Summary
Recent advances in bioelectronics and neural engineering allowed the development of brain machine interfaces and neuroprostheses, capable of facilitating or recovering functionality in people with neurological disability. To realize energy-efficient and real-time capable devices, neuromorphic computing systems are envisaged as the core of next-generation systems for brain repair. We demonstrate here a real-time hardware neuromorphic prosthesis to restore bidirectional interactions between two neuronal populations, even when one is damaged or missing. We used in vitro modular cell cultures to mimic the mutual interaction between neuronal assemblies and created a focal lesion to functionally disconnect the two populations. Then, we employed our neuromorphic prosthesis for bidirectional bridging to artificially reconnect two disconnected neuronal modules and for hybrid bidirectional bridging to replace the activity of one module with a real-time hardware neuromorphic Spiking Neural Network. Our neuroprosthetic system opens avenues for the exploitation of neuromorphic-based devices in bioelectrical therapeutics for health care.
Subject Areas: Neuroscience, Systems Neuroscience, Computer Science, Evolvable Hardware, Electronic Materials
Graphical Abstract
Highlights
-
•
We developed a novel real-time neuromorphic system acting as a neuroprosthesis
-
•
The neuroprosthesis can bidirectionally reconnect two disconnected networks
-
•
The neuroprosthesis implements a real-time spiking neural network
-
•
The spiking network can successfully replace the activity of a neural population
Neuroscience; Systems Neuroscience; Computer Science; Evolvable Hardware; Electronic Materials
Introduction
One of the greatest challenges of modern neuroscience is to find reliable and sustainable treatments for the disabling effects caused by many chronic and incurable brain conditions. With the greatest impact carried by stroke (Feigin et al., 2017) and traumatic brain injury (Maas et al., 2017), brain disorders are among the leading causes of disabilities worldwide. Owing to recent advances in bioelectronics and in neural and neuromorphic engineering, direct interfacing of artificial circuits with large neuronal networks is possible to develop novel “neurobiohybrid” systems (such as neuroprostheses Vassanelli and Mahmud, 2016), which are envisaged as potentially interesting clinical applications for brain lesions (Broccard et al., 2017). In this paper, we introduce an innovative bioelectronic system acting as a neuroprosthesis, which, thanks to a neuromorphic real-time hardware interface, can re-establish the communication between two disconnected neuronal populations.
Neural interfaces are promising solutions for brain repair (Soekadar et al., 2015). Modern neural interfaces are mainly designed to restore lost motor functions in only one direction, i.e., from the brain to the body (Abdulkader et al., 2015) or from the body to the brain (Flesher et al., 2016). Additionally, recent neuroprosthetic developments have shown the enormous potential of neural interfaces to aid and accelerate functional recovery (Bouton et al., 2016, Rosin et al., 2011). However, a major obstacle in developing novel neuroprostheses for bidirectional communication with and within the brain is the complex nature of interactions among different brain areas, which in turn presents a challenge for the development of appropriate stimulation protocols as well as for testing such devices using in vivo models (Kohler et al., 2017).
Despite very recent technological progress (Jun et al., 2017, Steinmetz et al., 2018), in vivo models still have two main bottlenecks. The first bottleneck is the technical challenge to faithfully reproduce specific/focal network lesions (mainly due to their complexity) that the neuroprosthesis aims to treat, whereas the second is the difficulty in disentangling the actual effect of the adopted electrical therapy from the complex activity of a brain constantly processing sensory inputs and producing behavior. Therefore, since in vivo models exhibit inherent complexity and low controllability, using in vitro reduced neuronal systems to model precise and reproducible neuronal network lesions and test neuroprosthetic devices for their treatment may be advantageous. This approach is also justified by a growing recognition that in vitro testing of both research and medical devices can be more effective in terms of cost, time consumption, and ethical issues and much more reliable than in vivo testing (Myers et al., 2017).
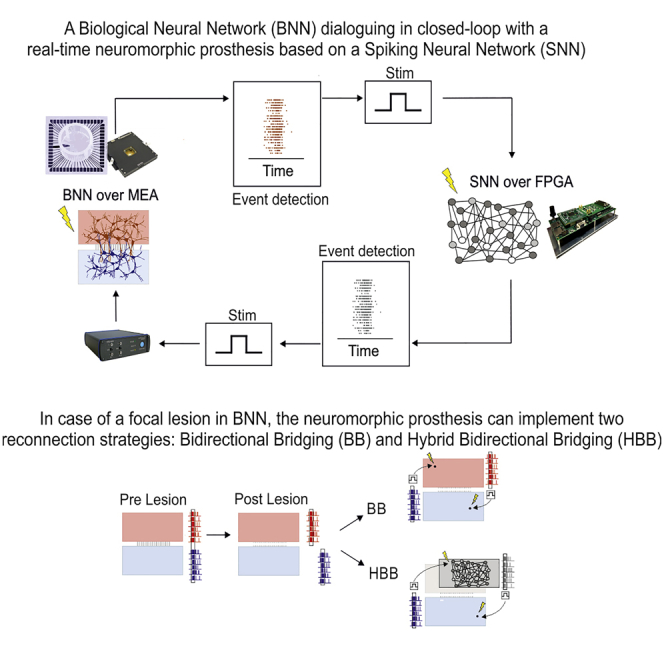
In this work, we bidirectionally interfaced in real-time a neuroprosthetic system with an in vitro culture constituted by interconnected “neuronal assemblies” (Hebb, 1949). Therefore, our first objective was to create a simplified yet plausible in vitro model of a focal brain lesion by using bimodular cultures grown onto micro-electrode arrays (MEAs) (Bisio et al., 2014, Bonifazi et al., 2013, Shein-Idelson et al., 2011) with reciprocal connections cut by a custom-made laser setup (Difato et al., 2011) to mimic the pathological effect of a traumatic brain injury (Hayes et al., 2016). We created a neurobiohybrid system connecting the biological element (the bimodular culture) following the lesion with a neuroprosthetic prototype. Our hardware neuroprosthesis could perform low-power computations in hard real-time (Pirog et al., 2018), collecting the inputs coming from neural recordings, processing those signals, and generating suitable electrical stimulation triggers as an output. With this experimental setup, we tested two specific applications, namely, bidirectional bridging (BB) to artificially reconnect two disconnected neuronal modules and hybrid bidirectional bridging (HBB) in which a real-time spiking neural network (SNN) replaced the activity of one of the two modules in real-time while implementing bidirectional connectivity with the remaining neuronal module.
The motivation of our research is to provide a new technological instrument as a novel form of neuroprosthesis aimed at treating disabling brain pathologies. The hardware choice (field-programmable gate array, FPGA) maximizes the real-time performances of the system and allows for a faster development of a future implantable bioelectronic device for biomedical applications. The adoption of bidirectional communication allows the development of a generalized non-specific approach that is applicable to the central nervous system (CNS) or peripheral nervous system. In particular, prostheses for the CNS should restore the communication between two or more neuronal assemblies whose functional and anatomical path could be distributed and sparse and not necessarily known a priori.
Indeed, our idea to develop a generalized approach comes from the future perspective of creating a cerebral neuroprosthesis for direct implantation in the brain that could be used by patients affected by stroke or brain injury. Our proof-of-principle results are the first for a next-generation neurobiohybrid system to restore brain functions (Broccard et al., 2017, Vassanelli and Mahmud, 2016).
Results
Neuroprosthetic Architecture
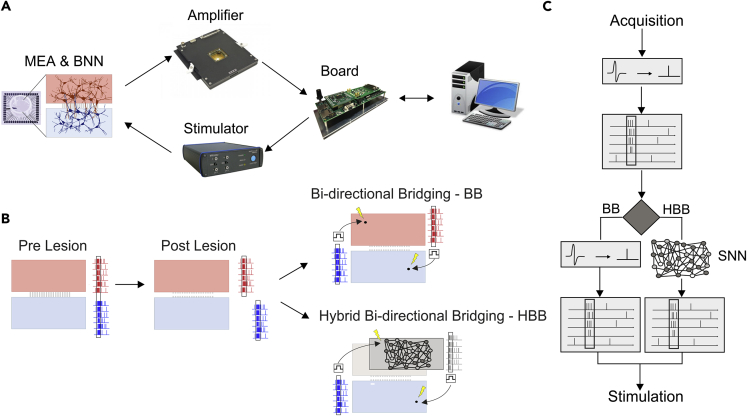
To create bimodular in vitro systems, we developed Polydimethylsiloxane (PDMS) masks with two connecting compartments that constrained the growth of neuronal cells in two precise areas over 60-electrode MEAs (Figure S1). The obtained bimodular neuronal culture constitutes the biological neuronal network (BNN) of our system (Figure 1A). The signal from the BNN was amplified by a commercial system and acquired by a custom-developed FPGA-based neuromorphic board (Figure S2) previously configured by a custom-made MATLAB code (MathWorks, Natick, MA, USA) running on a general-purpose personal computer. The neuromorphic board triggered a commercial stimulator to close the loop with the BNN. The general protocol designed for this study involved three steps. First, spontaneous activity in both neuronal modules was recorded (“pre-lesion condition”). Then, laser ablation of the biological connections between the two modules (see Video S1) was performed (see Transparent Methods), followed by recording of spontaneous activity in both modules (“post-lesion condition”) to assess the viability of the networks. Finally, we tested our neuroprosthetic device using two experimental frameworks. In the first case, we applied a reconnection strategy using a bidirectional activity-dependent stimulation (i.e., BB paradigm), whereas in the second case, we interfaced a hardware-implemented biomimetic SNN with one of the two neuronal modules (i.e., HBB paradigm) to simulate a “replacement” strategy that utilizes the bidirectional interaction between the biological system and its artificial counterpart (Figure 1B). The sequence of algorithms (e.g., spike detection, network burst detection) implemented on the board to realize both experimental approaches (BB or HBB) is schematically depicted in Figure 1C.
Figure 1.
Interfacing a Biological Neural Network and Neuromorphic Neuroprosthesis
(A) Schematic representation of the main elements of the setup: cartoon of an MEA coupled with a BNN; picture of the amplification system; picture of the custom FPGA board; picture of the stimulus generator. Out of the loop, we used a PC to configure the board.
(B) Schematic representation of the different phases of two experimental approaches that share a pre-lesion, a lesion (performed through laser ablation, not shown), and a post-lesion phase. The final experimental phase can be either bidirectional bridging (BB) or hybrid bidirectional bridging (HBB).
(C) Schematic of real-time data processing performed by the board: the first step is spike detection followed by network burst (NB) detection monitoring module 1. After NB detection, delivering stimulation to module 2 of the BNN (BB approach) or to the SNN is possible. In the second modality, there is also NB detection of the SNN, which can result in stimulation delivered to the BNN (HBB approach).
Video recorded during a laser ablation of the connections between two modules (visible on the left and right side) of a bimodular cell culture. Video recorded on November 16th 2016. Duration 18 s.
Control Experiments
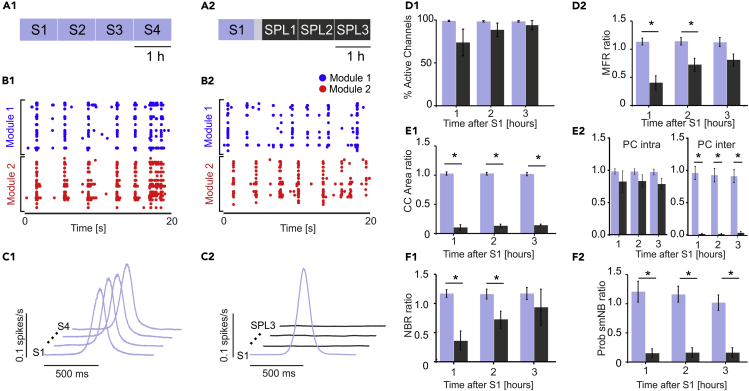
To evaluate the stability and effect of the focal lesion on bimodular BNN activity, we first performed two sets of control experiments. In the first set defined as “experiments with no lesion” (Figure 2A1), we recorded four consecutive hours of spontaneous activity (S1-S4, n = 9 cultures). In the second set defined as “experiments with a lesion” (Figure 2A2), we recorded 1 h of spontaneous activity (S1), followed by laser ablation of the connections between the two modules, which usually took less than 20 min. Next, we recorded 3 h of spontaneous activity post lesion (SPL1-SPL3) to quantify the effects of laser ablation (n = 4 cultures). As depicted in the raster plot of one representative experiment (Figure 2B1), bimodular neuronal networks exhibited spontaneous, synchronized, multi-unit activity composed of network-wide bursts (NBs) spreading over the two modules. Following laser ablation, the propagation between compartments was disrupted, as shown in Figure 2B2.
Figure 2.
A Laser Ablation Induced Lesion Can Disconnect Two Neuronal Modules
(A–F) (A1) Schematic of the first experimental protocol. Experiments with no lesion: we recorded four consecutive hours of spontaneous activity (S1–S4). (A2) Schematic of the second experimental protocol. Experiments with lesion: we recorded 1 h of spontaneous activity (S1) followed by laser ablation and three consecutive hours of spontaneous activity post lesion (SPL1-SPL3). The gray-shaded area indicates 20 min of no recording due to the execution of the lesion. (B1) A 20-s raster plot of the network bursting activity of one representative experiment during the S1 phase. (B2) A 20-s raster plot of the network bursting activity of one representative experiment during SPL3. (C1) Cross-correlation (CC) function for one representative experiment during the S1–S4 phases. The CC profiles between the spike trains of each module (light blue) in the four phases of the experiment were high and stable (lines shifted for the sake of clarity). Time axis [-500, +500] ms. (C2) CC profile between the spike trains of each module for one representative experiment with lesion. Before the lesion (light blue profile), CC was high; following the lesion (dark gray), CC collapsed to zero (lines shifted for the sake of clarity). Time axis [-500, +500] ms. (D1) Percentage of active channels with respect to S1 for the experiments with no lesions (light blue columns, n = 9) and with lesions (n = 4, dark gray columns). No significant difference was found using the Mann-Whitney test (S2 VS SPL1 p = 0.2042; S3 VS SPL2: p = 0.31608; S4 VS SPL3: p = 0.70769). (D2) Mean firing rate (MFR) ratio with respect to S1 for experiments without (light blue bars) and with lesions (dark gray bars). No significant difference was found during the last hour using the Mann-Whitney test (S2 vs SPL1: p = 0.0028; S3 vs SPL2: p = 0.01119; S4 vs SPL3: p = 0.10629). (E1) Comparison of the CC area ratio with respect to S1 for the experiments without (light blue bars) and with lesions (dark gray bars) (Mann-Whitney test; S2 vs SPL1: p = 0.0028; S3 vs SPL2: p = 0.0028; S4 vs SPL3: p = 0.0028). (E2) On the left, comparison of the intra-module correlation coefficient (i.e., Pearson Correlation, PC) ratio with respect to S1 for the experiments without (light blue bars) and with lesions (dark gray bars). No significant difference was found using the Mann-Whitney test (S2 VS SPL1 p = 0.71049; S3 VS SPL2: p = 0.14825; S4 VS SPL3: p = 0.07552). On the right, the same comparison regarding inter-module PC that showed clear differences between experiments without and with lesion (Mann-Whitney test; S2 vs SPL1: p = 0.0028; S3 vs SPL2: p = 0.0028; S4 vs SPL3: p = 0.0028). (F1) Network burst rate (NBR) ratio with respect to S1 showing significant differences during the first and second hour after the lesion (Mann-Whitney test; S2 vs SPL1: p = 0.0028; S3 vs SPL2: p = 0.01119; S4 vs SPL3: p = 0.26014). (F2) Probability of single-module NB (Prob smNB). The ratio with respect to S1 shows stability for experiments without (light blue bars) and with lesions (dark gray bars) (Mann-Whitney test; S2 vs SPL1: p = 0.0028; S3 vs SPL2: p = 0.0028; S4 vs SPL3: p = 0.0028). Data in the bar graphs is reported as mean ± standard error of the mean.
With no lesion, the percentage of active channels with respect to the first hour of recording (S1) was higher than 98% and was maintained for the entire duration of the experiment (Figure 2D1, light blue bars). The mean firing rate (MFR) was stable for all control experiments with no lesion (from S1 to S4, Figure S3A). Alternatively, control experiments with lesions showed a reduced number of active channels (close to 73%) during the first hour after ablation (SPL1). During the following 2 h (SPL2-SPL3), this value increased and reached 93% at the end of the recording (Figure 2C1, dark gray bars). No significant differences were found. The MFR was quite stable for all control experiments with lesions except between S1 and SPL1 (Figure S4A). The activity level with respect to the S1 phase, expressed by the MFR ratio with respect to S1, was stable during the control experiments without lesions (Figure 2D2, light blue bars). The lesion produced a clear decrease in activity in most cultures, especially during the first 2 h (SPL1-SPL2, Figure 2D2). We found a significant difference between the two experimental sets during the first two hours after S1 but not during the last hour. This result suggests that 2 h after a lesion, almost complete spontaneous recovery occurred in terms of the firing rate for the two neuronal modules.
To evaluate changes in the synchronicity between the two modules, we performed cross-correlation (CC) analysis between the collapsed spike trains of each module. The shape of the CC function was stable throughout the entire recordings in experiments without lesions, as reported in Figure 2C1 for a representative experiment. After a lesion, the CC function collapsed to zero and did not recover during the experiment (Figure 2C2: representative experiment). To quantify this difference, we integrated the CC function in a range of ±500 ms to obtain the CC area. We did not find any significant change in the CC area values for all experiments with no lesion (Figure S3B). By contrast, the CC area values showed a marked decrease following the lesion (SPL1). This decrease was due to the lack of anatomical connections between the compartments and did not recover by itself (Figure S4B). Comparing the CC area ratio between later phases and S1 resulted in a significant difference between matching periods in “lesion” and “no lesion” experiments (Figure 2E1). We also computed the correlation coefficient (i.e., Pearson Correlation, PC) among all the active channels both intra module and inter module (Figure 2E2). The intra-module PC was constant across experimental phases for controls with no lesion (light blue bars). On the other hand, for controls with lesion (dark gray bars) there was a drop in the intra-module PC, related to the reduced firing rate following the lesion, but no statistical difference was found (Figure 2E2, left panel). The inter-module CC was stable for controls with no lesion. On the other hand, following the lesion the inter-module PC collapsed and never recovered by itself (Figure 2E2, right panel) as already demonstrated with the previous analysis.
The network bursting rate (NBR) was stable during all experiments with no lesions (Figure S3C). For the experiments with lesions, this parameter was less stable but with no significant differences between phases (Figure S4C). When comparing the two experimental protocols with the NBR ratio with respect to S1, we found significant differences during the first and the second hour post lesion (Figure 2F1). The mean probability to have NBs composed of spikes belonging to a single module (i.e., Prob smNB, see Transparent Methods) was close to 0.2 in the experiments without lesions (Figure S3D), meaning that the majority of NBs in an intact bimodular network involved both modules. Alternatively, following the lesion, the probability became close to 1 (Figure S4D), meaning a total loss of functional communication between the two compartments. Using the Prob smNB ratio with respect to S1 (Figure 2F2), we found significant differences between the two experimental groups during all phases post lesion (Mann-Whitney test; p < 0.05). Thus, for the no lesion experiment, the Prob smNB remained very similar to the initial values, whereas for the lesion experiments, it changed abruptly due to the lesion. This result further confirmed that the lesion was effective in functionally disconnecting the two modules.
Experiment 1: Bidirectional Bridging
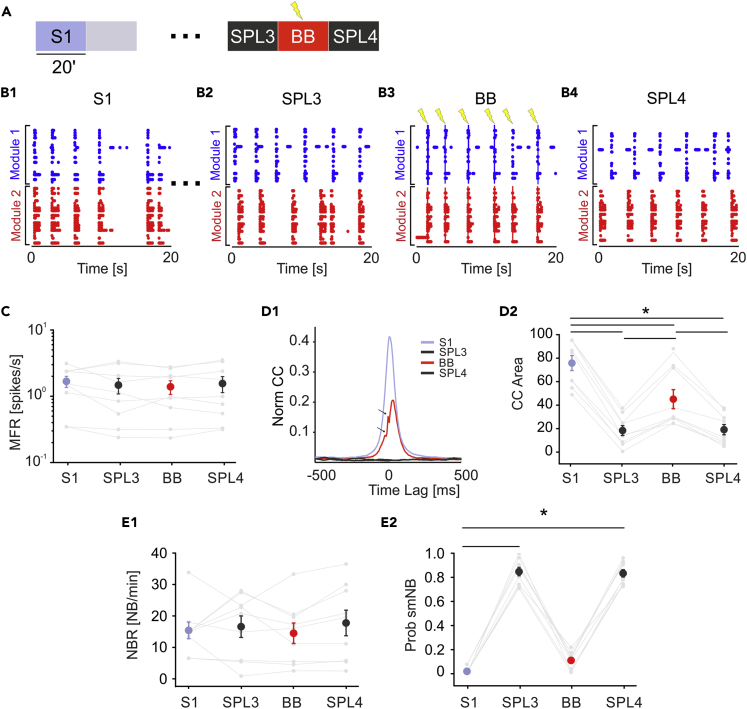
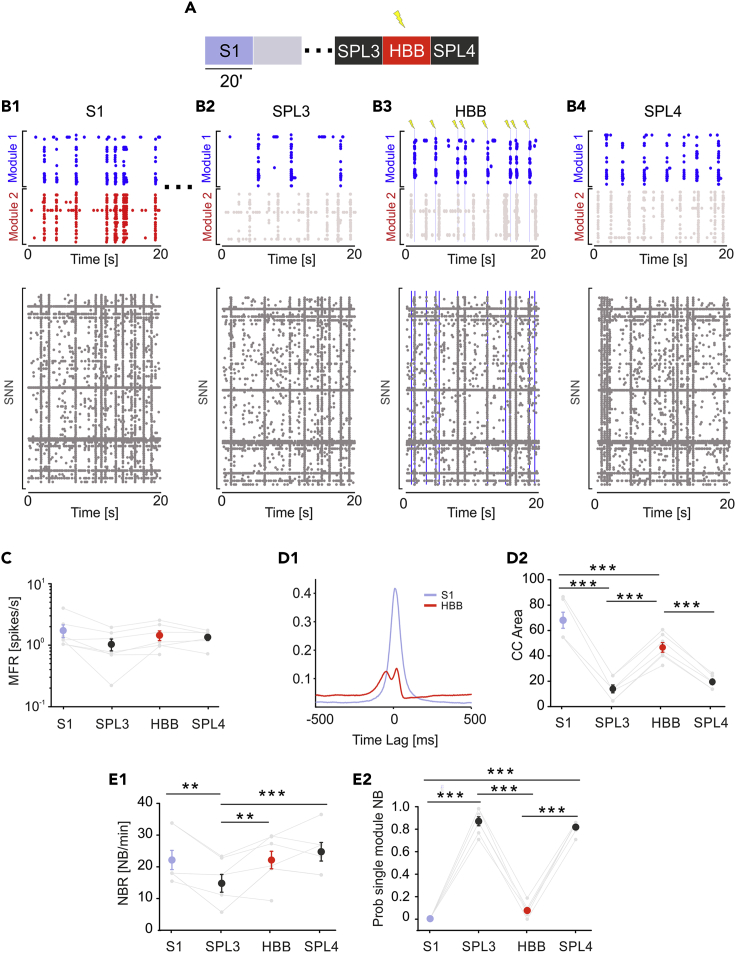
The goal of this experiment is to restore communication between two neuronal assemblies after lesion-induced separation. To achieve this goal, we designed and implemented a stimulation reactive paradigm inspired by the “activity-dependent stimulation” (ADS) described in Guggenmos et al. (2013) in our neuromorphic board. In contrast to the control experiments, the general protocol (Figure 3A) included 20-min recordings of spontaneous activity before the lesion (S1). Upon the lesion execution, we waited for 2 h to reach stable activity in both modules, as shown by the results of control experiments (see Figure 2). Then, we recorded 20 min of spontaneous activity (SPL3). The raster plot of a representative experiment is reported in Figure 3B. Before the lesion (S1), the bursting activity involved both modules (Figure 3B1), whereas after the lesion (SPL3), the activity was characterized by single-module NBs (Figure 3B2). To choose the best parameters (threshold and window time, see Transparent Methods) that allowed us to reliably detect NBs in both modules, we performed offline NB detection. After the FPGA was updated with these parameters, a 20-min session of BB was conducted (see Figures 1B and 1C for the description of the BB protocol). The BB approach implemented a reactive paradigm: every time an NB was detected in one module, a stimulation pulse was delivered to an electrode in the other module (see Transparent Methods) in both directions. During the BB phase, the bursting activity involved both modules similar to the intact condition due to the bidirectional stimulation pulses (Figure 3B3, blue and red lines represent electrical stimulation pulses delivered from module 1 to module 2 and vice versa). The last phase of the protocol involved 20 additional minutes of spontaneous activity (SPL4), which showed the same activity as SPL3 (Figure 3B4). We did not observe significant changes in spiking activity (i.e., MFR) throughout the recordings (Figure 3C).
Figure 3.
Bidirectional Bridging Is Effective in Reconnecting Functionally and Anatomically Disconnected Neuronal Modules
(A and B) (A) Schematic of the experimental protocol. We recorded 20 min of spontaneous activity (S1) followed by laser ablation. The gray-shaded area indicates 20 min of no recording during ablation. Dots represent 2 h of no recording after the lesion to maintain a stable activity in both modules. Then, we recorded 20 min of SPL activity (SPL3) followed by 20 min of the bidirectional bridging (BB) protocol and another 20 min of spontaneous activity (SPL4). (B1–B4) The 20-s-long raster plots of representative experiments (respectively, from phases S1, SPL1, BB, and SPL4). In (B3) blue and red lines represent electrical stimulation pulses delivered from module 1 to module 2 and vice versa, respectively.
(C–E) (C) MFR during the four experimental phases was stable (color code as in panel A: S1: light blue dot; SPL3, SPL4: dark gray dots; BB: red dot). No significant difference was found (one-way RM ANOVA, p = 0.469, DF = 3, F = 0.872). (D1) CC function during the four experimental phases. Small arrows indicate the blanking period of 8 ms following each stimulation. Color code, the same as that in panel A. Note that during BB, the cross-correlation function (red) recovers even if not completely with respect to the initial profile (light blue), whereas it stays at zero during the spontaneous activity phases post lesion (SPL3 and SPL4, dark gray profiles). (D2) CC area was highly reduced during the post-lesion phases. The CC area partially recovered during the BB protocol and collapsed again when stimulation was switched off (one-way repeated measures analysis of variance; degrees of freedom = 3; F = 101,832. S1 vs SPL3 p = 5.67 × 10−13; S1 vs SPL4 p = 7.54 × 10−13; S1 vs BB p = 1.60 × 10−7; BB vs SPL3 p = 1.77 × 10−6; BB vs SPL4 p = 2.81 × 10−6; SPL4 vs SPL3 p = 1). (E1) NBR remained stable during the experiments. No significant difference was found (one-way repeated measures analysis of variance: p = 0.501, DF = 3; F = 0.810). (E2) Probability of the single-module NB (Prob smNB) was close to one after the lesion. During the BB protocol, the probability was similar to the pre-lesion condition (Friedman's repeated measures analysis of variance; p < 0.001, DF = 3, Chi-square = 24.3. SPL4 vs S1 and SPL3 vs S1: p < 0.001). Data in the plots is reported as mean ± standard error of the mean.
Next, we evaluated the effect of this configuration in terms of CC (Figures 3D1 and 2). During spontaneous activity before the lesion (S1), the CC peak was high and stable due to the functional and anatomical connections between the two modules, which was also reported for the control experiments. After the lesion (SPL3), there was a decrease in CC that was not expected to recover without external intervention, as we demonstrated earlier (see Figure 2D). The bidirectional stimulation at least partially recovered the CC area and consequently the communication between modules (Figure 3D2), as demonstrated by statistical analysis. Regarding the number of NBs, we did not find any significant difference between the experimental phases (Figure 3E1). However, the probability of isolated NBs was not uniform; it reached the maximum value after the lesion, as we previously observed in the control experiments with lesions (see Figure 2D2). During bidirectional stimulation, these values became closer to the spontaneous recording (Figure 3E2), meaning that NBs mainly involved both modules. This finding further confirmed that the BB protocol could reconnect two disconnected modules through a real-time ADS acting in both directions (from module 1 to module 2 and from module 2 to module 1).
Experiment 2: Hybrid Bidirectional Bridging
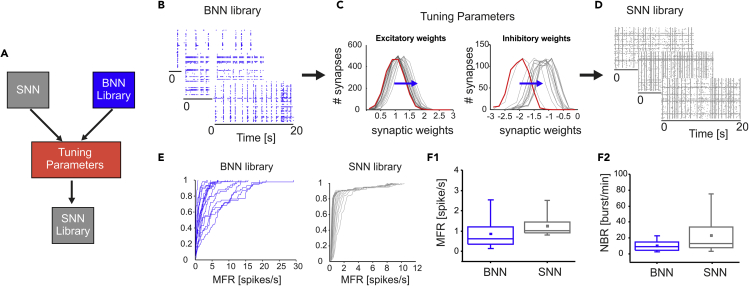
With an injury causing damage to an entire neuronal subnetwork, a reconnection strategy such as the BB illustrated earlier would not be feasible. For this reason, we developed a second reconnection strategy based on the use of a hardware SNN that can interact in real-time with its biological counterpart, HBB (see Figure 1). We created a set of SNNs (i.e., SNN library) by tuning the mean value of the synaptic weight distributions of our models to cover the variability of the BNNs (i.e., BNN library, Figure 4A). The biomimetic SNN (see Figure S5), working in hardware real-time to allow bidirectional communication with living neurons, was modeled as a network of 100 Izhikevich (IZH) neurons (Izhikevich, 2003), with 80 excitatory and 20 inhibitory neurons (see Transparent Methods), according to the biological composition of dissociated cultures (Bonifazi et al., 2005, Hayashi et al., 2003). Synaptic noise (Grassia et al., 2016), inhibitory and excitatory synapses (Izhikevich, 2004), short-term plasticity (Izhikevich and Edelman, 2008), and axonal delays were included in the model to recreate the network dynamics (see Transparent Methods and Figures S6A1 and S6A2). Regarding the connectivity rules, we set the outdegree (i.e., the number of post-synaptic neurons) to 25 for all neurons in the network, whereas the indegree (i.e., the number of pre-synaptic neurons) followed a normal distribution with a mean value of 25 and a standard deviation of 4.3 (Figure S6, R-Square = 0.806). The goal of creating an SNN library was to cover a wide range of NBRs because NB was chosen as the triggering event for our reconnection paradigm, as explained earlier. To this end, we tuned only the mean value of the normal distribution of synaptic weights (the standard deviation was kept constant at the value of 0.3). By increasing or decreasing the mean synaptic weights, we tuned the NBR. For excitatory synapses, the mean value ranged from 0.99 to 1.34 (Figure 4C left), whereas that for inhibitory synapses ranged from −2.02 to −1.02 (Figure 4C right and Table S1). As previously stated, our goal was to cover the NBR variability and not the MFR. The MFR variability in our BNN library was higher than that obtained with our SNN library (Figures 4E and 4F1). Nevertheless, the BNN variability in terms of NBR was completely covered by our SNN library, which also contains networks with a much higher NBR than that in the BNN library (Figures 4F2).
Figure 4.
Spiking Neural Network (SNN) Design and Characterization
(A) Schematic of the procedure used to create a library of SNNs. Starting from the Izhikevich model implemented on the FPGA and a library of 34 BNNs with a large spectrum of activity, we tuned the mean value of the synaptic weight distribution to obtain and select from a collection of SNNs (SNN library, comprising 27 different configurations).
(B) Representative 20-s-long raster plots of different BNNs showing different NB rates.
(C) Left, distribution of excitatory synaptic weights from the 27 SNNs. In red, the slower SNN of the library (SNN 1). The blue arrow indicates the shift of the mean value (from 0.99 to 1.34) of the normal distribution with standard deviation = 0.3 to obtain increasing NBR values. Right, distribution of inhibitory synaptic weights from the 27 SNNs. In red, the slower SNN of the library (SNN 1). The blue arrow indicates the shift of the mean value (from −2.02 to −1.02) of the normal distribution with standard deviation = 0.3 to obtain increasing NBR values.
(D) Representative 20-s-long raster plots of different SNNs showing different NB rates.
(E and F) (E) Left, cumulative MFR profile for the BNN library. Right, cumulative MFR profile for the SNN library. (F1) Box plot showing the comparison between BNN and SNN libraries in terms of network MFR (i.e., the mean value of all active electrodes for BNN and neurons for SNN). (F2) Box plot showing the comparison between BNN and SNN NBR, showing that the SNN library covers the BNN variability and contains networks with a higher NBR. For each box plot (F1- F2), the small square indicates the mean, the central line illustrates the median and the box limits indicate the 25th and 75th percentiles. Whiskers represent the 5th and the 95th percentiles.
The general HBB protocol (Figure 5A) is similar to the BB protocol. The HBB procedure included a 20-min recording of spontaneous activity before the lesion. This recording was used to quantify activity in terms of the NB rate of the network. This feature was used to choose one SNN from the SNN library that had an NB rate closer to its biological counterpart. We waited 2 h after the lesion to allow activity in both modules to stabilize, as shown by the results of control experiments (e.g., Figure 2). Then, we recorded 20 min of spontaneous activity. After setting FPGA detection parameters, we performed a 20-min HBB session (see Figures 1B and 1C for the description of the HBB protocol). As anticipated, the HBB approach also implemented an ADS paradigm; every time an NB was detected on the “surviving” module (i.e., when one of the two modules was completely damaged), a stimulation pulse was delivered to the SNN. The board implemented the corresponding paradigm in the opposite direction. Detection of NBs occurred in the SNN, whereas stimulation was delivered to the “surviving” module, thus avoiding the imposition of any predefined unidirectional communication. Next, we recorded 20 additional minutes of spontaneous activity.
Figure 5.
The Hybrid Bidirectional Bridging Approach Is Effective When a Neuronal Assembly Must be Replaced
(A and B) (A) Schematic of the experimental protocols. We recorded 20 min of spontaneous activity (S1) followed by laser ablation. The gray-shaded area indicates 20 min of no recording during ablation. Dots represent 2 h of no recording after the lesion to obtain stable activity in both modules and to test different stimulation channels. Then, we recorded 20 min of SPL activity (SPL3) followed by 20 min of a hybrid bidirectional bridging (HBB) protocol and another 20 min of spontaneous activity (SPL4). (B1) Top, 20-s-long raster plot depicting the BNN bursting activity involving both modules before lesion. Bottom, activity of SNN uncorrelated with the BNN. The networks are not linked. (B2) Top, 20-s-long raster plot after lesion showing uncorrelated bursting activity on BNN modules 1 and 2. Bottom, same as that in B1. (B3) Twenty-second-long raster plot during HBB depicting two hybrid events. The first event on the left was an NB detected on module 1 of the BNN. The detection resulted in a stimulation pulse delivered to 10 excitatory neurons of the SNN (blue line, bottom). An NB on the SNN was detected 18 ms after the stimulation and triggered the delivery of a stimulation pulse to module 1 of the BNN (gray line, top). (B4) Twenty-second-long raster plot depicting the uncorrelated activity of BNN modules (top) and SNN network (bottom).
(C–E) (C) MFR during the four experimental phases was stable (color code as in panel A: S1: light blue dot; SPL3, SPL4: dark gray dots; HBB: red dot). No significant difference was found (one-way repeated measures analysis of variance. p < 0.001, DF = 3, F = 3,16; Bonferroni test: all comparisons with p > 0.05). (D1) CC function during the four experimental phases. Color code the same as that in panel A. Note that during BB, the cross-correlation function (red) recovers even if not completely with respect to the initial profile (light blue). (D2) CC area was highly reduced during the post-lesion phases. The CC area partially recovered during the BB protocol and collapsed again when stimulation was switched off (one-way repeated measures analysis of variance. p < 0.001, DF = 3; F = 70,448; S1 vs SPL3: p = 5.80E-10; S1 vs SPL4 p = 2.72 × 10−9; S1 vs HBB: p = 9.16 × 10−4; HBB vs SPL3 p = 1.16 × 10−7; HBB vs SPL4 p = 1.02 × 10−6; SPL4 vs SPL3 p = 0.73643). (E1) NBR did not change during HBB with respect to the S1 phase (one-way repeated measures analysis of variance. p = 0.005, DF = 3; F = 6,069; S1 vs SPL3 p = 0.02482; S1 vs SPL4 p = 1; S1 vs HBB p = 1; HBB vs SPL3 p = 0.01022; HBB vs SPL4 p = 1; SPL4 vs SPL3 p = 0.00674). (E2) Probability of a single-module NB (Prob smNB) was close to one after the lesion. During the HBB protocol, the probability was similar to that in the pre-lesion condition (one-way repeated measures analysis of variance. p < 0.001, DF = 3; F = 453,439; S1 vs SPL3 p = 4.96 × 10−13; S1 vs SPL4 p = 1.03 × 10−12; S1 vs HBB p = 0.22606; HBB vs SPL3 p = 1.94 × 10−12; HBB vs SPL4 p = 4.30 × 10−12; SPL4 vs SPL3 p = 1). Data in the plots is reported as mean ± standard error of the mean.
We did not observe significant changes in terms of spiking activity (i.e., MFR) throughout the recordings (Figure 5C). Then, we evaluated the effect of this configuration in terms of CC (Figures 5D1 and 2). During spontaneous activity before the lesion (S1), the CC peak was high and stable due to the functional and anatomical connections between the two modules, which was also reported for the control experiments. As expected, with no external intervention, CC decreased sharply after the lesion (SPL3), as we previously observed. One of the two modules was damaged, whereas the correlation was evaluated between the SNN and the surviving module during the HBB phase. The bidirectional stimulation created a relevant correlation area between SNN and the surviving module, as demonstrated by statistical analysis. Regarding the number of NBs, we did not find a significant difference between the S1 and HBB phases (Figure 5E1). However, the probability of isolated NBs was not uniform; it reached the maximum value after the lesion, as we previously observed in the control experiments with lesions (see Figure 3E2). During the hybrid bidirectional stimulation, these values became closer to the spontaneous recording (Figure 5E2), meaning that NBs mainly involved both modules. This finding further confirmed that the HBB protocol created a hybrid system with the surviving biological module though real-time ADS acting in both directions (from BNN to SNN and from SNN to BNN).
Discussion
We presented an innovative neuromorphic prosthesis based on an FPGA board and demonstrated two successful reconnection paradigms for a lesion interrupting the communication between two neuronal populations in vitro.
According to previous reports, in vitro systems constitute a successful experimental model of neuronal dynamics (Javier et al., 2013, Johnson et al., 2010), thus providing an excellent test bed for adaptive closed-loop neural interfaces (Potter, 2010). Starting from our recently developed methodology (Kanner et al., 2015), we created custom bimodular cultures with the goal of reproducing two interacting neuronal populations, thus mimicking the intrinsic modularity of the brain (Bonifazi et al., 2013). Our bimodular cultures were highly temporally stable in terms of firing properties at the whole network level as the activity between the two populations remained highly correlated for the entire duration of the recording. A lesion produced via laser ablation was employed to physically cut the connections between the two modules. This methodology was proven to be safe because it produced localized damage by selectively ablating subcellular compartments without damaging adjacent structures (Difato et al., 2011, Habibey et al., 2015, Soloperto et al., 2016). We assume that such a focal damage, allowing to specifically cut few connections among those available in the network, together with possible intrinsic compensatory mechanisms of synaptic scaling (le Feber et al., 2017, Turrigiano, 2008), was responsible for the spontaneous recovery of the firing rate on a timescale of 2–3 h. This demonstrates the effectiveness of our technique in preserving the functionality of the two modules while decoupling their activity, as proven by the loss of correlation of bursting behavior.
Two different applications of our neuroprosthesis, BB and HBB, were tested. Our neuromorphic prosthesis, independently of the stimulation paradigm, works according to a closed-loop reactive policy as follows: each time a condition is met (i.e., an “event” is detected), a stimulus is delivered. The hardware architecture was designed to be flexible enough to allow the implementation of different experimental paradigms and the definition of different triggering events. In our study, we chose NBs as trigger events (see Transparent Methods). The choice to deliver a stimulation depending on a network-wide event has two main advantages as follows: first, NB frequency is low enough to avoid inducing plasticity phenomena by electrical stimulation in our cultures (Wagenaar et al., 2006), which could confound the final results and effectiveness of neuroprosthetic reconnection. The second point is anticipation of the following major issue that will emerge during in vivo recordings: monitoring single neurons presents problems at both theoretical (Guggenmos et al., 2013) and practical levels. Namely, how much information on complex functions can be obtained by single-neuron observation remains unclear (Luczak et al., 2015, Panzeri et al., 2017), whereas tracking the activity of the same neuron for extended periods of time is problematic (Kozai et al., 2015). Taking multiple input sources into account was also used in the work of Berger (Berger et al., 2012), but they employed a neuroprosthetic strategy different from ours. In particular, these authors used a generalized linear model to predict the CA1 activity from spikes in CA3 of the hippocampal circuit. Our system is considered more flexible and adaptable to networks with different connectivity, not just feedforward similar to that in the hippocampus. Moreover, we were interested in mimicking the overall spiking activity of the network and not mapping an input-output transformation only.
Another important novelty of our system regards directionality. To the best of our knowledge, this neuroprosthetic system is the first to implement a truly bidirectional interaction with an SNN through a hard real-time interface. We recorded activity from the first module (via multiple sources); when a criterion was met, the device stimulated the second module (this is how a “typical” closed-loop in neuroscience works; for a review see Greenwald et al., 2016). The novelty is simultaneous monitoring of multiple sources from another module and delivery of the stimulation when the triggering event is detected. To date and as far as we are aware of, only Jung and colleagues (Jung et al., 2001) have performed a bidirectional interface to a neuromorphic device, but their models were not precise at the spike level (modeling neuron populations) and they used non-configurable analogue electronics, which resulted in an experiment-specific setup. The other neuroprosthetic devices that have been proposed in the literature can implement a “unidirectional” artificial link only from one area to another (or maybe the same) but not double it. Here, we are not imposing any preferred directionality to the communication; networks are self-organizing on the basis of their intrinsic natural relationship (we are not imposing who is driving whom). This approach has the main advantage of informing both brain regions (i.e., neuronal modules, in our case) that an event occurred in the other region, given that interaction in the brain is intrinsically bidirectional (Roelfsema and Holtmaat, 2018). For example, in the sensorimotor system, sensory simulation can help motor recovery (Cuppone et al., 2018) and motor learning can enhance sensory functions (Ostry et al., 2010, Takeuchi and Izumi, 2013). Applications of our neuroprosthetic systems to conditions where the sensorimotor interaction is impaired would allow restoration of both communication channels, suggesting improvements in current rehabilitation therapies. Moreover, although tested on a bimodular system, the neuromorphic FPGA board can be easily upgraded to play the bridging role on an arbitrary number (within reason) of different neuronal circuits. A recent work (Forró et al., 2018) developed directional networks of primary hippocampal neurons on MEA and compared the information flow of these networks with respect to bidirectional networks (similar to our bimodular preparations). They found that, without physically imposing a unidirectional configuration, there is a continuous back and forth communication between nodes thus suggesting the importance of a bidirectional communication in a healthy network.
The second paradigm we tested was based on the use of a biomimetic SNN to “substitute” a missing/damaged neuronal population. Currently, SNN applications span different fields, including computational neuroscience (Markram, 2012, Melozzi et al., 2017), and very recently, they were used for sensory encoding in hand prosthesis for amputees (Osborn et al., 2018, Valle et al., 2018). SNNs can be simulated in software (Goodman and Brette, 2009) and/or neuromorphic hardware (Thakur et al., 2018). As time and energy consumption are fundamental in neuroprosthetic applications for translational purposes, the use of hardware-based computing systems becomes mandatory.
In general, hybrid systems composed of in vitro BNNs coupled to SNNs are rare. In one approach, the SNN served as a self-organizing classifier of activity patterns exhibited by the BNN, with output of the SNN being subsequently used to control the behavior of a robot (Pizzi et al., 2009). Other studies focused on the unidirectional or bidirectional influence of the two networks, investigating the dynamics of the interaction between the BNN and SNN in which the SNN played a role of an artificial counterpart of its biological original (Bruzzone et al., 2015, Chou et al., 2015). However, closed-loop effects in those hybrid networks were not thoroughly determined. In one of these studies, only unidirectional connectivity was considered with input from the SNN to the BNN, which was also simulated beforehand (Bruzzone et al., 2015). In this study, we established hybrid communication in the case of an entire neuronal population that needed substitution.
A study by Chou et al. (2015) implemented a bidirectional interface between an SNN and a retinal slice obtained from an adult rat and recorded by an MEA. This system is quite interesting, but there is a 1-s delay between the BNN and SNN interactions. Therefore, this delay in Chou's setup is three orders of magnitude larger than that in our work in which the sampling of biological activity is never interrupted and the step size of the SNN is 1 ms. The difference between the two systems is radical; bidirectional communication in real-time allows actual clinical application, whereas delays in the range of seconds prevent (or at least seriously reduce) the possibility of meaningful control of a biological system.
A recent study, inspired by a previous work (Hogri et al., 2015), implemented a hybrid interaction (Xu et al., 2018) between the cerebellum of a rat and an SNN implemented on FPGA. Their model involved 10k leaky integrate and fire (LIF) neurons and did not integrate other biomimetic behaviors, such as axonal delay, short-term plasticity, and synaptic noise, unlike the IZH neurons implemented in our system. Both the hard real-time processing and simplified neuronal model (which allow mimicking the richness of the electrophysiological patterns in vivo) are mandatory for reproducing the biological dynamics of living neural networks and for performing useful real-time hybrid experiments.
In this work, we demonstrated the possibility of designing a neuromorphic all-hardware prosthesis capable of artificially reconnecting two disconnected neuronal networks or artificially replacing one entire neuronal sub-network.
The use of a fully integrated hardware computing system allowed hard real-time performances and low power consumption, which are crucial for translational purposes related to therapeutic applications in humans (Kipke et al., 2008, Wang et al., 2010).
Limitations of the Study
A limitation of the present work is that we deliberately chose to downsample both the number of biological neurons recorded through a low-density MEA and the number of artificial neurons implemented on the FPGA. For the purpose of detecting network-wide activity, this oversimplification of biological complexity can be acceptable to test the functionality of the device and the feasibility of the approach, but if the goal is to functionally replace a biological network, a higher resolution would be preferable. It is worth underlining that this hardware implementation is not exploiting the full resources of the FPGA, thus in follow-up studies, also thanks to the flexibility of our system, we foresee to scale up the number of neurons and synapses and to upgrade the computational algorithms to deal with more complex experimental designs. From a technological point of view, the current state of the art makes possible the use of devices with a large number (thousands) of recording electrodes (Berdondini et al., 2009, Frey et al., 2009). Such an improvement would also allow one to have more information about functional connectivity of the biological network (Pastore et al., 2018) and thus develop more realistic, in terms of topology, artificial models. In this work, we arbitrarily modeled the network connectivity with a random adjacency matrix since the use of MEAs with 60 electrodes made impossible to correctly identify the topological properties (e.g., hubs, recurring connections, modules) of the network under investigation.
We are aware that the road is still long to target human applications. Despite this, we think that the extensive work performed represent an important milestone to start from. The next fundamental (and critical) step would be to test the neuromorphic prosthesis in vivo, for example, on animal models affected by ischemic or traumatic lesions (Guggenmos et al., 2013). Even if the adaptation to the new experimental setups will require time, we believe these are necessary steps to further push the translational potential of our system, which will be able to create real innovation in the clinical therapeutics.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
The presented research results have received funding from the European Union's Seventh Framework Programme (ICT-FET FP7/2007-2013, FET Young Explorers scheme) under grant agreement n° 284772 BRAIN BOW (www.brainbowproject.eu).
The authors would like to thank Dr Daisuke Ito, Dr Marina Nanni, Dr Claudia Chiabrera, and Dr Giacomo Pruzzo for precious technical support in performing the in vitro experiments at IIT. The authors are grateful to Dr Marianna Semprini for useful comments on the final drafts of the manuscript. The authors wish to thank Prof. Sergio Martinoia, Prof. Sylvie Renaud, Prof. Sylvain Saighi, and Prof. Ari Barzilai for their mentorship during the BrainBow project and for useful discussions on the final results.
Author Contributions
T.L., Y.B., P.M., P.B., and M. C. designed the study. Y.B. and T.L. designed and fabricated the hardware board. S.B., V.P., F.D., and M.C. designed the experiments. M.A., P.M., P.N., F.G., and T.L. designed and worked on SNN. I.C., M.B., and M.T. prepared the bimodular cultures. S.B., L.M., A.A., and F.D. performed the experiments. S.B., L.M., J.T., V.P., and M.C. designed the analyses. S.B. and L.M. performed the analyses. S.B., I.C., and V.P. performed the statistical analyses. S.B. and I.C. prepared the original figures. S.B., I.C., T.L., and M.C. wrote the manuscript. T.L., P.M., P.B., and M.C. acquired funding to conduct the research. All authors have read, corrected, and approved the final version of the manuscript.
Declaration of Interests
The authors report no competing interests.
Published: September 27, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.07.046.
Contributor Information
Timothée Levi, Email: timothee.levi@u-bordeaux.fr.
Michela Chiappalone, Email: michela.chiappalone@iit.it.
Supplemental Information
References
- Abdulkader S.N., Atia A., Mostafa M.-S.M. Brain computer interfacing: applications and challenges. Egypt. Inform. J. 2015;16:213–230. [Google Scholar]
- Berdondini L., Imfeld K., Maccione A., Tedesco M., Neukom S., Koudelka-Hep M., Martinoia S. Active pixel sensor array for high spatio-temporal resolution electrophysiological recordings from single cell to large scale neuronal networks. Lab Chip. 2009;9:2644–2651. doi: 10.1039/b907394a. [DOI] [PubMed] [Google Scholar]
- Berger T.W., Song D., Chan R.H., Marmarelis V.Z., LaCoss J., Wills J., Hampson R.E., Deadwyler S.A., Granacki J.J. A hippocampal cognitive prosthesis: multi-input, multi-output nonlinear modeling and VLSI implementation. IEEE Trans. Neural Syst. Rehabil. Eng. 2012;20:198–211. doi: 10.1109/TNSRE.2012.2189133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisio M., Bosca A., Pasquale V., Berdondini L., Chiappalone M. Emergence of bursting activity in connected neuronal sub-populations. PLoS One. 2014;9:e107400. doi: 10.1371/journal.pone.0107400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifazi P., Difato F., Massobrio P., Breschi G.L., Pasquale V., Levi T., Goldin M., Bornat Y., Tedesco M., Bisio M. In vitro large-scale experimental and theoretical studies for the realization of bi-directional brain-prostheses. Front. Neural Circuits. 2013;7:40. doi: 10.3389/fncir.2013.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifazi P., Ruaro M.E., Torre V. Statistical properties of information processing in neuronal networks. Eur. J. Neurosci. 2005;22:2953–2964. doi: 10.1111/j.1460-9568.2005.04464.x. [DOI] [PubMed] [Google Scholar]
- Bouton C.E., Shaikhouni A., Annetta N.V., Bockbrader M.A., Friedenberg D.A., Nielson D.M., Sharma G., Sederberg P.B., Glenn B.C., Mysiw W.J. Restoring cortical control of functional movement in a human with quadriplegia. Nature. 2016;533:247–250. doi: 10.1038/nature17435. [DOI] [PubMed] [Google Scholar]
- Broccard F.D., Joshi S., Wang J., Cauwenberghs G. Neuromorphic neural interfaces: from neurophysiological inspiration to biohybrid coupling with nervous systems. J. Neural Eng. 2017;14:041002. doi: 10.1088/1741-2552/aa67a9. [DOI] [PubMed] [Google Scholar]
- Bruzzone A., Pasquale V., Nowak P., Tessadori J., Massobrio P., Chiappalone M. Interfacing in silico and in vitro neuronal networks. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2015;2015:3391–3394. doi: 10.1109/EMBC.2015.7319120. [DOI] [PubMed] [Google Scholar]
- Chou Z., Lim J., Brown S., Keller M., Bugbee J., Broccard F., Khraiche M.L., Silva G.A., Cauwenberghs G. Bidirectional neural interface: closed-loop feedback control for hybrid neural systems. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2015;2015:3949–3952. doi: 10.1109/EMBC.2015.7319258. [DOI] [PubMed] [Google Scholar]
- Cuppone A.V., Semprini M., Konczak J. Consolidation of human somatosensory memory during motor learning. Behav. Brain Res. 2018;347:184–192. doi: 10.1016/j.bbr.2018.03.013. [DOI] [PubMed] [Google Scholar]
- Difato F., Dal Maschio M., Marconi E., Ronzitti G., Maccione A., Fellin T., Berdondini L., Chieregatti E., Benfenati F., Blau A. Combined optical tweezers and laser dissector for controlled ablation of functional connections in neural networks. J. Biomed. Opt. 2011;16:051306. doi: 10.1117/1.3560268. [DOI] [PubMed] [Google Scholar]
- Feigin V.L., Norrving B., Mensah G.A. Global burden of stroke. Circ. Res. 2017;120:439–448. doi: 10.1161/CIRCRESAHA.116.308413. [DOI] [PubMed] [Google Scholar]
- Flesher S.N., Collinger J.L., Foldes S.T., Weiss J.M., Downey J.E., Tyler-Kabara E.C., Bensmaia S.J., Schwartz A.B., Boninger M.L., Gaunt R.A. Intracortical microstimulation of human somatosensory cortex. Sci. Transl. Med. 2016;8:361ra141. doi: 10.1126/scitranslmed.aaf8083. [DOI] [PubMed] [Google Scholar]
- Forró C., Thompson-Steckel G., Weaver S., Weydert S., Ihle S., Dermutz H., Aebersold M.J., Pilz R., Demkó L., Vörös J. Modular microstructure design to build neuronal networks of defined functional connectivity. Biosens. Bioelectron. 2018;122:75–87. doi: 10.1016/j.bios.2018.08.075. [DOI] [PubMed] [Google Scholar]
- Frey U., Egert U., Heer F., Hafizovic S., Hierlemann A. Microelectronic system for high-resolution mapping of extracellular electric fields applied to brain slices. Biosens. Bioelectron. 2009;24:2191–2198. doi: 10.1016/j.bios.2008.11.028. [DOI] [PubMed] [Google Scholar]
- Goodman D.F., Brette R. The brian simulator. Front. Neurosci. 2009;3:192–197. doi: 10.3389/neuro.01.026.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassia F., Kohno T., Levi T. Digital hardware implementation of a stochastic two-dimensional neuron model. J. Physiol. Paris. 2016;110:409–416. doi: 10.1016/j.jphysparis.2017.02.002. [DOI] [PubMed] [Google Scholar]
- Greenwald E., Masters M.R., Thakor N.V. Implantable neurotechnologies: bidirectional neural interfaces–applications and VLSI circuit implementations. Med. Biol. Eng. Comput. 2016;54:1–17. doi: 10.1007/s11517-015-1429-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggenmos D.J., Azin M., Barbay S., Mahnken J.D., Dunham C., Mohseni P., Nudo R.J. Restoration of function after brain damage using a neural prosthesis. Proc. Natl. Acad. Sci. U S A. 2013;110:21177–21182. doi: 10.1073/pnas.1316885110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habibey R., Golabchi A., Latifi S., Difato F., Blau A. A microchannel device tailored to laser axotomy and long-term microelectrode array electrophysiology of functional regeneration. Lab Chip. 2015;15:4578–4590. doi: 10.1039/c5lc01027f. [DOI] [PubMed] [Google Scholar]
- Hayashi K., Kawai-Hirai R., Harada A., Takata K. Inhibitory neurons from fetal rat cerebral cortex exert delayed axon formation and active migration in vitro. J. Cell Sci. 2003;116:4419–4428. doi: 10.1242/jcs.00762. [DOI] [PubMed] [Google Scholar]
- Hayes J.P., Bigler E.D., Verfaellie M. Traumatic brain injury as a disorder of brain connectivity. J. Int. Neuropsychol. Soc. 2016;22:120–137. doi: 10.1017/S1355617715000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb D.O. John Wiley & Sons; 1949. The Organization of Behavior: A Neuropsychological Approach. [Google Scholar]
- Hogri R., Bamford S.A., Taub A.H., Magal A., Del Giudice P., Mintz M. A neuro-inspired model-based closed-loop neuroprosthesis for the substitution of a cerebellar learning function in anesthetized rats. Sci. Rep. 2015;5:8451. doi: 10.1038/srep08451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izhikevich E.M. Simple model of spiking neurons. IEEE Trans. Neural Netw. 2003;14:1569–1572. doi: 10.1109/TNN.2003.820440. [DOI] [PubMed] [Google Scholar]
- Izhikevich E.M. Which model to use for cortical spiking neurons? IEEE Trans. Neural Netw. 2004;15:1063–1070. doi: 10.1109/TNN.2004.832719. [DOI] [PubMed] [Google Scholar]
- Izhikevich E.M., Edelman G.M. Large-scale model of mammalian thalamocortical systems. Proc. Natl. Acad. Sci. U S A. 2008;105:3593–3598. doi: 10.1073/pnas.0712231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javier G.O., Soriano J., Alvarez-Lacalle E., Teller S., Casademunt J. Noise focusing and the emergence of coherent activity in neuronal cultures. Nat. Phys. 2013;9:582–590. [Google Scholar]
- Johnson H.A., Goel A., Buonomano D.V. Neural dynamics of in vitro cortical networks reflects experienced temporal patterns. Nat. Neurosci. 2010;13:917–919. doi: 10.1038/nn.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun J.J., Steinmetz N.A., Siegle J.H., Denman D.J., Bauza M., Barbarits B., Lee A.K., Anastassiou C.A., Andrei A., Aydin C. Fully integrated silicon probes for high-density recording of neural activity. Nature. 2017;551:232–236. doi: 10.1038/nature24636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung R., Brauer E.J., Abbas J.J. Real-time interaction between a neuromorphic electronic circuit and the spinal cord. IEEE Trans. Neural Syst. Rehabil. Eng. 2001;9:319–326. doi: 10.1109/7333.948461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner S., Bisio M., Cohen G., Goldin M., Tedesco M., Hanein Y., Ben-Jacob E., Barzilai A., Chiappalone M., Bonifazi P. Design, surface treatment, cellular plating, and culturing of modular neuronal networks composed of functionally inter-connected circuits. J. Vis. Exp. 2015 doi: 10.3791/52572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipke D.R., Shain W., Buzsaki G., Fetz E., Henderson J.M., Hetke J.F., Schalk G. Advanced neurotechnologies for chronic neural interfaces: new horizons and clinical opportunities. J. Neurosci. 2008;28:11830–11838. doi: 10.1523/JNEUROSCI.3879-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler F., Gkogkidis C.A., Bentler C., Wang X., Gierthmuehlen M., Fischer J., Stolle C., Reindl L.M., Rickert J., Stieglitz T. Closed-loop interaction with the cerebral cortex: a review of wireless implant technology. Brain Comput. Interfaces. 2017;4:146–154. [Google Scholar]
- Kozai T.D., Jaquins-Gerstl A.S., Vazquez A.L., Michael A.C., Cui X.T. Brain tissue responses to neural implants impact signal sensitivity and intervention strategies. ACS Chem. Neurosci. 2015;6:48–67. doi: 10.1021/cn500256e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- le Feber J., Erkamp N., Van Putten M.J., Hofmeijer J. Loss and recovery of functional connectivity in cultured cortical networks exposed to hypoxia. J. Neurophysiol. 2017;118:394–403. doi: 10.1152/jn.00098.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczak A., McNaughton B.L., Harris K.D. Packet-based communication in the cortex. Nat. Rev. Neurosci. 2015;16:745–755. doi: 10.1038/nrn4026. [DOI] [PubMed] [Google Scholar]
- Maas A.I.R., Menon D.K., Adelson P.D., Andelic N., Bell M.J., Belli A., Bragge P., Brazinova A., Buki A., Chesnut R.M. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16:987–1048. doi: 10.1016/S1474-4422(17)30371-X. [DOI] [PubMed] [Google Scholar]
- Markram H. The human brain project. Sci. Am. 2012;306:50–55. doi: 10.1038/scientificamerican0612-50. [DOI] [PubMed] [Google Scholar]
- Melozzi F., Woodman M.M., Jirsa V.K., Bernard C. The virtual mouse brain: a computational neuroinformatics platform to study whole mouse brain dynamics. eNeuro. 2017;4 doi: 10.1523/ENEURO.0111-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers D.K., Goldberg A.M., Poth A., Wolf M.F., Carraway J., McKim J., Coleman K.P., Hutchinson R., Brown R., Krug H.F. From in vivo to in vitro: the medical device testing paradigm shift. ALTEX. 2017;34:479–500. doi: 10.14573/altex.1608081. [DOI] [PubMed] [Google Scholar]
- Osborn L.E., Dragomir A., Betthauser J.L., Hunt C.L., Nguyen H.H., Kaliki R.R., Thakor N.V. Prosthesis with neuromorphic multilayered e-dermis perceives touch and pain. Sci. Robot. 2018;20:eaat3818. doi: 10.1126/scirobotics.aat3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostry D.J., Darainy M., Mattar A.A., Wong J., Gribble P.L. Somatosensory plasticity and motor learning. J. Neurosci. 2010;30:5384–5393. doi: 10.1523/JNEUROSCI.4571-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzeri S., Harvey C.D., Piasini E., Latham P.E., Fellin T. Cracking the neural code for sensory perception by combining statistics, intervention, and behavior. Neuron. 2017;93:491–507. doi: 10.1016/j.neuron.2016.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastore V.P., Massobrio P., Godjoski A., Martinoia S. Identification of excitatory-inhibitory links and network topology in large-scale neuronal assemblies from multi-electrode recordings. PLoS Comput. Biol. 2018;14:e1006381. doi: 10.1371/journal.pcbi.1006381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirog A., Bornat Y., Perrier R., Raoux M., Jaffredo M., Quotb A., Lang J., Lewis N., Renaud S. Multimed: an integrated, multi-application platform for the real-time recording and sub-millisecond processing of biosignals. Sensors (Basel) 2018;18:2099. doi: 10.3390/s18072099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzi R.M., Rossetti D., Cino G., Marino D., Vescovi A.L., Baer W. A cultured human neural network operates a robotic actuator. Biosystems. 2009;95:137–144. doi: 10.1016/j.biosystems.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Potter S.M. Closing the loop between neurons and neurotechnology. Front. Neurosci. 2010;4:15. doi: 10.3389/fnins.2010.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelfsema P.R., Holtmaat A. Control of synaptic plasticity in deep cortical networks. Nat. Rev. Neurosci. 2018;19:166–180. doi: 10.1038/nrn.2018.6. [DOI] [PubMed] [Google Scholar]
- Rosin B., Slovik M., Mitelman R., Rivlin-Etzion M., Haber S.N., Israel Z., Vaadia E., Bergman H. Closed-loop deep brain stimulation is superior in ameliorating parkinsonism. Neuron. 2011;72:370–384. doi: 10.1016/j.neuron.2011.08.023. [DOI] [PubMed] [Google Scholar]
- Shein-Idelson M., Ben-Jacob E., Hanein Y. Engineered neuronal circuits: a new platform for studying the role of modular topology. Front. Neuroeng. 2011;4:10. doi: 10.3389/fneng.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soekadar S.R., Birbaumer N., Slutzky M.W., Cohen L.G. Brain-machine interfaces in neurorehabilitation of stroke. Neurobiol. Dis. 2015;83:172–179. doi: 10.1016/j.nbd.2014.11.025. [DOI] [PubMed] [Google Scholar]
- Soloperto A., Bisio M., Palazzolo G., Chiappalone M., Bonifazi P., Difato F. Modulation of neural network activity through single cell ablation: an in vitro model of minimally invasive neurosurgery. Molecules. 2016;21:1018. doi: 10.3390/molecules21081018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz N.A., Koch C., Harris K.D., Carandini M. Challenges and opportunities for large-scale electrophysiology with Neuropixels probes. Curr. Opin. Neurobiol. 2018;50:92–100. doi: 10.1016/j.conb.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi N., Izumi S. Rehabilitation with poststroke motor recovery: a review with a focus on neural plasticity. Stroke Res. Treat. 2013;2013:128641. doi: 10.1155/2013/128641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur C.S.T., Molin J., Cauwenberghs G., Indiveri G., Kumar K., Qiao N., Schemmel J., Wang R.M., Chicca E., Olson Hasler J. Large-scale neuromorphic spiking array processors: a quest to mimic the brain. Front. Neurosci. 2018;12:891. doi: 10.3389/fnins.2018.00891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano G.G. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle G., Mazzoni A., Iberite F., D'Anna E., Strauss I., Granata G., Controzzi M., Clemente F., Rognini G., Cipriani C. Biomimetic intraneural sensory feedback enhances sensation naturalness, tactile sensitivity, and manual dexterity in a bidirectional prosthesis. Neuron. 2018;100:37–45. doi: 10.1016/j.neuron.2018.08.033. [DOI] [PubMed] [Google Scholar]
- Vassanelli S., Mahmud M. Trends and challenges in neuroengineering: toward “intelligent” Neuroprostheses through brain-“brain inspired systems” communication. Front. Neurosci. 2016;10:438. doi: 10.3389/fnins.2016.00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenaar D.A., Pine J., Potter S.M. Searching for plasticity in dissociated cortical cultures on multi-electrode arrays. J. Negat. Results Biomed. 2006;5:16. doi: 10.1186/1477-5751-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Collinger J.L., Perez M.A., Tyler-Kabara E.C., Cohen L.G., Birbaumer N., Brose S.W., Schwartz A.B., Boninger M.L., Weber D.J. Neural interface technology for rehabilitation: exploiting and promoting neuroplasticity. Phys. Med. Rehabil. Clin. N. Am. 2010;21:157–178. doi: 10.1016/j.pmr.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T., Xiao N., Zhai X., Kwan C.P., Tin C. Real-time cerebellar neuroprosthetic system based on a spiking neural network model of motor learning. J. Neural Eng. 2018;15:016021. doi: 10.1088/1741-2552/aa98e9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video recorded during a laser ablation of the connections between two modules (visible on the left and right side) of a bimodular cell culture. Video recorded on November 16th 2016. Duration 18 s.