Abstract
A 53-year-old woman was admitted with thyroid storm and severe behavioural problems. She had longstanding bipolar affective disorder. She was psychotic and obstructed in-patient medical management for thyroid storm. She required one-to-one psychiatric nursing and was placed under section 3 of the Mental Health Act meaning she could be detained in hospital for psychiatric treatment for up to 6 months. She underwent a total thyroidectomy. Due to her paranoid mental state, she refused treatment and the administration of thyroid hormone replacement was difficult. Postoperatively, intramuscular levothyroxine was used effectively to stabilise her thyroid function. There are no consensus guidelines on the use of parenteral levothyroxine and intramuscular levothyroxine is rarely used. This case uniquely illustrates its utility with bi-weekly blood tests showing a fast and stable response to intramuscular hormone replacement.
Keywords: endocrinology, thyroid disease, psychiatry (drugs and medicines), drugs: endocrine system, psychotic disorders (incl schizophrenia)
Background
Non-adherence among patients with hypothyroidism is a well-recognised and complex problem. Poor adherence to treatment is the most common cause of persistently elevated thyroid stimulating hormone (TSH) in those prescribed adequate doses of levothyroxine.1 Managing such patients is a therapeutic challenge for clinicians, particularly when psychiatric illness complicates the situation.
For those who lack mental capacity and refuse oral levothyroxine, weekly intramuscular levothyroxine can work well. It is, however, rarely used. This case illustrates its utility in such situations and shows that it can quickly and effectively address this therapeutic conundrum.
Case presentation
A 53-year-old woman was brought into the accident and emergency department following concerns from her family that she was increasingly withdrawn. Initial assessment found her to be feverish with a temperature of 38°C and tachycardia of 122 beats/min. She had longstanding bipolar affective disorder and HIV infection. She had a very abnormal mental state and when she was admitted she was catatonic and non-communicative.
Treatment was initiated for both sepsis and encephalitis. Despite this, she became increasingly tachycardic and tachypnoeic with ongoing fever in the 24 hours after admission and was transferred to the high dependency unit (HDU) for monitoring. Subsequent examination revealed exophthalmos, a smooth symmetrical rubbery goitre and brisk reflexes; blood tests showed that she was profoundly thyrotoxic.
Her psychiatric history included a previous in-patient admission for the treatment of bipolar affective disorder, however prior to this admission she had been stabilised on depot antipsychotic medication for 3 years with good symptom control. The psychiatric team felt that her catatonic state was precipitated by the hyperthyroidism.
Investigations
On admission, her HIV markers revealed an undetectable viral load and a CD4 count of 254×106/L. Her full blood count was within normal limits and C reactive protein was 22 mg/L (0–10). Her serum electrolytes showed a calcium of 2.73 mmol/L (2.2–2.6), sodium of 148 mmol/L (135–147) and potassium of 3.1 mmol/L (3.4–4.9) alongside normal renal function. She had mildly elevated transaminases (alanine transaminase 69 U/L (5–40), aspartate transaminase 60 U/L (5–35)) and raised creatine kinase (1220 U/L (1–165)). Multiple blood cultures were negative, although Escherichia coli was grown from her urine.
At the point of referral to the endocrine team, her TSH was <0.01 mU/L (0.3–5), free T4 47.5 pmol/L (9–19) and free T3 17.0 pmol/L (3.25–6.2); she was predominantly T3 toxic. Though thyroid peroxidase antibodies were within the normal range at 1.9 IU/mL (0–6), anti-TSH receptor antibodies were elevated at 32.0 U/mL (0–1). A diagnosis of Graves’ thyrotoxicosis was made.
Treatment
Abnormal thyroid function alongside raised muscle enzymes, marked tachycardia and fever led to the diagnosis of thyroid storm with a Burch and Wartofsky score of 45. A gradual improvement of her tachycardia, but not her mental state, occurred on HDU with 60 mg carbimazole, 120 mg propranolol and 60 mg prednisolone daily. High-dose lorazepam was initiated and antipsychotics were withheld as is recommended in catatonia.
The catatonic symptoms resolved to reveal underlying psychotic relapse, for which antipsychotics were reintroduced. When stepped-down to the ward, adherence with medications was poor. She was mostly mute but when she was able to engage with her surroundings she was frightened and paranoid, wary of staff and would hide tablets. It took one-to-one nursing, intravenous methylprednisolone and intramuscular lorazepam to manage her symptoms.
While acutely unwell with both thyroid storm and psychotic illness, the joint decision was made between the psychiatric and medical team that she lacked mental capacity and it was in her best interest to undergo a total thyroidectomy to manage the thyrotoxicosis.
Postoperatively she was commenced on intramuscular levothyroxine 500 μg/week to circumvent non-adherence. High doses of intramuscular levothyroxine put patients at risk of thyrotoxicosis and cardiac sequelae, particularly in the elderly.2 She was monitored clinically and biochemically and tolerated the treatment well. Her thyroid function and later her mood improved and she was transferred to the psychiatric ward for the management of her bipolar affective disorder.
Outcome and follow-up
During treatment with 500 μg intramuscular levothyroxine, free T4 and TSH levels were measured immediately before injection (trough) and 24 hours following injection (peak). This was done weekly for 4 weeks and then again at 7 weeks. At this point her mental health had improved such that she was able to comply with oral levothyroxine.
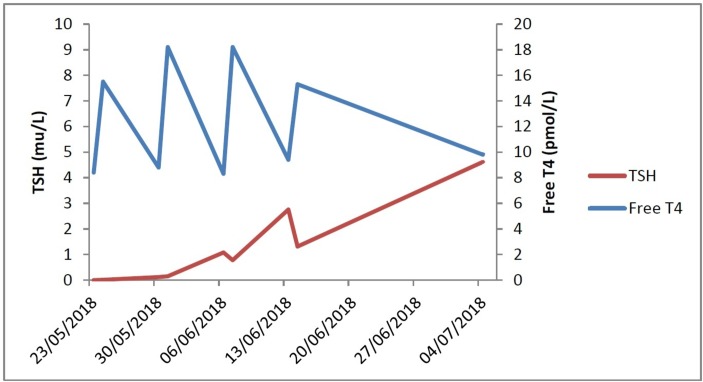
Weekly intramuscular levothyroxine delivered stable serum TSH and free T4 levels rapidly (figure 1).
Figure 1.
Trough (pre-dose) and peak (24 hours post-dose) serum TSH and free T4 response to intramuscular levothyroxine.
She has now been discharged home with regular community psychiatric input. She is adherent with oral levothyroxine.
Discussion
There are records of parenteral levothyroxine use as early as 1984,3 however just a handful of case studies since. Studies have demonstrated the successful use of intramuscular levothyroxine therapy in patients with both non-adherence4 and malabsorption.5 That being said, there are no well-established guidelines on its use6 and just five adult cases have been examined in the literature. Generally, intramuscular levothyroxine is a well-tolerated preparation but Peynirci et al described a case of reversible left-sided weakness in a patient on the treatment. In this case the prescription was 1200 μ g intramuscular given weekly and signs resolved with a dosereduction.7
The limited experience and information on the use of intramuscular levothyroxine may be the reason that this treatment is rarely utilised. In this case, her bi-weekly blood tests show the peaks and troughs in free T4 and TSH from the point of instigation up to 7 weeks. It shows that weekly dosage is adequate and that normal serum levels of free T4 and TSH can be achieved within 3 weeks and remain stable thereafter.
Learning points.
Intramuscular levothyroxine is a useful solution in non-adherent patients—especially those with mental health problems.
Stable levels of serum thyroid hormones can be achieved on weekly intramuscular levothyroxine preparations.
Footnotes
Contributors: KL wrote this article. JA, CB and CP were the consultants responsible for the care of this patient and provided feedback on drafts of this article.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Gaitonde DY, Rowley KD, Sweeney LB. Hypothyroidism: an update. Am Fam Physician 2012;86:244–51. [PubMed] [Google Scholar]
- 2. Levothyroxine Injection. 2019. https://www.drugs.com/pro/levothyroxine-injection.html.
- 3. Miyauchi A, Kataoka K, Suzuki Y, et al. [Parenteral replacement of thyroid hormones]. Nihon Naibunpi Gakkai Zasshi 1984;60:23–9. [DOI] [PubMed] [Google Scholar]
- 4. Taylor PN, Tabasum A, Sanki G, et al. Weekly intramuscular injection of levothyroxine following Myxoedema: a practical solution to an old crisis. Case Report 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kalathil D, Ranjeev S, Chattington P. Hypothyroidism treated with intramuscular thyroxine injections. Society for Endocrinology Conference. 2012.
- 6. Hays MT. Parenteral Thyroxine Administration. Thyroid 2007;17:127–9. 10.1089/thy.2006.0283 [DOI] [PubMed] [Google Scholar]
- 7. Peynirci H, Taskiran B, Erturk E, et al. Is parenteral levothyroxine therapy safe in intractable hypothyroidism? J Natl Med Assoc 2018;110:245–9. 10.1016/j.jnma.2017.05.007 [DOI] [PubMed] [Google Scholar]