Abstract
Background
The presence of extraintestinal manifestations may be associated with the development of pouchitis in patients with ulcerative colitis after ileal pouch–anal anastomosis. The aim of this study was to assess this correlation.
Methods
A systematic literature search was performed using MEDLINE and the Cochrane Library. Studies published in English up to 22 May 2017 investigating the association between extraintestinal manifestations and development of pouchitis in adults with ulcerative colitis were included. Case reports were excluded. The association of extraintestinal manifestations with the development of overall and chronic pouchitis was investigated using a random‐effects model.
Results
Of 1010 citations identified, 22 observational studies comprising 5128 patients were selected for analysis. The presence of extraintestinal manifestations was significantly associated with both chronic pouchitis (odds ratio 2·28, 95 per cent c.i. 1·57 to 3·32; P = 0·001) and overall pouchitis (odds ratio 1·96, 1·49 to 2·57; P < 0·001).
Conclusion
The presence of extraintestinal manifestations is associated with development of pouchitis after ileal pouch–anal anastomosis.
Introduction
Several treatment options are currently available for patients with ulcerative colitis1, 2. Many patients still require surgical resection for intractable disease or the development of colorectal cancer3. The standard surgical treatment for ulcerative colitis is restorative proctocolectomy and ileal pouch–anal anastomosis (IPAA). Anal function after surgery is generally satisfactory4. Minimally invasive surgery using a laparoscopic or robotic technique is also an option for IPAA5, 6. Pouchitis is a common complication after IPAA, developing in 10–58 per cent of patients according to previous reports7, 8.
Pouchitis is an idiopathic inflammation of the ileal pouch. First‐line treatment for pouchitis is antibiotics such as metronidazole and ciprofloxacin9. One‐third to one‐half of patients with pouchitis, however, experience chronic pouchitis. There are no evidence‐based treatments for chronic refractory pouchitis10, and treatments used for ulcerative colitis have been used empirically to treat chronic pouchitis. Consequently, in extreme cases of intractable chronic pouchitis, diversion or resection of the ileal pouch may be necessary.
Several risk factors for pouchitis have been reported in small retrospective cohorts; they include the presence of extraintestinal manifestations (EIMs)11, 12, primary sclerosing cholangitis (PSC)13, serological markers and smoking habit14. In a meta‐analysis15, the association between smoking habit and pouchitis was not confirmed. The presence of perinuclear antineutrophil cytoplasmic antibodies is a risk factor for the development of chronic pouchitis16. The present authors have reported previously12, 17 that the presence of EIMs was an independent risk factor for the development of pouchitis. Although some articles11, 13 have shown a similar association, this association has not been clarified. The aim of the present study was to assess the association between the presence of EIMs and the development of pouchitis in patients with ulcerative colitis after IPAA.
Methods
This systematic review and meta‐analysis was conducted according to PRISMA guidelines18.
Literature search and eligibility criteria
Ovid MEDLINE and Cochrane Library databases were searched systematically to identify relevant articles published to May 2017. Studies investigating preoperative risk factors for the development of pouchitis were searched for using the search terms ‘ulcerative colitis’ and ‘pouchitis’ or ‘pouch surgery’ or ‘ileal pouch anal anastomosis’ or ‘IPAA’ and ‘complication’.
The search was limited to RCTs and observational studies written in English. Studies with information on the presence of EIMs in relation to the development of pouchitis were included. Studies on familial adenomatous polyposis were excluded. The diagnostic criteria of pouchitis are essential, and it is recommended19, 20, 21 that the diagnosis of pouchitis is based on clinical symptoms and endoscopic confirmation of inflammation in the ileal pouch. Thus, publications in which the diagnosis was made without endoscopic features were excluded. Non‐English studies, case reports, and studies that focused only on children were also excluded.
PSC is one of the major EIMs in Western countries, and its association with pouchitis is the most frequently investigated among all EIMs. Thus, an additional attempt was made to elucidate the association of PSC and pouchitis.
Study selection and data extraction
Two authors independently checked the titles and abstracts to exclude irrelevant studies and retrieve relevant articles. In the case of multiple publications from one institution, only studies for which the study periods did not overlap with one another were included, to avoid the duplication of cases. The reference lists of included articles were screened for additional studies.
Data extraction was performed independently by two authors, and a third author revised and finalized any disagreements. The final data were presented in Excel® (Microsoft, Redmond, Washington, USA) format for statistical analysis. The corresponding author of one article22 was contacted for additional data.
Statistical analysis
EZR (Easy R)® for the R free software environment for statistical computing and graphics (https://www.R‐project.org/) was used for statistical analysis23. Summary statistic odds ratios (ORs) were calculated, and the risk of pouchitis was compared between patients with and without EIMs as well as between those with and without PSC. Both fixed‐effect and random‐effects models were employed, and a random‐effects model was accepted for a more conservative estimation of the effect of EIMs on the development of pouchitis. Forest plots were generated using pooled ORs from each study, and P < 0·050 was considered statistically significant for the pooled OR. Heterogeneity was calculated using I 2 values, and evaluated according to the method of Higgins et al.24. P < 0·100 was considered statistically significant for heterogeneity. To assess publication bias, funnel plots were created when ten or more studies were included, according to the recommendations of Sterne and colleagues25.
Results
Of 1010 citations identified, 22 articles were included (Fig. 1). No relevant RCTs were retrieved. Nine and 11 observational studies were selected for the analysis of chronic pouchitis and overall pouchitis respectively in relation to EIMs (Table S1, supporting information)7, 8, 12, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, and six and ten studies respectively in relation to PSC (Table S2, supporting information)13, 22, 28, 35, 36, 37, 38, 39, 40, 41, 42. One study42 included both ulcerative colitis and familial polyposis. Data on patients with ulcerative colitis were extracted. Sixteen of 22 studies incorporated histology scores (5 applied the pouchitis disease activity index (PDAI)19), and the remaining six did not (3 applied the modified PDAI20). Agreement between reviewers for the assessment of study eligibility was excellent (agreement 1006 of 1010; κ statistic 0·90).
Figure 1.
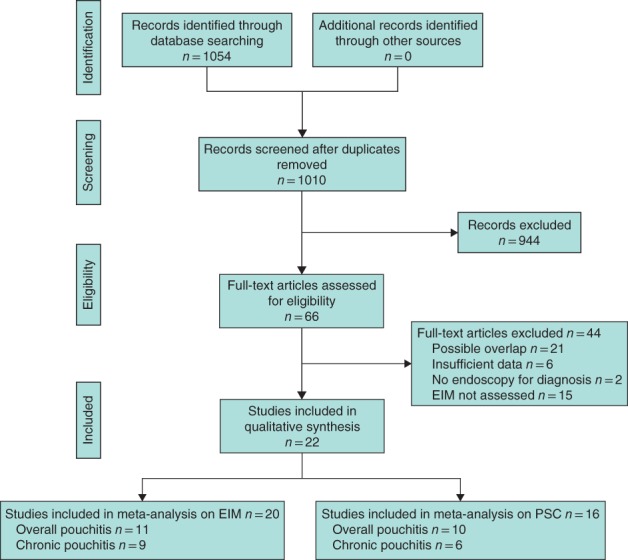
PRISMA flow diagram for the review. EIM, extraintestinal manifestation; PSC, primary sclerosing cholangitis
The definition of chronic pouchitis was diverse, with possible heterogeneity. EIMs included in each study varied from study to study. Most studies, however, included immune‐related EIMs such as joint disease (peripheral arthritis, ankylosing spondylitis and sacroiliitis), skin diseases (pyoderma gangrenosum and erythema nodosum), liver disease (PSC) and eye diseases (uveitis and episcleritis). In addition, some included thrombotic disorders and aphthous stomatitis. Regarding the presence of EIMs in relation to the timing of IPAA, 11 studies included exclusively preoperative EIMs, four included both preoperative and postoperative EIMs, and seven did not provide this information. The risk of bias of individual studies was assessed according to the Risk of Bias Assessment Tool for Nonrandomized Studies (RoBANS)43 (Table S3, supporting information).
Extraintestinal manifestations and overall pouchitis
The effect of EIMs on overall pouchitis was analysed using 11 papers comprising 2194 patients who received IPAA. Funnel and forest plots for overall pouchitis are shown in Fig. 2. The funnel plot was almost symmetrical, indicating limited publication bias.
Figure 2.
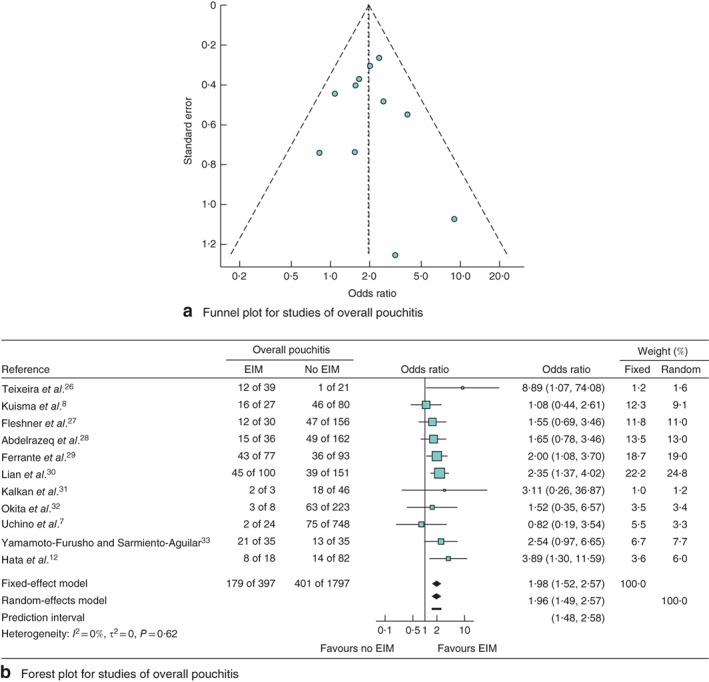
Meta‐analysis of the effect of extraintestinal manifestations (EIMs) on the development of overall pouchitis. a Funnel plot for studies of overall pouchitis. b Forest plot comparing the incidence of overall pouchitis in patients with ulcerative colitis who did or did not develop EIMs following ileal pouch–anal anastomosis. Odds ratios are shown with 95 per cent confidence intervals
An integrated analysis showed that the presence of EIMs was significantly associated with overall pouchitis (OR 1·96, 95 per cent c.i. 1·49 to 2·57; P < 0·001) with low heterogeneity (I 2 = 0 per cent, P = 0·62).
Extraintestinal manifestations and chronic pouchitis
The association of EIM with chronic pouchitis was analysed using nine papers comprising 2222 patients. The forest plot for the effect of EIMs on chronic pouchitis is shown in Fig. 3. An integrated analysis showed that the presence of EIMs was also significantly associated with chronic pouchitis (OR 2·28, 95 per cent c.i. 1·57 to 3·32; P = 0·001), with low heterogeneity (I 2 = 0 per cent, P = 0·53).
Figure 3.
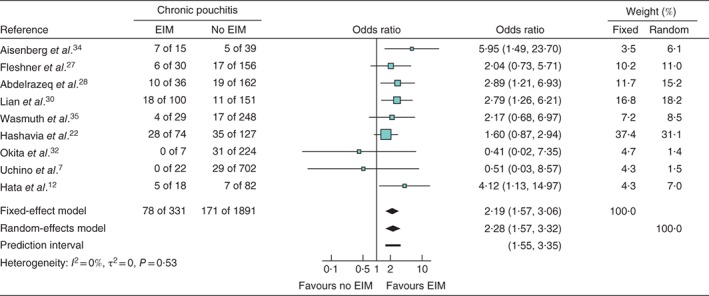
Meta‐analysis of the effect of extraintestinal manifestations (EIMs) on the development of chronic pouchitis. Forest plot comparing the incidence of chronic pouchitis in patients with ulcerative colitis who did or did not develop EIMs following ileal pouch–anal anastomosis. Odds ratios are shown with 95 per cent confidence intervals
Primary sclerosing cholangitis and overall pouchitis
Two papers provided detailed and sufficient data on PSC of the 13 articles in the analysis of the effect of EIMs on the development of overall pouchitis. Eight other articles investigating the association of PSC with pouchitis were included. These ten publications comprised 2877 patients with ulcerative colitis who underwent IPAA. Funnel and forest plots for the effect of PSC on overall pouchitis are shown in Fig. 4. The funnel plot for overall pouchitis was distributed asymmetrically, indicating the presence of publication bias. An integrated analysis showed that PSC was also significantly associated with overall pouchitis (OR 5·11, 95 per cent c.i. 2·42 to 10·78; P < 0·001), but with statistically significant heterogeneity (I 2 = 72 per cent, P < 0·01).
Figure 4.
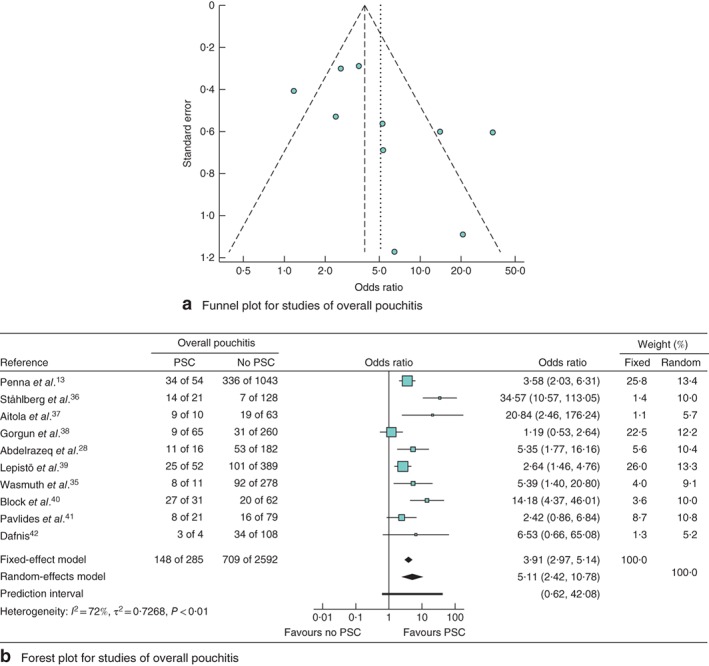
Meta‐analysis of the effect of primary sclerosing cholangitis (PSC) on the development of overall pouchitis. a Funnel plot for studies of overall pouchitis. b Forest plot comparing the incidence of overall pouchitis in patients with ulcerative colitis who did or did not develop PSC following ileal pouch–anal anastomosis. Odds ratios are shown with 95 per cent confidence intervals
Primary sclerosing cholangitis and chronic pouchitis
Six papers comprising 1179 patients were included to assess the association between PSC and chronic pouchitis. The forest plot for the effect of PSC on chronic pouchitis is shown in Fig. 5. An integrated analysis showed that the presence of PSC was significantly associated with chronic pouchitis (OR 5·45, 95 per cent c.i. 1·59 to 18·76; P = 0·017), with considerable heterogeneity (I 2 = 79 per cent, P < 0·01).
Figure 5.
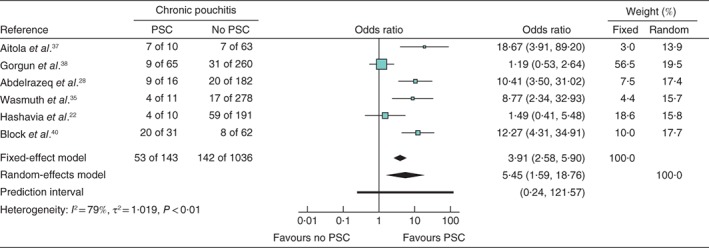
Meta‐analysis of the effect of primary sclerosing cholangitis (PSC) on the development of chronic pouchitis. Forest plot comparing the incidence of chronic pouchitis in patients with ulcerative colitis who did or did not develop PSC following ileal pouch–anal anastomosis. Odds ratios are shown with 95 per cent confidence intervals
Discussion
Both overall and chronic pouchitis occurred significantly more often when EIMs were present. Patients with EIMs may benefit from preoperative and postoperative counselling on the need for long‐term follow‐up. Some RCTs44, 45 have demonstrated that probiotics are effective for the prevention and maintenance of pouchitis in the general population with ulcerative colitis, and it may be beneficial to prescribe probiotics for the prevention of pouchitis in patients with EIMs after IPAA.
The authors have reported the association of EIMs and development of both overall and chronic pouchitis previously12. The present meta‐analysis supports these findings. The authors hypothesize that patients with EIMs may have an abnormal immune response to more diverse immune targets that may cause pouchitis, as well as EIMs such as arthritis, uveitis, pyoderma gangrenosum, PSC and erythema nodosum. Pouchitis is clearly associated with an abnormal immune response, as patients with familial adenomatous polyposis undergoing IPAA develop pouchitis less frequently than those with ulcerative colitis11. Antineutrophil cytoplasmic antibody‐positive patients with ulcerative colitis were reported to be at risk of chronic pouchitis16.
PSC was also associated with the development of pouchitis. Regarding other EIMs, such as arthritis and pyoderma gangrenosum, only a few publications have investigated the association with pouchitis, so their data could not be integrated. Although PSC is one of the most frequently occurring EIMs in Western countries, the incidence of PSC is reported to be lower in Japan46. In three articles from Japan7, 12, 32, the incidence of PSC was too low to be incorporated in the pooled analysis assessing the effect of PSC on the development of pouchitis. Two Western studies28, 35 investigated the effect of PSC and EIMs on the development of pouchitis. In the present pooled analysis, the heterogeneity of included studies was significant for both overall and chronic pouchitis.
It should be stressed that diagnostic criteria for pouchitis are essential for the precise elucidation of the risk of pouchitis, because symptoms did not necessarily correlate with endoscopic and histological findings in the pouch47. Symptoms of pouchitis may occur in patients with irritable pouch syndromes, Crohn's disease of the pouch, or cuffitis9, 21, 48. Therefore, pouchoscopy is unavoidable to make a definite diagnosis of pouchitis9.
There were several limitations of this study. The design of all included studies was observational. Selection bias and heterogeneity is therefore inherently present. In particular, heterogeneity was statistically significant in the analysis of PSC. Several RCTs investigated the preventive effectiveness of medications such as probiotics, but data on the presence of EIMs were not detailed. The authors could not access Embase for this meta‐analysis, which might have captured some relevant publications. Most included papers used logistic rather than Cox regression in multivariable analysis. As the event of pouchitis occurred over time, it would have been better if time had been taken into account and expressed as a hazard ratio. The observation period in most studies, however, was long enough with an average of 5–10 years. The diagnosis of chronic pouchitis was not uniform among the studies. Although most papers adopted long‐term antibiotic use (more than 4 weeks) or unresponsiveness to antibiotic treatment as the definition of chronic pouchitis, the definition differed slightly from study to study.
Supporting information
Table S1 Characteristics of included studies for extraintestinal manifestations and pouchitis
Table S2 Characteristics of included studies for primary sclerosing cholangitis and pouchitis
Table S3 Risk of bias of individual studies according to the RoBANS
Acknowledgements
This work was supported partly by the Research Group for Intractable Inflammatory Bowel Disease of the Ministry of Health, Labour and Welfare of Japan and Ministry of Education, Culture, Sports, Science and Technology (MEXT KAKENHI; grant number JP17K10622).
Disclosure: The authors declare no conflict of interest.
Presented in part to the Annual Scientific Meeting of the American Society of Colon and Rectal Surgeons, Los Angeles, California, USA, April/May 2016; published in abstract form as Dis Colon Rectum 2016; 59: e178
References
- 1. Komaki Y, Komaki F, Micic D, Yamada A, Suzuki Y, Sakuraba A. Pharmacologic therapies for severe steroid refractory hospitalized ulcerative colitis: a network meta‐analysis. J Gastroenterol Hepatol 2017; 32: 1143–1151. [DOI] [PubMed] [Google Scholar]
- 2. Cholapranee A, Hazlewood GS, Kaplan GG, Peyrin‐Biroulet L, Ananthakrishnan AN. Systematic review with meta‐analysis: comparative efficacy of biologics for induction and maintenance of mucosal healing in Crohn's disease and ulcerative colitis controlled trials. Aliment Pharmacol Ther 2017; 45: 1291–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hata K, Watanabe T, Kazama S, Suzuki K, Shinozaki M, Yokoyama T et al Earlier surveillance colonoscopy programme improves survival in patients with ulcerative colitis associated colorectal cancer: results of a 23‐year surveillance programme in the Japanese population. Br J Cancer 2003; 89: 1232–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ishii H, Kawai K, Hata K, Shuno Y, Nishikawa T, Tanaka T et al Comparison of functional outcomes of patients who underwent hand‐sewn or stapled ileal pouch–anal anastomosis for ulcerative colitis. Int Surg 2015; 100: 1169–1176. [DOI] [PubMed] [Google Scholar]
- 5. Hata K, Kazama S, Nozawa H, Kawai K, Kiyomatsu T, Tanaka J et al Laparoscopic surgery for ulcerative colitis: a review of the literature. Surg Today 2015; 45: 933–938. [DOI] [PubMed] [Google Scholar]
- 6. Anzai H, Ishihara S, Kiyomatsu T, Kishikawa J, Yasuda K, Otani K et al Robot‐assisted restorative proctocolectomy and ileal pouch–anal anastomosis for ulcerative colitis. Videoscopy 2017; 27 https://www.liebertpub.com/doi/10.1089/vor.2016.0383 [accessed 9 February 2019]. [Google Scholar]
- 7. Uchino M, Ikeuchi H, Matsuoka H, Bando T, Takesue Y, Tomita N. Clinical features and management of pouchitis in Japanese ulcerative colitis patients. Surg Today 2013; 43: 1049–1057. [DOI] [PubMed] [Google Scholar]
- 8. Kuisma J, Järvinen H, Kahri A, Färkkilä M. Factors associated with disease activity of pouchitis after surgery for ulcerative colitis. Scand J Gastroenterol 2004; 39: 544–548. [DOI] [PubMed] [Google Scholar]
- 9. Hata K, Ishihara S, Nozawa H, Kawai K, Kiyomatsu T, Tanaka T et al Pouchitis after ileal pouch–anal anastomosis in ulcerative colitis: diagnosis, management, risk factors, and incidence. Dig Endosc 2017; 29: 26–34. [DOI] [PubMed] [Google Scholar]
- 10. Segal JP, Ding NS, Worley G, McLaughlin S, Preston S, Faiz OD et al Systematic review with meta‐analysis: the management of chronic refractory pouchitis with an evidence‐based treatment algorithm. Aliment Pharmacol Ther 2017; 45: 581–592. [DOI] [PubMed] [Google Scholar]
- 11. Lohmuller JL, Pemberton JH, Dozois RR, Ilstrup D, van Heerden J. Pouchitis and extraintestinal manifestations of inflammatory bowel disease after ileal pouch–anal anastomosis. Ann Surg 1990; 211: 622–627. [PMC free article] [PubMed] [Google Scholar]
- 12. Hata K, Ishii H, Anzai H, Ishihara S, Nozawa H, Kawai K et al Preoperative extraintestinal manifestations associated with chronic pouchitis in Japanese patients with ulcerative colitis after ileal pouch–anal anastomosis: a retrospective study. Inflamm Bowel Dis 2017; 23: 1019–1024. [DOI] [PubMed] [Google Scholar]
- 13. Penna C, Dozois R, Tremaine W, Sandborn W, LaRusso N, Schleck C et al Pouchitis after ileal pouch–anal anastomosis for ulcerative colitis occurs with increased frequency in patients with associated primary sclerosing cholangitis. Gut 1996; 38: 234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Merrett MN, Mortensen N, Kettlewell M, Jewell DO. Smoking may prevent pouchitis in patients with restorative proctocolectomy for ulcerative colitis. Gut 1996; 38: 362–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. To N, Ford AC, Gracie DJ. Systematic review with meta‐analysis: the effect of tobacco smoking on the natural history of ulcerative colitis. Aliment Pharmacol Ther 2016; 44: 117–126. [DOI] [PubMed] [Google Scholar]
- 16. Singh S, Sharma PK, Loftus EV Jr, Pardi DS. Meta‐analysis: serological markers and the risk of acute and chronic pouchitis. Aliment Pharmacol Ther 2013; 37: 867–875. [DOI] [PubMed] [Google Scholar]
- 17. Hata K, Watanabe T, Shinozaki M, Nagawa H. Patients with extraintestinal manifestations have a higher risk of developing pouchitis in ulcerative colitis: multivariate analysis. Scand J Gastroenterol 2003; 38: 1055–1058. [DOI] [PubMed] [Google Scholar]
- 18. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sandborn WJ. Pouchitis following ileal pouch–anal anastomosis: definition, pathogenesis, and treatment. Gastroenterology 1994; 107: 1856–1860. [DOI] [PubMed] [Google Scholar]
- 20. Shen B, Achkar JP, Connor JT, Ormsby AH, Remzi FH, Bevins CL et al Modified pouchitis disease activity index: a simplified approach to the diagnosis of pouchitis. Dis Colon Rectum 2003; 46: 748–753. [DOI] [PubMed] [Google Scholar]
- 21. Shen B, Achkar JP, Lashner BA, Ormsby AH, Brzezinski A, Soffer EE et al Irritable pouch syndrome: a new category of diagnosis for symptomatic patients with ileal pouch–anal anastomosis. Am J Gastroenterol 2002; 97: 972–977. [DOI] [PubMed] [Google Scholar]
- 22. Hashavia E, Dotan I, Rabau M, Klausner JM, Halpern Z, Tulchinsky H. Risk factors for chronic pouchitis after ileal pouch–anal anastomosis: a prospective cohort study. Colorectal Dis 2012; 14: 1365–1371. [DOI] [PubMed] [Google Scholar]
- 23. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013; 48: 452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J et al Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011; 343: d4002. [DOI] [PubMed] [Google Scholar]
- 26. Teixeira WG, da Silva JH, Teixeira MG, Almeida M, Calache JE, Habr‐Gama A. Pouchitis: extracolonic manifestation of ulcerative colitis? Rev Hosp Clin Fac Med Sao Paulo 1999; 54: 155–158. [DOI] [PubMed] [Google Scholar]
- 27. Fleshner P, Ippoliti A, Dubinsky M, Ognibene S, Vasiliauskas E, Chelly M et al A prospective multivariate analysis of clinical factors associated with pouchitis after ileal pouch–anal anastomosis. Clin Gastroenterol Hepatol 2007; 5: 952–958. [DOI] [PubMed] [Google Scholar]
- 28. Abdelrazeq AS, Kandiyil N, Botterill ID, Lund JN, Reynolds JR, Holdsworth PJ et al Predictors for acute and chronic pouchitis following restorative proctocolectomy for ulcerative colitis. Colorectal Dis 2008; 10: 805–813. [DOI] [PubMed] [Google Scholar]
- 29. Ferrante M, Declerck S, De Hertogh G, Van Assche G, Geboes K, Rutgeerts P et al Outcome after proctocolectomy with ileal pouch–anal anastomosis for ulcerative colitis. Inflamm Bowel Dis 2008; 14: 20–28. [DOI] [PubMed] [Google Scholar]
- 30. Lian L, Fazio VW, Lavery IC, Hammel J, Remzi FH, Shen B. Evaluation of association between precolectomy thrombocytosis and the occurrence of inflammatory pouch disorders. Dis Colon Rectum 2009; 52: 1912–1918. [DOI] [PubMed] [Google Scholar]
- 31. Kalkan IH, Dağli Ü, Önder FO, Tunç B, Öztaş E, Ülker A et al Evaluation of preoperative predictors of development of pouchitis after ileal–pouch‐anastomosis in ulcerative colitis. Clin Res Hepatol Gastroenterol 2012; 36: 622–627. [DOI] [PubMed] [Google Scholar]
- 32. Okita Y, Araki T, Tanaka K, Hashimoto K, Kondo S, Kawamura M et al Predictive factors for development of chronic pouchitis after ileal pouch–anal anastomosis in ulcerative colitis. Digestion 2013; 88: 101–109. [DOI] [PubMed] [Google Scholar]
- 33. Yamamoto‐Furusho JK, Sarmiento‐Aguilar A. Frequency, clinical features and factors associated with pouchitis after proctocolectomy with ileo‐pouch–anal anastomosis in patients with ulcerative colitis: a Latin‐American Country Retrospective‐Cohort Study. Dig Surg 2015; 32: 489–495. [DOI] [PubMed] [Google Scholar]
- 34. Aisenberg J, Wagreich J, Shim J, Almer S, Peen E, Heimann T et al Perinuclear anti‐neutrophil cytoplasmic antibody and refractory pouchitis. A case–control study. Dig Dis Sci 1995; 40: 1866–1872. [DOI] [PubMed] [Google Scholar]
- 35. Wasmuth HH, Tranø G, Endreseth BH, Wibe A, Rydning A, Myrvold HE. Primary sclerosing cholangitis and extraintestinal manifestations in patients with ulcerative colitis and ileal pouch–anal anastomosis. J Gastrointest Surg 2010; 14: 1099–1104. [DOI] [PubMed] [Google Scholar]
- 36. Ståhlberg D, Gullberg K, Liljeqvist L, Hellers G, Löfberg R. Pouchitis following pelvic pouch operation for ulcerative colitis. Incidence, cumulative risk, and risk factors. Dis Colon Rectum 1996; 39: 1012–1018. [DOI] [PubMed] [Google Scholar]
- 37. Aitola P, Matikainen M, Mattila J, Tomminen T, Hiltunen KM. Chronic inflammatory changes in the pouch mucosa are associated with cholangitis found on peroperative liver biopsy specimens at restorative proctocolectomy for ulcerative colitis. Scand J Gastroenterol 1998; 33: 289–293. [DOI] [PubMed] [Google Scholar]
- 38. Gorgun E, Remzi FH, Manilich E, Preen M, Shen B, Fazio VW. Surgical outcome in patients with primary sclerosing cholangitis undergoing ileal pouch–anal anastomosis: a case‐control study. Surgery 2005; 138: 631–637. [DOI] [PubMed] [Google Scholar]
- 39. Lepistö A, Kärkkäinen P, Järvinen HJ. Prevalence of primary sclerosing cholangitis in ulcerative colitis patients undergoing proctocolectomy and ileal pouch–anal anastomosis. Inflamm Bowel Dis 2008; 14: 775–779. [DOI] [PubMed] [Google Scholar]
- 40. Block M, Jørgensen KK, Oresland T, Lindholm E, Grzyb K, Cvancarova M et al Colectomy for patients with ulcerative colitis and primary sclerosing cholangitis – what next? J Crohns Colitis 2014; 8: 421–430. [DOI] [PubMed] [Google Scholar]
- 41. Pavlides M, Cleland J, Rahman M, Christian A, Doyle J, Gaunt R et al Outcomes after ileal pouch anal anastomosis in patients with primary sclerosing cholangitis. J Crohns Colitis 2014; 8: 662–670. [DOI] [PubMed] [Google Scholar]
- 42. Dafnis G. Early and late surgical outcomes of ileal pouch–anal anastomosis within a defined population in Sweden. Eur J Gastroenterol Hepatol 2016; 28: 842–849. [DOI] [PubMed] [Google Scholar]
- 43. Kim SY, Park JE, Lee YJ, Seo HJ, Sheen SS, Hahn S et al Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol 2013; 66: 408–414. [DOI] [PubMed] [Google Scholar]
- 44. Gionchetti P, Rizzello F, Helwig U, Venturi A, Lammers KM, Brigidi P et al Prophylaxis of pouchitis onset with probiotic therapy: a double‐blind, placebo‐controlled trial. Gastroenterology 2003; 124: 1202–1209. [DOI] [PubMed] [Google Scholar]
- 45. Mimura T, Rizzello F, Helwig U, Poggioli G, Schreiber S, Talbot IC et al Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut 2004; 53: 108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tanaka A, Takikawa H. Geoepidemiology of primary sclerosing cholangitis: a critical review. J Autoimmun 2013; 46: 35–40. [DOI] [PubMed] [Google Scholar]
- 47. Ben‐Bassat O, Tyler AD, Xu W, Kirsch R, Schaeffer DF, Walsh J et al Ileal pouch symptoms do not correlate with inflammation of the pouch. Clin Gastroenterol Hepatol 2014; 12: 831–837.e2. [DOI] [PubMed] [Google Scholar]
- 48. Shen B, Remzi FH, Brzezinski A, Lopez R, Bennett AE, Lavery IC et al Risk factors for pouch failure in patients with different phenotypes of Crohn's disease of the pouch. Inflamm Bowel Dis 2008; 14: 942–948. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Characteristics of included studies for extraintestinal manifestations and pouchitis
Table S2 Characteristics of included studies for primary sclerosing cholangitis and pouchitis
Table S3 Risk of bias of individual studies according to the RoBANS


