Abstract
The surgical treatment of locally advanced mediastinal tumors invading the great vessels and other nearby structures still represent a tricky question, principally due to the technical complexity of the resective phase, the contingent need to carry out viable vascular reconstructions and, therefore, the proper management of pathophysiologic issues. Published large-number series providing oncologic outcomes of patients who have undergone extended radical surgery for invasive mediastinal masses are just a few. Furthermore, the wide variety of different histologies included in some of these studies, as well as the heterogeneity of chemo and radiation therapies employed, did not allow for the development of clear oncologic guidelines. Usually in the past, surgical resections of large masses along with the neighbouring structures were not offered to patients because of related morbidity and mortality and limited information available on the prognostic advantage for long term. However, in the last decades, advances in surgical technique and perioperative management, as well as increased oncologic experience in this field, have allowed radical exeresis in selected patients with invasive tumors requiring resections extended to the surrounding structures and complex vascular reconstructions. Such aggressive surgical treatment has been proposed in association or not with adjuvant chemo- or radiotherapy regimens, achieving encouraging oncologic results with limited morbidity and mortality in experienced institutions. Congestive heart failure or impending cardiovascular collapse due to the compression by the large mass are the most frequent immediately life-threatening problems that some of these patients can experience. In this setting, medical palliation is usually ineffective and an aggressive salvage surgical treatment may remain the only therapeutic option.
Keywords: Mediastinal surgery, superior vena cava replacement (SVC replacement), aorta resection
Introduction
Neoplasms originating in the anterior mediastinum usually include thymoma comprising thymic carcinoma, germ cell tumors, lymphoma, and metastatic tumors (1). More than 80% of patients with advanced mediastinal malignancies present with symptoms that may be due to compression or direct invasion of the neighboring structures or that may be related to paraneoplastic syndromes. Some of these tumors, because of their aggressive behavior, may extend to adjacent organs; further technical issues may be addressed concerning the resection and the choice of the surgical approach (2,3).
In the presence of invasive masses of the anterior mediastinum, surgery can be indicated for radical resection, more frequently as a part of a multimodality treatment; a curative surgical resection sometimes cannot be accomplished because anterior mediastinal tumor (usually not symptomatic in the early stages) at the time of diagnosis already invade extensively the surrounding organs, including major blood vessels, lung parenchyma and pericardium (3).
Because of related morbidity and mortality and limited experience available on the prognostic advantage for long term, usually in the past, surgical resections of large masses along with the neighboring structures were not offered to patients. Nevertheless, advances in surgical technique and perioperative management obtained in the last decades, as well as increased oncologic experience in this field, have allowed radical exeresis of invasive tumors requiring resections extended to the surrounding structures and complex vascular reconstructions in selected patients (4-6). Such aggressive surgical treatment has been proposed in association or not with adjuvant chemo- or radiotherapy regimens, achieving encouraging oncologic results with limited morbidity and mortality in experienced surgical institution (7-9). Moreover, some of these patients present with immediately life-threatening problems such as congestive heart failure or impending cardiovascular collapse due to the large mass compression. An aggressive surgical treatment may remain the only option, especially for chemoresistant germ-cell tumors, since medical palliation is usually ineffective in this setting. Thus, salvage surgery is offered in selected patients with advanced mediastinal tumors, results from few limited series have been reported; fewer reviews on this topic have been published. We hereby present the most recent relevant experiences about this.
Superior vena cava (SVC)
The resection and reconstruction of the SVC is more frequently required when anterior mediastinal tumors that infiltrate the surrounding vascular structures are amenable for radical en bloc exeresis
A partial SVC resection and vascular repair either by direct suture or by interposition of a patch, usually of biologic material (pericardium), can be accomplished if the vessel circumference is infiltrated for less than 30%. A complete resection of the vessel with prosthetic replacement is required if a larger circumferential involvement is present. SVC resection and reconstruction represent a major technical challenge, especially for the potential negative effect of clamping a patent vessel (10); a partial caval clamping or clamping of chronically obstructed SVC is usually well tolerated, while a complete clamping of a patent SVC could lead to a marked hemodynamic imbalance.
A SVC conduit replacement can be performed if the confluence of both the innominate veins is not infiltrated. After clamping and vascular resection, the anastomosis between the superior caval stump and the prosthetic conduit is performed first with 5-0 polypropylene suture. In order to avoid the kinking of the conduit, the length of the prosthesis must be meticulously adapted so that the distal anastomosis could be under tension. When the junction between the origin of the SVC and the confluence with the innominate veins is infiltrated by the tumour, the revascularization is usually performed between the left innominate vein and the inferior SVC stump (or the right atrium) with closure of the right innominate vein, or alternatively between the right innominate vein and the inferior SVC stump (or the right atrium) with closure of the left innominate vein, according to the local invasion. Reconstruction from the right innominate vein usually provide a lower risk of prosthetic kinking; in fact, the residual venous stump is shorter and the direction of the graft is almost vertical. Some authors report the revascularization of both the innominate veins implanted independently at the right atrium. The current author believes that it should be avoided because the blood flow through the graft is too low and exposes at high risk of thrombosis. Total resections of the SVC and both the innominate veins and reconstruction with a Y-shaped synthetic prosthesis (Dacron or PTFE) have been successfully realized and reported in literature (11).
Aorta and epiaortic vessels
Some large mediastinal masses on the left side can infiltrate the arch or the descending aorta, while some other tumors can infiltrate the ascending aorta, carotid (Figures 1-3) and subclavian artery from the super anterior mediastinum. Sometimes, large tumors even with radiologically extensive aortic involvement present intraoperatively with a limited aortic adventitia infiltration that requires eventually cross-clamping but certainly not cardiopulmonary bypass (CPB) for resection. Otherwise, CPB allows safe resection when, in selected cases, a conduit reconstruction is required (12) (Figure 1B, Figure 3B,C) Dacron is generally preferred among materials for reconstruction for stiffness and resistance at high-pressure blood flow.
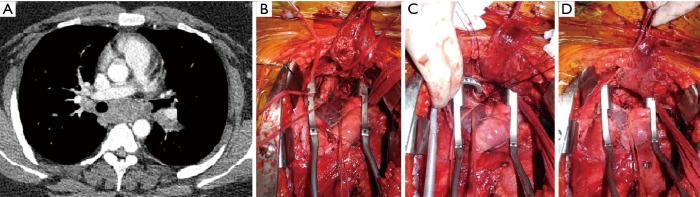
Figure 1.
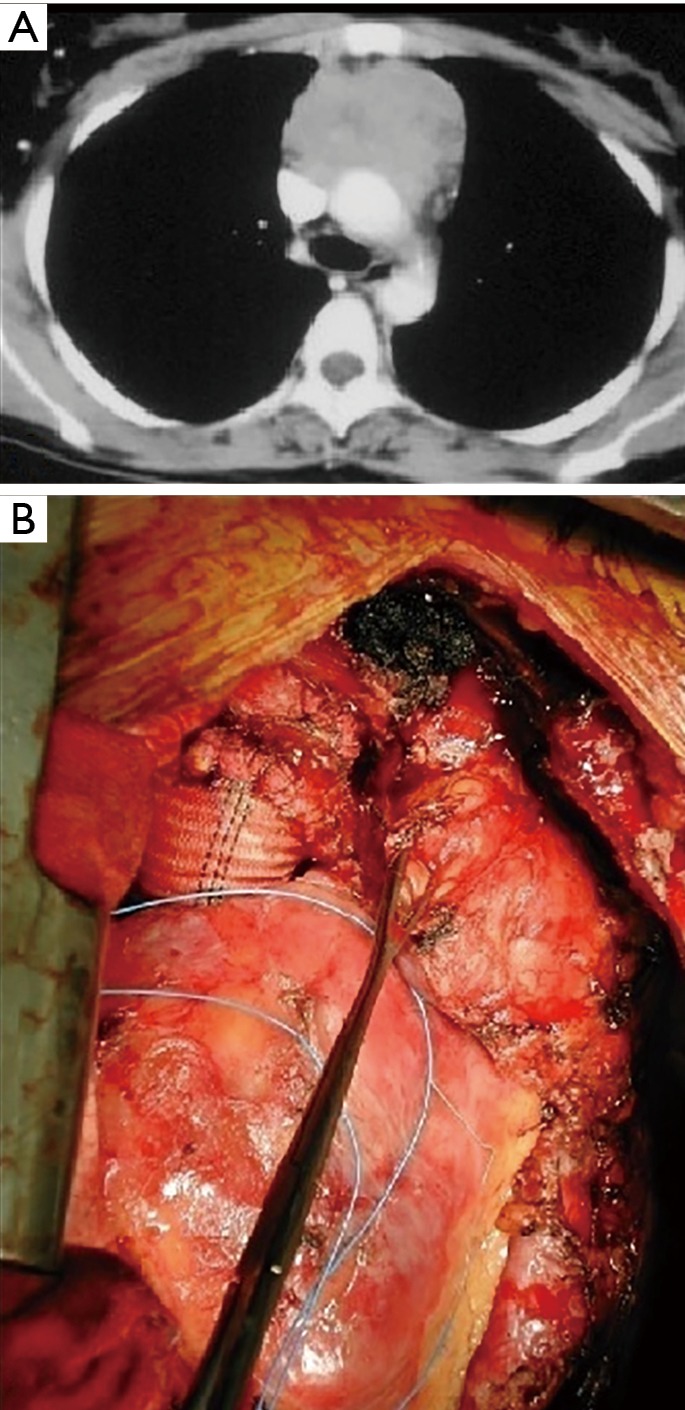
Anterior mediastinal mass infiltrating the aorta. (A) CT scan image illustrating extensive infiltration of the vessel; (B) intraoperative picture: the tumor has been resected and an aorta Dacron conduit reconstruction has been performed.
Figure 2.
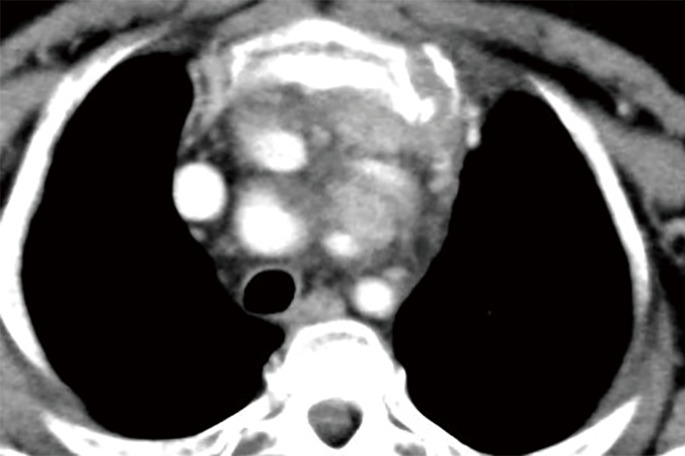
CT scan image showing anterior mediastinal tumor invading the carotid artery.
Figure 3.
Intraoperative picture showing a carotid artery prosthesis reconstruction for anterior superior mediastinal tumor. (A) Carotid artery is infiltrated by the super anterior mediastinal tumor; (B) the tumor has been resected en-bloc with carotid artery circumferential portion; (C) reconstructed carotid artery (dacron).
Materials for vascular reconstruction
Various biologic (autologous or heterologous) or synthetic materials have been proposed for these vascular reconstructions.
For reconstruction of low-pressure thoracic vessels, biologic materials, such as autologous or bovine pericardium, saphenous vein and azygos vein have gained large acceptance because of improved biocompatibility, lower risk of infection and thrombosis, and lower costs than synthetic materials (13-15).
Among the biologic materials, the bovine pericardium is currently the most used option. Its characteristics, such as stiff edges and the limited tendency to retract, facilitating its adaptation and suturing to the vascular wall, are an advantage. However, in the patch reconstructive procedures it has lower diffusion with respect to the autologous pericardium because of inferior biocompatibility and higher costs (16). Conversely, when total SVC replacement is required, bovine pericardium is preferred because the autologous tissue is generally not sufficient to create a long conduit. The pericardial conduit that has lower risk of thrombosis than synthetic materials does not necessitate long-term anticoagulation (15,17). The current authors presented an original technique for the intraoperative construction of a heterologous pericardial conduit to be used for SVC replacement (18).
Autologous venous grafts (i.e., saphenous) have a limited diameter that is sufficient only for the reconstruction of the brachiocephalic vein and is not suitable for SVC replacement. Saphenous vein graft of adequate diameter has been created by suturing the venous wall in a spiral fashion around a stent or a chest tube of appropriate size (13). Among the synthetic materials employed for graft reconstruction of the SVC (Dacron, PTFE, Gore-tex), the PTFE is the option of choice. It is the synthetic material showing the highest patency rate at long term and, shortly after its implantation, it becomes re-epithelialized with autogenous epithelial cells in humans (11,19). PTFE grafts (that are usually reinforced with external rings) have low risk of infection, less platelet deposition, and less thrombogenicity of the flow surface if compared with Dacron grafts.
For high-pressure vessels reconstruction such as aorta or carotid and subclavian artery, the risk of thrombosis is lower than in case of SVC reconstruction. In these conditions, the main issue that should be addressed is to obtain an adequate resistance of the reconstructed portion at the high-pressure flow. Available autologous or heterologous biologic materials (with exception of cadaveric graft) are not sufficiently responding to this requirement. Therefore, synthetic materials for vascular reconstruction are preferred. The current author believes that, among synthetic materials, the polyester such as Dacron are the suitable choice for aorta and epiaortic trunks prosthetic replacement.
Carina
Carinal resection for direct infiltration of an advanced mediastinal tumor is definitely even rarer than mediastinal great vessels resection. Carina is generally infiltrated by primary or recurrent neoplasms like lymphoma or germ-cell tumor (Figure 4). In this condition, where the carina is generally involved in the midline, the surgical approaches that should be preferred are median sternotomy or clamshell incision. These approaches provide wide exposure of the surgical field and facilitate the resection and reconstruction of the carina especially when the tumor extends towards the left side (Figure 4B).
Figure 4.
Recurrence of teratoma infiltrating the carina. (A) CT scan image; (B) median sternotomy had been performed and mediastinum is exposed; (C) left carina resection has been performed; stiches between trachea and right main bronchus are in place but still untied; (D) trachea-bronchial reconstruction is complete.
Some authors suggest the use of CPB/ECMO instead of cross-field ventilation with the aim to simplify the reconstruction phase and to improve results of surgery reducing the operative time (20,21). The current authors’ choice is for the cross-field ventilation, due to the potential increase of morbidity related to CPB/ECMO (4,17).
Airway reconstruction is performed with absorbable material, and we suggest the use of 3-0 monofilament (PDS) in an interrupted-sutures fashion to facilitate the adjustment of caliber discrepancy between the tracheal/bronchial stumps (22) (Figure 4B,C).
Anyway, the reports of these carinal reconstructive operations for resection of mediastinal tumors are quite anecdotal.
Comments
The surgical treatment of locally advanced mediastinal tumors invading the great vessels and other nearby structures still represent a tricky question, principally due to the technical complexity of the resective phase, the contingent need to carry out viable vascular reconstructions and, therefore, the proper management of pathophysiologic issues.
New technologies as 3D reconstruction or printing can be fascinating and useful for planning the operation, nevertheless these technologies even helpful are not conclusive and only intraoperative findings are reliable to establish the real infiltration of the organs; this even more true after neoadjuvant chemotherapy, when the scar tissue could be indistinguishable from neoplastic residual tissue.
Published large-number series providing oncologic outcomes of patients who have undergone extended radical surgery for invasive mediastinal masses are just a few (5,7,8,11,23-25). Furthermore, the wide variety of different histologies included in some of these studies (5,7,11,23), as well as the heterogeneity of chemo and radiation therapies employed (7,8), did not allow for the development of clear oncologic guidelines.
On the other hand, the implementation over time of the oncologic treatments that now include immunotherapy could represent a great chance for patients with advanced mediastinal tumours. Novel oncologic regimens may reduce the neoplastic tissue increasing the rate of patients receiving an “adjuvant” R0 resection. However, when considering patients affected by advanced mediastinal tumours that involve the surrounding vascular structures with immediate life-treating problems due to the invasion or compression of the vessel, surgery should be taken into account as first-line treatment.
Bacha et al. (7), reported results from the Marie Lannelongue Hospital long-term experience of surgical resection of invasive primary mediastinal neoplasms. They included a total of 89 patients with different pathologic diagnosis: advanced thymoma (n=35), thymic carcinoma (n=12), germ cell tumors (n=17), lymphomas (n=16), neurogenic tumors (n=3), thyroid carcinoma (n=3), radiation induced sarcomas (n=2), and mediastinal mesothelioma (n=1). Seventy-four percent of these patients had a tumor located in the anterior mediastinum, so sternotomy was the preferred approach. In three cases of chemoresistant germ cell masses a clamshell incision was required. Concerning the surrounding infiltrated organs, pericardium was resected in most cases (73%). A concurrent pulmonary resection was required in 55% of patients; among these, 28 wedge resections, 16 lobectomies and 5 pneumonectomies. Impairment of the major vascular structures was reported in 40% of cases, including 21 SVC and 13 brachiocephalic veins involvement. In all cases of SVC resection, vascular reconstruction was performed by using a synthetic conduit (PTFE). Instead, patients in whom the infiltration was limited to a single innominate branch didn’t require any vascular replacement. Complete removal of the tumor (R0) was achieved in 79% of cases. Major complications occurred in 17% of patients, requiring reoperation in 4 cases (4.5%). Operative mortality was 6%. In patients who have undergone SVC replacement, the patency of the PTFE conduit at 3 months was preserved in all the cases except for one. One more graft occlusion was reported after 15 months in a patient who had inserted a central venous catheter. About the oncologic cares, adjuvant radiation treatments were administered in 85% of thymomas and in 58% of thymic carcinomas, while 67% of patients with a diagnosis of thymic carcinoma received postoperative chemotherapy. Fourteen of the patients with germ cell carcinoma (82%) underwent cisplatin-based chemotherapy regimen in the preoperative period; ten of these even received postoperative chemotherapy. Eight patients who had a preoperative diagnosis of lymphoma received chemotherapy followed by removal of the residual tumor. When the diagnosis of lymphoma was achieved under the operation, postoperative chemotherapy was administered. Five-year overall survival rate was 63%. When considering survival related to histology, it was 69% for thymomas (stage III + IV), 42% for thymic carcinomas, 48% for patients with a germ cell tumor and 83% for those with lymphoma at 5-year. Recurrence rate was higher in the thymic carcinoma group (75% at 5-year).
A paper from Chen and colleagues (26) focused on the surgical management of complex malignant anterior mediastinal tumors invading the SVC-innominate veins system. A total of 15 patients with heterogeneous histologies were enrolled, including 9 malignant thymomas and one case of each between thymic carcinoma, teratoma, embryonal carcinoma, Hodgkin’s lymphoma, non-Hodgkin’s lymphoma and mixed teratoma with thymoma. A median sternotomy was performed in all patients. In the event of limited invasion of the SVC or a brachio-cephalic vein, non-circumferential resection of the vascular wall was accomplished by a partial clamping of the vessel and then repaired through a running suture (n=7). Three further cases required the tailoring of a pericardial patch to repair the vascular defect; a complete caval clamping was performed in all of these patients. Rest of the patients underwent the complete resection of the SVC and both the brachio-cephalic veins at the confluence (n=3). Reconstruction was performed in 2 cases by the interposition of a Dacron conduit between the right innominate vein and the right atrium (with ligature of the left innominate), and by the use of a “Y” shaped Dacron graft in one case. Two patients in whom the vascular involvement was limited to the left brachio-cephalic vein, underwent the tumor resection and ligature of vein stumps without need for a vascular reconstruction. Major complication rate was 13%. No perioperative mortality was observed. Four patients (26%) received chemotherapy before surgery. At a median follow-up of 35-month all patients were alive except for one. Upon publication, disease-free survival ranged between 10 and 43 months.
Over the past few years, some attractive series proposed the use of CPB in the surgical treatment of anterior and middle mediastinum neoplasms, most of them invading the heart or major vessels, with the aim to secure the complex removal of locally advanced masses or to allow safe manipulation of the tumor during resection (4,27-29). On the subject, a study published in 2002 (4), retrospectively reviewed results of 19 patients with thoraco-mediastinal malignancies involving the heart or great vessels, or resulting in substantial cardiac compression, who had undergone resection under CPB at The University of Texas M.D. Anderson Cancer Center. Only 20% among the 19 cases had a diagnosis of primary mediastinal neoplasm. Median sternotomy and clamshell incision were the preferred approaches (68% and 15% of cases respectively). After resection of the tumor, over half of the patients had no need for any vascular reconstruction other than direct suture of the defect. The remaining patients (42%) required different kinds of reconstruction by the use of autologous and heterologous pericardial tissues or synthetic prosthesis, such as Dacron conduits and Gore-tex patches. Overall/whole morbidity rate was 58%, instead perioperative mortality was 11%. Radical resection (R0) of the tumor was achieved in 79% of cases. Median overall survival was 62.4±25.4 months. In general, overall survival was significantly improved in those patients who have undergone an R0 resection. On the same topic, in 2004 Park et al. published the experience from the Memorial Sloan-Kettering Cancer Center (23). Ten patients with different histotype of neoplasms invading great vessels or part of the cardiac chambers were included in this study. In 7 cases CPB was employed. Three patients had a tumor from the anterior mediastinum causing SVC obstruction, including one from each of advanced thymoma, malignant teratoma and synovial cell sarcoma. The stage IV thymoma required a hemi-clamshell approach, while sternotomy was used in the other two patients. SVC replacement was performed through the use of a ringed Gore-tex prosthesis. With regard to these three operations, CPB was required only in the one for teratoma. No perioperative mortality was recorded. No postoperative oncologic treatments were administered in the anterior mediastinum subset of patients, nevertheless, the one with malignant teratoma received neoadjuvant chemotherapy before surgery. Despite the non-complete (R1) resection margins, both patients with malignant teratoma and synovial sarcoma were still alive at the time of publication and after a follow-up period of 33 months and 69 months respectively. By contrast, the patient with thymoma who has accomplished an R0 excision of the tumor, died of disease after 29 months due to brain metastasis progression. Although the patient with thymoma received a radical (R0) resection, he died due to brain relapse of the disease after 29 months.
If you look only at patients with Masaoka stage III and IV thymic malignancies, one of the most powerful variables affecting prognosis is the achievement of a radical resection (8,30,31). Surgical debulking or incomplete resections (R1, R2) usually do not modify long-term outcomes (32). Nevertheless, due to the very extensive involvement of nearby structures, radical surgery for invasive thymic masses may not be praticable in 30% for 40% of cases. The largest published surgical experience from the Massachusetts General Hospital (30), which included 179 patients with epithelial thymic tumors, showed a higher rate of recurrence in patients with Masaoka stage III–IV tumors than in all other patients (stage III: 31%, stage IV: 45% vs. other stages: 2%).
In 2003, the present authors reported results of a multimodality approach of treatment in 45 stage III thymic tumors, including 11 patients with a diagnosis of thymic carcinoma (8). Patients presenting with SVC involvement, pericardial invasion or limited infiltration of the lung and mediastinal pleura (66.6%; n=30) were considered eligible and then were included for primary surgery. All the other patients (33.4%; n=15), whose tumors were considered not completely resectable due to the extensive invasion of the surrounding organs, received induction chemotherapy followed by resection of the residual tumor. Median sternotomy was the approach of choice in all cases. When a radical tumor resection was achieved, adjuvant chemotherapy and 40 Gy dosage of radiotherapy were administered in the postoperative period, whereas in case of incomplete resection the radiation dose was increased at 50 to 60 Gy. Eleven patients underwent vascular reconstruction through a bovine pericardial conduit interposition: in 9 cases, it was completed after resection of the SVC, in the remaining two cases, it followed resection of the left innominate vein. Radical resection was achieved in 82% of thymic carcinomas, whereas in patients with other histologies the R0 resection rate was 91%. Major complications occurred in 6.7% of cases. No operative mortality was observed. Ten-year overall actuarial survival rate was 78%, while the cumulative disease-free survival rate was 53%. Considering patients who had undergone a complete resection, 10-year survival rate was 80%, compared with 60% for patients with R1–R2 resections. In the group of patients receiving preoperative chemotherapy, 10-year survival rate was 90%, although it was 71% for patients undergoing primary surgery.
Our recent homogenous case series (25) including only patients undergoing SVC conduit replacement for thymoma or thymic carcinoma (n=27; 21 thymoma, 6 thymic carcinoma) reports results after vascular reconstruction always performed by the cross-clamping technique. Twelve patients had undergone previous oncology treatment. Vascular reconstruction was synthetic (PTFE) in 13 cases in the first study period and biological (n=14) more recently; with a 100% long-term patency after pericardial conduit reconstruction. Mortality and major complication rates were 7.4% and 11.1%, respectively. Long-term patency of Cumulative 3- and 5-year cancer-specific survival of both thymoma and thymic carcinoma patients (including 5 associated pneumonectomy) were 90.5% and 75.4%.
Petrella and colleagues (33) reported results from a series of 21 patients that, up to 2005, had undergone salvage resection of mediastinal tumors with different histologies including thymoma (n=8) and thymic carcinoma (n=3), synovial sarcoma, neuroendocrine mediastinal tumor, teratoblastoma, dysgerminoma, thyroid carcinoma and Hodgkin lymphoma. All the patients underwent surgery for persistent or recurrent tumor after previous local treatment with curative intent or exclusive chemotherapy for bulky tumor.
Extended vascular resection was required in 10 cases and in 1 under CBP. They reported mortality rate of 4.7% and a morbidity rate of 19%. At median follow-up of 30.6 months, cumulative survival that included all the histology types showed a 5-year survival of 56.6%; prognosis was better for thymic malignancies than for other (mediastinal tumor) histology (5-year survival: 87.5% vs. 26.7%). In conclusion, mediastinal salvage surgery should be taken into account as therapeutic option in selected cases because of good -short and long-term results.
Acknowledgments
We wish to thank Dr. Marta Silvi for data management and editorial work.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Duwe BV, Sterman DH, Musani AI. Tumors of the mediastinum. Chest 2005;128:2893-909. 10.1378/chest.128.4.2893 [DOI] [PubMed] [Google Scholar]
- 2.Aigner C, Hoda MA, Klepetko W. Combined cervicothoracic approaches for complex mediastinal masses. Thorac Surg Clin 2009;19:107-12. 10.1016/j.thorsurg.2008.09.011 [DOI] [PubMed] [Google Scholar]
- 3.D'Andrilli A, Venuta F, Rendina EA. Surgical approaches for invasive tumors of the anterior mediastinum. Thorac Surg Clin 2010;20:265-84. 10.1016/j.thorsurg.2010.02.002 [DOI] [PubMed] [Google Scholar]
- 4.Vaporciyan AA, Rice D, Correa AM, et al. Resection of advanced thoracic malignancies requiring cardiopulmonary bypass. Eur J Cardiothorac Surg 2002;22:47-52. 10.1016/S1010-7940(02)00204-X [DOI] [PubMed] [Google Scholar]
- 5.Gómez-Caro A, Martinez E, Rodríguez A, et al. Cryopreserved arterial allograft reconstruction after excision of thoracic malignancies. Ann Thorac Surg 2008;86:1753-61; discussion 1761. [DOI] [PubMed]
- 6.Tanaka Y, Hokka D, Ogawa H, et al. Surgery for malignant lesions of the chest which extensively involved the mediastinum, lung, and heart. Gen Thorac Cardiovasc Surg 2017;65:365-73. 10.1007/s11748-017-0782-0 [DOI] [PubMed] [Google Scholar]
- 7.Bacha EA, Chapelier AR, Macchiarini P, et al. Surgery for invasive primary mediastinal tumors. Ann Thorac Surg 1998;66:234-9. 10.1016/S0003-4975(98)00350-6 [DOI] [PubMed] [Google Scholar]
- 8.Venuta F, Rendina EA, Longo F, et al. Long-term outcome after multimodality treatment for stage III thymic tumors. Ann Thorac Surg 2003;76:1866-72; discussion 1872. [DOI] [PubMed]
- 9.Magois E, Guigay J, Blancard PS, et al. Multimodal treatment of thymic carcinoma: Report of nine cases. Lung Cancer 2008;59:126-32. 10.1016/j.lungcan.2007.05.016 [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Fajardo JA, Garcia-Yuste M, Florez S, et al. Hemodynamic and cerebral repercussions arising from surgical interruption of the superior vena cava. Experimental model. J Thorac Cardiovasc Surg 1994;107:1044-9. [PubMed] [Google Scholar]
- 11.Okereke IC, Kesler KA, Rieger KM, et al. Results of superior vena cava reconstruction with externally stented-polytetrafluoroethylene vascular prostheses. Ann Thorac Surg 2010;90:383-7. 10.1016/j.athoracsur.2010.04.004 [DOI] [PubMed] [Google Scholar]
- 12.Takahashi T, Suzuki K, Ito Y, et al. Aortic arch resection under temporary bypass grafting for advanced thymic cancer. Jpn J Thorac Cardiovasc Surg 2002;50:302-4. 10.1007/BF03032300 [DOI] [PubMed] [Google Scholar]
- 13.Chiu CJ, Terzis J, MacRae ML. Replacement of superior vena cava with spiral composite vein graft A versatile technique. Ann Thorac Surg 1974;17:555-60. 10.1016/S0003-4975(10)65697-4 [DOI] [PubMed] [Google Scholar]
- 14.Warren WH, Piccione WJ, Jr, Faber LP. As originally published in 1990: Superior vena caval reconstruction using autologous pericardium. Updated in 1998. Ann Thorac Surg 1998;66:291-2. 10.1016/S0003-4975(98)00324-5 [DOI] [PubMed] [Google Scholar]
- 15.Spaggiari L, Veronesi G, D’Aiuto M, et al. Superior vena cava reconstruction using heterologous pericardial tube after extended resection for lung cancer. Eur J Cardiothorac Surg 2004;26:649-51. 10.1016/j.ejcts.2004.05.021 [DOI] [PubMed] [Google Scholar]
- 16.D’Andrilli A, Ibrahim M, Venuta F, et al. Glutaraldehyde preserved autologous pericardium for patch reconstruction of the pulmonary artery and superior vena cava. Ann Thorac Surg 2005;80:357-8. 10.1016/j.athoracsur.2004.02.012 [DOI] [PubMed] [Google Scholar]
- 17.Ciccone AM, Venuta F, D’Andrilli A, et al. Long-term patency of the stapled bovine pericardial conduit for replacement of the superior vena cava. Eur J Cardiothorac Surg 2011;40:1487-91. [DOI] [PubMed] [Google Scholar]
- 18.D’Andrilli A, Ciccone AM, Ibrahim M, et al. A new technique for prosthetic reconstruction of the superior vena cava. J Thorac Cardiovasc Surg 2006;132:192-4. 10.1016/j.jtcvs.2006.03.021 [DOI] [PubMed] [Google Scholar]
- 19.Dartevelle PG, Chapelier AR, Pastorino U, et al. Long-term follow-up after prosthetic replacement of the superior vena cava combined with resection of mediastinal-pulmonary malignant tumors. J Thorac Cardiovasc Surg 1991;102:259-65. [PubMed] [Google Scholar]
- 20.Horita K, Itoh T, Furukawa K, et al. Carinal reconstruction under veno-venous bypass using a percutaneous cardiopulmonary bypass system. Thorac Cardiovasc Surg 1996;44:46-9. 10.1055/s-2007-1011982 [DOI] [PubMed] [Google Scholar]
- 21.Bellier J, Sage E, Gonin F, et al. Radical Carinal Resection for a Glomic Tumor. Ann Thorac Surg 2016;102:e143-5. 10.1016/j.athoracsur.2016.01.023 [DOI] [PubMed] [Google Scholar]
- 22.Rendina EA, Venuta F, Ciriaco P, et al. Bronchovascular sleeve resection. Technique, perioperative management, prevention, and treatment of complications. J Thorac Cardiovasc Surg 1993;106:73-9. [PubMed] [Google Scholar]
- 23.Park BJ, Bacchetta M, Bains MS, et al. Surgical management of thoracic malignancies invading the heart or great vessels. Ann Thorac Surg 2004;78:1024-30. 10.1016/j.athoracsur.2004.02.043 [DOI] [PubMed] [Google Scholar]
- 24.Sun Y, Gu C, Shi J, et al. Reconstruction of mediastinal vessels for invasive thymoma: a retrospective analysis of 25 cases. J Thorac Dis 2017;9:725-33. 10.21037/jtd.2017.03.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maurizi G, Poggi C, D'Andrilli A, et al. Superior Vena Cava Replacement for Thymic Malignancies. Ann Thorac Surg 2019;107:386-92. 10.1016/j.athoracsur.2018.08.060 [DOI] [PubMed] [Google Scholar]
- 26.Chen KN, Xu SF, Gu ZD, et al. Surgical treatment of complex malignant anterior mediastinal tumors invading the superior vena cava. World J Surg 2006;30:162-70. 10.1007/s00268-005-0009-x [DOI] [PubMed] [Google Scholar]
- 27.Zhang H, Miao Q, Liu J, et al. Complete resection of a mediastinal solitary extramedullary plasmacytoma and reconstruction of right pulmonary artery and superior vena cava. Ann Thorac Surg 2011;92:2244-6. 10.1016/j.athoracsur.2011.05.047 [DOI] [PubMed] [Google Scholar]
- 28.De Giacomo T, Patella M, Mazzesi G, et al. Successful resection of thymoma directly invading the right atrium under cardiopulmonary bypass. Eur J Cardiothorac Surg 2015;48:332-3. 10.1093/ejcts/ezu376 [DOI] [PubMed] [Google Scholar]
- 29.Turbendian H, Seastedt KP, Shavladze N, et al. Extended resection of sarcomas involving the mediastinum: a 15-year experience. Eur J Cardiothorac Surg 2016;49:829-34. 10.1093/ejcts/ezv222 [DOI] [PubMed] [Google Scholar]
- 30.Wright CD, Wain JC, Wong DR, et al. Predictors of recurrence in thymic tumors: importance of invasion, World Health Organization histology and size. J Thorac Cardiovasc Surg 2005;130:1413-21. 10.1016/j.jtcvs.2005.07.026 [DOI] [PubMed] [Google Scholar]
- 31.Moser B, Scharitzer M, Hacker S, et al. Thymomas and thymic carcinomas: prognostic factors and multimodal management. Thorac Cardiovasc Surg 2014;62:153-60. [DOI] [PubMed] [Google Scholar]
- 32.Wilkins KB, Sheikh E, Green R, et al. Clinical and pathologic predictors of survival in patients with thymoma. Ann Surg 1999;230:562-72; discussion 572-4. 10.1097/00000658-199910000-00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petrella F, Leo F, Veronesi G, et al. “Salvage” surgery for primary mediastinal malignancies: is it worthwhile? J Thorac Oncol 2008;3:53-8. 10.1097/JTO.0b013e31815e6d54 [DOI] [PubMed] [Google Scholar]