Abstract
Individuals born very preterm (VPT; <32 weeks' gestational age) are at increased risk of impaired mathematics and word reading performance, as well as widespread white matter microstructural alterations compared with individuals born full term (FT; ≥37 weeks' gestational age). To date, the link between academic performance and white matter microstructure is not well understood. This study aimed to investigate the associations between mathematics and reading performance with white matter microstructure in 114 VPT and 36 FT 13-year-old children. Additionally, we aimed to investigate whether the association of mathematics and reading performance with white matter microstructure in VPT children varied as a function of impairment. To do this, we used diffusion tensor imaging and advanced diffusion modelling techniques (Neurite Orientation Dispersion and Density Imaging and the Spherical Mean Technique), combined with a whole-brain analysis approach (Tract-Based Spatial Statistics). Mathematics performance across VPT and FT groups was positively associated with white matter microstructural measurements of fractional anisotropy and neurite density, and negatively associated with radial and mean diffusivities in widespread, bilateral regions. Furthermore, VPT children with a mathematics impairment (>1 standard deviation below FT mean) had significantly reduced neurite density compared with VPT children without an impairment. Reading performance was not significantly associated with any of the white matter microstructure parameters. Additionally, the associations between white matter microstructure and mathematics and reading performance did not differ significantly between VPT and FT groups. Our findings suggest that alterations in white matter microstructure, and more specifically lower neurite density, are associated with poorer mathematics performance in 13-year-old VPT and FT children. More research is required to understand the association between reading performance and white matter microstructure in 13-year-old children.
Keywords: Very preterm, Prematurity, Academic performance, Diffusion imaging, White matter
Highlights
-
•
Diffusion tensor and neurite density metrics were associated with mathematics.
-
•
Associations were present in very preterm and full-term children.
-
•
Associations were widespread throughout the white matter microstructure.
-
•
Decreased neurite density was evident in children with a mathematics impairment.
-
•
Limited evidence of associations between white matter microstructure and reading.
1. Introduction
Very preterm (VPT) birth (<32 weeks' gestational age) occurs in approximately 1.5% of all live births in Australia (Australian Institute of Health and Welfare, 2018). Up to 75% of VPT children exhibit macrostructural cerebral white matter abnormalities, such as cystic and punctate lesions, delayed myelination, loss of white matter volume or thinning of the corpus callosum (Anderson et al., 2015; Inder et al., 2003; Mangin et al., 2017). Consistent with these findings, diffusion weighted imaging (DWI) studies have reported that VPT infants also exhibit white matter microstructural alterations in various white matter regions compared with full-term (FT) infants, including reduced fibre density and fractional anisotropy (FA) and increased radial diffusivity (RD) and mean diffusivity (MD), likely reflecting delayed brain development (Pannek et al., 2018; Thompson et al., 2019; Thompson et al., 2014). Similar white matter microstructural alterations have been reported in adolescence and young adulthood (Kelly et al., 2016; Murray et al., 2016; Nosarti et al., 2014; Thompson et al., 2015; Vangberg et al., 2006).
VPT children are also at increased risk for neurodevelopmental impairments across a range of domains, including mathematics and reading, compared with children born FT (Anderson, 2014; Anderson and Doyle, 2008; Mangin et al., 2017). The rate of academic problems increases with decreasing gestational age (Aarnoudse-Moens et al., 2011; Chan and Quigley, 2014; Simms et al., 2013), with the majority of 11-year-olds born <25 weeks' gestation reported to have a mathematics (70%) or reading (52%) impairment (Johnson et al., 2009).
The variability in academic performance outcomes for VPT children is significantly related to general cognitive ability (Twilhaar et al., 2018). In turn, general cognitive ability (IQ) has been associated with white matter microstructure in several brain regions in preterm individuals, including the inferior and superior longitudinal fasciculi, inferior fronto-occipital fasciculi, uncinate fasciculi and corpus callosum (Eikenes et al., 2011; Feldman et al., 2012a; Kelly et al., 2016; Skranes et al., 2007). Mathematics and reading performance in children born VPT may also be associated with white matter microstructure, however few studies have investigated this. In typically developing children, better mathematics and reading performance has been associated with increased FA in several brain regions. Specifically, mathematics has been positively associated with FA in the left anterior superior longitudinal fasciculus (also referred to as the anterior arcuate fasciculus; Li et al., 2013; Tsang et al., 2009; Van Beek et al., 2014), the left inferior longitudinal fasciculus (Li et al., 2013; van Eimeren et al., 2008), the left superior corona radiata (van Eimeren et al., 2008) and the bilateral inferior fronto-occipital fasciculus (Li et al., 2013) in children of varying ages from 7 to 15 years. Reading performance has been positively associated with FA in typical children varying in age from 6 to 13 years in a small left temporo-parietal region, speculated to correspond with the superior corona radiata and centrum semiovale (Niogi and McCandliss, 2006), the posterior limb of the internal capsule (Beaulieu et al., 2005) and a region at the border between the left corona radiata and the superior longitudinal fasciculus (Deutsch et al., 2005). We have previously explored the association between FA and academic performance in 7-year-old VPT children using a whole-brain exploratory analysis (Tract-Based Spatial Statistics (TBSS)), and reported positive relationships between FA and mathematics and reading performance in tracts consistent with other studies (Kelly et al., 2016).
Most DWI studies investigating the relationship between academic performance and white matter microstructure have focused on FA. FA reflects the degree of diffusion anisotropy, with low values reflecting isotropic diffusion and high values reflecting anisotropic diffusion. Water diffusion in the white matter can be influenced by numerous factors, including axonal myelination, density, diameter and dispersion, but FA cannot delineate amongst these (Jones et al., 2013). More advanced techniques are now available that can assess white matter microstructure with greater specificity than FA, allowing a deeper understanding of the neurobiological relationship between white matter and functional outcomes. One technique is Neurite Orientation Dispersion and Density Imaging (NODDI), which assesses neurite orientation dispersion and neurite density (Zhang et al., 2012). Another technique is multi-compartment microscopic diffusion imaging based on the Spherical Mean Technique (SMT), which measures neurite density and intrinsic diffusivity, with the effects of neurite orientation dispersion factored out (Kaden et al., 2016). We have previously applied the NODDI technique to explore the relationships between academic performance and neurite orientation dispersion and density in VPT 7-year-olds, but did not find any significant associations (Kelly et al., 2016). These findings, however, need further investigation in older children following several years of formal education.
The current study aimed to examine associations between mathematics and single word reading performance and white matter microstructure in a sample of VPT and FT 13-year-old children, using a whole-brain approach and advanced diffusion modelling techniques. A further aim was to investigate whether the association between mathematics and reading performance with white matter microstructure in VPT children varied as a function of impairment. It was expected that white matter microstructure in the superior longitudinal fasciculus, inferior longitudinal fasciculus, superior corona radiata and inferior fronto-occipital fasciculus would be most strongly associated with mathematics performance (Li et al., 2013; Tsang et al., 2009; Van Beek et al., 2014; van Eimeren et al., 2008), while white matter microstructure in the temporo-parietal region, corona radiata, centrum semiovale and posterior limb of the internal capsule would be most strongly associated with word reading performance (Beaulieu et al., 2005; Deutsch et al., 2005; Niogi and McCandliss, 2006). We expected these associations to be similar between the VPT and FT groups, based on previous research in both VPT and FT populations (Beaulieu et al., 2005; Deutsch et al., 2005; Kelly et al., 2016; van Eimeren et al., 2008).
2. Materials and methods
2.1. Participants
A cohort of 224 VPT infants (born <30 weeks' gestational age or < 1250 g) and 46 FT infants (born ≥37 weeks' gestational age) born between 2001 and 2003 at the Royal Women's Hospital, Melbourne, were recruited shortly after birth into the prospective longitudinal Victorian Infant Brain Study (VIBeS). Children have been followed up at 2, 5, 7 and 13 years of age. A further 31 FT participants were recruited from the community at age 2 and matched to the VPT sample based on socio-demographic information. This study included the data of 114 VPT and 36 FT children collected at the 13-year follow-up (see section 3.1 for inclusion/exclusion criteria and Table 1 for demographic details). Ethics approval was granted by the Human Research Ethics Committee at the Royal Children's Hospital in Melbourne. Parents/guardians provided written informed consent and children provided assent to participate in each follow-up.
Table 1.
Characteristics of the VPT and FT groups.
| VPT, n = 114 | FT, n = 36 | Unadjusted mean difference (95% CI) | p-Value | |
|---|---|---|---|---|
| Male, n (%) | 63 (55.2) | 17 (47.2) | χ2 = 0.71 | 0.39 |
| Age (years) at assessment, M (SD) | 13.22 (0.36) | 13.24 (0.47) | −0.04 (−0.21, 0.13) | 0.64 |
| Gestational age (weeks), M (SD) | 27.4 (1.9) | 39.1 (1.4) | NA | NA |
| Birthweight (g), M (SD) | 972 (239) | 3263 (575) | NA | NA |
| Multiple pregnancy, n (%) | 56 (49.1) | 4 (11.1) | OR 7.7 (2.6, 23.26) | <0.001 |
| BPD, n (%) | 40 (35.1) | 0 | NA | NA |
| PDA, n (%) | 53 (46.5) | 0 | NA | NA |
| Sepsis (proven), n (%) | 35 (30.7) | 0 | NA | NA |
| NEC (proven), n (%) | 5 (4.4) | 0 | NA | NA |
| Grade III/IV IVH, n (%) | 4 (3.5) | 0 | NA | NA |
| Moderate to severe WMA, n (%) | 12 (10.5) | 0 | NA | NA |
| Cystic PVL, n (%) | 3 (2.6) | 0 | NA | NA |
| General cognitive ability, M (SD) | 102.9 (14.6) | 109.9 (12.8) | −6.7 (−11.6, −1.7) | 0.008 |
| Higher social risk background, n (%) | 65 (57) | 11 (31) | OR 3.0 (1.35, 6.71) | 0.007 |
| Grade repetition, n (%) | 13 (12) | 1 (3) | OR 4.7 (0.59, 37.32) | 0.043 |
| Mathematics score, M (SD) | 94.7 (14.6) | 102.6 (15.8) | −8.1 (−14.1, −2.1) | 0.009 |
| Mathematics impairment, n (%) | 41 (36) | 8 (22) | OR 1.96 (0.82, 4.71) | 0.13 |
| Reading score, M (SD) | 104.3 (15.0) | 108.4 (16.8) | −3.9 (−10.2, 2.3) | 0.22 |
| Reading impairment, n (%) | 22 (20) | 3 (8) | OR 2.6 (0.74, 9.37) | 0.13 |
CI = confidence interval, n = number of participants, M = mean, SD = standard deviation, NA = not applicable, OR = odds ratio, BPD = bronchopulmonary dysplasia, PDA = patent ductus arteriosus, NEC = necrotising enterocolitis, IVH = intraventricular haemorrhage, WMA = white matter abnormality, PVL = periventricular leukomalacia.
2.2. Procedure
As part of the VIBeS 13-year follow-up, children completed a neuropsychological assessment and MRI scan (after completing mock MRI training), usually over two days. Assessments were administered by trained child psychologists blinded to group membership and previous assessment results. Parents completed questionnaires to gather socio-demographic information.
2.3. Measures
2.3.1. Mathematics and word reading
The Wide Range Achievement Test – 4th edition (WRAT-4) was used to assess mathematics and single word reading performance (Wilkinson and Robertson, 2006). The Math Computation subtest consisted of 40 written mathematics problems of increasing difficulty, including addition, subtraction, multiplication, division and algebra. Participants were instructed to complete as many problems as possible in 15 min. The Single Word Reading subtest required participants to read aloud a list of words of increasing complexity. The WRAT-4 has US norms stratified to be representative of the 2001 US census, with all scales having a mean of 100 and standard deviation of 15 (higher scores reflect better performance). The WRAT-4 is appropriate for individuals aged 5 years and older and has strong reliability and validity (Wilkinson and Robertson, 2006). Given the availability of a contemporaneous sample of FT Australian children, mathematics and reading impairment were defined as scores that were 1 standard deviation or more below the FT group's mean score (Johnson et al., 2009), i.e. ≤87 for mathematics and ≤92 for word reading.
2.3.2. General cognitive ability
The Kaufman Brief Intelligence Test, Second Edition (KBIT-2) was used to estimate general cognitive ability based on a composite score of three subtests assessing verbal and non-verbal IQ (Kaufman and Kaufman, 2004). The Composite IQ score has a normative mean of 100 and standard deviation of 15.
2.3.3. Social risk
Social risk information was collected in a parent questionnaire, including family structure, primary caregiver education level, employment status and occupation of the primary income earner, maternal age at birth and whether English is spoken at home. A score was calculated for each domain, with 0 representing the lowest risk and 2 the highest (Roberts et al., 2008). The maximum possible Social Risk Index score was 12. Children were considered to be from either a lower social risk background (a Social Risk Index score of 0–1) or higher social risk background (a Social Risk Index score of 2–12).
2.4. MRI acquisition
MRI was completed at the Royal Children's Hospital, Melbourne, using a Siemens Trio System 3 T scanner. Two diffusion MRI sequences were acquired. The first sequence, a CUbe and SPhere (CUSP) sequence (Scherrer and Warfield, 2012), was acquired with multiple non-zero b-values between 400 and 3000 s/mm2, 78 diffusion-weighted gradient directions, 12 b = 0 s/mm2 volumes, multi-band factor of 2, repetition time (TR) = 3500 ms, echo time (TE) = 73 ms, field of view (FOV) = 230 × 230 mm, matrix = 116 × 116 and an isotropic voxel size of 2mm3. The second diffusion sequence was acquired with a b-value = 2800 s/mm2, 60 diffusion-weighted gradient directions, 4 b = 0 s/mm2 images, TR = 3200 ms, TE =110 ms, FOV = 260 × 260 mm, matrix = 110 × 110 and an isotropic voxel size of 2.4 mm3. Along with each diffusion sequence, a pair of b = 0 s/mm2 images were acquired with reversed phase encoding.
2.5. Diffusion pre-processing
Each of the diffusion sequences were separately corrected for susceptibility-induced distortions using the reversed phase-encoding images as well as movement and eddy current-induced distortions using the Functional MRI of the Brain Software Library (FSL), version 5.0.10 (‘topup’ and ‘eddy’ tools; Andersson et al., 2003; Andersson and Sotiropoulos, 2016; Smith et al., 2004). This included b-vector reorientation (Leemans and Jones, 2009). Image quality was evaluated based on visual inspection of the diffusion images and final parameter maps (FA, AD, RD, MD and NODDI orientation dispersion and density and SMT density and intrinsic diffusivity) by an experienced MRI researcher (C·K). Participants were excluded if their images exhibited excessive motion artefact. The diffusion tensor imaging (DTI) model was fitted to the first diffusion sequence using the weighted linear least squares method. Several additional steps were required prior to NODDI and SMT model fitting. The second diffusion sequence was registered to the first diffusion sequence using the FSL Linear Image Registration Tool (FLIRT; Jenkinson et al., 2002; Jenkinson and Smith, 2001). Next, each sequence was normalised separately by its b = 0 images to attempt to account for the difference in TE and TR between the two sequences. Finally, the two sequences were merged together. The NODDI model was fitted to the combined sequences using the NODDI Matlab toolbox, version 0.9 (Zhang et al., 2012). The SMT model was fitted to the combined sequences using the developer's software package (available at https://github.com/ekaden/smt; Kaden et al., 2016).
2.6. Tract-based spatial statistics (TBSS)
The diffusion images were analysed using FSL's TBSS (Smith et al., 2006). The FA images were eroded slightly to remove likely outliers. Each participant's FA image was non-linearly aligned to every other participant's FA image (Andersson et al., 2007a; Andersson et al., 2007b), enabling the most representative ‘target’ image to be selected, which was the FA image with the minimum mean displacement required to align it to all the other FA images. The target image was affine aligned to MNI152 space and all the other FA images were brought into MNI152 space by combining the non-linear registration to the target with the affine registration to the MNI template. A mean FA image was created, along with a mean FA skeleton, with a threshold of 0.2. Each FA image was projected onto the mean FA skeleton. The original non-linear registrations were applied to the other diffusion images (axial diffusivity (AD), RD, MD, neurite density and orientation dispersion from NODDI, and neurite density and intrinsic diffusivity from SMT), which were then projected onto the mean FA skeleton.
2.7. Statistical analyses
Demographic and behavioural data were analysed using Stata 14.2 (StataCorp, 2011). Group differences on demographic variables for VPT and FT participants were examined using linear regression models for continuous variables, and logistic regression models and chi-square analyses for categorical variables. These analyses were also used to examine group differences on demographic variables between participants and non-participants (all children from the wider cohort who did not meet inclusion criteria for the current study or declined follow up). Differences in mathematics and word reading performance scores between the VPT and FT groups were examined using linear regression models. Secondary analyses were run adjusting for age at assessment (in years) and social risk. All linear regression models were fitted using generalised estimating equations reported with robust standard errors to allow for clustering of multiples within a family (i.e., twins/triplets; Carlin et al., 2005).
Voxel-wise non-parametric permutation testing was undertaken using FSL's ‘randomise’ tool, version 2.9 (Nichols and Holmes, 2002; Winkler et al., 2014). Firstly, general linear models were constructed to examine relationships between the diffusion parameters and academic performance across the total sample of VPT and FT children, adjusting for age at scan (in years). Secondly, we investigated whether the relationships between diffusion parameters and mathematics or reading scores differed between the VPT and FT groups. We did this by constructing a model with an interaction between mathematics or reading score and birth group. To explore whether the effect of VPT birth on white matter microstructure varies as a function of impairment, we performed secondary analyses using general linear models to compare diffusion parameters between VPT children with versus without an impairment in mathematics and reading. As there were only a small number of FT children with a mathematics or reading impairment and useable MRI data, we were underpowered to examine any potential group-impairment interaction.
All voxel-wise analyses were performed with 5000 permutations, family-wise error rate (FWE) correction and threshold-free cluster enhancement (Smith and Nichols, 2009). We ran both positive and negative contrasts to determine whether the microstructure measures were positively or negatively associated with mathematics and reading performance. Statistically significant voxels (p ≤.05, FWE-corrected) were labelled as being in the approximate location of particular anatomical white matter regions using the John Hopkins University (JHU) White Matter Tractography Atlas and the JHU International Consortium of Brain Mapping (ICBM) DTI-81 White Matter Labels atlas (Hua et al., 2008).
3. Results
3.1. Participant characteristics
The 13-year follow-up included 179 VPT and 61 FT children (80% and 79% retention, respectively). To ensure the relationship between academic performance and white matter microstructure was not influenced by those with low general cognitive ability (<70) at 13 years, these individuals were excluded, resulting in 16 VPT children being excluded. A further 33 VPT and 14 FT children did not have a MRI (did not consent or did not pass the mock MRI training). Of those scanned, 12 VPT and 11 FT children were excluded due to having incomplete or incorrectly acquired diffusion MRI data, and 4 VPT children were excluded due to excessive motion or other artefacts on diffusion images. Thus, the final study sample included 114 VPT children and 36 FT children who were assessed for academic performance and had useable diffusion MRI data at 13 years.
Perinatal characteristics of VPT participants were generally similar to non-participants. However, participants were more likely to have been from a multiple pregnancy and less likely to have had moderate-severe white matter abnormalities compared with non-participants (Supplementary Table 1). FT participants (n = 36) were similar to non-participants (n = 41) across all perinatal characteristics (data not shown).
At 13 years, VPT and FT groups were similar in age at assessment and sex (Table 1). Neonatal medical characteristics for VPT and FT participants are reported in Table 1. Compared with FT children, VPT children had lower general cognitive ability, were from families of a higher social risk background, and were more likely to have repeated a school grade. VPT children performed below their FT peers in mathematics (Table 1), even after controlling for age and social risk [mean difference (95% confidence interval (CI)) = −5.8 (−11.3, −0.3), p = .04]. For single word reading, the VPT group performed on average 4 points below the FT group, however, this difference was not significant (p ≥ .05). The VPT group was 2 times more likely to have a mathematics impairment and 2.6 times more likely to have a reading impairment than the FT group, though these were not significant (p ≥ .05; Table 1).
3.2. Associations between white matter microstructure and mathematics
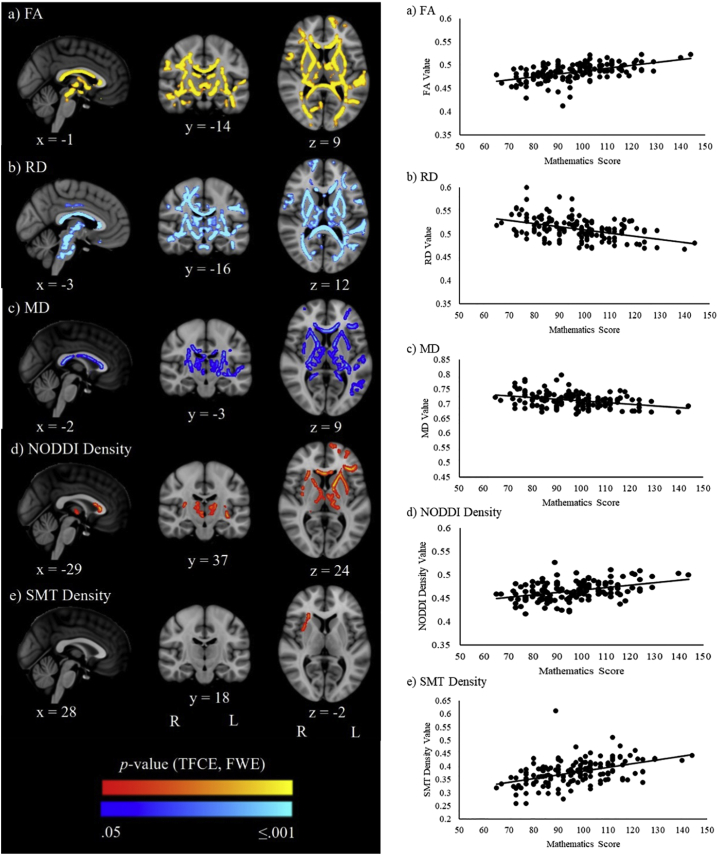
TBSS analyses revealed that higher FA, and lower RD and MD, were associated with better mathematics scores in the total sample (Fig. 1; Supplementary Fig. 1). These associations were located in many white matter regions, including the bilateral inferior fronto-occipital fasciculi, inferior longitudinal fasciculi, external and internal capsules, thalamic radiations, superior longitudinal fasciculi and corona radiata (Supplementary Table 2). Additionally, higher neurite density from NODDI and SMT were associated with better mathematics performance in several regions, including the corona radiata, corpus callosum (for NODDI and SMT), and the cerebral peduncles and left sagittal stratum (for NODDI only; Fig. 1; Supplementary Fig. 1; Supplementary Table 2). There were no associations between AD, neurite orientation dispersion or intrinsic diffusivity and mathematics performance at p ≤.05, FWE-corrected. There were also no voxels that had an interaction at p ≤.05, FWE-corrected between birth group (VPT or FT) and mathematics score for the associations with the diffusion parameters.
Fig. 1.
Left panel: TBSS results illustrating regions where white matter microstructure parameters were significantly associated with mathematics performance in the total sample (VPT and FT groups) at p ≤ .05, following family-wise error rate (FWE) correction and threshold-free cluster enhancement (TFCE). P-values in red-yellow indicate positive correlations and dark blue-light blue indicate negative correlations. P-value maps have been overlaid on the standard space (MNI152) T1-weighted image and the coordinates reported indicate standard space coordinates in mm. Right panel: The average diffusion value across all significant voxels for each participant plotted against mathematics scores. FA = fractional anisotropy, RD = radial diffusivity, MD = mean diffusivity, NODDI = neurite orientation dispersion and density imaging, SMT = spherical mean technique.
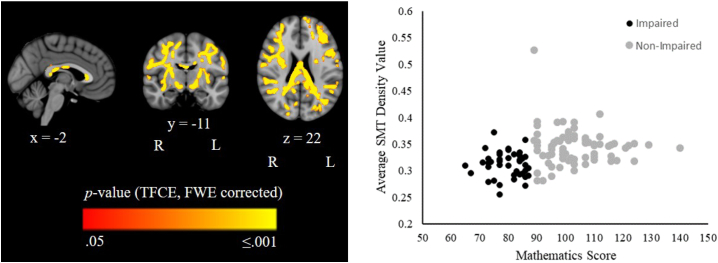
VPT children with a mathematics impairment had lower neurite density (from SMT only) in the corpus callosum, the bilateral cingulum, internal and external capsules, corona radiata, posterior thalamic radiations, sagittal stratum, cerebral peduncles and superior longitudinal fasciculi, the left fornix/stria terminalis, and the right superior fronto-occipital fasciculus and uncinate fasciculus than VPT children without a mathematics impairment (Fig. 2; Supplementary Fig. 1). There were no voxels in which FA, RD, MD, AD, neurite orientation dispersion and intrinsic diffusivity differed between VPT children with and without a mathematics impairment at p ≤.05, FWE-corrected. Average diffusion values across the mean FA skeleton for children with versus without a mathematics impairment are reported in Supplementary Table 3.
Fig. 2.
Left panel: TBSS results illustrating regions where neurite density from the spherical mean technique (SMT) was significantly lower in VPT children with versus without a mathematics impairment, at p = <.05, following family-wise error (FWE) rate correction and threshold-free cluster enhancement (TFCE). P-value maps have been overlaid on the standard space (MNI152) T1-weighted image and the coordinates reported indicate standard space coordinates in mm. Right panel: The average neurite density value across all the significant voxels, plotted separately for impaired and non-impaired VPT children.
3.3. Associations between white matter microstructure and word reading
We found no associations between any of the diffusion parameters and word reading performance in the total sample of VPT and FT children, and there were no birth group interactions, at p ≤.05, FWE-corrected. Similarly, there were no voxels in which the diffusion parameters differed between VPT children with versus without a word reading impairment at p ≤.05, FWE-corrected.
4. Discussion
This study investigated associations between whole-brain white matter microstructure using advanced models of diffusion, and academic performance in VPT and FT children at 13 years of age. For mathematics, there were widespread associations with FA, RD, MD and neurite density across VPT and FT children, however, for reading, there were no significant associations with diffusion measures. The associations between white matter microstructure, mathematics and word reading performance did not differ by birth group. Additionally, VPT children with a mathematics impairment had lower neurite density in a number of white matter regions compared to those without an impairment, but there were no differences in white matter microstructure between VPT children with and without a reading impairment.
4.1. White matter microstructural associations with mathematics
Better mathematics performance was associated with increased FA and neurite density and decreased RD and MD in widespread and bilateral white matter regions, including the hypothesised regions of the inferior fronto-occipital and longitudinal fasciculi, superior longitudinal fasciculi and corona radiata, as well as the internal and external capsules and anterior corona radiata, in VPT and FT children. These regions have previously been associated with mathematics performance in the same VPT cohort at age 7 years (Kelly et al., 2016) and in healthy individuals across a range of ages and types of mathematical tasks (Moeller et al., 2015).
In addition to DTI, we have also utilised advanced models of diffusion imaging, NODDI and SMT. The use of NODDI and SMT measures is advantageous as they provide increased specificity to white matter microstructural properties than DTI measures. The associations between DTI measures (FA, RD and MD) and mathematics performance in the total sample were more widespread across the white matter than the associations between NODDI density and mathematics performance. Given that DTI is a non-specific technique, and DTI may be more influenced by cerebrospinal fluid contamination than NODDI, due to the separate modelling of the cerebrospinal fluid compartment in NODDI (Zhang et al., 2012), it is possible that DTI may be more prone to false positive associations with mathematics performance than NODDI. It is also possible that DTI may be more sensitive than NODDI to microstructural features other than neurite density that may be related to mathematics performance, for example, myelination. While both DTI and NODDI measures may be influenced by myelination (Zhang et al., 2012), the use of additional techniques that are potentially more sensitive or specific to myelination than DTI and NODDI may improve our understanding of the microstructural factors related to mathematics performance (Deoni et al., 2008; Glasser and Van Essen, 2011; Stikov et al., 2015).
There was some agreement between the NODDI and SMT findings in the total sample, with mathematics performance being associated with neurite density from both NODDI and SMT in similar regions, including the corona radiata, external capsule and corpus callosum. However, while the SMT analyses suggested those VPT children with an impairment had lower neurite density in widespread bilateral regions compared with those without an impairment, the NODDI analyses did not. This could suggest that SMT may have greater sensitivity than NODDI for detecting differences between VPT children with and without a mathematics impairment. While NODDI and SMT both conceptually estimate neurite density, there are differences in the underlying models which may influence the estimation of neurite density. For instance, the NODDI model is based on three tissue compartments (intra- and extra-neurite and cerebrospinal fluid) and sets fixed diffusivities in the parameter estimation, whereas the SMT model is based on two tissue compartments (intra- and extra-neurite) and does not set fixed diffusivities (Zhang et al., 2012; Kaden et al., 2016; Zucchelli et al., 2018). Additionally, NODDI estimates neurite orientation distributions using a Watson distribution, while SMT is free of neurite orientation distribution models and factors out neurite orientation distribution in the parameter estimation (Zhang et al., 2012; Kaden et al., 2016; Zucchelli et al., 2018). In a comparative study of multiple microstructure models, Zucchelli et al. (2018) found that NODDI may underestimate neurite density while SMT may overestimate neurite density, and that SMT may have less fitting error than NODDI (Zucchelli et al., 2018). Differences such as these between NODDI and SMT may contribute to the differences in the NODDI and SMT results in the current study.
Because the parameters derived from DTI, NODDI and SMT are indirect measures of white matter microstructure, the mechanisms underlying the relationships between academic performance and white matter microstructure can only be speculated. The neurite density measure from NODDI and SMT is thought to reflect axonal packing, but could also be influenced by myelination (Kaden et al., 2016; Zhang et al., 2012). Indeed, both axon density and myelination have been reported to increase during childhood (Lebel and Deoni, 2018), and are speculated to be adversely influenced by injury and developmental disturbances associated with preterm birth (Volpe, 2009). Thus, better mathematics performance in childhood may be associated with higher axon density or more myelination per axon, which could enable the transmission of more information, or faster transmission of information, between brain regions involved in mathematics (Lazar, 2017).
Previous theoretical research has hypothesised a fronto-parietal network involved in mathematics tasks (Dehaene et al., 2003), which has subsequently been supported by studies using DTI and functional MRI (Klein et al., 2013; Klein et al., 2016). Within this network, higher FA and lower RD in the external and internal capsules and the left inferior fronto-occipital and longitudinal fasciculi have been related to better performance on measures of approximate arithmetic, magnitude processing, mathematical fact retrieval, computation and mathematical giftedness (Kelly et al., 2016; Klein et al., 2013; Matejko and Ansari, 2015; Navas-Sánchez et al., 2014; Rykhlevskaia et al., 2009). The external capsules have been shown to connect brain regions that are thought to be used specifically for mathematical tasks (intraparietal sulci and angular gyri), to regions that are involved in general cognitive processing (dorsolateral prefrontal cortex and the inferior frontal gyrus; Moeller et al., 2015; Navas-Sánchez et al., 2014). Further, the left inferior fronto-occipital and longitudinal fasciculi are hypothesised to relate to the visual and verbal representation of numbers, while the corona radiata has been associated with multiple types of mathematical skills (Matejko and Ansari, 2015; Moeller et al., 2015). Consistent with previous findings, we found associations between mathematics and white matter microstructure in relatively widespread regions, including these fronto-parietal white matter regions. In addition to the proposed fronto-parietal network, increased FA in the corpus callosum has been consistently associated with better mathematics performance (Barnea-Goraly et al., 2005; Navas-Sánchez et al., 2014) and decreased FA with impaired performance (Barnea-Goraly et al., 2005; Rykhlevskaia et al., 2009), which is consistent with our findings.
The current results are consistent with findings in our previous paper reporting on children in the same cohort of VPT children at 7 years of age that found associations between mathematics performance and FA in widespread white matter regions (Kelly et al., 2016). However, in contrast to the previous findings at 7 years (Kelly et al., 2016), we found an association between mathematics and neurite density (from NODDI) at 13 years of age. This discrepancy could be due to differences in sample compositions and image pre-processing methods between studies. For example, in the 7-year study, the analysis of associations between NODDI density and mathematics included VPT children only (n = 144; Kelly et al., 2016), whereas the current paper included both VPT and FT children (n = 114 and 36 respectively). Additionally, the current paper excluded participants with an IQ score < 70 and included susceptibility-induced distortion correction for the diffusion images, whereas the 7-year paper did not (Kelly et al., 2016).
4.2. White matter microstructural associations with reading
Unexpectedly we found no significant associations between reading performance and white matter microstructure in our total sample of VPT and FT children, and no significant differences when we compared microstructure measures between impaired and non-impaired VPT children. Previous research in this area is conflicting. Some studies using TBSS have found associations between FA in various white matter regions and reading in preterm children (Kelly et al., 2016; Feldman et al., 2012b) and in typically developing children (de Moura et al., 2016), whereas other studies using TBSS have not found associations between FA and reading in typically developing children (Bathelt et al., 2018). A previous study that used an alternative whole-brain approach, Voxel-Based Morphometry, found that FA in the left temporo-parietal white matter was associated with reading in typically developing children (Beaulieu et al., 2005). Other previous studies have used region of interest approaches, and have found associations between FA in various white matter regions and reading performance in typically developing (Deutsch et al., 2005; Niogi and McCandliss, 2006), and preterm children (Andrews et al., 2010; Travis et al., 2015), including the superior corona radiata, centrum semiovale (Niogi and McCandliss, 2006), the superior longitudinal fasciculus (Deutsch et al., 2005), the corpus callosum (Andrews et al., 2010) and cerebellar peduncles (Travis et al., 2015). The different results between previous studies are likely due to differing sample sizes, sample demographics (particularly age and age range), methods for measuring reading ability, MRI acquisitions, and/or image processing methods between studies. One major difference between previous studies is that some were based on typically developing children (Bathelt et al., 2018; Beaulieu et al., 2005; de Moura et al., 2016; Deutsch et al., 2005; Niogi and McCandliss, 2006), while others were based on preterm children (Kelly et al., 2016) or a combination of preterm and typically developing children (the current paper; Andrews et al., 2010; Travis et al., 2015). In the current paper, we found no significant birth group interactions on the white matter microstructure-reading associations, suggesting that associations between white matter microstructure parameters and reading score do not differ significantly between the VPT and FT groups. However, we acknowledge that we had more VPT participants than FT participants and thus, we may have been underpowered to detect birth group interactions. Further research on the neural factors related to reading performance in children is warranted.
4.3. Strengths and limitations
This study applied the advanced diffusion modelling techniques of NODDI and SMT to data from a large, prospective cohort of VPT and FT-born children. These techniques provide increased specificity to white matter microstructural features compared with traditional DTI (Zhang et al., 2012). However, NODDI has been criticised for using a Watson distribution, which may not accurately model all neurite orientation distributions, and for setting a fixed intrinsic diffusivity. This motivated our use of also including SMT, which attempts to overcome these particular limitations to NODDI (Kaden et al., 2016).
While TBSS is a well-established method for voxel-based analysis of DWI data (Bach et al., 2014), our results must be interpreted in line with the known limitations of TBSS. TBSS is dependent on accurate registration and some studies have suggested more advanced registration methods may improve alignment between images (Schwarz et al., 2014; Zhang et al., 2006). While the skeletonisation used in TBSS mitigates some of the effects of misalignment, removes the need for data smoothing, and reduces the dimensionality of the data, increasing statistical power (Bach et al., 2014; Jones et al., 2005; Smith et al., 2006), it also introduces some difficulties in interpreting results. The skeletonisation and projection steps cause the statistical sensitivity to vary by spatial location and fibre orientation (Edden and Jones, 2011), and have limited ability to assign diffusion values to the correct anatomical tract within individuals and to the same tract between participants (Bach et al., 2014). We also acknowledge that it is possible that our results were driven by the larger VPT cohort and we were underpowered to identify birth group interactions.
5. Conclusions
Widespread white matter microstructural alterations were associated with poorer mathematics performance in 13-year-old VPT and FT children. However, this may not be the case for reading performance. This study expands on the current knowledge of neurobiological correlates associated with mathematics performance, and variability in performance, in childhood.
The following are the supplementary data related to this article.
Perinatal and demographic characteristics of VPT participants and non-participants.
White matter regions associated with mathematics performance in the total sample.
Average diffusion values across the mean FA skeleton for VPT children with versus without a mathematics impairment.
TBSS results illustrating regions where white matter microstructure parameters were significantly associated with mathematics performance, at p ≤.05, following family-wise error rate (FWE) correction and threshold-free cluster enhancement (TFCE). Slices represented begin at slice z = −48, and show every 8th slice thereafter. P-values in red-yellow indicate positive correlations, with yellow representing a stronger association, and dark blue-light blue indicates negative correlations, with light blue representing a stronger association. P-value maps have been overlaid on the standard space (MNI152) T1-weighted image. Images a) through e) illustrate regions associated in the total sample (VPT and FT groups); image f) illustrates regions where neurite density from SMT was significantly lower in VPT children with versus without a mathematics impairment. FA = fractional anisotropy, RD = radial diffusivity, MD = mean diffusivity, NODDI = neurite orientation dispersion and density imaging, SMT = spherical mean technique.
Scatterplots show each participants' average diffusion value across the mean FA skeleton against their reading performance. FA = fractional anisotropy, RD = radial diffusivity, MD = mean diffusivity, NODDI = neurite orientation dispersion and density imaging, SMT = spherical mean technique.
Acknowledgements
We would like to extend our gratitude to the families that continuously support our research. We would also like to thank the Victorian Infant Brain Studies (VIBeS) team, the Developmental Imaging team and the Melbourne Children's MRI Center at the Murdoch Children's Research Institute.
This work was supported in part by the Australian National Health and Medical Research Council (NHMRC) (Project Grant 1028822 and 1024516; Centre of Clinical Research Excellence Grant 546519; Centre of Research Excellence Grant 1060733; Senior Research Fellowship 1081288 to P.A.; Early Career Fellowships 1053787 to J.C., 1012236 to D.T.; Career Development Fellowship 1085754 to D.T.), Murdoch Children's Research Institute Clinical Sciences Theme Grant, the Royal Children's Hospital, the Department of Paediatrics at the University of Melbourne, the Victorian Government Operational Infrastructure Support Program, and The Royal Children's Hospital Foundation.
Contributor Information
Simonne E. Collins, Email: simonne.collins@monash.edu.
Peter J. Anderson, Email: peter.j.anderson@monash.edu.
References
- Aarnoudse-Moens C.S., Oosterlaan J., Duivenvoorden H.J., van Goudoever J.B., Weisglas-Kuperus N. Development of preschool and academic skills in children born very preterm. J. Pediatr. 2011;158(1):51–56. doi: 10.1016/j.jpeds.2010.06.052. [DOI] [PubMed] [Google Scholar]
- Anderson P.J. Neuropsychological outcomes of children born very preterm. Seminar. Fetal Neonatal Med. 2014;19(2):90–96. doi: 10.1016/j.siny.2013.11.012. [DOI] [PubMed] [Google Scholar]
- Anderson P.J., Doyle L.W. Cognitive and educational deficits in children born extremely preterm. Seminars Perinatol. 2008;32(1):51–58. doi: 10.1053/j.semperi.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Anderson P.J., Cheong J.L., Thompson D.K. The predictive validity of neonatal MRI for neurodevelopmental outcome in very preterm children. Semin. Perinatol. 2015;39(2):147–158. doi: 10.1053/j.semperi.2015.01.008. [DOI] [PubMed] [Google Scholar]
- Andersson J.L.R., Sotiropoulos S.N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063–1078. doi: 10.1016/j.neuroimage.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J.L.R., Skare S., Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage. 2003;20(2):870–888. doi: 10.1016/S1053-8119(03)00336-7. [DOI] [PubMed] [Google Scholar]
- Andersson J.L.R., Jenkinson M., Smith S.M. FMRIB Centre; Oxford, United Kingdom: 2007. Non-linear Optimisation. [Google Scholar]
- Andersson J.L.R., Jenkinson M., Smith S.M. FMRIB Centre; Oxford, United Kingdom: 2007. Non-linear Registration Aka Spatial Normalisation. [Google Scholar]
- Andrews J.S., Ben-Shachar M., Yeatman J.D., Flom L.L., Luna B., Feldman H.M. Reading performance correlates with white-matter properties in preterm and term children. Dev. Med. Child Neurol. 2010;52(6):e94–e100. doi: 10.1111/j.1469-8749.2009.03456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Australian Institute of Health and Welfare Australia's Mothers and Babies 2016—In Brief. 2018. https://www.aihw.gov.au/getmedia/7a8ad47e-8817-46d3-9757-44fe975969c4/aihw-per-97.pdf.aspx?inline=true Retrieved from Canberra.
- Bach M., Laun F.B., Leemans A., Tax C.M.W., Biessels G.J., Stieltjes B., Maier-Hein K.H. Methodological considerations on tract-based spatial statistics (TBSS) Neuroimage. 2014;100:358–369. doi: 10.1016/j.neuroimage.2014.06.021. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N., Eliez S., Menon V., Bammer R., Reiss A.L. Arithmetic ability and parietal alterations: a diffusion tensor imaging study in velocardiofacial syndrome. Cogn. Brain Res. 2005;25(3):735–740. doi: 10.1016/j.cogbrainres.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Bathelt J., Gathercole S.E., Butterfield S., the CALM team, Astle D.E. Children's academic attainment is linked to the global organization of the white matter connectome. Dev. Sci. 2018;21(5) doi: 10.1111/desc.12662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C., Plewes C., Paulson L.A., Roy D., Snook L., Concha L., Phillips L. Imaging brain connectivity in children with diverse reading ability. Neuroimage. 2005;25(4):1266–1271. doi: 10.1016/j.neuroimage.2004.12.053. [DOI] [PubMed] [Google Scholar]
- Carlin J.B., Gurrin L.C., Sterne J.A.C., Morley R., Dwyer T. Regression models for twin studies: a critical review. Int. J. Epidemiol. 2005;34(5):1089–1099. doi: 10.1093/ije/dyi153. [DOI] [PubMed] [Google Scholar]
- Chan E., Quigley M.A. School performance at age 7 years in late preterm and early term birth: a cohort study. Arch. Dis. Child Fetal Neonatal Ed. 2014;99(6):F451–F457. doi: 10.1136/archdischild-2014-306124. [DOI] [PubMed] [Google Scholar]
- de Moura L.M., Cogo-Moreira H., de Avila C.R., Pan P.M., Gadelha A., Moriyama T.…Jackowski A.P. Children with poor reading skills at the word level show reduced fractional anisotropy in white matter tracts of both hemispheres. Brain Connect. 2016;6(7):519–523. doi: 10.1089/brain.2016.0430. [DOI] [PubMed] [Google Scholar]
- Dehaene S., Piazza M., Pinel P., Cohen L. Three parietal circuits for number processing. Cogn. Neuropsychol. 2003;20(3):487–506. doi: 10.1080/02643290244000239. [DOI] [PubMed] [Google Scholar]
- Deoni S., Rutt B., Arun T., Pierpaoli C., Jones D. Gleaning multicomponent T 1 and T 2 information from steady-state imaging data. Magn. Reson. Med. 2008;60(6):1372–1387. doi: 10.1002/mrm.21704. [DOI] [PubMed] [Google Scholar]
- Deutsch G.K., Dougherty R.F., Bammer R., Siok W.T., Gabrieli J.D.E., Wandell B. Children's reading performance is correlated with white matter structure measured by diffusion tensor imaging. Cortex. 2005;41(3):354–363. doi: 10.1016/s0010-9452(08)70272-7. [DOI] [PubMed] [Google Scholar]
- Edden R.A., Jones D.K. Spatial and orientational heterogeneity in the statistical sensitivity of skeleton-based analyses of diffusion tensor MR imaging data. J. Neurosci. Methods. 2011;201(1):213–219. doi: 10.1016/j.jneumeth.2011.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikenes L., Løhaugen G.C., Brubakk A.-M., Skranes J., Håberg A.K. Young adults born preterm with very low birth weight demonstrate widespread white matter alterations on brain DTI. Neuroimage. 2011;54(3):1774–1785. doi: 10.1016/j.neuroimage.2010.10.037. [DOI] [PubMed] [Google Scholar]
- Feldman H.M., Lee E.S., Loe I.M., Yeom K.W., Grill-Spector K., Luna B. White matter microstructure on diffusion tensor imaging is associated with conventional magnetic resonance imaging findings and cognitive function in adolescents born preterm. Dev. Med. Child Neurol. 2012;54(9):809–814. doi: 10.1111/j.1469-8749.2012.04378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman H.M., Lee E.S., Yeatman J.D., Yeom K.W. Language and reading skills in school-aged children and adolescents born preterm are associated with white matter properties on diffusion tensor imaging. Neuropsychologia. 2012;50(14):3348–3362. doi: 10.1016/j.neuropsychologia.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser M., Van Essen D. Mapping human cortical areas in vivo based on myelin content as revealed by T1- and T2-weighted MRI. J. Neurosci. 2011;31(32):11597–11616. doi: 10.1523/JNEUROSCI.2180-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua K., Zhang J., Wakana S., Jiang H., Li X., Reich D.S.…Mori S. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39(1):336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inder T.E., Wells S.J., Mogridge N.B., Spencer C., Volpe J.J. Defining the nature of the cerebral abnormalities in the premature infant: a qualitative magnetic resonance imaging study. J. Pediatr. 2003;143(2):171–179. doi: 10.1067/S0022-3476(03)00357-3. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Smith S. Global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P.R., Brady M., Smith S.M. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Johnson S., Hennessy E., Smith R., Trikic R., Wolke D., Marlow N. Academic attainment and special educational needs in extremely preterm children at 11 years of age: the EPICure study. Arch. Dis. Child Fetal Neonatal Ed. 2009;94(4):F283–F289. doi: 10.1136/adc.2008.152793. [DOI] [PubMed] [Google Scholar]
- Jones D.K., Symms M.R., Cercignani M., Howard R.J. The effect of filter size on VBM analyses of DT-MRI data. Neuroimage. 2005;26(2):546–554. doi: 10.1016/j.neuroimage.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Jones D.K., Knösche T.R., Turner R. White matter integrity, fiber count, and other fallacies: the do's and don'ts of diffusion MRI. Neuroimage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Kaden E., Kelm N.D., Carson R.P., Does M.D., Alexander D.C. Multi-compartment microscopic diffusion imaging. Neuroimage. 2016;139:346–359. doi: 10.1016/j.neuroimage.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman A.S., Kaufman N.L. Second edition. Pearson, Inc.; Bloomington, MN: 2004. Kaufman Brief Intelligence Test. [Google Scholar]
- Kelly C.E., Thompson D.K., Chen J., Leemans A., Adamson C.L., Inder T.E.…Anderson P.J. Axon density and axon orientation dispersion in children born preterm. Hum. Brain Mapp. 2016;37(9):3080–3102. doi: 10.1002/hbm.23227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein E., Moeller K., Glauche V., Weiller C., Willmes K. Processing pathways in mental arithmetic—evidence from probabilistic fiber tracking. PLoS One. 2013;8(1) doi: 10.1371/journal.pone.0055455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein E., Suchan J., Moeller K., Karnath H.O., Knops A., Wood G.…Willmes K. Considering structural connectivity in the triple code model of numerical cognition: differential connectivity for magnitude processing and arithmetic facts. Brain Struct. Funct. 2016;221(2):979–995. doi: 10.1007/s00429-014-0951-1. [DOI] [PubMed] [Google Scholar]
- Lazar M. Working memory: how important is white matter? Neuroscientist. 2017;23(2):197–210. doi: 10.1177/1073858416634298. [DOI] [PubMed] [Google Scholar]
- Lebel C., Deoni S. The development of brain white matter microstructure. Neuroimage. 2018 doi: 10.1016/j.neuroimage.2017.12.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans A., Jones D.K. The B-matrix must be rotated when correcting for subject motion in DTI data. Magn. Reson. Med. 2009;61(6):1336–1349. doi: 10.1002/mrm.21890. [DOI] [PubMed] [Google Scholar]
- Li Y., Hu Y., Wang Y., Weng J., Chen F. Individual structural differences in left inferior parietal area are associated with school childrens' arithmetic scores. Front. Hum. Neurosci. 2013;7:844. doi: 10.3389/fnhum.2013.00844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangin K.S., Horwood L.J., Woodward L.J. Cognitive development trajectories of very preterm and typically developing children. Child Dev. 2017;88(1):282–298. doi: 10.1111/cdev.12585. [DOI] [PubMed] [Google Scholar]
- Matejko A.A., Ansari D. Drawing connections between white matter and numerical and mathematical cognition: a literature review. Neurosci. Biobehav. Rev. 2015;48:35–52. doi: 10.1016/j.neubiorev.2014.11.006. [DOI] [PubMed] [Google Scholar]
- Moeller K., Willmes K., Klein E. A review on functional and structural brain connectivity in numerical cognition. Front. Hum. Neurosci. 2015;9:227. doi: 10.3389/fnhum.2015.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A.L., Thompson D.K., Pascoe L., Leemans A., Inder T.E., Doyle L.W.…Anderson P.J. White matter abnormalities and impaired attention abilities in children born very preterm. Neuroimage. 2016;124(Pt A):75–84. doi: 10.1016/j.neuroimage.2015.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas-Sánchez F.J., Alemán-Gómez Y., Sánchez-Gonzalez J., Guzmán-De-Villoria J.A., Franco C., Robles O.…Desco M. White matter microstructure correlates of mathematical giftedness and intelligence quotient. Hum. Brain Mapp. 2014;35(6):2619–2631. doi: 10.1002/hbm.22355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T.E., Holmes A.P. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum. Brain Mapp. 2002;15(1):1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niogi S.N., McCandliss B.D. Left lateralized white matter microstructure accounts for individual differences in reading ability and disability. Neuropsychologia. 2006;44(11):2178–2188. doi: 10.1016/j.neuropsychologia.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Nosarti C., Nam K.W., Walshe M., Murray R.M., Cuddy M., Rifkin L., Allin M.P.G. Preterm birth and structural brain alterations in early adulthood. NeuroImage. 2014;6:180–191. doi: 10.1016/j.nicl.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannek K., Fripp J., George J.M., Fiori S., Colditz P.B., Boyd R.N., Rose S.E. Fixel-based analysis reveals alterations is brain microstructure and macrostructure of preterm-born infants at term equivalent age. Neuroimage Clin. 2018;18:51–59. doi: 10.1016/j.nicl.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts G., Howard K., Spittle A.J., Brown N.C., Anderson P.J., Doyle L.W. Rates of early intervention services in very preterm children with developmental disabilities at age 2 years. J. Paediatr. Child Health. 2008;44(5):276–280. doi: 10.1111/j.1440-1754.2007.01251.x. [DOI] [PubMed] [Google Scholar]
- Rykhlevskaia E., Uddin L.Q., Kondos L., Menon V. Neuroanatomical correlates of developmental dyscalculia: combined evidence from morphometry and tractography. Front. Hum. Neurosci. 2009;3:51. doi: 10.3389/neuro.09.051.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer B., Warfield S.K. Parametric representation of multiple white matter fascicles from cube and sphere diffusion MRI. PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0048232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz C.G., Reid R.I., Gunter J.L., Senjem M.L., Przybelski S.A., Zuk S.M.…Alzheimer's Disease Neuroimaging I. Improved DTI registration allows voxel-based analysis that outperforms tract-based spatial statistics. Neuroimage. 2014;94:65–78. doi: 10.1016/j.neuroimage.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms V., Cragg L., Gilmore C., Marlow N., Johnson S. Mathematics difficulties in children born very preterm: current research and future directions. Archiv. Dis. Childhood. 2013;98(5):F457–F463. doi: 10.1136/archdischild-2013-303777. [DOI] [PubMed] [Google Scholar]
- Skranes J., Vangberg T.R., Kulseng S., Indredavik M.S., Evensen K.A.I., Martinussen M.…Brubakk A.M. Clinical findings and white matter abnormalities seen on diffusion tensor imaging in adolescents with very low birth weight. Brain. 2007;130(3):654–666. doi: 10.1093/brain/awm001. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Nichols T.E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E., Johansen-Berg H.…Matthews P.M. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E.…Behrens T.E.J. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- StataCorp . 2011. Stata/IC 12.0 for Windows. (College Station, TX) [Google Scholar]
- Stikov N., Campbell J.S., Stroh T., Lavelée M., Frey S., Novek J., Nuara S., Ho M.K., Bedell B.J., Dougherty R.F., Leppert I.R., Boudreau M., Narayanan S., Duval T., Cohen-Adad J., Picard P.A., Gasecka A., Côté D., Pike G.B. In vivo histology of the myelin g-ratio with magnetic resonance imaging. NeuroImage. 2015;118:397–405. doi: 10.1016/j.neuroimage.2015.05.023. [DOI] [PubMed] [Google Scholar]
- Thompson D.K., Lee K.J., Egan G.F., Warfield S.K., Doyle L.W., Anderson P.J., Inder T.E. Regional white matter microstructure in very preterm infants: predictors and 7 year outcomes. Cortex. 2014;52:60–74. doi: 10.1016/j.cortex.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D.K., Lee K.J., van Bijnen L., Leemans A., Pascoe L., Scratch S.E.…Anderson P.J. Accelerated corpus callosum development in prematurity predicts improved outcome. Hum. Brain Mapp. 2015;36(10):3733–3748. doi: 10.1002/hbm.22874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D.K., Kelly C.E., Chen J., Beare R., Alexander B., Seal M.L.…Cheong J.L.Y. Early life predictors of brain development at term-equivalent age in infants born across the gestational age spectrum. Neuroimage. 2019;185:813–824. doi: 10.1016/j.neuroimage.2018.04.031. [DOI] [PubMed] [Google Scholar]
- Travis K.E., Leitner Y., Feldman H.M., Ben-Shachar M. Cerebellar white matter pathways are associated with reading skills in children and adolescents. Hum. Brain Mapp. 2015;36(4):1536–1553. doi: 10.1002/hbm.22721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang J.M., Dougherty R.F., Deutsch G.K., Wandell B.A., Ben-Shachar M. Frontoparietal white matter diffusion properties predict mental arithmetic skills in children. Proc. Natl. Acad. Sci. U. S. A. 2009;106(52):22546–22551. doi: 10.1073/pnas.0906094106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twilhaar E.S., de Kieviet J.F., Aarnoudse-Moens C.S., van Elburg R.M., Oosterlaan J. Academic performance of children born preterm: a meta-analysis and meta-regression. Arch. Dis. Child Fetal Neonatal Ed. 2018;103(4):F322–F330. doi: 10.1136/archdischild-2017-312916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beek L., Ghesquiere P., Lagae L., De Smedt B. Left fronto-parietal white matter correlates with individual differences in children's ability to solve additions and multiplications: a tractography study. Neuroimage. 2014;90:117–127. doi: 10.1016/j.neuroimage.2013.12.030. [DOI] [PubMed] [Google Scholar]
- van Eimeren L., Niogi S.N., McCandliss B.D., Holloway I.D., Ansari D. White matter microstructures underlying mathematical abilities in children. Neuroreport. 2008;19(11):1117–1121. doi: 10.1097/WNR.0b013e328307f5c1. [DOI] [PubMed] [Google Scholar]
- Vangberg T.R., Skranes J., Dale A.M., Martinussen M., Brubakk A.-M., Haraldseth O. Changes in white matter diffusion anisotropy in adolescents born prematurely. Neuroimage. 2006;32(4):1538–1548. doi: 10.1016/j.neuroimage.2006.04.230. [DOI] [PubMed] [Google Scholar]
- Volpe J.J. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8(1):110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson G.S., Robertson G.J. Psychological Assessment Resources; Lutz, FL: 2006. Wide Range Achievement Test 4. [Google Scholar]
- Winkler A.M., Ridgway G.R., Webster M.A., Smith S.M., Nichols T.E. Permutation inference for the general linear model. Neuroimage. 2014;92:381–397. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Yushkevich P.A., Alexander D.C., Gee J.C. Deformable registration of diffusion tensor MR images with explicit orientation optimization. Med. Image Anal. 2006;10(5):764–785. doi: 10.1016/j.media.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Zhang H., Schneider T., Wheeler-Kingshott C.A., Alexander D.C. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2012;61(4):1000–1016. doi: 10.1016/j.neuroimage.2012.03.072. [DOI] [PubMed] [Google Scholar]
- Zucchelli M., Descoteaux M., Menegaz G. Computational Diffusion MRI. 2018. A generalized SMT-based framework for diffusion mri microstructural model estimation; pp. 51–63. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Perinatal and demographic characteristics of VPT participants and non-participants.
White matter regions associated with mathematics performance in the total sample.
Average diffusion values across the mean FA skeleton for VPT children with versus without a mathematics impairment.
TBSS results illustrating regions where white matter microstructure parameters were significantly associated with mathematics performance, at p ≤.05, following family-wise error rate (FWE) correction and threshold-free cluster enhancement (TFCE). Slices represented begin at slice z = −48, and show every 8th slice thereafter. P-values in red-yellow indicate positive correlations, with yellow representing a stronger association, and dark blue-light blue indicates negative correlations, with light blue representing a stronger association. P-value maps have been overlaid on the standard space (MNI152) T1-weighted image. Images a) through e) illustrate regions associated in the total sample (VPT and FT groups); image f) illustrates regions where neurite density from SMT was significantly lower in VPT children with versus without a mathematics impairment. FA = fractional anisotropy, RD = radial diffusivity, MD = mean diffusivity, NODDI = neurite orientation dispersion and density imaging, SMT = spherical mean technique.
Scatterplots show each participants' average diffusion value across the mean FA skeleton against their reading performance. FA = fractional anisotropy, RD = radial diffusivity, MD = mean diffusivity, NODDI = neurite orientation dispersion and density imaging, SMT = spherical mean technique.