Abstract
Introduction
Adolescents with perinatally acquired HIV (PHIV) are at risk of chronic disease due to long‐standing immune suppression, HIV disease and antiretroviral therapy (ART) exposure. However, there are few data on multisystem disease in this population. We investigated the overlapping burden of neurocognitive, cardiovascular, respiratory and/or renal impairment among PHIV positive (PHIV+) adolescents.
Methods
In this cross‐sectional analysis, participants aged 9 to 14 years on ART for >6 months were recruited from seven sites across Cape Town from July 2013 through March 2015, together with age‐matched HIV‐negative (HIV‐) adolescents. Impairment at enrolment was assessed across neurocognitive functioning (using the youth‐International HIV Dementia Scale); cardiac function (echocardiogram abnormality); respiratory function (abnormal spirometry) and renal function (abnormal glomerular filtration rate).
Results and Discussion
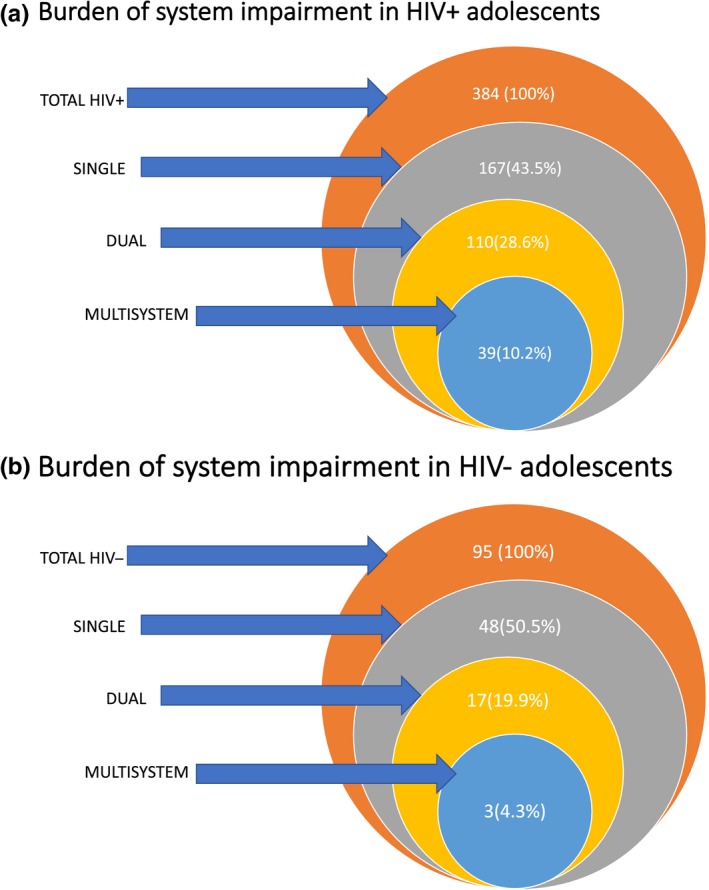
Overall, 384 PHIV+ and 95 HIV‐ adolescents were included (mean age, 11.9 years; 49% female). Median age of ART initiation was 4.2 years (IQR: 1.7 to 7.6) and median CD4 count was 709 (IQR: 556 to 944) with 302 (79%) of PHIV+ adolescents virologically suppressed. Abacavir and Zidovudine were the most commonly used nucleoside reverse transcriptase inhibitors (NRTIs) with 60% of adolescents on non‐nucleoside reverse transcriptase inhibitors (NNRTI) and 38% on a protease inhibitor (PI). Among PHIV+ adolescents, 167 (43.5%) had single system impairment only, 110 (28.6%) had two systems involved, and 39 (10.2%) had three or four systems involved. PHIV+ participants had more 2‐system and 3‐system impairment than HIV‐, 110 (28.6%) versus 17 (17.9%), p = 0.03 and 39 (10.2%) versus 3 (4.3%), p = 0.03. PHIV+ participants who had failed a year of school (73.8% vs. 46.4%, p = 0.00) and with a viral load >1000 copies/mL at enrolment (16.8% vs. 8.1%, p = 0.03) were more likely to have dual or multisystem impairment. Of those with cardiac impairment, 86.7% had an additional system impaired. Similarly, in those with neurocognitive impairment, almost 60% had additional systems impaired and of those with respiratory impairment, 74% had additional systems impaired.
Conclusions
Despite relatively early ART initiation, there is a substantial burden of multisystem chronic impairment among PHIV+ adolescents. This phenomenon needs to be further explored as this population ages and begins to engage in adult lifestyle factors that may compound these impairments.
Keywords: perinatally HIV‐positive adolescents, multisystem morbidity, antiretroviral therapy, chronic disease, Sub‐Saharan Africa
1. Introduction
There are 2.1 million adolescents aged 10 to 19 years living with HIV, the majority of whom live in sub‐Saharan Africa and have perinatally acquired infection 1, 2. This population is growing as most children on antiretroviral therapy (ART) are surviving into adolescence, due to increased access and earlier initiation of ART 3. Single system morbidity in perinatally HIV‐positive (PHIV+) adolescents is well described but there is limited data on multimorbidity in this population.
Cardiac, respiratory, neurocognitive or chronic renal impairment are common long‐term sequelae of perinatal HIV infection 4, 5, 6, 7. PHIV+ adolescents in the United States (USA) and Europe have high rates of chronic lung disease, kidney disease and neuropsychiatric problems 8. In sub‐Saharan Africa, however, diagnosis and initiation of ART occur much later than in the USA, with the median ages at first visit and at ART initiation of 7.1 and 7.9 years compared to 0.7 and 0.9 years respectively 9. HIV+ children in sub‐Saharan Africa are therefore at greater risk of chronic morbidity due to untreated HIV in childhood, long‐standing immune suppression and associated infection, suboptimal ART formulations and regimens or lack of access to care 10. The interplay between these factors along with chronic inflammation result in PHIV+ adolescents being at risk for multisystem impairment 11.
Single system morbidity has been described in resource limited settings, mostly focusing on cardiac or chronic lung disease 12, 13. A recent study from Asia showed that infectious HIV‐related morbidity was more common in younger adolescence (10 to 14 years of age) with a trend toward non‐infectious and treatment‐ related morbidity in later adolescence, however, this study did not specifically explore chronic or multisystem morbidity 14.
Despite the high prevalence of single system morbidity, there are surprisingly few data on prevalence of and risk factors for multisystem involvement in PHIV+ adolescents on ART, especially in Africa. Multisystem morbidity may be associated with worse clinical outcomes, increased healthcare utilization and more difficulty in adhering to ART due to a high pill burden. Many studies of single organ impairment are limited by small sample sizes, with no comparison group of HIV‐ adolescents and many are from the pre‐ART era. As growing numbers of PHIV+ adolescents present to overburdened health systems in resource limited countries, optimizing strategies to best care for them is crucial. The aim of this study was to investigate the prevalence of and risk factors for overlapping multisystem (neurocognitive, cardiovascular, respiratory and renal) impairment in PHIV+ adolescents in the Cape Town Antiretroviral Cohort (CTAAC).
2. Methods
2.1. Study population
This was a cross‐sectional study of PHIV+ children and adolescents enrolled in CTAAC, a longitudinal cohort study in Cape Town, South Africa. Children between 9 and 14 years on ART for more than six months were enrolled from seven sites in the Western Cape Province, South Africa with age matched HIV‐ youth of similar ancestry from July 2013 to March 2015. Children and adolescents between the ages of nine to fourteen years were considered to be perinatally infected 15. Ethical approval was given by the Faculty of Health Sciences, University of Cape Town and Stellenbosch University, Human Research Ethics Committee (051/2013). Parents gave informed consent and assent was obtained from all adolescents. All participants knew their HIV status as a pre‐requisite to study enrolment.
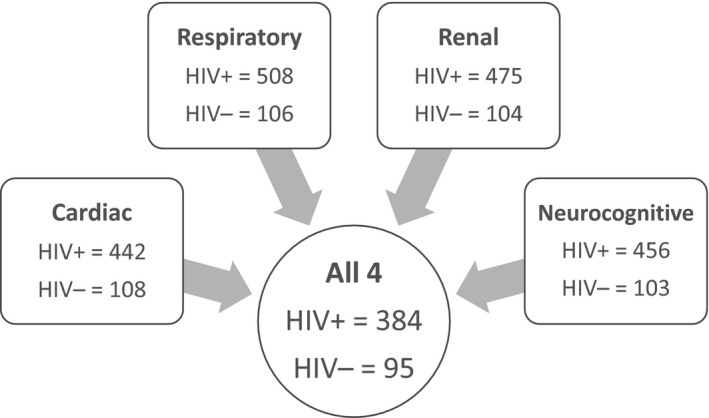
For this analysis, we included only those participants with complete respiratory, cardiac, neurological and renal assessments from the enrolment visit (Figure 1).
Figure 1. Participants with completed study measures from a total of 625 participants.
2.2. Study measures
2.2.1. Sociodemographic data and other health information
Routine sociodemographic data were collected at enrolment and each participant's clinical record was reviewed at their primary treatment facility.
Participants were screened for cardiac and respiratory symptoms such as wheeze, cough, shortness of breath and a validated respiratory questionnaire derived from the International Study of Asthma and Allergies in Childhood study was performed at enrolment 16.
A physical examination including Tanner staging, WHO HIV staging, blood pressure (BP) and anthropometry was performed at enrolment. Body Mass Index (BMI) was calculated as weight in kilogrammes divided by height in metres squared (kg/m2). BMI was classified according to WHO reference standards 17. BP was measured using an electronic sphygmomanometer (Spot Vital Signs, Welch Allyn, New York, NY, USA). All anthropometric measures were performed by one of two trained study nurses to ensure standardization of measures.
Laboratory measures performed at enrolment included viral load (COBAS Ampliprep system; Roche Molecular Systems, Branchburg, NJ, USA) and CD4 count (Beckman Coulter®, Brea, CA, USA) in HIV+ participants. Abnormal total cholesterol (TC), high‐density lipoprotein (HDL), and low‐density lipoprotein (LDL) were defined as >5.18, <1.03 and >3.37 mmol/L respectively. Abnormal triglycerides were defined as >2.85 mmol/L if age <10 years or >3.89 mmol/L if age ≥ 10 years at the time of baseline investigations 18. The Homeostatic Model Assessment (HOMA) (fasting insulin [mIU/L]×fasting glucose [mmol/L] divided by 22.5) was used to assess insulin resistance (IR) 19.
2.2.2. Cardiac measures
Echocardiograms were performed by two trained research echocardiographers using either a Philips iE33 or CX50 (Phillips, Eindhoven, The Netherlands) using standardized techniques. All echocardiograms were interpreted by a single paediatric cardiologist and a random subset was read by a second blinded paediatric cardiologist. Left ventricular shortening fraction was measured by M‐mode and ejection fraction was derived using standard methods. The ejection fraction was also measured using the modified Simpson's method 20. Left ventricular diastolic function was measured using Doppler assessment of mitral inflow. Tissue Doppler techniques were used to measure mitral annular velocity. Tricuspid annular plane excursion was measured using M‐mode 20. Pulmonary artery pressures (systolic and diastolic) were estimated using standard continuous and pulse wave Doppler methods.
Cardiac dimensions were assessed either using direct measurement of 2‐D images or M‐mode recordings.
Body surface area (BSA) was estimated using the Mosteller formula 21. Echocardiographic structural parameters were expressed as raw means as well as a deviation from the BSA‐corrected mean (z‐scores), based on normal values 22.
Cardiac impairment was defined as any one or more of the following six findings: (i) Left ventricular (LV) hypertrophy defined as a LV mass >88 g/m2 for females and >102 g/m2 for males 23 (ii) LV systolic dysfunction defined by LV shortening fraction (LVSF) ≤25% 20, (iii) LV diastolic dysfunction defined by the early to late ventricular filling ratio (E/A ratio) 24, (vi) right ventricular (RV) systolic dysfunction determined using a tricuspid annular plane systolic excursion (TAPSE) z‐score <2 25, (v) fractional RV area change (FAC) ≤34% 26 and (vi) mean pulmonary arterial pressure (mPAP) >25 mmHg 27.
2.2.3. Respiratory measures
Spirometry was done using the NDD EasyOne Pro LAB® (NDD, Switzerland). Testing adhered to the American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines 28, 29, 30. Lower limit of normal (LLN) for spirometry outcome variables was calculated using the African‐American reference cohort in global lung initiative (GLI) software, −1.64 standard deviations (SD) below the mean 31. Lung function testing was deferred if the participant had an acute respiratory illness.
Respiratory impairment was defined as a forced expiratory volume (FEV1) below the lower limit of normal (FEV1) <LLN) or FEV1/forced vital capacity (FEV1/FVC<LLN) 32.
2.2.4. Neurocognitive measures
The youth‐International HIV Dementia Scale (y‐IHDS), a sensitive screening test for neurocognitive disorders, was used to screen for cognitive impairment 33. The y‐IHDS is a 3‐part test that includes timed finger tapping, a time alternating hand sequence test and a two‐minute delayed recall of four words 34. Each participant is asked about a history of repeating a grade/grades at school, with one point subtracted from the total score for positive response. The test was conducted in the participant's home language by trained study doctors.
Neurocognitive impairment was defined as a y‐IHDS less than or equal to 10 33.
2.3. Renal measures
Enrolment blood was taken to assess creatinine and standing height was measured using a stadiometer with a moveable headboard in centimetres.
Serum creatinine was measured in μmol per litre by the enzymatic method. The modified Schwarz formula was used to estimate glomerular filtration rate (GFR) 35. Renal impairment was defined as glomerular filtration rate (GFR) below 90 mL/min/1.73 m2.
2.4. Statistical analysis
2.4.1. Primary outcomes
The primary outcomes were the number of participants with single, dual and multisystem impairment.
Single, dual and multisystem impairment were defined as having impairment of only one (single), only two (dual) or three or more (multisystem) of the following systems: cardiac, respiratory, neurological or renal.
Baseline variables and outcomes for PHIV + and HIV− adolescents were compared using t‐tests, Wilcoxon and chi‐square tests as appropriate. Among PHIV+ participants, logistic regression was performed to evaluate factors associated with having dual or multisystem impairment. Covariates considered for associations with multisystem impairment included anthropometry, a history of having had Tuberculosis (TB), HIV laboratory parameters, and duration and type of ART.
Statistical analysis was performed using Stata version 14.1. StataCorpInc. College Station, TX, USA.
3. Results
Four hundred and seventy‐nine participants (384 PHIV+ and 95 HIV−) had complete respiratory, cardiac, neurological and renal assessments from the enrolment visit.
3.1. Characteristics of participants
Of 479 participants, 384 (80%) were PHIV+ and 95 (20%) were HIV−. The mean age was 11.9 years (SD 1.6); 234 (49%) were female and 456 (95.2%) were Black African. PHIV+ participants were more likely to have a history of TB treatment (p ≤ 0.01), a history of asthma (p = 0.03), or to have failed a grade at school (p ≤ 0.01) compared to HIV−. Respiratory rate and blood pressure were significantly different although these differences were not clinically important. PHIV+ participants also had a lower BMI, height and were more stunted than HIV‐ participants (p ≤ 0.01 for all). Almost half (46.4%) PHIV+ participants were prepubertal (Tanner Stage 1) versus 32.6% of HIV‐ participants, p ≤ 0.01. Lipid abnormalities were more frequent in PHIV+ participants with 56 (14.6%) versus 3 (3.2%) having hypercholesterolaemia, p ≤ 0.01 (Table 1).
Table 1.
Clinical and laboratory characteristics of study participants
| PHIV+ (n = 384)a | HIV− (n = 95) | p‐value | |
|---|---|---|---|
| Clinical characteristics | |||
| Age (years) | 12.0 (1.7) | 11.8 (1.6) | 0.28 |
| Female | 183 (47.7) | 51 (53.7) | 0.29 |
| Black African | 361 (94.0) | 95 (100) | 0.01 |
| Previous TB treatment | 234 (61.6) | 2 (2.2) | 0.00 |
| Hospital admissions in past 12 months | 8 (2.1) | 1 (1.1) | 1.00 |
| History of wheeze | 43 (11.2) | 6 (6.3) | 0.19 |
| History of asthma diagnosis | 49 (12.8) | 4 (4.4) | 0.03 |
| History of dyspnoea | 12 (3.1) | 2 (2.1) | 1.00 |
| History of cough | 52 (13.7) | 8 (8.6) | 0.23 |
| History of failing a grade at school | 219 (57.0) | 33 (34.7) | 0.00 |
| BMI (kg/m2) | 17.1 (15.9 to 18.8) | 18.8 (16.6 to 21.5) | 0.00 |
| Height | 140.6 (10.4) | 144.6 (11.1) | 0.00 |
| Stunting | 101 (26.3) | 4 (4.2) | 0.00 |
| Puberty | |||
| Pre‐pubertal (Tanner stage 1) | 175 (46.4) | 31 (32.6) | 0.02 |
| Pubertal (Tanner stage 2 to 5) | 202 (53.6) | 64 (67.4) | |
| Laboratory measures | |||
| Triglycerides | 0.9 (0.7 to 1.1) | 0.7 (0.5 to 0.8) | 0.00 |
| Hypertriglyceridaemia | 12 (3.1) | 1 (1.1) | 0.48 |
| Total cholesterol | 4.1 (0.8) | 3.8 (0.7) | 0.00 |
| Hypercholesterolaemia | 56 (14.6) | 3 (3.2) | 0.00 |
| LDL | 2.2 (0.7) | 2.0 (0.6) | 0.02 |
| HDL | 1.5 (1.3 to 1.7) | 1.40 (1.2 to 1.7) | 0.28 |
| HOMA | 2.0 (1.3 to 3.0) | 1.9 (1.2 to 3.6) | 0.97 |
| Log HOMA | 0.3 (0.3) | 0.3 (0.4) | 0.46 |
| Viral load (copies/mL) | |||
| <50 | 302 (79.0) | ‐ | ‐ |
| 50 to 1000 | 37 (9.7) | ‐ | ‐ |
| >1000 | 44 (11.3) | ‐ | ‐ |
| CD4 count (cells/mm³) | |||
| <200 | 8 (2.1) | ‐ | ‐ |
| 200 to 499 | 54 (14.1) | ‐ | ‐ |
| ≥500 | 320 (83.8) | ‐ | ‐ |
| WHO HIV staging | |||
| Stage I | 28 (7.7) | ‐ | ‐ |
| Stage II | 38 (10.4) | ‐ | ‐ |
| Stage III | 217 (59.4) | ‐ | ‐ |
| Stage IV | 82 (22.5) | ‐ | ‐ |
| Age at initiation of ART (years) | |||
| Median age | 4.2 (1.7 to 7.6) | ‐ | ‐ |
| 0 to 2 | 151 (40.1) | ‐ | ‐ |
| 3 to 5 | 95 (25.3) | ‐ | ‐ |
| 6 to 14 | 136 (34.6) | ‐ | ‐ |
| Current ART regimen | |||
| 2 × NRTI + NNRTI | 225 (60.0) | ‐ | ‐ |
| 2 × NRTI + PI | 144 (38.0) | ‐ | ‐ |
| Other | 8 (2.1) | ‐ | ‐ |
All continuous variables expressed as median (interquartile range) or mean (SD) and categorical variables as number (%). ART, antiretroviral treatment; BMI, body mass index; HDL, high‐density lipoprotein cholesterol; HOMA, Homeostatic Model Assessment; LDL, low‐density lipoprotein cholesterol; NNRTI, non‐nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; WHO, World Health Organization.
Total number may vary according to missing data for each variable.
PHIV+ participants initiated ART at a median age of 4.2 years (IQR: 1.7 to 7.6) with a median duration of ART of 7.9 years (IQR: 4.7 to 9.5). The median CD4 count was 709 (IQR: 556 to 944) with 79% of HIV+ participants having a viral load of less than 50 copies/mL.
Two hundred and twenty‐five (60%) participants were on a non‐nucleoside reverse transcriptase inhibitor (NNRTI) based regimen and 144 (38%) were on a protease inhibitor (PI)‐based regimen with only eight (2.1%) on an alternate regimen (either lamivudine monotherapy or a combination of darunavir/ritonavir and raltegravir).
PHIV+ participants had more cardiac (46.1% vs. 33.7%, p = 0.03), respiratory (27.1%) versus 14.7%, p = 0.01) or neurocognitive impairment (56.3% vs. 45.3%, p = 0.05) than HIV‐ participants. There were few participants with renal impairment (2.3% vs. 2.1%, p = 0.89) in both groups (Table 2).
Table 2.
Types of impairment (N = 479)
| PHIV+ (N = 384) | HIV− (N = 95) | p‐value | |
|---|---|---|---|
| Any Impairment | |||
| Any cardiac, renal, respiratory or neurocognitive impairment | 316 (82.3%) | 68 (72.6) | 0.02 |
| Cardiac | 177 (46.1) | 32 (33.7) | 0.03 |
| Renal | 9 (2.3) | 2 (2.1) | 0.89 |
| Respiratory | 104 (27.1) | 14 (14.7) | 0.01 |
| Neurocognitive | 216 (56.3) | 43 (45.3) | 0.05 |
| Single system impairment | 167 (43.5) | 48 (50.5) | |
| Cardiac | 51 (13.3) | 14 (14.7) | 0.71 |
| Renal | 1 (0.3) | 1 (1.1) | 0.36 |
| Respiratory | 27 (7.0) | 8 (8.4) | 0.66 |
| Neurocognitive | 88 (22.9) | 25 (26.3) | 0.50 |
| Dual system impairment | 110 (28.6) | 17 (17.9) | 0.03 |
| Renal cardiac | 0 (0) | 0 (0) | ‐ |
| Renal respiratory | 0 (0) | 0 (0) | ‐ |
| Renal neurocognitive | 0 (0) | 0 (0) | ‐ |
| Cardiac respiratory | 20 (5.2) | 2 (2.1) | 0.20 |
| Cardiac neurocognitive | 67 (17.5) | 13 (13.7) | 0.44 |
| Respiratory neurocognitive | 23 (6.0) | 2 (2.1) | 0.20 |
| 3 system impairment | 39 (10.2) | 3 (4.3) | 0.03 |
| Renal cardiac respiratory | 1 (0.3) | 0 (0) | ‐ |
| Renal neurocognitive respiratory | 0 (0) | 0 (0) | ‐ |
| Renal neurocognitive cardiac | 5 (1.3) | 1 (1.1) | 1.00 |
| Cardiac neurocognitive respiratory | 31 (8.1) | 2 (2.1) | 0.04 |
| 4 system impairment | |||
| Renal respiratory neurocognitive cardiac | 2 (0.5) | 0 (0) | 1.000 |
Two hundred and fifteen (44.9%), of all adolescents had single system impairment only. One hundred and twenty‐seven (26.5%) and 42 (8.8%) had dual and multisystem (three or four systems) involved respectively. PHIV+ participants had more “dual system” (28.6% vs. 17.9%, p = 0.03) and “multisystem” impairment (10.2% vs. 4, p = 0.03) than HIV‐ (Table 2 and Figure 2).
Figure 2. Burden of system impairment in (A) HIV+ and (B) HIV− adolescents.
3.2. PHIV+ participants
Neurocognitive impairment was the most common type of single system impairment.
Overall, a low TAPSE and FAC, indicative of right ventricular dysfunction, accounted for the majority of echocardiogram abnormalities with 104 (27.1%) and 42 (10.9%) of those PHIV+ adolescents that had cardiac impairment having an abnormal TAPSE and FAC respectively. Left heart dysfunction was rare with only one PHIV+ adolescent having a decreased LVSF and 32 (8.3%) having evidence of left ventricular diastolic dysfunction. No participant had dilated cardiomyopathy and 2 (0.5%) PHIV+ adolescents had raised pulmonary artery pressure. Ninety ‐seven (25.3%) PHIV+ participants had an abnormal FEV1 and 35 (9.1%) had a FEV 1/FVC<LLN.
The most common patterns of dual and multisystem impairment were “cardiac and neurocognitive” and “cardiac, neurocognitive and respiratory” impairment. Only 2 (0.5%) PHIV+ participants had impairment of all four systems. In participants with any impairment, the majority had additional system impairment: Of those found to have cardiac impairment 126/177 (86.7%) had an additional system impaired. Similarly, in those with neurocognitive impairment almost 60% had additional systems impaired and of those with respiratory impairment 74% had additional systems impaired.
In univariate analysis having failed a grade or having a viral load >1000 copies/mL at enrolment, were more common in those with dual or multisystem impairment compared to those with no impairment or single system impairment, 73.8% versus 46.4%, p = 0.00 and 16.8% versus 8.1%, p = 0.03 (Table 3). Participants with dual and multisystem disease were also older 12.2 years versus 11.8 years, p = 0.02.
Table 3.
No impairment or single system impairment versus dual or multisystem impairment in HIV‐infected participants
| None/single system (n = 235) | Dual/multisystem (n = 149) | p‐value | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 11.8 (1.6) | 12.2 (1.6) | 0.02 |
| Female | 121 (51.5) | 62 (41.6) | 0.06 |
| History and symptoms | |||
| Wheeze | 21 (8.9) | 22 (14.8) | 0.08 |
| Shortness of breath | 7 (3.0) | 5 (3.4) | 1.00 |
| Cough | 26 (11.1) | 26 (17.8) | 0.07 |
| Repeated a year at school | 109 (46.4) | 110 (73.8) | 0.00 |
| Previous TB | 135 (58.2) | 99 (66.9) | 0.09 |
| Age at ART initiation | |||
| Median age | 4.0 (1.7 to 7.7) | 4.4 (2.1 to 7.0) | 0.60 |
| 0 to 2 | 97 (42.4) | 54 (36.7) | 0.16 |
| 3 to 5 | 50 (21.8) | 45 (30.6) | |
| 6 to 14 | 82 (35.8) | 48 (32.7) | |
| Current ART regimen | |||
| 2 × NRTI + NNRTI | 134 (58.3) | 91 (61.9) | 0.09 |
| 2 × NRTI + PI | 94 (40.9) | 50 (34.0) | |
| Other | 2 (0.9) | 6 (4.1) | |
| Growth measures | |||
| BMI (kg/m2) | 17.2 (16.0 to 19.0) | 16.9 (15.7 to 18.6) | 0.12 |
| Height | 140.3 (10.0) | 141.2 (10.9) | 0.41 |
| Stunted | 54 (23.0) | 47 (31.5) | 0.06 |
| Puberty | |||
| Pre‐pubertal (Tanner stage 1) | 109 (47.4) | 66 (44.9) | 0.64 |
| Pubertal (Tanner stage 2 to 5) | 121 (52.6) | 81 (55.1) | |
| WHO stage | |||
| Stage I | 18 (8.0) | 10 (7.2) | 0.90 |
| Stage II | 25 (11.1) | 13 (9.4) | |
| Stage III | 131 (58.0) | 86 (61.9) | |
| Stage IV | 52 (23.6) | 30 (21.6) | |
| Laboratory measures | |||
| Viral Load (copies/mL) | |||
| <50 | 192 (82.1) | 110 (73.8) | 0.03 |
| 50 to 1000 | 23 (9.8) | 14 (9.4) | |
| >1000 | 19 (8.1) | 25 (16.8) | |
| CD4 count <200 (cells/mm³) | 5 (2.2) | 3 (2.0) | 0.50 |
In multivariable analysis failing a grade was associated with dual or multisystem disease (OR = 3.2, CI 2.1 to 5.1, p ≤ 0.01).
4. Discussion
This is the first study to report on multisystem impairment in African PHIV+ children and young adolescents on ART. The study found the prevalence of any cardiac, respiratory or neurocognitive impairment in PHIV+ participants was significantly higher than in HIV− participants. Similar findings of individual system involvement have been reported in other studies of adolescents with perinatally acquired HIV with a high prevalence of cardiac, respiratory, neurocognitive or less commonly renal impairment 5, 36, 37.
Neurocognitive impairment occurring most commonly, is perhaps the most concerning morbidity, impacting on PHIV+ adolescents as it influences all spheres of health including treatment adherence and school performance. The measure used, the y‐IHDS, is only a screening test and cannot definitively diagnose the type or extent of neurocognitive impairment. The score has been validated previously in a subset of this cohort and shown to be sensitive for neurocognitive disorder screening 33. In addition, it is quick and easily performed in busy clinic settings. We found a prevalence of neurocognitive impairment of 56% using y‐IHDS, similar to the 45% of HIV+ youth that met criteria for neurocognitive disorder diagnosis through an extensive battery of neurocognitive tests in the same setting 38. However, in other African settings, the adult IHDS score overestimated the burden of neurocognitive impairment 39. Follow‐up data from our adolescent cohort will be valuable to assess whether the y‐IHDS screening tool correlates with confirmed impairment.
Cardiac impairment was the second most commonly affected system. This was based on echocardiogram parameters reflecting subtle right ventricular dysfunction. The majority of these participants were asymptomatic with no difference between PHIV+ and HIV− participants. These results are consistent with a Spanish study showing subtle cardiac abnormalities found on echocardiogram and no difference in the control population 4. but the authors did not report on right heart dysfunction. Lower TAPSE has been reported in HIV+ young adults but not in HIV− controls 40. Follow‐up of all participants in our study may indicate if these findings are clinically relevant as both TAPSE and FAC are more useful in longitudinal studies 41, 42.
Respiratory single system impairment was reflected by subtle reduction in lung function that may impact on adult lung health. Our findings are consistent with the prevalence of abnormal spirometry, reported as between 24% and 38% in various African adolescent cohorts from Zimbabwe, Malawi or South Africa 32, 43, 44.
Renal impairment was surprisingly rare given the genetic predisposition to HIV nephropathy in Black Africans but may reflect survivor bias as those with more severe kidney disease may have died or already being followed at specialized renal clinics. The prevalence of proteinuria and microalbuminuria, risk factors for chronic kidney disease has been shown to be high in HIV+ adolescents, however, we previously reported no difference in these measurements between PHIV+ and HIV− adolescents in this cohort 45.
Dual system impairment affected about a quarter of participants but multisystem involvement was relatively uncommon. This may reflect that the cohort were ambulatory and relatively well, were on ART for several years and were adherent to therapy. Despite this, there remains a small but clinically significant proportion of adolescents that will need complex clinical services to ensure optimum care. In addition, a significant proportion of those with impairment in one system had another system involved.
Multivariable analysis found that failing a grade at school and age at enrolment was significantly associated with dual or multisystem disease. School failure may be due to neurocognitive impairment or chronic or prolonged illness and hospitalization resulting in missing significant periods of the school year 46. As this was a cross‐sectional study we were unable to infer that multisystem disease had a causal relationship with school failure. Checking for school failure may be helpful in deciding whom to screen for multisystem impairment.
There was no evidence for the association of duration or for the relatively later start of ART and dual or multisystem impairment. A possible explanation for later start of ART not being associated with multisystem impairment is that historically children that were started on early ART prior to the guidelines recommending early start for all were severely ill and this illness may have resulted in multisystem impairment despite early access to ART. In addition, treatment histories were often not available or difficult to interpret. Reasons for switching ART regimens were poorly documented and it was not possible to accurately assess viral suppression prior to study enrolment.
The study is limited by the cross‐sectional analysis that limited the ability to detect longitudinal changes. Only four systems were included, and a more comprehensive assessment of system involvement including hearing, dermatological complications of HIV and musculoskeletal abnormalities may be useful.
An additional limitation is that the measures of impairment that we chose for each system differ in their ability to assess severity of impairment with the y‐IHDS score being a relatively crude estimate of neurocognitive impairment compared to detailed assessment of lung function that is obtained with spirometry. Severity of impairment across different systems thus cannot be directly compared.
5. Conclusions
In those PHIV+ adolescents that had one system impaired a significant proportion had another system involved. Although multisystem impairment is relatively rare, a small minority of youth will require clinical attention for complex multisystem issues. Young adolescents with system impairment will need close observation as they transition to adulthood and are increasingly at risk for engaging in adult lifestyle factors such as smoking or recreational drug use. Adult onset diabetes and hypertension may also compound these impairments. Longitudinal follow‐up is needed to ascertain whether system impairment may impact long‐term morbidity.
Competing interest
All authors declare no competing interest.
Authors’ contributions
LJF contributed to the initial concept of the paper, did the statistical analysis and wrote the manuscript. KB did statistical analysis. SM contributed towards data management and did statistical analysis. LG, DG performed and interpreted spirometry and were involved in the initial pulmonology concept of CTAAC, LZ read echocardiograms and contributed to the initial cardiology concept of CTAAC, PN gave input on renal measures, DJS and JH gave input on neurocognitive concepts, MFC, LM, HJZ were involved in the initial concept of the paper and obtained funding for CTAAC. All authors have read and approved the final manuscript.
Acknowledgements
The authors acknowledge the CTAAC co‐investigators: Helena Rabie, James Nuttall, Brian Eley, Linda Gail‐Bekker, Paul Roux and the CTAAC study staff, caregivers and adolescents.
Funding
Study was funded by NIH RO1HD074051. LJF is supported by the South African Medical Research Council. HZ is supported by the South African Medical Research Council. The Cape Town Adolescent Antiretroviral Cohort is supported by National Institutes of Health grant R01HD074051 and the South African Medical Research Council.
Frigati, L. J. , Brown, K. , Mahtab, S. , Githinji, L. , Gray, D. , Zühlke, L. , Nourse, P. , Stein, D. J. , Hoare, J. , Cotton, M. F. , Myer, L. and Zar, H. J. Multisystem impairment in South African adolescents with Perinatally acquired HIV on antiretroviral therapy (ART). J Int AIDS Soc. 2019; 22:e25386
References
- 1. Slogrove AL, Sohn AH. The global epidemiology of adolescents living with HIV: time for more granular data to improve adolescent health outcomes. Curr Opin HIV AIDS. 2018;13(3):170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. UNAIDS . 2017 Estimates. Geneva, Switzerland: UNAIDS; 2017. [Google Scholar]
- 3. Armstrong A, Nagata JM, Vicari M, Irvine C, Cluver L, Sohn AH, et al. A global research agenda for adolescents living With HIV. J Acquir Immune Defic Syndr. 2018;78(1):S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sainz T, Álvarez‐Fuente M, Fernández‐Jiménez R, González‐Tomé MI, de José MI, Ramos JT, et al. Cardiac function in vertically HIV‐infected children and adolescents in the era of highly active antiretroviral therapy. Pediatr Infect Dis J. 2015;34(5):e125–31. [DOI] [PubMed] [Google Scholar]
- 5. Rylance J, McHugh G, Metcalfe J, Mujuru H, Nathoo K, Wilmore S, et al. Chronic lung disease in HIV‐infected children established on antiretroviral therapy. Aids. 2016;30(18):2795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Laughton B, Cornell M, Boivin M, Van Rie A. Neurodevelopment in perinatally HIV‐infected children: a concern for adolescence. J Int AIDS Soc. 2013;16(1):18603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leão FVF, de Menezes Succi RC, Machado DM, Gouvêa AdFTB, do Carmo FB, Beltrão SV, et al. Renal abnormalities in a cohort of HIV‐infected children and adolescents. Pediatr Nephrol. 2016;31(5):773–8. [DOI] [PubMed] [Google Scholar]
- 8. Sohn AH, Hazra R. The changing epidemiology of the global paediatric HIV epidemic: keeping track of perinatally HIV‐infected adolescents. J Int AIDS Soc. 2013;16(1):18555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Slogrove AL, Schomaker M, Davies M‐A, Williams P, Balkan S, Ben‐Farhat J, et al. The epidemiology of adolescents living with perinatally acquired HIV: a cross‐region global cohort analysis. PLoS Med. 2018;15:e1002514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lowenthal ED, Bakeera‐Kitaka S, Marukutira T, Chapman J, Goldrath K, Ferrand RA. Perinatally acquired HIV infection in adolescents from sub‐Saharan Africa: a review of emerging challenges. Lancet Infect Dis. 2014;14(7):627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mofenson LM, Cotton MF. The challenges of success: adolescents with perinatal HIV infection. J Int AIDS Soc. 2013;16(1):18650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Majonga ED, Rehman AM, Simms V, Mchugh G, Mujuru HA, Nathoo K, et al. High prevalence of echocardiographic abnormalities in older HIV‐infected children taking antiretroviral therapy. AIDS. 2018;32(18):2739–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Desai SR, Nair A, Rylance J, Mujuru H, Nathoo K, McHugh G, et al. HIV‐associated chronic lung disease in children and adolescents in Zimbabwe: chest radiographic and high‐resolution computed tomography findings. Clin Infect Dis. 2018;66(2):274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bartlett AW, Mohamed TJ, Sudjaritruk T, Kurniati N, Nallusamy R, Hansudewechakul R, et al. Disease‐and Treatment‐Related Morbidity in Adolescents With Perinatal HIV Infection in Asia. Pediatr Infect Dis J. 2019;38(3):287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tsondai P, Sohn A, Phiri S, Vinikoor M, Sawry S, Chimbete C, et al., editor. An algorithm to determine likely mode of infection in adolescents living with HIV enrolling in care at age 10‐15 years. International Workshop on HIV Paediatrics, 20‐21 July 2018; Amsterdam, the Netherlands; 2018.
- 16. Asher M, Keil U, Anderson H, Beasley R, Crane J, Martinez F, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8(3):483–91. [DOI] [PubMed] [Google Scholar]
- 17. Onis MD, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school‐aged children and adolescents. Bull World Health Organ. 2007;85(9):660–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. National Heart, Lung and Blood Institute . Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(Suppl 5):S213–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R. Homeostasis model assessment: insulin resistance and β‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. [DOI] [PubMed] [Google Scholar]
- 20. Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK, et al. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr. 2010;23(5):465–95. [DOI] [PubMed] [Google Scholar]
- 21. Mosteller RD. Simplified calculation of body‐surface area. N Engl J Med. 1987;317(17):1098 10.1056/NEJM198710223171717 [DOI] [PubMed] [Google Scholar]
- 22. Pettersen MD, Du W, Skeens ME, Humes RA. Regression equations for calculation of z scores of cardiac structures in a large cohort of healthy infants, children, and adolescents: an echocardiographic study. J Am Soc Echocardiogr. 2008;21(8):922–34. [DOI] [PubMed] [Google Scholar]
- 23. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7(2):79–108. [DOI] [PubMed] [Google Scholar]
- 24. Eidem BW, McMahon CJ, Cohen RR, Wu J, Finkelshteyn I, Kovalchin JP, et al. Impact of cardiac growth on Doppler tissue imaging velocities: a study in healthy children. J Am Soc Echocardiogr. 2004;17(3):212–21. [DOI] [PubMed] [Google Scholar]
- 25. Koestenberger M, Ravekes W, Everett AD, Stueger HP, Heinzl B, Gamillscheg A, et al. Right ventricular function in infants, children and adolescents: reference values of the tricuspid annular plane systolic excursion (TAPSE) in 640 healthy patients and calculation of z score values. J Am Soc Echocardiogr. 2009;22(6):715–9. [DOI] [PubMed] [Google Scholar]
- 26. Lai WW, Gauvreau K, Rivera ES, Saleeb S, Powell AJ, Geva T. Accuracy of guideline recommendations for two‐dimensional quantification of the right ventricle by echocardiography. Int J Cardiovasc Imaging. 2008;24(7):691–8. [DOI] [PubMed] [Google Scholar]
- 27. Chemla D, Castelain V, Provencher S, Humbert M, Simonneau G, Hervé P. Evaluation of various empirical formulas for estimating mean pulmonary artery pressure by using systolic pulmonary artery pressure in adults. Chest. 2009;135(3):760–8. [DOI] [PubMed] [Google Scholar]
- 28. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–38. [DOI] [PubMed] [Google Scholar]
- 29. Pellegrino R, Viegi G, Brusasco V, Crapo R, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–68. [DOI] [PubMed] [Google Scholar]
- 30. Wanger J, Clausen J, Coates A, Pedersen O, Brusasco V, Burgos F, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26(3):511. [DOI] [PubMed] [Google Scholar]
- 31. Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi‐ethnic reference values for spirometry for the 3–95‐yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Githinji LN, Gray DM, Hlengwa S, Myer L, Zar HJ. Lung function in South African adolescents infected perinatally with HIV and treated long‐term with antiretroviral therapy. Ann Am Thorac Soc. 2017;14(5):722–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Phillips NJ, Thomas KG, Myer L, Sacktor N, Zar HJ, Stein DJ, et al. Screening for HIV‐associated neurocognitive disorders in perinatally infected adolescents: y‐IHDS validation. AIDS. 2019;33:5–824. [DOI] [PubMed] [Google Scholar]
- 34. Sacktor NC, Wong M, Nakasujja N, Skolasky RL, Selnes OA, Musisi S, et al. The International HIV Dementia Scale: a new rapid screening test for HIV dementia. AIDS. 2005;19(13):1367–74. [PubMed] [Google Scholar]
- 35. Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Namuyonga J, Lubega S, Musiime V, Lwabi P, Lubega I. Cardiac dysfunction among Ugandan HIV‐infected children on antiretroviral therapy. Pediatr Infect Dis J. 2016;35(3):e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Phillips NJ, Hoare J, Stein DJ, Myer L, Zar HJ, Thomas KG. HIV‐associated cognitive disorders in perinatally infected children and adolescents: a novel composite cognitive domains score. AIDS Care. 2018;30:1–9. [DOI] [PubMed] [Google Scholar]
- 38. Hoare J, Phillips N, Joska JA, Paul R, Donald KA, Stein DJ, et al. Applying the HIV‐associated neurocognitive disorder diagnostic criteria to HIV‐infected youth. Neurology. 2016;87(1):86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Milanini B, Paul R, Bahemana E, Adamu Y, Kiweewa F, Langat R, et al. Limitations of the International HIV Dementia Scale in the current era. AIDS. 2018;32(17):2477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Junsirimongkol B, Cheewatanakornkul S, Jamulitrat S, Theres L, Huebscher A, Stangl K, et al. HIT Poster session 2. Eur Heart J Cardiovasc Imaging. 2019;20 Supplement_1:i363–81. [Google Scholar]
- 41. Sato T, Tsujino I, Ohira H, Oyama‐Manabe N, Yamada A, Ito YM, et al. Validation study on the accuracy of echocardiographic measurements of right ventricular systolic function in pulmonary hypertension. J Am Soc Echocardiogr. 2012;25(3):280–6. [DOI] [PubMed] [Google Scholar]
- 42. Raj R, Puri GD, Jayant A, Thingnam SKS, Singh RS, Rohit MK. Perioperative echocardiography‐derived right ventricle function parameters and early outcomes after tetralogy of Fallot repair in mid‐childhood: a single‐center, prospective observational study. Echocardiography. 2016;33(11):1710–7. [DOI] [PubMed] [Google Scholar]
- 43. Rylance J, Mwalukomo T, Rylance S, editors. Lung function and bronchodilator response in perinatally HIV‐infected Malawian adolescents. 19th Conference on Retroviruses and Opportunistic Infections; 2012.
- 44. Mwalukomo T, Rylance SJ, Webb EL, Anderson S, O'hare B, van Oosterhout JJ, et al. Clinical characteristics and lung function in older children vertically infected with human immunodeficiency virus in Malawi. J Pediatr Infect Dis Soc. 2015;5(2):161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Frigati L, Mahtab S, Nourse P, Ray P, Perrazzo S, Machemedze T, et al. Prevalence of risk factors for chronic kidney disease in South African youth with perinatally acquired HIV. Pediatr Nephrol. 2019;34:313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nachman S, Chernoff M, Williams P, Hodge J, Heston J, Gadow KD. Human immunodeficiency virus disease severity, psychiatric symptoms, and functional outcomes in perinatally infected youth. Arch Pediatr Adolesc Med. 2012;166(6):528–35. [DOI] [PMC free article] [PubMed] [Google Scholar]


