Abstract
During an infectious disease modeling study, a rhesus macaque (Macaca mulatta), experienced acute transient tachypnea including transient severe motion during the 70-second phases of serial contrast-enhanced magnetic resonance imaging of the abdomen. This same animal experienced transient severe motion during all but 2 of the 8 scans of the year-long study. This animal was the only animal in the study (1 of 12) to have this reaction to gadoxetate; the animal also vomited after the contrast injection once on day 146 of the study. On day 86, a different contrast agent (gadobutrol) was used, and the reaction did not occur. No treatment was required for any conditions relating to the reaction due to the self-limited nature. This type of reaction has not yet been reported in veterinary subjects before and is likely to be idiosyncratic after first exposure. However, this reaction should not be life threatening, and other contrast agents can be used if acute transient tachypnea does occur.
Keywords: Magnetic resonance imaging, Acute transient dyspnea, Contrast agent, Transient severe motion, Acute transient tachypnea, Gadoxetate disodium
Introduction
Acute transient dyspnea (ATD) with transient severe motion (TSM) is an adverse event described in humans during magnetic resonance imaging (MRI) acquisition shortly after injection of gadolinium contrast agents. Most reactions of this nature occur during the arterial phase of the MRI acquisition and are self-limited and mild with no need for medical intervention. However, cases of ATD as part of severe, systemic reactions in humans do occur [1]. First described in humans in the United States in 2013 (albeit rare) [2], [3], ATD to our knowledge has not been reported in the literature for veterinary subjects. Human patients who experience this side effect have difficulty breathing, and the resulting TSM can cause deterioration of image quality during breath-hold phases of MRI acquisition [3].
Several studies [4], [2] reported non-ATD adverse reactions to gadolinium-based contrast agents, most notably off-label use of gadobenate dimeglumine in dogs [5] and gadoxetate disodium in humans [6]. The US Food and Drug Administration package insert for gadoxetate use in humans, indicated for intravenous use in MRI of the liver to detect and characterize lesions with known or suspected focal liver disease, lists adverse reactions such as hypertension, respiratory disorders, vomiting, and nausea [6]. Anaphylactoid reactions (cardiovascular collapse) have also been described by veterinarians during gadolinium-enhanced MRI [4]. This case indicates an acquired sensitivity to the agent, which was likely an idiosyncratic reaction that developed after the first exposure to the contrast material.
Case report
As part of development of an infectious disease model in rhesus macaques (Macaca mulatta), 12 subjects underwent abdominal 3 T MRI (Philips Healthcare, Cleveland, OH) with a hepatocyte-specific contrast agent at least monthly to detect involvement of abdominal organs, particularly liver and spleen. The subjects underwent imaging at baseline, day 17, day 28, then every 4 weeks up to 1-year postexposure to infection. During imaging, animals on this study were anesthetized intramuscularly with ketamine (15 mg/kg IM 100 mg/mL) [7], intubated, and maintained with isoflurane 1%-4% [8] using a ventilator.
During all except 2 of the monthly timepoints in this study, a single animal (8-year-old female) displayed conspicuous body movement (TSM) during the portovenous phase (70 seconds after start of injection of the standard human dose (0.1 mL/kg body weight) of off-label intravenous gadoxetate disodium) of the dynamic T1-high resolution isotropic volume excitation MRI acquisitions [6]. The abnormal motion was detected only at the 70-second phase (Fig. 1C) during the serial MRI sequences with contrast administration; no motion occurred before contrast administration or during earlier or later phases (Fig. 1). Because motion was self-limited and never accompanied by other severe clinical signs or complications, the remaining sequences for each examination were completed as stated in the MRI scan protocol.
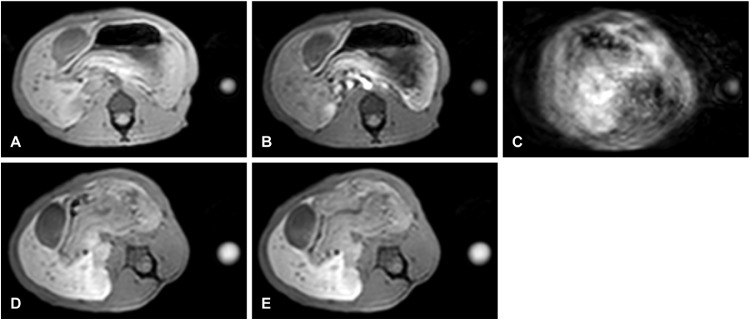
Fig. 1.
Axial, dynamic T1-high resolution isotropic volume excitation (THRIVE) MRI images of a female rhesus macaque at 182 days postchallenge with an infectious agent were acquired precontrast (A), 10 seconds (B), 70 seconds (C), 3 minutes (D), and 5 minutes (E) postcontrast with gadoxetate disodium intravenous injection. Severe motion artifact is demonstrated on the 70-second image. The subject also rolled onto the right side (D and E).
This motion artifact was seen on all the imaging days except for the baseline and day 86 postexposure. Importantly, the motion was associated with transiently increased respiratory and heart rates that were contemporaneous with the movement. The only other concomitant adverse reaction was vomiting once after gadoxetate injection (day 148 postexposure). Interestingly, on day 86, 0.1 mmol/kg of off-label gadobutrol contrast [9], another type of gadolinium-based agent, was given instead of gadoxetate, and the motion was not seen (Fig. 2). After a pattern was discerned after TSM occurred over several examinations, a diagnosis of repeated acute transient tachypnea (ATT) was made. Imaging findings were consistent with TSM artifact associated with ATD reactions in humans noticed after gadolinium-based intravenous injections [2]. From observation of animal signs, we labeled the reaction ATT rather than ATD.
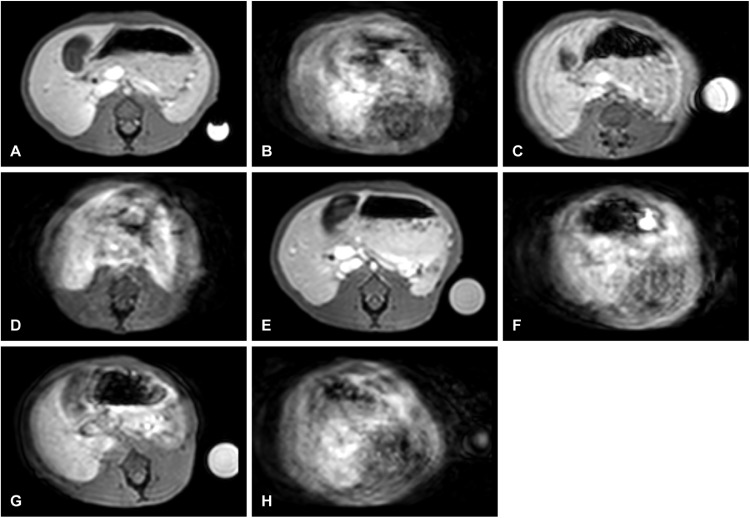
Fig. 2.
MRI images taken from approximately monthly scans made from a rhesus macaque, each from the 70-second phase of the axial T1-high resolution isotropic volume excitation (THRIVE) sequence following gadoxetate injection. Scans from all dates (A-H) reveal the transient severe motion artifact except baseline (A), and on day 86 (E) when gadobutrol contrast agent was administered in place of gadoxetate.
This animal did not require or receive treatment for any condition related to the episodes, because the transiently increased heart rate, respiratory rate, and motion all resolved at the same time and in a self-limited manner. No short- or long-term clinical sequelae were observed despite the repeated occurrences.
Discussion
ATD most characteristically occurs with gadoxetate administration according to studies investigating different gadolinium-based contrast agents and their adverse effects [10], [11]. The causal association in our case between the gadoxetate administration and TSM was not initially suspected on the early MRI image examinations, and the motion was originally considered related to level of anesthesia or other reaction. After noticing repeated TSM artifacts and rapid respiratory rate that occurred during the 70-second phase, a diagnosis of ATT was made. This case is interesting considering the motion artifact only occurred in this animal and that it did not occur on the baseline scan, but every scan thereafter except day 86 when gadobutrol was administered.
Results from one study in humans found that larger injection volumes of gadoxetate reduced the ability to breath hold during the portovenous phase of the MRI, and that a larger off-label dose significantly increased the incidence of TSM artifact [11]. The animals in our study were given the usual human dose off-label based on weight, and no publications stated that a dose adjustment would be required in rhesus macaques. Therefore, the normal human dosage administered should not be the cause of the TSM artifact. Because the reactions were rarely severe in this study and in the literature [4], [11], we were able to use the agent repeatedly without overt complications.
The ATT reaction described here is likely an idiosyncratic reaction after first exposure to gadoxetate (or any other agent) and has not been reported in the literature for veterinary subjects to our knowledge. Other types of anaphylactoid and non-ATD adverse reactions have been reported in veterinary subjects exposed to gadolinium-based contrast materials. This effect is rare yet will cause deteriorated image quality. Alternative contrast agents or administration methods should be considered if a reaction occurs. Gadoxetate should still be considered safe for use in subjects with prior ATD/ATT when needed. However, repeat imaging, reduced acquisition time, using dosing recommendations, longer administration intervals between dosing, and alternative contrast agents should be considered to avoid or reduce the potential motion artifact when appropriate [10], [2].
Footnotes
Declaration of Competing Interest: The authors have declared that no competing interests exist.
Acknowledgments: The authors would like to acknowledge Dr Lisa Hensley of the Integrated Research Facility, National Institute of Allergy and Infectious Diseases (NIAID), and National Institutes of Health (NIH), and Dr William Dowling of the Division of Microbiology and Infectious Diseases, NIH for their contribution to work done on this study, and Jiro Wada of Integrated Research Facility for formatting the figures. This work was supported by NIAID Division of Intramural Research and Division of Clinical Research (DCR) and was performed under Battelle Memorial Institute Contract (No. HHSN272200700016I with NIAID. This work was also partially supported by NIAID Interagency Agreement NOR15003-001-0000.
Animals were housed in an Association for Assessment and Accreditation of Laboratory Animal Care, International, accredited facility. All experimental procedures were approved by the NIAID DCR Animal Care and Use Committee and were in compliance with the Animal Welfare Act regulations, Public Health Service policy, and the Guide for the Care and Use of Laboratory Animals recommendations.
References
- 1.Davenport M.S., Viglianti B.L., Al-Hawary M.M., Caoili E.M., Kaza R.K., Liu P.S. Comparison of acute transient dyspnea after intravenous administration of gadoxetate disodium and gadobenate dimeglumine: effect on arterial phase image quality. Radiology. 2013;266:452–461. doi: 10.1148/radiol.12120826. [DOI] [PubMed] [Google Scholar]
- 2.Okigawa T., Utsunomiya D., Tajiri S., Okumura S., Sasao A., Wada H. Incidence and severity of acute adverse reactions to four different gadolinium-based MR contrast agents. Magn Reson Med Sci. 2014;13:1–6. doi: 10.2463/mrms.2012-0051. [DOI] [PubMed] [Google Scholar]
- 3.Pietryga J.A., Burke L.M., Marin D., Jaffe T.A., Bashir M.R. Respiratory motion artifact affecting hepatic arterial phase imaging with gadoxetate disodium: examination recovery with a multiple arterial phase acquisition. Radiology. 2014;271:426–434. doi: 10.1148/radiol.13131988. [DOI] [PubMed] [Google Scholar]
- 4.Girard N.M., Leece E.A. Suspected anaphylactoid reaction following intravenous administration of a gadolinium-based contrast agent in three dogs undergoing magnetic resonance imaging. Vet Anaesth Analg. 2010;37:352–356. doi: 10.1111/j.1467-2995.2010.00545.x. [DOI] [PubMed] [Google Scholar]
- 5.Bracco Diagnostics . National center for biotechnology information. DailyMed. Bracco Diagnostics, Monroe Township; Rockville Pike, MD: 2018. Gadobenate dimeglumine (Multihance) injection solution package insert.https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=f63abe56-46d9-4e32-afb1-f4e22370717d&type=display NJ. Accessed July 8, 2019. [Google Scholar]
- 6.Bayer HealthCarePharamaceuticals gadoxetate disodium (Eovist) injection solution for intravenous use package insert. In: National center for biotechnology information, ed Rockville Pike, MD: DailyMed. Bayer HealthCare, Wayne, NJ. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=6218b1e1-cbc3-4c14-bec7-528ac163a561&type=display. Accessed July 8, 2019
- 7.Henry Schein Animal Health . National center for biotechnology information. DailyMed Henry Schein Animal Health; Rockville Pike, MD: 2018. Ketamine (Ketathesia) hydrochloride veterinary injection for intramuscular use in cats and subhuman primates only pacakage insert.https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4dcc52aa-9c2b-457f-9cb2-af4cc25d9b6f Dublin, OH. Accessed July 8, 2019. [Google Scholar]
- 8.Patterson Veterinary . National center for biotechnology information. DailyMed. Patterson Veterinary; Rockville Pike, MD: 2017. Isoflurane inhalant anesthetic for use in horses and dogs package insert.https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3b8ca543-814d-465b-9dd0-6f5b75c215d0 Greeley, CO. Accessed July 8, 2019. [Google Scholar]
- 9.Bayer HealthCare Pharmaceuticals . National center for biotechnology information. DailyMed. Bayer HealthCare Pharmaceuticals; Rockville Pike, MD: 2016. Gadobutrol (Gadavist) injection for inravenous use package insert.https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=bd28560f-de4d-4140-874e-31df823f4dbb&type=display Wayne, NJ. Accessed July 8, 2019. [Google Scholar]
- 10.Dillman J.R., Ellis J.H., Cohan R.H., Strouse P.J., Jan S.C. Frequency and severity of acute allergic-like reactions to gadolinium-containing IV contrast media in children and adults. Am J Roentgenol. 2007;189:1533–1538. doi: 10.2214/AJR.07.2554. [DOI] [PubMed] [Google Scholar]
- 11.Davenport M.S., Bashir M.R., Pietryga J.A., Weber J.T., Khalatbari S., Hussain H.K. Dose-toxicity relationship of gadoxetate disodium and transient severe respiratory motion artifact. Am J Roentgenol. 2014;203:796–802. doi: 10.2214/AJR.13.11587. [DOI] [PubMed] [Google Scholar]