Abstract
We present the case of a 65-year-old woman who was referred urgently from primary care with worsening breathlessness for 3 weeks, associated with tachycardia and left bundle branch block (LBBB). She had a background of type 2 diabetes, asthma and hypertension. Initial ECG revealed atrial fibrillation with the fast ventricular rate on the background of LBBB. ECHO findings were consistent with systolic impairment. Initial testing including checking thyroid function test revealed hyperthyroidism. It became evident that this patient had thyrotoxic cardiomyopathy. Early advice from the endocrine team was sought and the patient was treated with a combination of carbimazole and ivabradine. After a hospital stay, she made a remarkable recovery.
Keywords: arrhythmias, heart failure, thyroid disease
Background
Heart failure is a clinical syndrome, not pathological entity.1 Causes of heart failure (HF) should be carefully looked for. Though most cases of HF are caused by issues within the heart itself (pathology within the coronaries, valves, electrical pathways or myocardium), rarely HF can be caused by a non-cardiac pathology.1
In this case report, we describe a patient who presented for urgent care with worsening shortness of breath and was found to have congestive HF secondary to hyperthyroidism.
Although thyroid disorders are not uncommon, they remain a challenge to diagnose and treat effectively. In the context of cardiac disease caused by thyroid disorders, hyperthyroidism should not be missed—without proper treatment of the underlying cause, outcomes are inevitably poor. The consequences of excess thyroid hormones on the myocardium have been well described. There is a predictable increase in heart rate and contractility which leads to increased cardiac output,2 ultimately resulting in undue strain on the heart. Thus, hyperthyroidism, if left untreated, significantly increases the risk of atrial fibrillation (AF) and HF. The importance of detecting hyperthyroidism in these patients when they first present cannot be overstated. When the patient is restored to a euthyroid state and the ventricular rate is slowed the outcomes are always excellent.3 4
Every medicine physicians need to have an awareness of thyrotoxic cardiomyopathy as its presentation can mimic other common diagnoses such as acute coronary syndrome and tachycardia-induced cardiomyopathy, but management priorities are often different. Second, arrhythmic abnormalities on ECG are common, even in asymptomatic patients and any such abnormalities should be meticulously correlated with the patient clinical presentation in order to avoid erroneous judgements.
Case presentation
A 65-years-old woman was referred urgently from primary care with a history of progressively increasing shortness of breath and cough for the last 3 weeks. Her breathlessness initially started on exertion which had now progressed to being present even at rest. This was associated with a cough which was productive and contained the scanty amount of whitish sputum without any diurnal variation. She also complained of three pillow orthopnoea (she normally used one pillow to sleep) and paroxysmal nocturnal dyspnoea.
On systemic review, she revealed three stone weight loss over a period of 4 months but there was an intentional element to it. She admitted to more frequent bowel opening for the last 4–6 weeks.
She was known to have type II diabetes mellitus and occupational asthma. She was postmenopausal, a social drinker and was an ex-smoker. She was independent, fit and well, recently back from a holiday in Australia. She was not known to have any drug allergies and was on metformin, steroid inhalers and valsartan although admitted to poor medication compliance. She denied any previous hospitalisation and there was no history of exposure to asbestosis. Her family history was significant for ischaemic heart disease (father had myocardial infarction).
On examination she was alert, pale but not icteric, and was struggling to finish sentences due to shortness of breath, her respiratory rate being 24 breaths/minute. She was apyrexial, tachycardiac at 120 beats per minute, the pulse being irregularly irregular with a blood pressure of 140/100 mm Hg. Her oxygen saturation was 97% on room air. She had bibasal crackles in her lungs. The remaining clinical examinations remained unremarkable.
Her ECG showed AF with a fast ventricular rate of 116 beats with a left bundle branch block morphology (figure 1).
Figure 1.
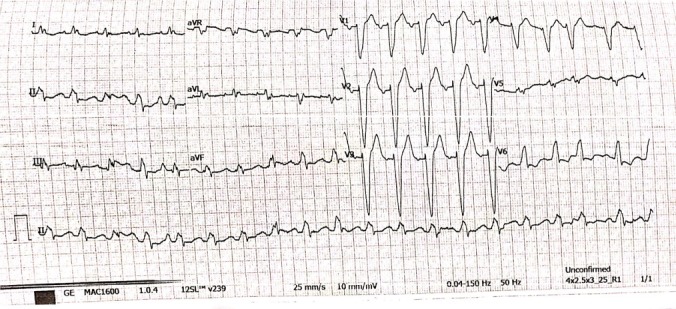
ECG.
With pending laboratory results, a preliminary diagnosis of heart failure was made. It was thought that this HF might be secondary to ischaemic heart disease, as she has significant risk factors for atherosclerosis.
Investigations
Her initial investigations were as follow.
Complete blood count: haemoglobin: 134 g/L (♀ 115–165 g/L), white cell count: 7.6×109/L (3.6–11.0×109/L), platelets 203×109/L (140–400×109/L).
Electrolytes: sodium: 138 mmol/L (133–146 mmol/L), potassium: 4.2 mmol/L (3.5–5.3 mmol/L), urea: 9.0 mmol/L (2.5–7.8 mmol/L), creatinine: 77 μmol/L (♀ 45–84 μmol/L).
Liver panel: bilirubin: 40 μmol/L (<21 μmol/L), alkaline phosphatase: 118 U/L (30–130 U/L), alanine aminotransferase: 61 U/L (♀<33 U/L), albumin: 33 g/L (♀<33 U/L).
Troponins: 20.10 ng/L and 21.60 (0 to 15.6 ng/L).
C reactive protein: 11 mg/L (<5 mg/L).
Blood glucose: 12.6 mmol (below 11.1 mmol/L).
Haemoglobin A1C: 55 mmol/mol (below 42 mmol/mol).
Lipid profile: total cholesterol 2.9 mmol/L (3.6–5 mmol/L), HDL-cholesterol: 0.6 mmol/L (1.2–9999 mmol/L).
Chest x-ray: cardiomegaly, left-sided pleural effusion, prominent pulmonary hila, appearance suggestive of early pulmonary oedema (figure 2).
Figure 2.
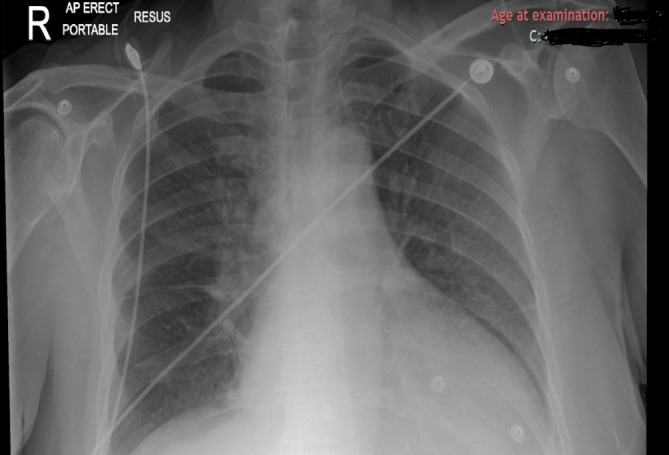
Chest x-ray.
Echocardiogram: showed severely dilated left atrium with severe impairment to overall left ventricle systolic contractility (left ventricle internal diameter (LVIDd)=5.5 cm (3.9–5.3 cm) with a severe increase in left ventricle end systolic volume and left ventricle end diastolic volume). Ejection fraction was 14% (biplane Simpson method). Moderate tricuspid regurgitation and mild mitral regurgitation (video 1).
Video 1.
Initial ECHO.
Differential diagnosis
This case is significant because it demonstrates a relatively rare clinical entity with common clinical cardiac symptoms. Initial differential diagnosis were
Silent myocardial infarction resulting in acute ischaemic cardiomyopathy.
Dilated cardiomyopathy.
Tachycardia-induced cardiomyopathy.
Hypertensive HF.
Treatment
The patient was initially treated with diuretics and transferred to cardiology ward for further management. As the patient had a new diagnosis of HF and AF, screening for wider causes of cardiomyopathy was requested including thyroid function test, serum ACE and ferritin levels.
The thyroid function test revealed:
Thyroid-simulating hormone (TSH): <0.01 (0.35–3.50 mU/L)
Thyroxine (free T4): 28.5 (7.5–21.1 pmol/L)
Triiodothyronine(free T3): 8 (3.8–6.0 pmol/L).
Thyrotoxic cardiomyopathy was diagnosed. Her Burch and Warthofsky’s Score was 40 (15 points for pulmonary oedema, 10 points each for AF and rate around 110 and 5 points for temperature) suggesting of impending thyroid storm, therefore early consultation with endocrine team was made. The patient was started on antithyroid medications (carbimazole 20 mg × once daily) beta-blocker (bisoprolol 2.5 mg × once daily), ramipril 2.5 mg × once daily and intravenous furosemide 80 mg × twice daily.
As the patient developed bronchospasm, bisoprolol was later switched to ivabradine 2.5 mg × twice daily which slowly uptitrated to 7.5 mg × twice daily. The dose of intravenous furosemide was decreased and switched to bumetanide 1 mg × once daily.
In the further course of hospitalisation, the patient’s condition improved over the next 3–4 days, with complete resolution of fluid overload and heart rate slowed down to 70 beats per minute.
Later on, further testing found to have antithyroid peroxidase antibody: 147.7 kU/L (0–34) and thyroid-stimulating antibody: 3.52 IU/L (<0.56).
Outcome and follow-up
She was followed by both cardiology and endocrinology in an outpatient clinic after 3 months and was found to have considerable improvement in her symptoms.
She was back in sinus rhythm, maintaining her heart around 50–60 beats per minute.
Her repeat echocardiogram showed moderate to severe left ventricular (LV) impairment with a decrease in tricuspid and mitral regurgitation. Her ejection fraction was improved to 37% (biplane Simpson method) (video 2).
Video 2.
Repeat ECHO.
Her repeat thyroid function test revealed TSH: 3.42 (0.35–3.50 mU/L); T4: 9.3 (7.5–21.1 pmol/L) and T3: 3.3 (3.8–6.0 pmol/L).
Her carbimazole dose was reduced to 10 mg × once daily and the patient was discharged back to primary care.
Discussion
Thyrotoxic cardiomyopathy is a rare complication of thyrotoxicosis with a high risk of fatal outcome. Cardiomyopathy has been reported as an initial presentation in 6% of patients3 though <1% developed severe LV dysfunction.5 However, it is very difficult to assess the real incidence of HF entirely related to hyperthyroidism, as ischaemic, hypertensive or other valvular heart disease have not been comprehensively excluded.
To understand the pathophysiology of HF in cases of hyperthyroidism, it is important to know that cardiovascular manifestations of thyrotoxicosis may be due to both direct effects of thyroid hormones on cardiac myocyte and on the systemic vasculature that alter haemodynamics.4 Thyroid hormones have positive inotropic and chronotropic effects on the heart along with increased adrenergic sensitivity, this may account for the increased heart rate and contractility in hyperthyroidism.6
In thyrotoxic cardiomyopathy, myocardial damage is caused by excessive thyroid hormones, in particular triiodothyronine (T3). There are a number of ways in which excessive levels of circulating T3 affect the cardiovascular system. Increased metabolic rate in myocytes leads to an increase in cardiac output due to increased myofibril contractility (stroke volume) as well as increased heart rate.7 In addition to an increase in stroke volume, there is an increase in total blood volume. T3 acts on vascular smooth muscle to cause peripheral vasodilatation2 which in turns lowers systemic vascular resistance. This then activates the renin–angiotension system (RAS) which causes fluid and salt retention. Furthermore, T3 promotes erythropoiesis resulting overall in a net increase in total blood volume in addition to stroke volume. These mechanisms all lead to a high output cardiac state which can lead to signs and symptoms of HF.
The incidence of arrhythmias in thyrotoxicosis is varied. Sinus tachycardia is the most common occurring in 42%–73% of cases8 followed by AF. There are a wide range of figures quoted from different studies about the prevalence of AF in hyperthyroidism. The figures range between 2% and 20% in comparison with 2.3% in the general population (ie, with normal thyroid function). The chances of developing AF due to hyperthyroidism increase with age and with comorbidities such as ischaemic heart disease and valvular disease.9 When viewed from another angle, it has recently been estimated that 1%–1.5% of patients with AF have evidence of hyperthyroidism.
The first-line treatment of the thyrotoxic heart is to reduce heart rate with beta-adrenergic blockade. In addition, in patients with HF, the use of digoxin and diuretics are appropriate.10 However, the definitive treatment for the hyperthyroidism is radioiodine. This is both safe and effective especially when used in conjunction with beta-adrenergic blockade. Anticoagulation of patients with hyperthyroidism and AF is controversial. The risk of systemic or cerebral embolisation must be weighed against the potential for bleeding and other complications of this therapy.11 12
The case report presented by us is unique in three distinct features when compared with previously published case reports on thyrotoxic cardiomyopathy. First, there were no signs or symptoms at initial presentation to indicate hyperthyroidism. Second, in hyperthyroidism, overt signs and symptoms of HF develop later in disease course and are more commonly seen in patients with pre-existing heart disease which was not present in our patient. Third, we successfully used ivabradine (a selective sodium channel blocker) for rate control in this patient as a second-line option after beta-blockers caused bronchospasm. This raises questions about the suitability of other second-line rate-control options, including non-dihydropyridine calcium channel blockers and digoxin. Recent studies evaluating digoxin use in AF have been associated with increased mortality.13 Preclinical studies of ivabradine in animal models demonstrated ivabradine attenuates thyroid hormone-induced reduction of myocardial deformation and altered intracellular calcium handling without modification of the myocyte hypertrophy with fibrosis.14 Although the mechanism of action of ivabradine is yet to be fully understood, there is research to suggest that it reduces conduction through the atrioventricular node without adverse haemodynamic effects (potentially found with other drugs) in patients with AF.15 Although we were able to demonstrate successful use of ivabradine in our context, further studies are necessary to further evaluate its use as a treatment option in patients with thyrotoxic cardiomyopathy.
Learning points.
Consider hyperthyroidism as an aetiological cause of cardiac dysfunction especially in the absence of overt ventricular structural abnormalities.
The ability to restore patients with thyrotoxicosis to a euthyroid state and sinus rhythm justifies thyroid function testing in most patients with a recent onset of otherwise unexplained atrial fibrillation or other supraventricular arrhythmias
Having a sound understanding of the mechanisms involved in heart failure as well as in hyperthyroidism will help the clinician in formulating a differential diagnosis based on clinical presentation and guide further management. This is important in thyrotoxic cardiomyopathy which carries significant morbidity and mortality.
Further study of the usefulness of ivabradine for rate control in atrial fibrillation is warranted
Acknowledgments
Dr Hasan Shamsi and Dr Ramalingham Srinivasan have provided valuable input in writing and submission of this manuscript. Dr Ramalingham Srinivasan was also involved in treating the patient.
Footnotes
Contributors: STA and JZ: contributed to the design and implementation of the case report and to the writing of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787–847. 10.1093/eurheartj/ehs104 [DOI] [PubMed] [Google Scholar]
- 2. Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med 2001;344:501–9. 10.1056/NEJM200102153440707 [DOI] [PubMed] [Google Scholar]
- 3. Siu CW, Yeung CY, Lau CP, et al. Incidence, clinical characteristics and outcome of congestive heart failure as the initial presentation in patients with primary hyperthyroidism. Heart 2007;93:483–7. 10.1136/hrt.2006.100628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Klein I. Endocrine disorders and cardiovascular disease : Zipes DP, Libby P, Bonow R, Mann DL, Tomaselli GF, Braunwald E, et al, Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 11th edn Philadelphia, Pa: W.B.Saunders, 2018:1813–20. [Google Scholar]
- 5. Nayak B, Burman K. Thyrotoxicosis and thyroid storm. Endocrinol Metab Clin North Am 2006;35:663–86. 10.1016/j.ecl.2006.09.008 [DOI] [PubMed] [Google Scholar]
- 6. Vargas-Uricoechea H, Bonelo-Perdomo A, Sierra-Torres CH. Effects of thyroid hormones on the heart. Clin Investig Arterioscler 2014;26:296–309. 10.1016/j.arteri.2014.07.003 [DOI] [PubMed] [Google Scholar]
- 7. Biondi B. Mechanisms in endocrinology: Heart failure and thyroid dysfunction. Eur J Endocrinol 2012;167:609–18. 10.1530/EJE-12-0627 [DOI] [PubMed] [Google Scholar]
- 8. Faizel O, Michael DG, Michael CS, et al. Cardiac dysrhythmias and thyroid dysfunction-The hidden menace? J Clin Endocrinol Metab 2002;87:963–7. [DOI] [PubMed] [Google Scholar]
- 9. Biondi B, Kahaly GJ. Cardiovascular involvement in patients with different causes of hyperthyroidism. Nat Rev Endocrinol 2010;6:431–43. 10.1038/nrendo.2010.105 [DOI] [PubMed] [Google Scholar]
- 10. Alina YU, Alekbar AB, Elena NG, et al. Thyrotoxic cardiomyopathy Russia Cardiomyopathies. Russia, 2012:553–81. [Google Scholar]
- 11. Frost L, Vestergaard P, Mosekilde L. Hyperthyroidism and risk of atrial fibrillation or flutter: a population-based study. Arch Intern Med 2004;164:1675 10.1001/archinte.164.15.1675 [DOI] [PubMed] [Google Scholar]
- 12. Danzi S, Klein I. Thyroid hormone and blood pressure regulation. Curr Hypertens Rep 2003;5:513–20. 10.1007/s11906-003-0060-7 [DOI] [PubMed] [Google Scholar]
- 13. Turakhia MP, Santangeli P, Winkelmayer WC, et al. Increased mortality associated with digoxin in contemporary patients with atrial fibrillation: findings from the TREAT-AF study. J Am Coll Cardiol 2014;64:660–8. 10.1016/j.jacc.2014.03.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim BH, Cho KI, Kim SM, et al. Heart rate reduction with ivabradine prevents thyroid hormone-induced cardiac remodeling in rat. Heart Vessels 2013;28:524–35. 10.1007/s00380-012-0304-z [DOI] [PubMed] [Google Scholar]
- 15. Turley SL, Francis KE, Lowe DK, et al. Emerging role of ivabradine for rate control in atrial fibrillation. Ther Adv Cardiovasc Dis 2016;10:348–52. 10.1177/1753944716669658 [DOI] [PMC free article] [PubMed] [Google Scholar]


