Abstract
Piles of evidence have supported the relationship between miR-618 rs2682818 polymorphism and tumorigenesis, but the conclusion remains inconsistent. In the present study, we conducted a meta-analysis to sniff out the potential risk between miR-618 rs2682818 and overall cancers. Crude odds ratios (ORs) and 95% confidence intervals (CIs) analyzed by Z-test were employed to estimate the potential interrelation in five genetic models. We also prospected how the rs2682818 affects the second structure of miR-618. Finally, 10 independent studies meet the enrolled criteria, along with 4099 cancer cases and 5057 healthy controls. Overall, no exceeding interrelation was sniffed out in the pooled data among five inherited models, as well as stratified analyses. Whereas, the enhanced cancer risk of miR-618 rs2682818 variant stratified by breast cancer was revealed, in heterozygote genetic model (AC vs. CC: OR = 1.291, 95%CI = 1.012–1.648, P = 0.040) and dominant contrast model (AA + AC vs. CC: OR = 1.280, 95%CI = 1.009–1.623, P = 0.042). The second structure prediction result shown that the mutant A allele might change the first stem-loop of miR-618, and the free energy of it would turn from –39.1 to –35.1 kcal/mol. All in all, our meta-analysis had successfully chased down that miR-618 rs2682818 polymorphism is not linked with overall cancer risk, but in the dominant genotype of breast cancer.
Keywords: cancer, meta-analysis, miR-618, polymorphism, rs2682818
Introduction
MicroRNAs (miRNAs), a series of 17–25 length small nucleotides, have been widely studied about its function in several types of cancer. In recent days, the method of miRNA expression profiling has been applied to reveal whether it promotes or suppresses tumorigenesis, and also exposed to be the biomarkers of predicting the occurrence or prognosis of tumors [1–5]. Although the size and number of miRNAs are less than messenger RNAs (mRNAs), they play a pivotal role in the regulation of mRNAs through binding to the 3′-UTRs of mRNA, furthermore affect the expression and function of tumor associate genes [6–8]. miRNAs could also regulate several mechanisms through the antagonization of the translation promoter of mRNA, result in the changing process of proliferation, differentiation, and apoptosis [9,10].
Recent studies have demonstrated the association between aberrant miR-618 expression and tumorigenesis; miR-618 is up-regulated in hepatocellular carcinoma, breast cancer, bladder cancer and Barrett’s esophageal cancer [11–14], while down-regulated in thyroid cancer [15]. Single nucleotide polymorphisms (SNPs) are the usual form of gene variants in genetic materials, it could also occur in the DNA sequence which encodes pre-miRNAs and miRNAs. rs2682818 polymorphism located on the precursor’s stem-loop of the miR-618 sequence, which could both affect the secondary structure of pre-miR-618 and the releasing of the mature miR-618 [16]. Whether rs2682818 polymorphism of miR-618 is associated with cancer susceptibility, we planned to verify and obtain the precise result in the current study.
Materials and methods
We established the evaluate process of the meta-analysis along with PRISMA statement, the preferred reporting items for systematic reviews and meta-analyses compliant statement [17]. No ethics problems need to be concerned in this study, while all the data were extracted and recorded from published articles.
Search strategy
We performed an extensive literature research about the relationship between miRNA-618 rs2682818 and cancer susceptibility up to May 2019. PubMed, Medline, Google Scholar, Wanfang and CNKI databases were all enrolled. The search items listed as following: (‘miR-618’ OR ‘microRNA-618’ OR ‘miRNA-618’ OR ‘rs2682818’) AND (‘tumor’ OR ‘cancer’ OR ‘neoplasm’) AND (‘polymorphism’ OR ‘mutation’ OR ‘variant’). The screen process of eligible studies is displayed in Figure 1.
Figure 1. Flow chart showing the study selection process.
Eligible criteria include: 1. Original studies, about cases and their matched controls, focused on the correlation of miR-618 polymorphism and cancer susceptibility. 2. Studies provided the sufficient data of SNP allele frequency in both cancer group and control group. Exclusion criteria include: 1. Studies unrelated to miRNA-618 or cancer; 2. Duplicated or repeated publication; 3. Case-only, conference abstract, review, animal or cell line articles; 4. Missing of allele frequency data.
Extraction of data sources
Extractions of the allele frequency data from enrolled studies were completed by two authors independently. First author, published year, ethnicity of involvers, genotyping method, cancer type, and allele distribution in cancer patients and control group were all recorded. As the two reviewers independently extracted the date of different items, any inconsistent record was discussed and re-confirmed by search the original article with the two reviewers together.
Methods of meta-analysis
We carried out the meta-analysis in pooled overall date, as well as in different stratified sub-groups. Crude odds ratios (ORs) and their 95% confidence intervals (CIs) calculated by Z-test were employed to estimate the potential interrelation between miR-618 rs2682818 variant and cancer risks in five common hereditary models, including allele genetic model (A vs. C), homozygote genetic model (AA vs. CC), heterozygote genetic model (AC vs. CC), recessive contrast model (AA vs. AC + CC) and dominant contrast model (AA + AC vs. CC). The model used for Z-test was depended upon the result of heterogeneity associated Q-test, if the P value of Q-test <0.1 indicated that there was meaningful heterogeneity among enrolled data, thus the random-effects model (Der Simonian and Laird method) was applied. Otherwise, the P value of Q-test ≥0.1 indicated the fixed-effects model (the Mantel–Haenszel method) [18]. Moreover, Hardy–Weinberg equilibrium (HWE) formula assessed by χ2 were made in control group of each study, P>0.05 were considered to have reliable and representative controls [19]. In order to evaluate the quality of each enrolled study, we applied Newcastle–Ottawa scale (NOS) [20].
Evaluation of the results
Begg’s funnel plot and Egger’s test were used to appraising any publication bias in the results [21,22]. On the other way, the underlying effects of each single study to overall results were evaluated by sensitivity analyses, with the method of deletion one independent study each time. All the statistic results shown in the current study were two-tailed, while P-values ≤0.05 were considered to be a remarkable difference.
In silico analysis of miR-618 rs2682818
In order to explore the underlying effect of rs2682818 to miR-618, we use several online tools. First, we observed the different allele frequency about rs2682818 around the world, with the help of Ensembl website based on the data extract from 1000 Genomes Project (http://asia.ensembl.org/Homo_sapiens/Variation/Population?db=core;g=ENSG00000208022;r=12:80935736-80935833;t=ENST00000385287;v=rs2682818;vdb=variation;vf=471664594#population_freq_SAS), the project unites multidisciplinary research teams from institutes around the world, including China, Italy, Japan, Kenya, Nigeria, Peru, the United Kingdom, and the United States. On the other way, the possible impact of rs2682818 located on miR-618 region on the structure of its stem-loop with the wild type and mutant allele was evaluated with an RNA structure website (http://rna.urmc.rochester.edu/RNAstructureWeb/Servers/Predict1/Predict1.html).
Results
Study characteristics
Along with the pre-set search items, as much as 124 articles were firstly taken into consideration from different databases. In the next two steps, we screened the relativity of each article by reading its abstract or whole manuscript. Finally, there are only seven articles met the inclusion criteria with 10 independent case–control studies [13,16,23–28]. The process of study selection is shown as a flow chart in Figure 1. Of the remaining 10 case–control studies, the details are listed in Table 1, there are concerns about 4099 cancer patients and 5057 controls. All the sources of control are population based, and accompanied by the P value of HWE is higher than 0.05, these two results ensured that the control group is representative and conformed to the law of genetic heredity. Meanwhile, we also assessed the quality of each study with NOS method, the result fulfilled in Supplementary Table S1 shown all the studies maintained the high quality.
Table 1. Characteristics of the enrolled studies on miR-618 rs2682818 polymorphism and cancer.
| First author | Year | Ethnicity | Genotyping method | Source of control | Cancer type | Cases | Controls | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | A allele (%) | CC | AC | AA | Total | A allele (%) | CC | AC | AA | HWE | ||||||
| Li et al. [25] | 2011 | Asian | TaqMan | PB | Hepatocellular carcinoma | 339 | 26.0 | 186 | 130 | 23 | 352 | 23.7 | 203 | 131 | 18 | 0.594 |
| Li et al. [25] | 2011 | Asian | TaqMan | PB | Hepatocellular carcinoma | 107 | 26.2 | 55 | 48 | 4 | 105 | 27.1 | 57 | 39 | 9 | 0.533 |
| Li et al. [25] | 2011 | Asian | TaqMan | PB | Nasopharyngeal carcinoma | 799 | 26.2 | 444 | 292 | 63 | 1021 | 26.4 | 551 | 401 | 69 | 0.731 |
| Wang et al. [28] | 2012 | Asian | TaqMan | PB | Bladder cancer | 336 | 29.5 | C = 474 | A = 198 | 454 | 30.1 | C = 635 | A = 273 | 0.256 | ||
| Zhang et al. [26] | 2012 | Asian | PCR-RFLP | PB | Breast cancer | 244 | 25.6 | 132 | 99 | 13 | 232 | 24.4 | 130 | 91 | 11 | 0.325 |
| Zhang et al. [27] | 2012 | Asian | PCR-RFLP | PB | Colorectal cancer | 444 | 25.3 | 249 | 165 | 30 | 455 | 24.3 | 262 | 165 | 28 | 0.767 |
| Fu et al. [16] | 2014 | Caucasian | MassARRAY | PB | Lymphoma | 349 | 14.9 | 256 | 82 | 11 | 511 | 13.4 | 383 | 119 | 9 | 0.945 |
| Navarro et al. [24] | 2016 | Caucasian | PCR | PB | Chronic lymphocytic leukemia | 163 | 8.6 | 138 | 22 | 3 | 236 | 14.6 | 172 | 59 | 5 | 0.982 |
| Morales et al. [13] | 2016 | Caucasian | TaqMan | PB | Breast cancer | 440 | 9.5 | 359 | 78 | 3 | 807 | 7.1 | 699 | 102 | 6 | 0.290 |
| Chen et al. [23] | 2018 | Asian | TaqMan | PB | Colorectal cancer | 878 | 25.8 | 475 | 353 | 50 | 884 | 30.1 | 436 | 363 | 85 | 0.457 |
H-B: hospital based; P-B: population based; HWE: Hardy–Weinberg equilibrium, P>0.05 means conformed to HWE.
Overall and subgroup analyses
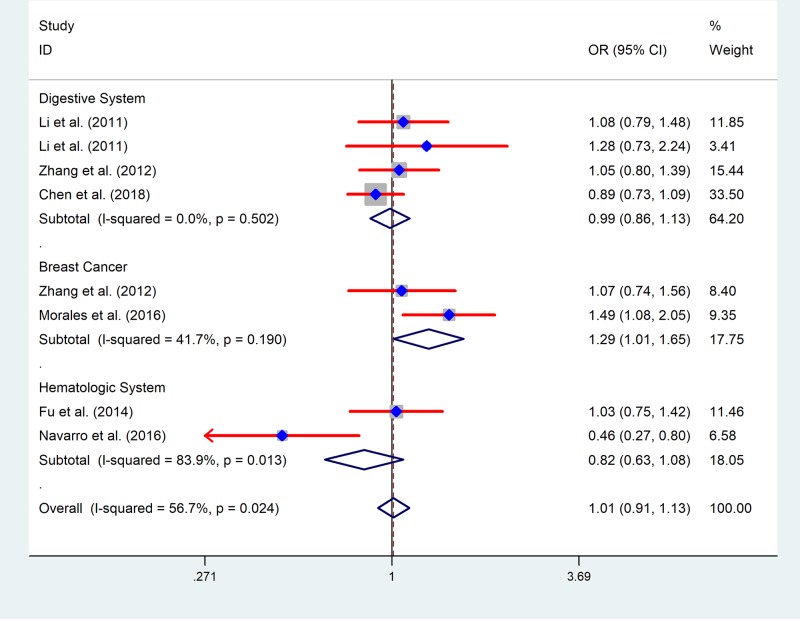
Analysis was handled to evaluate the feasible association between miR-618 rs2682818 variant and cancer risk, the results are shown in Table 2. Overall, no exceeding interrelation was sniffed out in the pooled data in all five genetic models (A vs. C: OR = 0.997, 95%CI = 0.886–1.122, P = 0.956; AA vs. CC: OR = 0.975, 95%CI = 0.711–1.338, P = 0.877; AC vs. CC: OR = 1.004, 95%CI = 0.863–1.167, P = 0.964; AA vs. AC + CC: OR = 0.979, 95%CI = 0.721–1.329, P = 0.891; AA + AC vs. CC: OR = 1.001, 95%CI = 0.860–1.165, P = 0.990) (Supplementary Figure S1). In the stratified calculate carried out by ethnicity, there are no vital effects of miR-618 rs2682818 variant to cancer risk on both Asian people and Caucasian people. We also tried to chase down the connection between miR-618 rs2682818 variant and different cancer type subgroup, there is no increased or decreased risk affected by it in digestive system cancer and hematologic system cancer. However, in the stratified analysis of breast cancer, we discovered an enhanced cancer risk caused by miR-618 rs2682818 variant in heterozygote genetic model (AC vs. CC: OR = 1.291, 95%CI = 1.012–1.648, P = 0.040) and dominant contrast model (AA + AC vs. CC: OR = 1.280, 95%CI = 1.009–1.623, P = 0.042) (Figure 2).
Table 2. Results of pooled analysis for miR-618 rs2682818 polymorphism and cancer susceptibility.
| Genetic model | Analysis group | N | PH | PZ | Effects model | OR (95% CI) |
|---|---|---|---|---|---|---|
| A vs. C | Overall | 10 | 0.011 | 0.956 | Random | 0.997 (0.886–1.122) |
| AA vs. CC | Overall | 9 | 0.057 | 0.877 | Random | 0.975 (0.711–1.338) |
| AC vs. CC | Overall | 9 | 0.028 | 0.964 | Random | 1.004 (0.863–1.167) |
| AA+AC vs. CC | Overall | 9 | 0.016 | 0.990 | Random | 1.001 (0.860–1.165) |
| AA vs. AC+CC | Overall | 9 | 0.065 | 0.891 | Random | 0.979 (0.721–1.329) |
| A vs. C | Asian | 7 | 0.200 | 0.265 | Fixed | 1.080 (0.899–1.297) |
| AA vs. CC | Asian | 6 | 0.027 | 0.690 | Random | 0.927 (0.640–1.344) |
| AC vs. CC | Asian | 6 | 0.672 | 0.544 | Fixed | 0.967 (0.868–1.077) |
| AA+AC vs. CC | Asian | 6 | 0.430 | 0.395 | Fixed | 0.956 (0.863–1.060) |
| AA vs. AC+CC | Asian | 6 | 0.029 | 0.687 | Random | 0.929 (0.648–1.332) |
| A vs. C | Caucasian | 3 | 0.004 | 0.953 | Random | 0.987 (0.626–1.554) |
| AA vs. CC | Caucasian | 3 | 0.526 | 0.435 | Fixed | 1.299 (0.674–2.504) |
| AC vs. CC | Caucasian | 3 | 0.001 | 0.800 | Random | 0.929 (0.527–1.640) |
| AA+AC vs. CC | Caucasian | 3 | 0.002 | 0.860 | Random | 0.953 (0.557–1.630) |
| AA vs. AC+CC | Caucasian | 3 | 0.582 | 0.407 | Fixed | 0.930 (0.767–1.129) |
| A vs. C | Digestive system | 4 | 0.059 | 0.705 | Random | 0.965 (0.803–1.160) |
| AA vs. CC | Digestive system | 4 | 0.026 | 0.495 | Random | 0.829 (0.483–1.422) |
| AC vs. CC | Digestive system | 4 | 0.502 | 0.847 | Fixed | 0.986 (0.859–1.132) |
| AA+AC vs. CC | Digestive system | 4 | 0.223 | 0.447 | Fixed | 0.950 (0.833–1.084) |
| AA vs. AC+CC | Digestive system | 4 | 0.037 | 0.442 | Random | 1.070 (0.528–2.168) |
| A vs. C | Hepatocellular carcinoma | 2 | 0.502 | 0.468 | Fixed | 1.082 (0.875–1.338) |
| AA vs. CC | Hepatocellular carcinoma | 2 | 0.119 | 0.789 | Fixed | 1.080 (0.616–1.892) |
| AC vs. CC | Hepatocellular carcinoma | 2 | 0.618 | 0.396 | Fixed | 1.126 (0.856–1.481) |
| AA+AC vs. CC | Hepatocellular carcinoma | 2 | 0.995 | 0.764 | Fixed | 1.121 (0.862–1.458) |
| AA vs. AC+CC | Hepatocellular carcinoma | 2 | 0.090 | 0.764 | Random | 0.840 (0.270–2.618) |
| A vs. C | Colorectal cancer | 2 | 0.040 | 0.501 | Random | 0.913 (0.699–1.191) |
| AA vs. CC | Colorectal cancer | 2 | 0.029 | 0.452 | Random | 0.759 (0.370–1.558) |
| AC vs. CC | Colorectal cancer | 2 | 0.342 | 0.471 | Fixed | 0.943 (0.804–1.106) |
| AA+AC vs. CC | Colorectal cancer | 2 | 0.126 | 0.169 | Fixed | 0.899 (0.771–1.047) |
| AA vs. AC+CC | Colorectal cancer | 2 | 0.043 | 0.428 | Random | 0.769 (0.401–1.473) |
| A vs. C | Hematologic system | 2 | 0.009 | 0.554 | Random | 0.808 (0.398–1.639) |
| AA vs. CC | Hematologic system | 2 | 0.303 | 0.364 | Fixed | 1.415 (0.669–2.994) |
| AC vs. CC | Hematologic system | 2 | 0.013 | 0.396 | Random | 0.713 (0.327–1.555) |
| AA+AC vs. CC | Hematologic system | 2 | 0.009 | 0.468 | Random | 0.747 (0.341–1.640) |
| AA vs. AC+CC | Hematologic system | 2 | 0.393 | 0.312 | Fixed | 1.472 (0.696–3.113) |
| A vs. C | Breast cancer | 2 | 0.219 | 0.066 | Fixed | 1.216 (0.987–1.497) |
| AA vs. CC | Breast cancer | 2 | 0.829 | 0.776 | Fixed | 1.110 (0.542–2.270) |
| AC vs. CC | Breast cancer | 2 | 0.190 | 0.040* | Fixed | 1.291 (1.012–1.648) |
| AA+AC vs. CC | Breast cancer | 2 | 0.219 | 0.042* | Fixed | 1.280 (1.009–1.623) |
| AA vs. AC+CC | Breast cancer | 2 | 0.799 | 0.851 | Fixed | 1.070 (0.528–2.168) |
PH: P value of Q test for heterogeneity test; PZ: means statistically significant (P < 0.05); SCCHN: squamous cell carcinoma of the head and neck; P-B: population based; HWE: Hardy–Weinberg equilibrium; Y: polymorphisms conformed to HWE in the control group; N: polymorphisms did not conform to HWE in the control group; *P value less than 0.05 was considered as statistically significant.
Figure 2. The forest plot of the meta-analysis for miR-618 rs2682818 polymorphism (AC vs. CC).
Analysis of sensitivity and publication bias
Sensitivity analysis was performed by eliminating each study one by one at a time, and the results indicated that no prominent effect from a single study would influence the stability of the above results (Supplementary Figure S2 and Table S2). As to assess the publication bias of all the enrolled studies, we deal it with statistical Egger’s test and graphical Begg’s funnel plot. We could see that the P value of Egger’s test in different models is higher than 0.05, and the distribution of studies in Begg’s funnel plot was symmetrical, which means there is no remarkable publication bias (Supplementary Figure S3 and Table S3).
In silico analysis
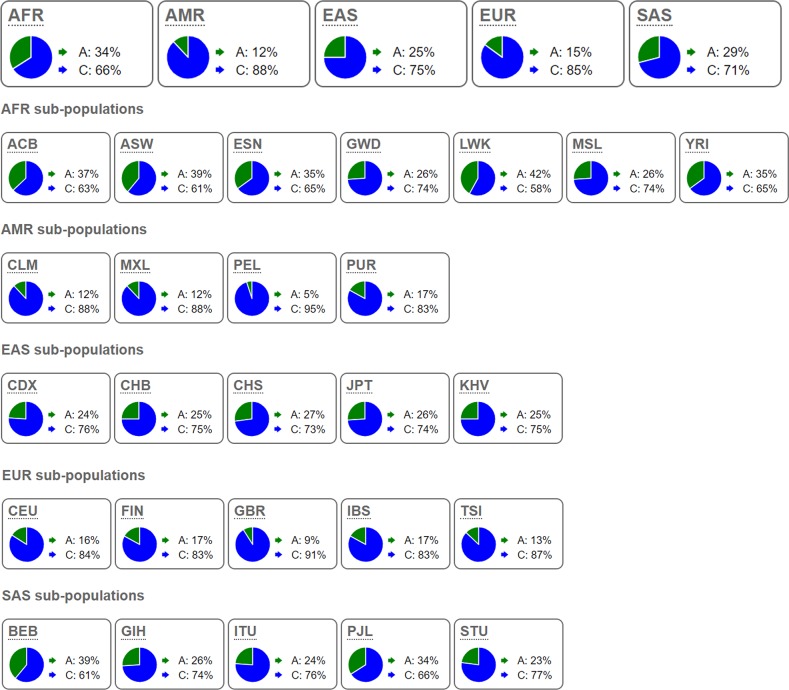
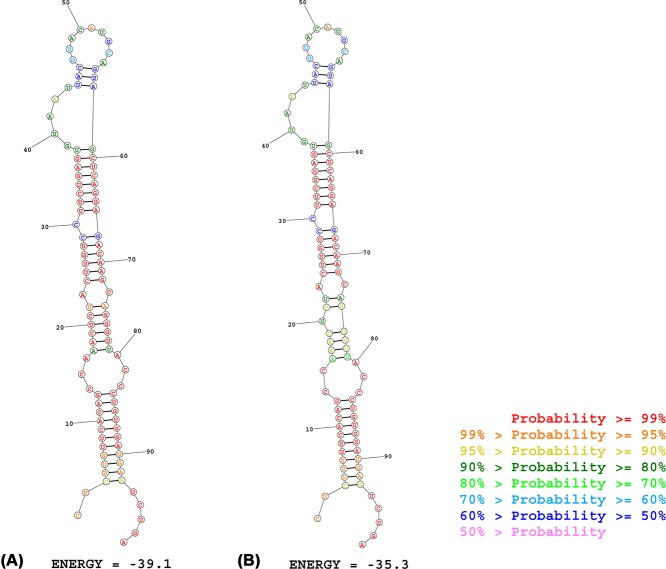
The results obtained from online tools make us understand how the rs2682818 variant affects miR-618 more deeply. From the data extracted from 1000 Genomes Project, we learned that the distribution of C allele and A allele in rs2682818 is inconsistent in different zones. As shown in Figure 3 and Supplementary Table S4, the rate of A allele in AMR and EUR is about 5–17%, consist with the data of three Caucasian base studies in current study (Fu et al.: 13.4%; Navarro et al.: 14.6%; Morales et al.: 7.1%). On the meanwhile, the rate of A allele in EAS and SAS is about 23–39%, also consist with the data of seven Caucasian base studies in the current study (Li et al. (a): 23.7%; Li et al. (b): 27.1%; Li et al. (c): 26.4%; Wang et al.: 30.1%; Zhang et al. (a): 24.4%; Zhang et al. (b): 24.3%; Chen et al.: 30.1%) (Supplementary Table S4). The allele frequency obtained from 1000 Genomes Project supported that all the enrolled studies comforted to nature inheritance in different zones and ethnicities. The second structure of miR-618 might be changed with or without the mutant of rs2682818. As illustrated in Figure 4, the mutant A allele might change the first stem-loop of miR-618, and the free energy of it would turn from −39.1 to −35.1 kcal/mol.
Figure 3. The allele frequencies of miR-618 rs2682818 in 1000 Genomes Project Phase 3.
AFR: African; AMR: American; EAS: East Asian; EUR: European; SAS: South Asian; ACB: African Caribbean in Barbados; ASW: African Ancestry in Southwest US; ESN: Esan in Nigeria; GWD: Gambian in Western Division, The Gambia; LWK: Luhya in Webuye, Kenya; MSL: Mende in Sierra Leone; YRI: Yoruba in Ibadan, Nigeria; CLM: Colombian in Medellin, Colombia; MXL: Mexican Ancestry in Los Angeles, California; PEL: Peruvian in Lima, Peru; PUR: Puerto Rican in Puerto Rico; CDX: Chinese Dai in Xishuangbanna, China; CHB: Han Chinese in Beijing, China; CHS: Southern Han Chinese, China; JPT: Japanese in Tokyo, Japan; KHV: Kinh in Ho Chi Minh City, Vietnam; CEU: Utah residents with Northern and Western European ancestry; FIN: Finnish in Finland; GBR: British in England and Scotland; IBS: Iberian populations in Spain; TSI: Toscani in Italy; BEB: Bengali in Bangladesh; GIH: Gujarati Indian in Houston, TX; ITU: Indian Telugu in the UK; PJL: Punjabi in Lahore, Pakistan; STU: Sri Lankan Tamil in the UK.
Figure 4. Predicted the secondary structure of miR-618 with the wild C allele (A) and mutant A allele (B).
Discussion
miR-618, the 98-nucleotide composed small molecule is the single transcription product of MIR618, which located on chromosome 12. In recent days, miR-618 has been reported associated with different types of cancer. Song et al. [29] reported that miR-618 could induce the transition of prostate cell from epithelial to mesenchymal through the target combined way to forkhead box p2 (FOXP2), finally lead to the inhibition of cell migration and invasion. On the meanwhile, Ivanovic et al. [30] found out that the overexpression of miR-618 could suppress the function of MMP-9 to prevent the progress of tumorigenesis, on the other hand, they also obtained the links that the expression level of miR-618 is negatively associated with the high Gleason score and advanced stages. Shi et al. [31] also revealed that the expression level of miR-618 in gastric cancer tissues is down-regulated compared with the adjacent non-tumor tissues, while overexpression of miR-618 could suppress the migration and invasion capacity of gastric cells.
Cause miR-618 plays an essential role in suppressing mRNA function through the combined effect of its second structure, the rs2682818 polymorphism on it is part of its precursor’s stem-loop sequence. Fu et al. [16] reported that rs2682818 increased the susceptibility of follicular lymphoma (OR = 1.65, 95% CI = 1.05–2.50), and the variant A allele is associated with the decrease the level of mature miR-618. In the contrast, Navarro et al. [24] gave the evidence that rs2682818 polymorphism plays a protective role in chronic lymphocytic leumkemia (CLL) (OR = 0.49, 95% CI = 0.29–0.81). For colorectal cancer (CRC), Chen et al. [23] sniff out that individuals who carry the AA or AC+AA genotype take along a lower CRC risk than CC (AA vs. CC: OR = 0.54, 95%CI = 0.37–0.79; AC+AA vs. CC: OR = 0.82, 95% CI = 0.68–0.99). Whereas, Wang et al. [28] presented the result that rs2682818 is not associated with the sensibility of CRC (AC vs. CC: OR = 1.03, 95%CI = 0.77–1.36; OR = 1.05, AA vs. CC: 95% CI = 0.60–1.84). Due to the misleading puzzle about rs2682818 and cancer risks, we managed this meta-analysis to remove the gaps. Results from our study show that rs2682818 of miR-618 is not associated with cancer sensibility, but it may act as the promoter of breast cancer. The in silico analysis from 1000 Genomes Project supported that all the enrolled studies comforted to the nature inheritance genetic rate in different zones and ethnicities. The second structure of miR-618 might be changed by rs2682818 polymorphism with the mutant A allele located at the first stem-loop of miR-618.
This study is the first meta-analysis concerned about the relationship between miR-618 rs2682818 polymorphism and cancer risk, we obtain some meaningful results, but the results should also be cautiously handled. There is some limitation that should not be covered up. First of all, the insufficient capacity of data occurred in the subgroup analysis, it might cause slight effects on the results of cancer risks. Besides, we ignored the feasible effect of complex factors, which rejects us to further evaluate the influence of gene–environment relations. On the meanwhile, there are also some obviously advantages. On the one hand, this is the first meta-analysis to talk about whether miR-618 rs2682818 would influence tumorigenicity. On the other hand, we completed a comprehensive literature search to enroll the correct studies, and NOS scale was used to eliminate the low-quality studies, so the results are reliable and unmistakable. What’s more, as the results showing, miR-618 rs2682818 is associated with the tumorigenesis of breast cancer, it could be a potential biomarker to remind people who with the polymorphism of rs2682818 pay more attention to the occurrence of breast cancer, and solve the problem as soon as possible.
In conclusion, our meta-analysis successfully chased down that miR-618 rs2682818 polymorphism is not linked with overall cancer risk, but in the dominant genotype of breast cancer.
Supporting information
Supplementary Figure S1.
Supplementary Figure S2.
Supplementary Figure S3.
Supplementary Table S1. Methodological quality of the includeds studies according to the Newcastle-Ottawa Scale.
Supplementary Table S2. Details of the sensitivity analyses for miR-618 rs2612818 polymorphism and cancer risk.
Supplementary Table S3. P values of the Egger’s test for miR-618 rs2612818 polymorphism.
Supplementary Table S4. The allele frequencies of miR-618 rs2612818 in 1000 Genomes Project Phase 3.
Abbreviations
- CLL
chronic lymphocytic leukemia
- CRC
colorectal cancer
- FOXP2
forkhead box p2
- HWE
Hardy–Weinberg equilibrium
- miRNA
microRNA
- mRNA
messenger RNA
- NOS
Newcastle–Ottawa scale
- ORs
odds ratios
- SNP
single nucleotide polymorphism
Author contribution
Xingliang Feng and Dan Ji performed the literature search, data extraction, and statistical analysis and wrote the manuscript. Chaozhao Liang and Song Fan supervised the literature search, data extraction, and analysis, and Song Fan reviewed the manuscript.
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
The National Science Foundation for Young Scientists [grant number 81400757].
References
- 1.Liu T., Zhang Q., Zhang J.. et al. (2019) EVmiRNA: a database of miRNA profiling in extracellular vesicles. Nucleic Acids Res. 47, D89–D93 10.1093/nar/gky985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lokeshwar S.D., Talukder A., Yates T.J.. et al. (2018) Molecular characterization of renal cell carcinoma: a potential three-microRNA prognostic signature. Cancer Epidemiol. Biomarkers Prev. 27, 464–472 10.1158/1055-9965.EPI-17-0700 [DOI] [PubMed] [Google Scholar]
- 3.Heublein S., Albertsmeier M., Pfeifer D.. et al. (2018) Association of differential miRNA expression with hepatic vs. peritoneal metastatic spread in colorectal cancer. BMC Cancer 18, 201 10.1186/s12885-018-4043-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ratert N., Meyer H.A., Jung M.. et al. (2013) miRNA profiling identifies candidate mirnas for bladder cancer diagnosis and clinical outcome. J. Mol. Diagn. 15, 695–705 10.1016/j.jmoldx.2013.05.008 [DOI] [PubMed] [Google Scholar]
- 5.White N.M., Khella H.W., Grigull J.. et al. (2011) miRNA profiling in metastatic renal cell carcinoma reveals a tumour-suppressor effect for miR-215. Br. J. Cancer 105, 1741–1749 10.1038/bjc.2011.401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erhard F., Haas J., Lieber D.. et al. (2014) Widespread context dependency of microRNA-mediated regulation. Genome Res. 24, 906–919 10.1101/gr.166702.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Brien J., Hayder H., Zayed Y. and Peng C. (2018) Overview of MicroRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol. (Lausanne) 9, 402 10.3389/fendo.2018.00402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quevillon Huberdeau M. and Simard M.J. (2019) A guide to microRNA-mediated gene silencing. FEBS J. 286, 642–652 10.1111/febs.14666 [DOI] [PubMed] [Google Scholar]
- 9.Liu B., Shyr Y., Cai J. and Liu Q. (2019) Interplay between miRNAs and host genes and their role in cancer. Brief Funct. Genomics 18, 255–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas J., Ohtsuka M., Pichler M. and Ling H. (2015) MicroRNAs: clinical relevance in colorectal cancer. Int. J. Mol. Sci. 16, 28063–28076 10.3390/ijms161226080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdalla M.A. and Haj-Ahmad Y. (2012) Promising candidate urinary microRNA biomarkers for the early detection of hepatocellular carcinoma among high-risk hepatitis C virus Egyptian patients. J. Cancer 3, 19–31 10.7150/jca.3.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fassan M., Volinia S., Palatini J.. et al. (2011) MicroRNA expression profiling in human Barrett’s carcinogenesis. Int. J. Cancer 129, 1661–1670 10.1002/ijc.25823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morales S., Gulppi F., Gonzalez-Hormazabal P.. et al. (2016) Association of single nucleotide polymorphisms in Pre-miR-27a, Pre-miR-196a2, Pre-miR-423, miR-608 and Pre-miR-618 with breast cancer susceptibility in a South American population. BMC Genet. 17, 109 10.1186/s12863-016-0415-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tolle A., Jung M., Rabenhorst S., Kilic E., Jung K. and Weikert S. (2013) Identification of microRNAs in blood and urine as tumour markers for the detection of urinary bladder cancer. Oncol. Rep. 30, 1949–1956 10.3892/or.2013.2621 [DOI] [PubMed] [Google Scholar]
- 15.Cheng Q., Zhang X., Xu X. and Lu X. (2014) MiR-618 inhibits anaplastic thyroid cancer by repressing XIAP in one ATC cell line. Ann. Endocrinol. (Paris) 75, 187–193 10.1016/j.ando.2014.01.002 [DOI] [PubMed] [Google Scholar]
- 16.Fu A., Hoffman A.E., Liu R., Jacobs D.I., Zheng T. and Zhu Y. (2014) Targetome profiling and functional genetics implicate miR-618 in lymphomagenesis. Epigenetics 9, 730–737 10.4161/epi.27996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D., Liberati A., Tetzlaff J., Altman D.G. and Group P. (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339, b2535 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DerSimonian R. and Laird N. (1986) Meta-analysis in clinical trials. Control. Clin. Trials 7, 177 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 19.Lau J., Ioannidis J.P. and Schmid C.H. (1997) Quantitative synthesis in systematic reviews. Ann. Intern. Med. 127, 820–826 10.7326/0003-4819-127-9-199711010-00008 [DOI] [PubMed] [Google Scholar]
- 20.Wells M., Chande N., Adams P.. et al. (2012) Meta-analysis: vasoactive medications for the management of acute variceal bleeds. Aliment. Pharmacol. Ther. 35, 1267–1278 10.1111/j.1365-2036.2012.05088.x [DOI] [PubMed] [Google Scholar]
- 21.Begg C.B. and Mazumdar M. (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 22.Higgins J.P. and Thompson S.G. (2002) Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539–1558 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 23.Chen Y., Du M., Chen W.. et al. (2018) Polymorphism rs2682818 in miR-618 is associated with colorectal cancer susceptibility in a Han Chinese population. Cancer Med. 7, 1194–1200 10.1002/cam4.1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Díaz Navarro A. (2017) Variantes genéticas en miRNAs implicadas en la susceptibilidad a desarrollar Leucemia Linfática Crónica. Grado en Bioquímica y Biología Molecular [Google Scholar]
- 25.Li P. (2011) Genetic Associations of miRNA-SNPs with the Common Diseases and Integrative Analysis of “OMICS” Data of Hepatocellular Carcinoma, Military Medical Academy of China, Beijing [Google Scholar]
- 26.Zhang M., Jin M., Yu Y.. et al. (2012) Associations of miRNA polymorphisms and female physiological characteristics with breast cancer risk in Chinese population. Eur. J. Cancer Care (Engl.) 21, 274–280 10.1111/j.1365-2354.2011.01308.x [DOI] [PubMed] [Google Scholar]
- 27.Zhang M. (2012) Study on the Associations of Lifestyle-related Factor, Genetic Viariantsin miRNA Encoding Regions and miRNA Binding Sites with Colorectal Cancer Risk. Hangzhou, Zhejiang University [Google Scholar]
- 28.Wang M., Chu H., Li P.. et al. (2012) Genetic variants in miRNAs predict bladder cancer risk and recurrence. Cancer Res. 72, 6173–6182 10.1158/0008-5472.CAN-12-0688 [DOI] [PubMed] [Google Scholar]
- 29.Song X.L., Tang Y., Lei X.H., Zhao S.C. and Wu Z.Q. (2017) miR-618 inhibits prostate cancer migration and invasion by targeting FOXP2. J. Cancer 8, 2501–2510 10.7150/jca.17407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ivanovic R.F., Viana N.I., Morais D.R.. et al. (2018) miR-618: possible control over TIMP-1 and its expression in localized prostate cancer. BMC Cancer 18, 992 10.1186/s12885-018-4930-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi J., Gong L., Chen L.. et al. (2019) MiR-618 suppresses metastasis in gastric cancer by downregulating the expression of TGF-beta2. Anat. Rec. (Hoboken) 10.1002/ar.24083 [DOI] [PubMed] [Google Scholar]