% carbohydrate, 20.9% protein, and 4.4% fat, with a metabolizable energy content of 1.18 MJ/100 g (282 kcal/100 g)) and filtered drinking water throughout the experiment. During tests that required fasting, only water was supplied.
Diabetes induction
A single intraperitoneal injection of 90 mg/kg, b/w of streptozotocin was administered to 48-h old rats (average weight 7 g; 40 rats of both sex) to induce type 2 diabetes [28]. The experiments were carried out 3 months after the injection of streptozotocin. Rats with a blood glucose level of 8–9 mmol/l at a fasted condition and >10 mmol/l at a postprandial state were selected as a type 2 diabetic model for the following experiments.
Effects of N. sativa on glucose tolerance
Glucose (2.5 g/kg, b/w) was orally administered with or without plant extract (500 mg/kg, b/w) to 12 hr fasted type 2 diabetes rats (n=8). The blood was extracted and sampled from the tip of the tail before and after extract ingestion within time periods of 0, 30, 60, 90, and 120 min. The blood glucose was measured using an Ascencia Contour Blood Glucose Meter (Bayer, Newbury, U.K.).
Effects of N. sativa on residual gut sucrose content
Glucose absorption was determined by the method as described by Hannan et al. [29]. Twenty-four hour fasted type 2 diabetic rats were provided with sucrose (2.5 g/kg of body mass) orally, with or without plant extract (0.5 g/kg, b/w). Following sucrose administration, rats were killed at 30, 60, 120, and 240 min, respectively, to measure the malabsorption of sucrose contents from six different parts of the GI tract. The GI tract was excised and six different segments including the stomach, the upper 20 cm, middle and lower 20 cm of the small intestine, the cecum and the large intestine were separated. Each segment was rinsed with acidified, ice-cold, saline and then centrifuged at 3000 rpm (1000×g) for 10 min. The supernatant was pipetted off and boiled for 2 h in H2SO4 to hydrolyze the sucrose content. The acid residue was then neutralized by 1 M NaOH solution. Both the plasma glucose concentration and the amount of glucose released from residual sucrose in the GI tract was determined. The GI sucrose content was calculated from the amount of liberated glucose [25].
Effects of methanol extract of N. sativa intestinal glucose absorption
An in situ intestinal perfusion technique [30] was implemented for the estimation of intestinal glucose absorption impeding the effect of N. sativa in normal rats. Subjected animals were fasted for 36 h and anesthetized with sodium pentobarbital (50 g/kg, b/w) solution. The extract of N. sativa (10 mg/ml), equivalent to 0.5 g/kg, made up in Krebs–Ringer buffer containing glucose (54 g/l). The solution was passed through the pylorus, and the perfusate was collected at the ileum from a catheter inserted at the end. The control group received only Krebs’ solution containing glucose. Perfusion was carried out at a constant rate of 0.5 ml/min for 30 min at a maintained temperature of 37°C. Results were calculated as a percentage of absorbed glucose, of the glucose within the solution before and after the perfusion.
Effects of N. sativa on gut motility
GI motility was determined using BaSO4 as described previously by Azad et al. [25]. The treatment group received the extract 1 h before consuming 10% BaSO4 (W/V of 0.5% Na-CMC). After providing BaSO4, animals were killed at 15 min after its administration and the distance travelled by BaSO4 was measured and calculated as a percentage of a total length of the small intestine (from the pylorus to ileocecal junction).
Effects of N. sativa on intestinal disaccharidase enzyme activity
This experiment was carried out as previously described by Hannan et al. [29]. In brief, non-diabetic rats were fasted for 20 h, and then the methanol extract of N. sativa (500 mg/kg, b/w) was introduced by gastric gavage. The rats were then killed at 60 min after gavaging and the small intestine was extracted and cut lengthwise, bathed in ice-cold saline, and then homogenized in 10 ml of saline (0.9% NaCl).
The aliquots of homogenate were incubated at 37°C within a 40 mM sucrose solution for 60 min. A DCTM Protein Kit (Bio-Rad, U.S.A.) was used to measure the protein load. The concentration of sucrose transformation into glucose, represents the disaccharidase enzyme activity and was obtained as μmol/mg protein/h.
Effects of N. sativa on insulin secretion from isolated islets
On the day of the experiment, islets were isolated and harvested from Long–Evans rats (180–250 g) pancreata using a collagenase digestion as described by Hannan et al. [31]. After preincubating the islets in KRB buffer containing 3 mM glucose for 40 min, islets (in groups of 8–10) were incubated for 1 h at 37°C in 500 μl KRB buffer containing 3 and 11 mM glucose along with methanol extract of N. sativa (Table 1). Aliquots of the resulting supernatant were stored at −20°C for insulin assay analysis using Rat Insulin ELISA Kit (Crystal Chem, U.S.A.).
Table 1. Effects of methanol extract of N. sativa on insulin secretion from isolated rat islets.
| Groups | Insulin secretion (ng/mg islet protein) | |
|---|---|---|
| 3 mM Glucose | 11 mM Glucose | |
| Control (Glucose alone) | 2.23 ± 0.65 | 5.31 ± 0.74 |
| N. sativa (25 μg/ml) | 2.61 ± 0.33 | 6.37 ± 0.47* |
| N. sativa (50 μg/ml) | 3.67 ± 0.33* | 6.91 ± 0.33* |
| N. sativa (100 μg/ml) | 5.87 ± 0.45† | 8.49 ± 0.85* |
| N. sativa (200 μg/ml) | 6.77 ± 0.35† | 9.97 ± 0.45† |
| Glibenclamide (10 μg/ml) | 7.55 ± 0.25‡ | 10.31 ± 0.65† |
Isolated rat islets were incubated for 60 min with methanol extract of N. sativa (25–200 μg/ml) in the presence of 3 or 11 mM glucose; whereas Glibenclamide (10 μg/ml) used as a reference control, respectively. Values are Mean ± SEM with n=4.
*P<0.05.
†P<0.01.
‡P<0.001 compared with control (3 and 11 mM glucose alone).
Statistical analysis
All data were presented as mean ± standard deviation and statistical analysis was prepared on GraphPad Prism v5.0. A one-way ANOVA was carried out with a non-parametric Dunnett’s test for adjustment and interpretation; P<0.05 was considered as the minimum level of significance.
Results
Effects of N. sativa powder on in vitro glucose-adsorption capacity
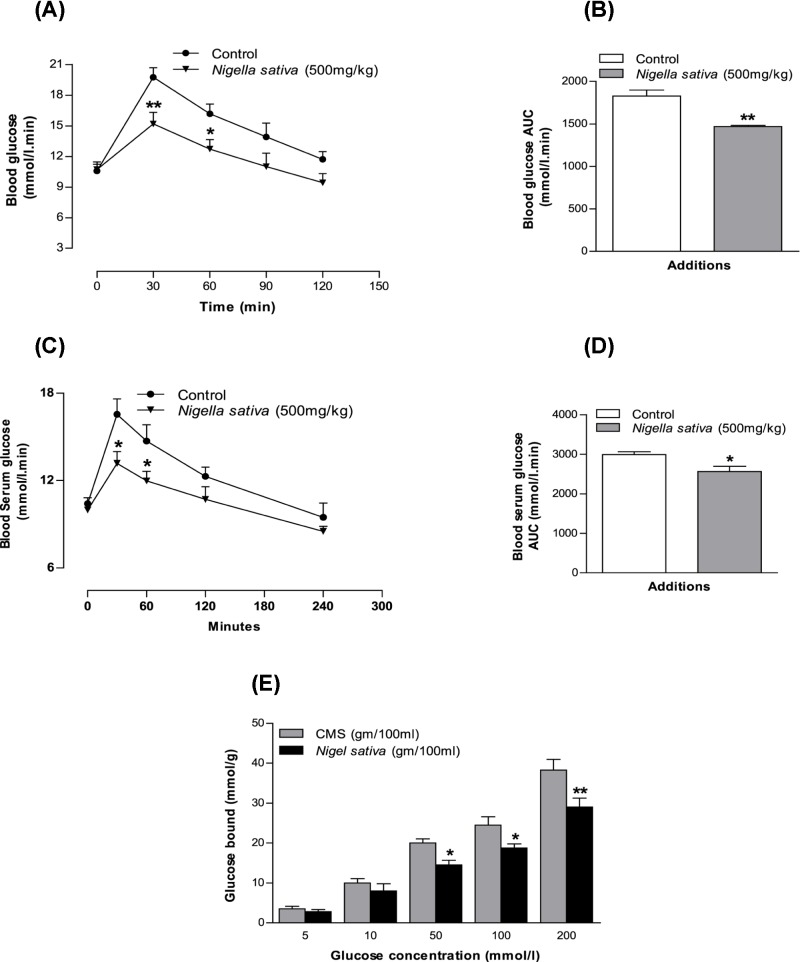
N. sativa powder illustrated the capacity of glucose adsorption at different concentrations of glucose within the solutions. This activity of adsorption continued from high concentrations of glucose to low concentrations of glucose (P<0.05, P<0.01; Figure 1E).
Figure 1. Effects of methanol extract of N. sativa on (A,B) glucose tolerance (GTT), (C,D) serum glucose after sucrose load (SGASL) in type 2 diabetic rats and (E) glucose adsorption capacity (GAC) in vitro.
Rats were fasted for 12 and 24 h and administered glucose or sucrose solution (2.5 g/kg, body weight) by oral gavage in presence or absence of methanol extract of N. sativa (500 mg/kg, body weight). Values are means and standard deviations represented by vertical bars (n=6, for GTT and SGASL and n=4 for GAC. The mean values that are marked with an asterisk (*) were substantially different from those of respective type 2 diabetic control rats (*P<0.05 and **P<0.01) alone (this was derived from repeated-measures ANOVA and adjusted using Bonferroni correction).
Effect of N. sativa on glucose tolerance
Figures 1A,B show the effects of N. sativa extracts (500 mg/kg, body weight) on glucose tolerance. A significant decline was noted (P<0.05 and P<0.01) in the blood glucose concentration at 30 and 60 min following glucose ingestion (2.5g/kg, b/w). Type 2 diabetic rats that received N. sativa treatment had efficiently lowered glucose levels in comparison with rats that received glucose alone (P<0.05 and P<0.01; Figure 1A,B).
Effects of N. sativa on serum glucose after the sucrose load
The glucose level of type 2 diabetic rats reached a peak 30 min after sucrose ingestion (Figure 1C,D). This rise in blood glucose due to sucrose load was suppressed by the methanol extract efficiently at both 30 min (P<0.05) and 60 min (P<0.05) in type 2 diabetic rats. This result may be a reflection of extracts activity on insulin secretion/action but also evidence of delaying the absorption of sucrose in the gut.
Effects of N. sativa on unabsorbed sucrose content in the gut
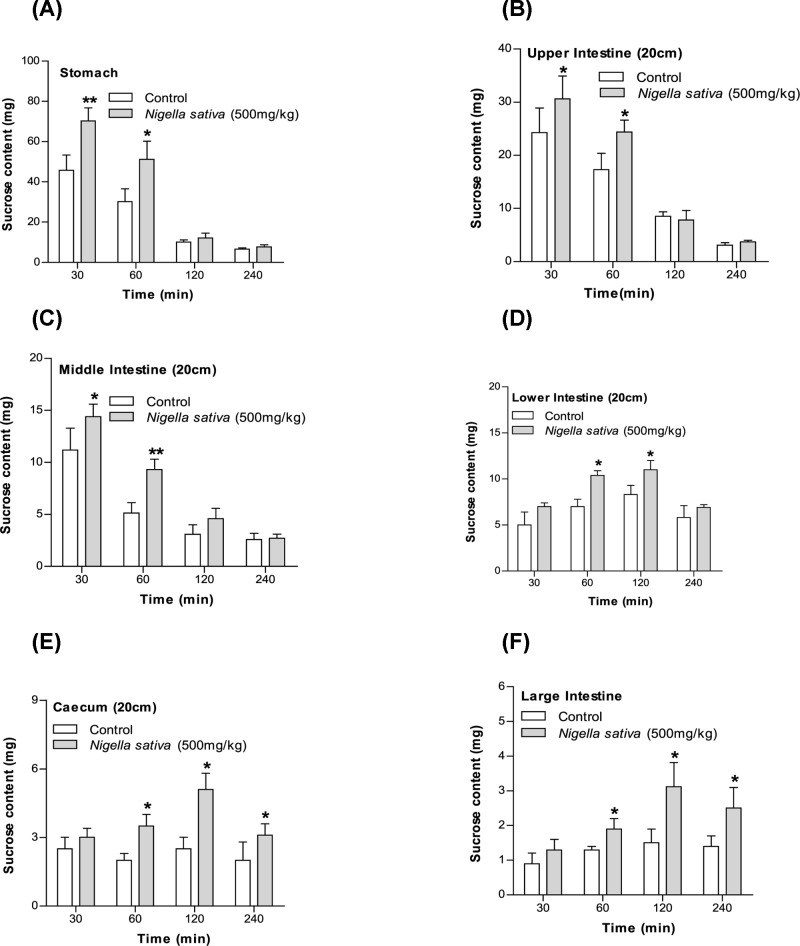
The unabsorbed sucrose content after the administration of sucrose (2.5g/kg, b/w) with methanol extract (500mg/kg, body weight) had increased significantly (P<0.05 and P<0.01; Figure 2) in the (A) stomach, (B) upper and (C) middle intestine after 30 min, in the whole small intestine after 1 h, and in the (D) lower intestine, (E) cecum, and (F) large intestine after 2 h. After 4 h, the sucrose content was detected in trace amounts in the control group, however, at the same time, sucrose was detected in the cecum as well as in the large intestine in the rat group that received the extract treatment (Figure 2).
Figure 2. Effects of methanol extract of N. sativa on (A–F) GI sucrose content after oral sucrose loading in type 2 diabetic rats.
Type 2 diabetic rats were fasted for 24 h prior to the oral administration of sucrose solution (2.5 g/kg body weight) in the presence (treated group) or absence of (control group) methanol extract of N. sativa (500 mg/kg body weight). The values are means and standard deviations represented by vertical bars (n=6). The mean values that are marked with an asterisk (*) were substantially different from those of respective type 2 diabetic control rats (*P<0.05 and **P<0.01) (this was derived from repeated-measures ANOVA and adjusted using Bonferroni correction).
Effects of N. sativa on intestinal glucose absorption
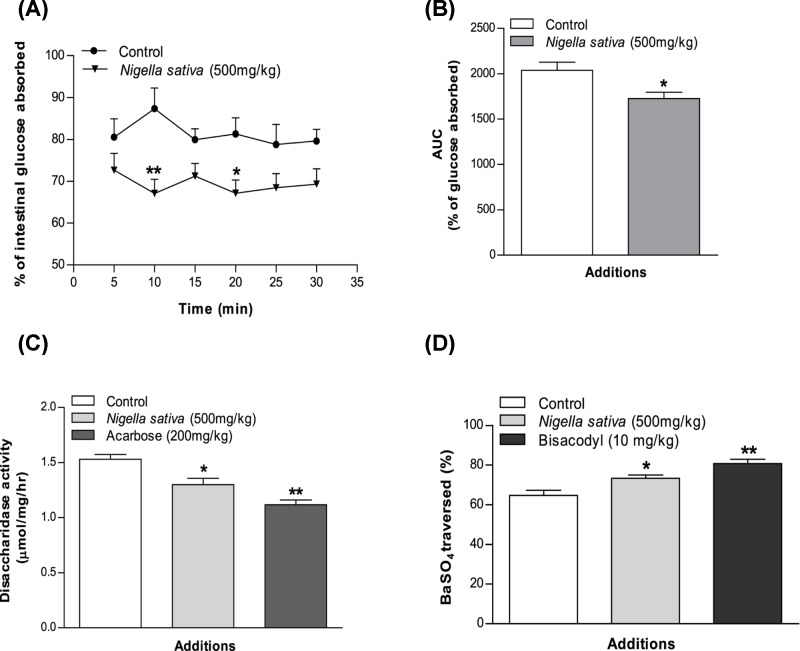
Intestinal glucose absorption was almost constant during the 30-min period of perfusion with glucose. The glucose solution containing the extract decreased intestinal glucose absorption significantly (P<0.05 to P<0.01) at both 10 and 20 min of the perfusion period (Figure 3A,B).
Figure 3. Effects of methanol extract of N. sativa on (A,B) intestinal glucose absorption, (C) disaccharidase enzyme activity, and (D) GI motility (by BaSO4 traversed) in non-diabetic rats.
Rats were fasted for 36 h (gut perfusion) and 20 h (enzyme activity and gut motility), and intestine was perfused with glucose (54 g/l) in the presence (treated group) or absence of (control group) methanol extract of N. sativa (10 mg/ml; with every individual obtaining 15 ml of perfusion). Enzyme activity was determined and BaSO4 administered at 60 min. Motility was measured over the following 15 min. Acarbose (ACB) (200 mg/kg) and bisacodyl (10 mg/kg) were used as standard drugs for disaccharidase activity and GI motility test correspondingly. The values are means and standard deviations represented by vertical bars (n=8). The mean values that are marked with an asterisk (*) were substantially different from those of respective control rats (*P<0.05 and **P<0.01) (this was derived from repeated-measures ANOVA and adjusted using Bonferroni correction).
Effects of N. sativa on intestinal disaccharidase activity and GI motility
The methanol extract of N. sativa inhibited disaccharidase enzyme activity significantly (P<0.05, Figure 3C) in normal rats. Additionally, the extract showed potential to increase GI motility at a dose of 500 mg/kg, body weight (P<0.05, Figure 3D).
Effects of N. sativa on insulin secretion from isolated islets
N. sativa extract effects on insulin secretion has been presented in Table 1. Extracts effect were assayed using isolated rat islets and compared in presence of 3 and 11 mM glucose. Extract concentration increases of 25–200 μg/ml had also increased the secretion of glucose-inducing insulin by 1.3–3 times in comparison with 3 and 11 mM glucose alone (P<0.01–0.001, Table 1). While, the dose-dependent increment in insulin release was 14–67% by the methanol extract in comparison with the 3 mM glucose alone (P<0.05 and P<0.01; Table 1), which has been increased to 2.3-fold with the increase in glucose concentration (11 mM). Whereas a positive control, Glibenclamide induced the insulin release from 1.9 to 3.4-folds in presence of 3 and 11 mM glucose.
Discussion
The current study at hand used a streptozotocin-induced type 2 diabetes animal model; streptozotocin causes DNA damage and generates superoxide radicals to destroy the pancreatic β-cells [26]. N. sativa has established background as a potential antioxidant and anti-diabetic natural product due to its alkaloid derivatives, mostly thymohydroquinone and TQ [32,33]. It has been reported several times that TQ can protect β-cells from ROS damage and alleviate DM [34–37]. It was also indicated that supplementation with TQ (20 mg/kg, body weight/d, p.o.) during the gestation and lactation periods of diabetic mice protected their offspring from diabetes and its associated complications via decreasing the levels of blood glucose [38,39].
Hyperglycemia causes cellular damage that hinders the homeostatic regulation of internal glucose concentration, resulting in acutely altered cellular metabolism and long-term changes in cellular macromolecular content [40,41]. A postprandial glucose spike causes perturbation in endothelial cell function [42,43], and increases the risk of blood coagulation [43]. Hyperglycemic state also increases products of glycosylation, which in turn has a significant influence on the development of diabetes-induced vascular disease [44]. Consequently, management of hyperglycemic states is an essential method of diabetes control. There are some primary pathways used in anti-diabetic drugs that include enhanced insulin secretion, enhanced sensitivity to insulin, improved peripheral glucose utilization, inhibition of glucose absorption, and inhibition of carbohydrate digestion [45]. N. sativa presented promising glucose-lowering effect in the recent study with chemically induced diabetic rats. The methanol extract showed high efficiency in stimulating insulin secretion from the isolated rat islets. This effect was enhanced further with the increase in glucose concentration from 3 to 11 mM. Thus, the extract is increasing glucose sensitivity that leads into the increased insulin release and causes hypoglycemia [46].
An in situ intestinal perfusion of the GI tract showed a marked reduction in glucose absorption. Within the GI motility assay using BaSO4 milk, the intestinal motility was enhanced significantly by the methanolic seed extract. The Six Segment study of the GI tract accounted for a high amount of unabsorbed sucrose contents in the stomach, upper, middle, and lower intestines in groups that received extracts. The last three parts of the GI tract are the most important for the absorption of nutrients, including sugars [47]. N. sativa ability to slow sucrose absorption widely across the GI tract has been shown by the high amount of unabsorbed sucrose content left in the GI tract. Resulting from this, a substantial concentration of sucrose reached the large intestine and cecum, and remained unabsorbed and egested with feces.
A significant reduction in hyperglycemia after an oral sucrose load and an increased level of residual sucrose content throughout the gut was observed, including the critical last three parts of the GI tract. Disaccharides are not absorbed from gut unless converted into monosaccharides due to structural complexity. A high level of unabsorbed sucrose in the GI tract is a clear indication of reduced sucrose digestion. This decrement is further justified by the study with intestinal disaccharidase activity where the similar low-level absorption of sucrose was observed and this indicates a partial inhibition of the intestinal disaccharidase enzyme activity.
N. sativa seed extract showed a promising decline regarding disaccharidase enzyme activity. As complex carbohydrates require this enzyme to breakdown into simpler monosaccharides before absorption, any inhibition of this enzyme would interfere with sugar absorption, lowering the glycemic peak. In addition, the methanol extracts also showed improvement in glucose sensitivity and insulin release. However, precise mechanism of this inhibitory action remains to be studied.
Conclusion
To conclude, the present study has shown that the effects of N. sativa seeds extract on anti-hyperglycemic activities in normoglycemic and diabetic animals are associated with a decreased intestinal glucose absorption and enhanced tissue glucose utilization that is mediated by the improvement in insulin release from islet. Also, a sound scientific basis appears to exist for the use of N. sativa as a dietary adjuvant for type 2 diabetes. Altogether, these findings suggesting a new mode of action of N. sativa in the treatment of DM that is co-related with its previous claims.
Acknowledgments
The authors are grateful to all staff belonging to the East West University and the International Centre for Diarrhoeal Disease Research, Dhaka, Bangladesh (Icddr, b). Their technical support and collaboration made the present study possible.
Abbreviations
- Aβ
Amyloid β
- AD
Alzheimer’s Disease
- b/w
Body Weight
- DM
Diabetes mellitus
- GAC
Glucose adsorption capacity
- GI
Gastrointestinal
- GOD-PAP
Glucose oxidase-phenol amino phenazone
- KRB
Krebs Ringer Bicarbonate
- OGTT
Oral glucose tolerance test
- p.o.
Oral gavage
- ROS
Reactive oxygen species
- TQ
Thymoquinone
Animal ethical statement
All authors declare that ‘Principles of laboratory animal care’ (NIH publication No. 85-23, revised 1985) were followed, as well as the U.K. Animals (Scientific Procedures) Act 1986 for animal experiments. All experiments were examined and approved by the appropriate ethics committee.
Funding
The authors declare that there are no sources of funding to be acknowledged.
Author Contribution
J.M.A.H. and P.A. designed the whole project including its supervision and direction. A.H., A.S., A.H., A.R.M. and A.G. participated in experiments and statistical analysis. J.M.A.H. and P.A. wrote the manuscript. P.A. and S.A. edited the final manuscript. All authors read and approved the final version of the manuscript.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Roglic G.et al. (2005) The burden of mortality attributable to diabetes: realistic estimates for the year 2000. Diabetes Care 28, 2130–2135 10.2337/diacare.28.9.2130 [DOI] [PubMed] [Google Scholar]
- 2.Booth G.L.et al. (2006) Relation between age and cardiovascular disease in men and women with diabetes compared with non-diabetic people: a population-based retrospective cohort study. Lancet 368, 29–36 10.1016/S0140-6736(06)68967-8 [DOI] [PubMed] [Google Scholar]
- 3.Sicree R, Shaw J. and Zimmet P (2006) Diabetes and impaired glucose tolerance. Chapter 1: Diabetes Atlas, 2nd edn., pp.15–103, International Diabetes Federation, Brussels, Belgium [Google Scholar]
- 4.Trivedi N.A.et al. (2004) Effect of shilajit on blood glucose and lipid profile in alloxaninduced diabetic rats, Indian J. Pharmacol. 36, 373–376 [Google Scholar]
- 5.Zimmet P.et al. (1990) The epidemiology and natural history of NIDDM–lessons from the South Pacific. Diabetes Metab. Rev. 6, 91–124 10.1002/dmr.5610060203 [DOI] [PubMed] [Google Scholar]
- 6.Toeller M. (1994) Alpha-glucosidase inhibitors in diabetes: efficacy in NIDDM subjects. Eur. J. Clin. Invest. 24, 31–35 [DOI] [PubMed] [Google Scholar]
- 7.Davis S.N. and Granner D.K. (1996) Insulin, oral hypoglycemic agents, and the pharmacology of endocrine pancreas. In The Pharmacological Basis of Therapeutics(Gilman G.A., ed.), McGraw-Hill, New York [Google Scholar]
- 8.Ahmad A.et al. (2013) A review on therapeutic potential of Nigella sativa: a miracle herb. Asian Pac. J. Trop. Biomed. 3, 337–352 10.1016/S2221-1691(13)60075-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yimer E.M.et al. (2019) Nigella sativa L. (black cumin): a promising natural remedy for wide range of illnesses. Evid. Based Complement. Alternat. Med. 2019, 16 10.1155/2019/1528635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boskabady M.H.et al. (2007) The possible prophylactic effect of Nigella sativa seed extract in asthmatic patients. Fundam. Clin. Pharmacol. 21, 559–566 10.1111/j.1472-8206.2007.00509.x [DOI] [PubMed] [Google Scholar]
- 11.Akhtar M.et al. (2012) Ameliorating effects of two extracts of Nigella sativa in middle cerebral artery occluded rat. J. Pharm. Bioallied Sci. 4, 70–75 10.4103/0975-7406.92740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bakathir H.A. and Abbas N.A. (2011) Detection of the antibacterial effect of Nigella sativa ground seeds with water. Afr. J. Tradit. Complement. Altern. Med. 8, 159–164 10.4314/ajtcam.v8i2.63203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdelmeguid N.E.et al. (2010) Effects of Nigella sativa and thymoquinone on biochemical and subcellular changes in pancreatic beta-cells of streptozotocin-induced diabetic rats. J. Diabetes 2, 256–266 10.1111/j.1753-0407.2010.00091.x [DOI] [PubMed] [Google Scholar]
- 14.Abel-Salam B.K. (2012) Immunomodulatory effects of black seeds and garlic on alloxan-induced diabetes in albino rat. Allergol. Immunopathol. (Madr.) 40, 336–340 10.1016/j.aller.2011.07.002 [DOI] [PubMed] [Google Scholar]
- 15.Al-Ali A.et al. (2008) Oral and intraperitoneal LD50 of thymoquinone, an active principle of Nigella sativa, in mice and rats. J. Ayub Med. Coll. Abbottabad 20, 25–27 [PubMed] [Google Scholar]
- 16.Noorbakhsh M.-F.et al. (2018) Protective effects of thymoquinon on pulmonary disorders in experimental studies. Tanaffos 17, 211–222 [PMC free article] [PubMed] [Google Scholar]
- 17.Bimonte S.et al. (2019) Dissecting the roles of thymoquinone on the prevention and the treatment of hepatocellular carcinoma: an overview on the current state of knowledge. Infect. Agent Cancer 14, 10 10.1186/s13027-019-0226-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L.et al. (2019) A review on advanced microencapsulation technology to enhance bioavailability of phenolic compounds: based on its activity in the treatment of Type 2 Diabetes. Trends Food Sci. Technol. 85, 149–162 [Google Scholar]
- 19.Kanter M. (2008) Nigella sativa and derived thymoquinone prevents hippocampal neurodegeneration after chronic toluene exposure in rats. Neurochem. Res. 33, 579–588 10.1007/s11064-007-9481-z [DOI] [PubMed] [Google Scholar]
- 20.Kanter M. (2011) Protective effects of thymoquinone on the neuronal injury in frontal cortex after chronic toluene exposure. J. Mol. Histol. 42, 39–46 10.1007/s10735-010-9305-3 [DOI] [PubMed] [Google Scholar]
- 21.Jakaria M.et al. (2018) Neuropharmacological potential and delivery prospects of thymoquinone for neurological disorders. Oxid. Med. Cell. Longev. 2018, 17 10.1155/2018/1209801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alhebshi A.H., Gotoh M. and Suzuki I. (2013) Thymoquinone protects cultured rat primary neurons against amyloid beta-induced neurotoxicity. Biochem. Biophys. Res. Commun. 433, 362–367 10.1016/j.bbrc.2012.11.139 [DOI] [PubMed] [Google Scholar]
- 23.Azam S.et al. (2019) Regulation of Toll-Like Receptor (TLR) signaling pathway by polyphenols in the treatment of age-linked neurodegenerative diseases: focus on TLR4 signaling. Front. Immunol. 10, 1000 10.3389/fimmu.2019.01000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanter M., Akpolat M. and Aktas C. (2009) Protective effects of the volatile oil of Nigella sativa seeds on beta-cell damage in streptozotocin-induced diabetic rats: a light and electron microscopic study. J. Mol. Histol. 40, 379–385 10.1007/s10735-009-9251-0 [DOI] [PubMed] [Google Scholar]
- 25.Azad S.B.et al. (2017) Anti-hyperglycaemic activity of Moringa oleifera is partly mediated by carbohydrase inhibition and glucose-fibre binding. Biosci. Rep. 37, BSR20170059 10.1042/BSR20170059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ou S.et al. (2001) In vitro study of possible role of dietary fiber in lowering postprandial serum glucose. J. Agric. Food Chem. 49, 1026–1029 10.1021/jf000574n [DOI] [PubMed] [Google Scholar]
- 27.Ambade V.N., Sharma Y.V. and Somani B.L. (1998) Methods for estimation of blood glucose: a comparative evaluation. Med. J. 54, 131–133 10.1016/S0377-1237(17)30502-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lenzen S. (2008) The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 51, 216–226 10.1007/s00125-007-0886-7 [DOI] [PubMed] [Google Scholar]
- 29.Hannan J.M.et al. (2007) Soluble dietary fibre fraction of Trigonella foenum-graecum (fenugreek) seed improves glucose homeostasis in animal models of type 1 and type 2 diabetes by delaying carbohydrate digestion and absorption, and enhancing insulin action. Br. J. Nutr. 97, 514–521 10.1017/S0007114507657869 [DOI] [PubMed] [Google Scholar]
- 30.Doluisio J.T.et al. (1969) Drug absorption I: an in situ rat gut technique yielding realistic absorption rates. J. Pharm. Sci. 58, 1196–1200 10.1002/jps.2600581006 [DOI] [PubMed] [Google Scholar]
- 31.Hannan J.M.et al. (2006) Ocimum sanctum leaf extracts stimulate insulin secretion from perfused pancreas, isolated islets and clonal pancreatic beta-cells. J. Endocrinol. 189, 127–136 10.1677/joe.1.06615 [DOI] [PubMed] [Google Scholar]
- 32.El Rabey H.A., Al-Seeni M.N. and Bakhashwain A.S. (2017) The antidiabetic activity of Nigella sativa and propolis on streptozotocin-induced diabetes and diabetic nephropathy in male rats. Evid. Based Complement. Alternat. Med. 2017, 5439645 10.1155/2017/5439645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alimohammadi S.et al. (2013) Protective and antidiabetic effects of extract from Nigella sativa on blood glucose concentrations against streptozotocin (STZ)-induced diabetic in rats: an experimental study with histopathological evaluation. Diagn. Pathol. 8, 137–137 10.1186/1746-1596-8-137 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Hamdy N.M. and Taha R.A.J.P. (2009) Effects of Nigella sativa oil and thymoquinone on oxidative stress and neuropathy in streptozotocin-induced diabetic rats. Pharmacology 84, 127–134 [DOI] [PubMed] [Google Scholar]
- 35.Omran O.M.J.U.p. (2014) Effects of thymoquinone on STZ-induced diabetic nephropathy: an immunohistochemical study. Ultrastruct. Pathol. 38, 26–33 [DOI] [PubMed] [Google Scholar]
- 36.Al-Trad B.et al. (2016) Nigella sativa oil and thymoquinone ameliorate albuminuria and renal extracellular matrix accumulation in the experimental diabetic rats, Eur. Rev. Med. Pharmacol. Sci. 20, 2680–2688 [PubMed] [Google Scholar]
- 37.Pei X.et al. (2016) Thymoquinone inhibits angiotensin II-induced proliferation and migration of vascular smooth muscle cells through the AMPK/PPARγ/PGC-1α pathway. DNA Cell Biol. 35, 426–433 [DOI] [PubMed] [Google Scholar]
- 38.Farkhondeh T., Samarghandian S. and Borji A. (2017) An overview on cardioprotective and anti-diabetic effects of thymoquinone. Asian Pac. J. Trop. Med. 10, 849–854 10.1016/j.apjtm.2017.08.020 [DOI] [PubMed] [Google Scholar]
- 39.Badr G.et al. (2013) Maternal supplementation of diabetic mice with thymoquinone protects their offspring from abnormal obesity and diabetes by modulating their lipid profile and free radical production and restoring lymphocyte proliferation via PI3K/AKT signaling. Lipids Health Dis. 12, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.UK Prospective Diabetes Study (UKPDS) Group (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352, 837–853 10.1016/S0140-6736(98)07019-6 [DOI] [PubMed] [Google Scholar]
- 41.Heilig C.W.et al. (1995) Overexpression of glucose transporters in rat mesangial cells cultured in a normal glucose milieu mimics the diabetic phenotype. J. Clin. Invest. 96, 1802–1814 10.1172/JCI118226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haller H.J.D.M. (1997) Postprandial glucose and vascular disease. Diabet. Med. 14, S50–S56 [DOI] [PubMed] [Google Scholar]
- 43.Koya D. and King G.L.J.D. (1998) Protein kinase C activation and the development of diabetic complications. Diabetes 47, 859–866 [DOI] [PubMed] [Google Scholar]
- 44.Ceriello A.et al. (1996) Post-meal coagulation activation in diabetes mellitus: the effect of acarbose. Diabetologia 39, 469–473 10.1007/BF00400679 [DOI] [PubMed] [Google Scholar]
- 45.Thornalley P.J.E.M. (1996) Advanced glycation and the development of diabetic complications. Unifying the involvement of glucose, methylglyoxal and oxidative stress. Endocrinol. Metab. 3, 149–166 [Google Scholar]
- 46.Hannan J.M.A.et al. (2007) Insulin secretory actions of extracts of Asparagus racemosus root in perfused pancreas, isolated islets and clonal pancreatic β-cells. J. Endocrinol. 192, 159. [DOI] [PubMed] [Google Scholar]
- 47.Wilson P.W., Cupples L.A. and Kannel W.B. (1991) Is hyperglycemia associated with cardiovascular disease? The Framingham Study. Am. Heart J. 121, 586–590 10.1016/0002-8703(91)90729-2 [DOI] [PubMed] [Google Scholar]