Abstract
Purpose
To describe a choroidal mass that proved to be histiocytic choroidal infiltration in Erdheim-Chester disease.
Observations
A 54-years-old Caucasian male presented to our Retina Clinic with a suspect of choroidal melanoma in the left eye. Dilated fundus exam of the left eye showed a yellow-grey lesion along the inferior arcade, with sub-retinal fluid clinically visible. Enhanced depth imaging-OCT (EDI-OCT) showed a dome-shaped choroidal lesion with hyperreflective exudation present between the inner retina and the retinal pigment epithelium (RPE). On fundus autofluorescence the lesion appeared to have a diffuse speckled hyper-autofluorescent pattern secondary to the exudative subretinal material. On ultrasound, the lesion appeared hyper-echoic and dome-shaped, with a baseline thickness of 6.13 mm. Indocyanine green angiography (ICGA) was performed and showed hypocyanescence of the lesion from the early phases that persisted through the whole exam. Chest CT with contrast showed an abnormal, non-calcific, eccentric thickening of segments of the aorta (“coated aorta”) and PET an abnormally strong labeling of the distal ends of the long bones. An additional proximal tibial biopsy was performed to confirm the diagnosis on histology of Erdheim-Chester disease and the patient was started on oral prednisone. The choroidal mass progressively shrunk and the subretinal exudative material on top partially reabsorbed.
Conclusions and importance
Intraocular involvement in Erdheim-Chester disease is extremely rare but as a result of recent better awareness the number of new diagnosis is increasing. Erdheim-Chester disease should be considered in the differential of every choroidal mass.
Keywords: Choroidal mass, Subretinal fluid, Histiocytosis, Aortic plaques, Erdheim-chester disease
1. Introduction
Erdheim-Chester disease (ECD) is a rare form of non-Langerhans cell histiocytosis that was initially described by William Chester and his tutor, the Viennese pathologist Jakob Erdheim, in 1930.1 ECD clinical spectrum is particularly broad,2 and depends on the distribution and extent of the lesions. The most common presentation includes diffuse sclerotic lesions,3 which reveal CD68 positive foamy histiocytes surrounded by fibrosis.4 The disease primarily affects the bone,5 but may involve virtually every organ and tissue with protean clinical manifestations,2 ranging from asymptomatic bone involvement to multisystemic, life-threatening forms.
Visual symptoms in patients with ECD have been reported in about 3% of all ECD patients6 and 23% of patients with CNS involvement.7 The most common form of ocular involvement is diffuse, intraconal, infiltrative orbital infiltrates in 25%–30% of ECD patients,8 with risk of compressive optic neuropathy9 and exophthalmos. Intraocular involvement is extremely rare and to our knowledge only 6 previous case reports have been published in literature.10, 11, 12
We hereby report a case of unilateral choroidal infiltration as the presenting sign of ECD.
2. Case report
A 54-years-old Caucasian male presented to our Retina Clinic with a suspect of choroidal melanoma in the left eye. His past medical history included hypertension and unspecific pain in his lower extremities. The patient sought medical attention after experiencing photopsias in the left eye, and upon examination of the fundus in the left eye, his ophthalmologist detected the presence of a solid lesion along the inferior arcades (Fig. 1A). An ultrasound was performed measuring the lesion with a thickness of 6 mm and a longer axis of 11 mm; fluorescein angiography detected early staining of the lesion (Fig. 1B) with late leakage (Fig. 1C) in the context of subretinal fluid surrounding it. A brain and orbit MRI did not detect any satellite lesions, and the lesion appeared hyperintense in T1- (Fig. 1D) and hypointense in T2-weighted (Fig. 1E) images. The patient was referred to Cleveland Clinic Abu Dhabi with a diagnosis of choroidal melanoma.
Fig. 1.
Baseline imaging of the left eye of a patient referred for choroidal melanoma evaluation. Fundus photo (A) performed by a local ophthalmologist shows a yellow choroidal mass along the inferior arcade, with early staining on fluorescein angiography (B) and late leakage of the margin (C) due to the presence of subretinal fluid. An MRI brain and orbit with contrast was obtained to rule out satellite lesions and showed hyperintensity of the choroidal mass in T1- (D) and hypointensity in T2-weighted (E) images.
At baseline visit, the patient's best corrected visual acuity was 20/25 in the right eye and 20/70 in the left eye. Intraocular pressure was within normal limits. Slit lamp examination was unremarkable in the right eye, and showed stellate keratic precipitates and 1 + anterior chamber cells in the left eye. Dilated fundus exam of the right eye was within normal limits, while the left eye had 1 + vitreous cells, blunting of the foveal reflex, and a yellow-grey lesion along the inferior arcade, with sub-retinal fluid clinically visible (Fig. 2A).
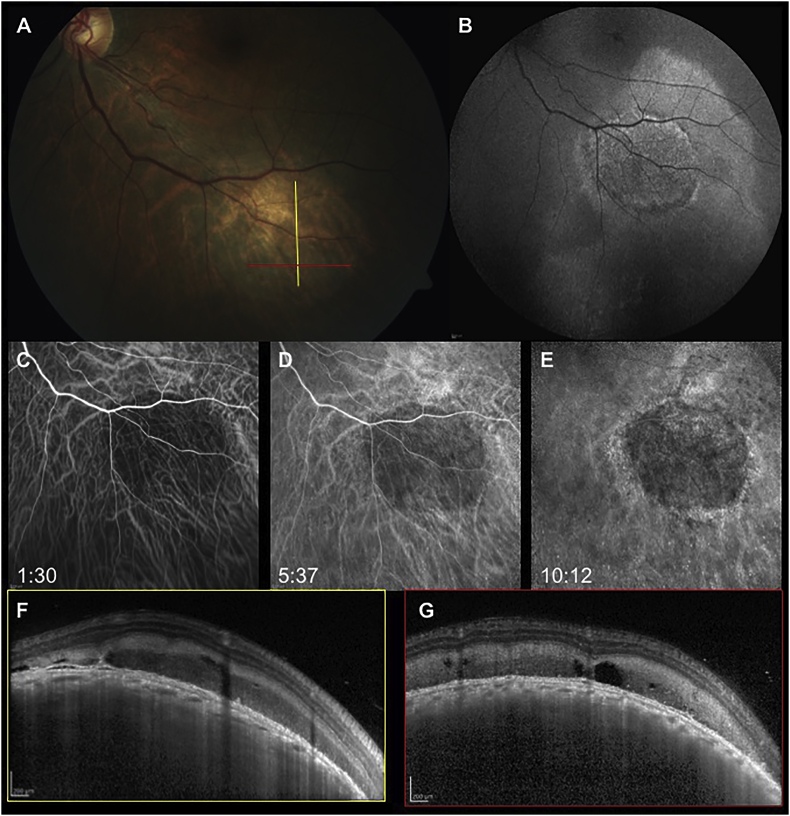
Fig. 2.
When the patient presented to our attention, the choroidal lesion had not progressed (A). Fundus autofluorescence (B) showed a speckled hyperautofluorescent pattern of the mass with diffuse hyperautofluorescence surrounding it, secondary to RPE alterations from subretinal fluid. On indocyanine green angiography the mass masked the underlying choroidal cyanescence in the early (C), mid (D) and late phases (D) of the exam. Enhanced depth imaging-OCT (F,G) of the mass revealed a dome-shaped elevation of the choroid with effacement of the choroidal vessels and a preserved choriocapillaris; hyperreflective subretinal exudation could be detected, corresponding to the hyperautofluorescent material seen on FAF.
Spectral domain-optical coherence tomography (SD-OCT) of the left eye showed cystoid macular edema with a central retinal thickness of 533 μm, SD-OCT scans along the inferior arcade confirmed the presence of subretinal fluid surrounding a dome-shaped choroidal elevation. Hyperreflective exudation was present between the inner retina and the retinal pigment epithelium (RPE) on top of the solid lesion (Fig. 2F), and irregularities of the RPE with loss of the outer retina bands were detected in correspondence with the lesion. Enhanced depth imaging-OCT (EDI-OCT) of the choroidal lesion showed an intact choriocapillaris but a complete effacement of the choroidal vasculature (Fig. 2F). On fundus autofluorescence the lesion appeared to have a diffuse speckled hyper-autofluorescent patter secondary to the exudative subretinal material (Fig. 2B). On ultrasound, the lesion appeared hyper-echoic and dome-shaped, with a baseline thickness of 6.13 mm (Fig. 3, top row). Indocyanine green angiography (ICGA) was performed and showed hypocyanescence of the lesion from the early phases that persisted through the whole exam (Fig. 2C–E).
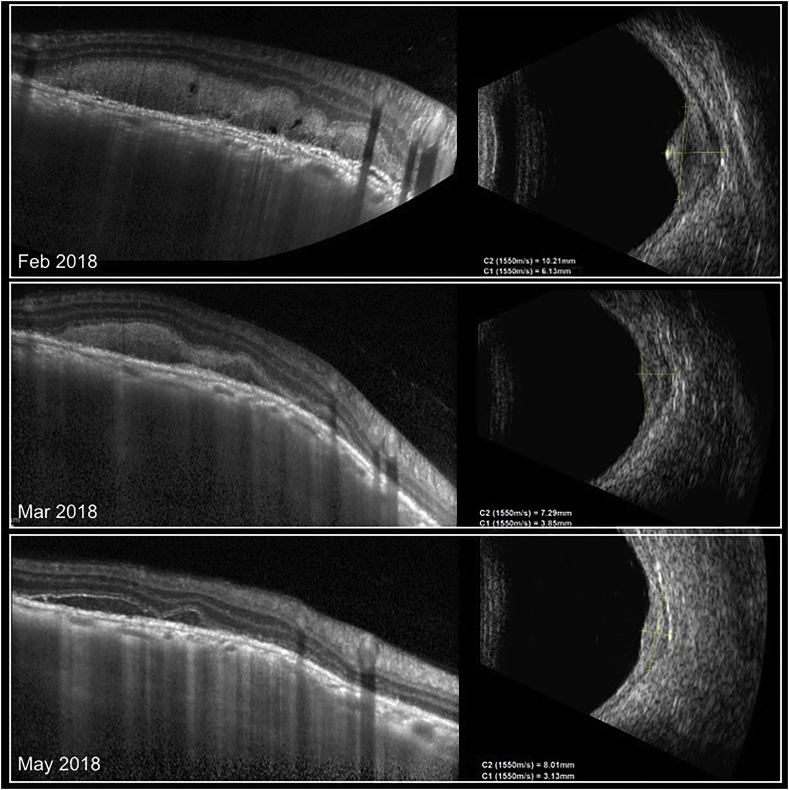
Fig. 3.
Oral corticosteroid treatment (1 mg/kg) led to a progressive flattening of the choroidal mass, evident in EDI-OCT but even more on b-scan ultrasound. Baseline ultrasound thickness of the choroidal mass (top right) was 6.13 mm, and decreased to 3.13 mm (bottom right) after 4 months of treatment. The subretinal hyperreflective exudative material progressively reabsorbed as well (left panel) in EDI-OCT along with the decrease in the size of the choroidal mass.
Based on clinical and imaging findings, the presenting diagnosis of choroidal melanoma was discarded. The presence of anterior chamber and anterior vitreous cells, along with cystoid macular edema, pointed towards a uveitic process, and the choroidal mass was identified as “granuloma”. A complete laboratory work-up was performed. ACE and Quantiferon GOLD turned out negative, and a chest x-ray showed only smoking-related changes. Before establishing a treatment plan, a chest computerized tomography (CT) was requested to exclude any granulomatous lung disease. While on CT the lungs were clear of granulomas, the exam showed an abnormal eccentric thickening of segments of the aorta (Fig. 4, yellow asterisks) and an asymmetric posterior thickening of the origin of the left subclavian artery. None of these thickening had a calcific component. The differential diagnosis proposed by the radiologist excluded sarcoidosis or tuberculosis, including instead large vessel vasculitis disorders (such as Takayasu and giant cells arteritis), and Erdheim-Chester disease. The ocular findings were not consistent with vasculitis, therefore a diagnosis of Erdheim-Chester disease was pursued.19 Fluorodeoxyglucose (FDG)-positron emission tomography (PET) showed diffuse FDG uptake in long bones (humeri and tibias). In order to make a definitive diagnosis, a proximal tibial biopsy of a sclerotic osseous lesion was performed and showed non Langerhans' foamy histiocytes with xanthogranulomatosis that stained positive for Cluster of Differentiation (CD) 68 and negative for CD1a. Genetic analysis showed no mutations in BRAF and ARAF. Based on these findings, a final diagnosis of Erdheim-Chester disease was posed.
Fig. 4.
Contrast chest CT revealed an abnormal eccentric thickening of the aortic arch (superior panel, yellow asterisk) and a segmental non-calcific thickening of the descending aorta (bottom panel, yellow asterisk). This peculiar ECD feature, described in up to two-thirds of patients is termed ‘coated aorta’.
The patient was started on a course of oral prednisone 1 mg/kg with a tapering schedule and topical difluprednate three times a day in the left eye.
During the course of 3 months, the cystoid macular edema progressively decreased, and at the final follow-up central retinal thickness was 324 μm with mild residual intraretinal fluid. The subretinal exudative material on top of the choroidal lesion had partially reabsorbed but was still present at the last follow up (Fig. 3, bottom row). On sequential ultrasound, the choroidal mass progressively shrunk, with a final thickness of 3.13 mm upon completion of the steroid treatment course (Fig. 3, bottom row). At the last follow-up, BCVA improved to 20/20 and inflammation in the anterior chamber and vitreous had resolved completely. Upon retinal examination, the choroidal lesion along the inferior arcade had consolidated and become more atrophic.
3. Discussion
Since the first description by Jakob Erdheim's pupil William Chester in 19301, more than 500 cases of ECD have been reported in the English literature.2,3,5,7,13 This makes ECD definitely rare, but, as a result of recent findings and a better awareness among the medical community, the number of new diagnosis is dramatically increasing.
ECD is a non-Langerhans cell histiocytosis,14 stemming from an abnormal monocyte-macrophage ancestry.
Since virtually every tissue and system can be involved by pathologic histiocytes, phenotypically ECD has many faces, making the diagnostic procedure particularly challenging. Presentation may vary from indolent focal disease to multiorgan involvement with fatal outcome. A very common characteristic is bone involvement,5,15 which is the first manifestation of ECD in half of all patients. Bone pain represents the most common symptom and usually involves the lower limbs. Positron emission tomography scan16 in these cases is paramount in identifying bilateral symmetric sclerosis of peripheral long bones. Cardiovascular manifestations17 are the major cause of death in ECD patients, since about 60% of patients perish due to a cardiac complication. They are often silent and detected incidentally by CT or MRI. Cardiovascular anomalies include “pseudo-tumor” of the right side of the heart, pericardial fibrosis and valvular infiltration. The fibrous encasement of the aorta that was detected through contrast-enhanced CT scan in our patient (Fig. 4), is a peculiar ECD feature, described in up to two-thirds of patients and termed ‘coated aorta’.18 Many patients have central nervous system (CNS) involvement,7,19 resulting in central diabetes insipidus.
Space-occupying orbital ECD,8 which is often bilateral and manifests as proptosis, is found in 27% of ECD patients.20 Occasionally it may be resistant to treatment, requiring surgical debulking. Much rarer is intraocular involvement, with only 1 case in the literature reporting subretinal infiltration21 and 4 choroidal infiltration.10, 11, 12
When intraocular ECD shows choroidal infiltration, such as in our case, it is accompanied by overlying subretinal fluid (SRF) and progression of these lesions may result in pigmentary clumping and subretinal fibrosis. If bilateral, such in the case presented by Tan et al.,11 the FAF appearance of these histiocytic ECD lesions are similar to those seen in bilateral diffuse uveal melanocytic proliferation. Tan et al.11 also reported the development of a secondary choroidal neovascularization (CNV) in 2 of their cases. In the absence of a CNV such as in our case, exudative SRF may be caused by a secondary inflammatory process owing to histiocytic infiltration of the choroid.
Diagnosing ECD is challenging, as the most common clinical manifestations usually lack adequate specificity. Tissue biopsy,22 preferably of an osteosclerotic bone lesion if possible, is crucial for diagnosis. Histopathological examination usually shows foamy S-100-negative and cluster of differentiation 68 (CD68)-positive histiocytes as the principal cells, surrounded by fibrosis and inflammatory cells, such as lymphocytes, plasma cells, and Touton-type giant cells.23 The only chorioretinal biopsy reported in literature demonstrates histiocytic infiltration within the choroid of ECD11 and supports the theory that the choroidal lesions in ECD with serous retinal detachment (SRD) are most likely not secondary to a paraneoplastic inflammatory response. A combination of clinical assessment, pathological examination and immunohistochemical staining is used to differentiate ECD from the other rare types of non-Langerhans' histiocytosis. Biopsy is also necessary to establish BRAF mutational status.
Treatment, which has often been palliative rather than curative, is not necessarily needed for asymptomatic patients with an indolent disease course. In our case, we treated the choroidal infiltration with oral corticosteroids, also obtaining an associated reduction in the overlying SRF and exudation. The literature reports short-term resolution of SRF with ocular therapies such as intravitreal anti-vascular endothelial growth factor (VEGF), intravitreal methotrexate, and photodynamic therapy in combination with systemic therapies such as oral corticosteroids and chemotherapy agents10,11,21; however, recurrences are common.
If treatment for the systemic disease is warranted, most of the therapeutic literature is anecdotal, without clear-cut guidelines. Until recently, treatment included diverse approaches such as steroids, cladribine-based chemotherapy and cytokine-based agents.24,25 More recently, interferon (IFN)-α and the interleukin (IL)-1 receptor antagonist anakinra have been used to treat ECD patients,26,27 as well as imatinib, cladribine, zoledronic acid, and transplantation.
Of special interest, the V600E BRAF mutation28 has been identified in some patients with ECD but not in patients with other non-Langerhans histiocytosis. This is a drug-responsive mutation, with excellent response rates in other diseases to BRAF inhibitors vemurafenib and dabrafenib.11,28,29 However, the benefit of BRAF inhibitors in ECD has not been confirmed, and our patient had negative genetic testing for BRAF mutations.
The patient that we reported had negative genetic testing for BRAF mutations. However, his choroidal infiltration responded very well to oral steroids. Furthermore, the lesion and the associated SRF were not macular, and did not show any associated CNV, and thus did not cause permanent visual deterioration.
In conclusion, we report a case of a choroidal mass that led to the diagnosis of Erdheim-Chester disease. Despite the rarity of intraocular ECD, it should be kept in the differential diagnosis of every choroidal granuloma or mass.
Patient consent
Written consent to publish this case has not been obtained. This report does not contain any personal identifying information.
Acknowledgements and disclosures
Funding
No funding or grant support
Conflicts of interest
The following authors have no financial disclosures: FP.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajoc.2019.100539.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Haroche J., Cohen-Aubart F., Arnaud L. Erdheim-Chester disease. Rev Med Interne. 2014;35(11):715–722. doi: 10.1016/j.revmed.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Cavalli G., Guglielmi B., Berti A. The multifaceted clinical presentations and manifestations of Erdheim-Chester disease: comprehensive review of the literature and of 10 new cases. Ann Rheum Dis. 2013;72(10):1691–1695. doi: 10.1136/annrheumdis-2012-202542. [DOI] [PubMed] [Google Scholar]
- 3.Campochiaro C., Tomelleri A., Cavalli G., Berti A., Dagna L. Erdheim-Chester disease. Eur J Intern Med. 2015;26(4):223–229. doi: 10.1016/j.ejim.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Kerstetter J., Wang J. Adult orbital xanthogranulomatous disease: a review with emphasis on etiology, systemic associations, diagnostic tools, and treatment. Dermatol Clin. 2015;33(3):457–463. doi: 10.1016/j.det.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 5.White T.V., Silvester N.C., Otero H.J. Non-sclerotic bone involvement in Erdheim-Chester: PET/CT and MRI findings in a 15-year-old boy. Pediatr Radiol. 2016;46(9):1345–1349. doi: 10.1007/s00247-016-3594-y. [DOI] [PubMed] [Google Scholar]
- 6.Munoz J., Janku F., Cohen P.R., Kurzrock R. Erdheim-Chester disease: characteristics and management. Mayo Clin Proc. 2014;89(7):985–996. doi: 10.1016/j.mayocp.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 7.Drier A., Haroche J., Savatovsky J., Godenèche G., Dormont D, Chiras J, Amoura Z, Bonneville F. Cerebral, facial, and orbital involvement in Erdheim-Chester disease: CT and MR imaging findings. Radiology. 2010;255(2):586–594. doi: 10.1148/radiol.10090320. [DOI] [PubMed] [Google Scholar]
- 8.Merritt H., Pfeiffer M.L., Richani K., Phillips M.E. Erdheim-Chester disease with orbital involvement: case report and ophthalmic literature review. Orbit. 2016;35(4):221–226. doi: 10.1080/01676830.2016.1176211. [DOI] [PubMed] [Google Scholar]
- 9.Manousaridis K., Casper J., Schittkowski M.P., Nizze H., Guthoff R.F. Erdheim-Chester disease of the orbit with compressive optic neuropathy. Der Ophthalmologe. 2010;107(3):266–269. doi: 10.1007/s00347-009-2041-y. [DOI] [PubMed] [Google Scholar]
- 10.Abdellatief A., Mason C.M., Ytterberg S.R. Choroidal involvement in erdheim-chester disease. Ophthalmic Surg Lasers Imag Retina. 2015;46(6):674–676. doi: 10.3928/23258160-20150610-13. [DOI] [PubMed] [Google Scholar]
- 11.Tan A.C.S., Yzer S., Atebara N. Three cases of erdheim-chester disease with intraocular manifestations: imaging and histopathology findings of a rare entity. Am J Ophthalmol. 2017;176:141–147. doi: 10.1016/j.ajo.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 12.Huang L.C., Topping K.L., Gratzinger D. Orbital and chorioretinal manifestations of Erdheim-Chester disease treated with vemurafenib. Am J Ophthalmol Case Rep. 2018 Jul 25;11:158–163. doi: 10.1016/j.ajoc.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cives M., Simone V., Rizzo F.M. Erdheim-Chester disease: a systematic review. Crit Rev Oncol Hematol. 2015;95(1):1–11. doi: 10.1016/j.critrevonc.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Haroche J., Cohen-Aubart F., Charlotte F. The histiocytosis Erdheim-Chester disease is an inflammatory myeloid neoplasm. Expert Rev Clin Immunol. 2015;11(9):1033–1042. doi: 10.1586/1744666X.2015.1060857. [DOI] [PubMed] [Google Scholar]
- 15.Ceulemans G., Keyaerts M., Verbruggen L. Erdheim-Chester disease detected with 99mTc MDP bone SPECT/CT. JBR-BTR. 2012;95(4):245–248. doi: 10.5334/jbr-btr.630. [DOI] [PubMed] [Google Scholar]
- 16.García-Gómez F.J., Acevedo-Báñez I., Martínez-Castillo R. The role of 18FDG, 18FDOPA PET/CT and 99mTc bone scintigraphy imaging in Erdheim-Chester disease. Eur J Radiol. 2015;84(8):1586–1592. doi: 10.1016/j.ejrad.2015.04.022. [DOI] [PubMed] [Google Scholar]
- 17.Berti A., Ferrarini M., Ferrero E., Dagna L. Cardiovascular manifestations of Erdheim-Chester disease. Clin Exp Rheumatol. 2015;33(2 suppl 89) S-155-163. [PubMed] [Google Scholar]
- 18.Nicolazzi M.A., Carnicelli A., Fuorlo M., Favuzzi A.M., Landolfi R. Cardiovascular involvement in erdheim-chester disease: a case report and review of the literature. Medicine (Baltim) 2015;94(43):e1365. doi: 10.1097/MD.0000000000001365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Abreu M.R., Chung C.B., Biswal S. Erdheim-Chester disease: MR imaging, anatomic, and histopathologic correlation of orbital involvement. Am J Neuroradiol. 2004;25(4):627–630. [PMC free article] [PubMed] [Google Scholar]
- 20.Beylergil V., Carrasquillo J.A., Hyman D.M., Diamond E.L. Visualization of orbital involvement of Erdheim-Chester disease on PET/CT. Clin Nucl Med. 2014;39(7):660–661. doi: 10.1097/RLU.0000000000000452. [DOI] [PubMed] [Google Scholar]
- 21.Biccas Neto L., Zanetti F. Intraocular involvement in Erdheim-Chester disease–first report in the literature: case report. Arq Bras Oftalmol. 2007;70(5):862–867. doi: 10.1590/s0004-27492007000500025. [DOI] [PubMed] [Google Scholar]
- 22.Bosco J., Allende A., Varikatt W., Lee R., Stewart G.J. Does the BRAF(V600E) mutation herald a new treatment era for Erdheim-Chester disease? A case-based review of a rare and difficult to diagnose disorder. Intern Med J. 2015;45(3):348–351. doi: 10.1111/imj.12685. [DOI] [PubMed] [Google Scholar]
- 23.Arceci R.J. Biological and therapeutic implications of the BRAF pathway in histiocytic disorders. Am Soc Clin Oncol Educ. 2014:e441–e445. doi: 10.14694/EdBook_AM.2014.34.e441. [DOI] [PubMed] [Google Scholar]
- 24.Diamond E.L., Dagna L., Hyman D.M. Consensus guidelines for the diagnosis and clinical management of Erdheim-Chester disease. Blood. 2014;124(4):483–492. doi: 10.1182/blood-2014-03-561381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broccoli A., Stefoni V., Faccioli L. Bilateral orbital Erdheim-Chester disease treated with 12 weekly administrations of VNCOP-B chemotherapy: a case report and a review of literature. Rheumatol Int. 2012;32(7):2209–2213. doi: 10.1007/s00296-011-1998-4. [DOI] [PubMed] [Google Scholar]
- 26.Hervier B., Arnaud L., Charlotte F. Treatment of Erdheim-Chester disease with long-term high-dose interferon-α. Semin Arthritis Rheum. 2012;41(6):907–913. doi: 10.1016/j.semarthrit.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Cohen P.R., Kurzrock R. Anakinra-responsive lichen planus in a woman with Erdheim-Chester disease: a therapeutic enigma. Dermatol Online J. 2014;20(1):21241. [PubMed] [Google Scholar]
- 28.Janku F., Vibat C.R., Kosco K. BRAF V600E mutations in urine and plasma cell-free DNA from patients with Erdheim-Chester disease. Oncotarget. 2014;5(11):3607–3610. doi: 10.18632/oncotarget.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta A., Yeganeh A., Rootman D., Goldberg R. Vemurafenib (BRAF inhibitor) therapy for orbital erdheim-chester disease. Ophthalmic Plast Reconstr Surg. 2017;33(6):e138–e139. doi: 10.1097/IOP.0000000000000866. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.