Abstract
Objectives
This study aims to examine the effect of backward walking (BW) and forward walking (FW) on the myoelectric activity ratio of the vastus medialis oblique (VMO)/vastus lateralis (VL) in females with patellofemoral pain syndrome (PFPS).
Patients and methods
Between September 2016 and December 2016, a total of 40 female participants (mean age 20.9±1.9 years; range, 19 to 26 years) were included in the study. The participants were divided into two groups as those with unilateral PFPS (PFPS group, n=20) and healthy controls (Control group, n=20). Surface electromyography (EMG) from VMO and VL muscles were collected during FW and BW at a speed of 3 km/h using the Myomonitor® IV EMG system.
Results
There was a significant increase in the EMG activities of the VMO and VL muscles during BW compared to FW in PFPS and healthy groups (p=0.001). During BW, the VMO activity of PFPS was significantly higher than the healthy controls (p=0.013) without any significant difference in the VL activity (p=0.916). During FW, there was no significant difference in the VMO and VL activities between the groups (p=0.348 and p=0.705), respectively. The VMO/VL ratio of the PFPS group during BW was significantly higher than the FW ratio (p=0.001) without any significant difference between BW and FW of the healthy group (p=0.841). During BW, the ratio of the PFPS group was significantly higher than compared to the healthy controls (p=0.016) without any significant difference between the groups during FW (p=0.100).
Conclusion
Our study results show that BW increases the VMO muscle activation and preserve the ideal VMO/VL ratio in PFPS patients. Therefore, clinicians should consider BW training when developing rehabilitation programs for females with PFPS.
Keywords: Backward walking, electromyography, knee muscles, patellofemoral pain
Introduction
Patellofemoral pain syndrome (PFPS) is a relatively common disorder encountered in the clinical setting, affecting an estimated 7 to 40% of adolescents and active young adults.[1] It has a negative effect on human function,[2] and is associated with the development of patellofemoral osteoarthritis.[3] Overuse, local joint impairments, and altered lower extremity biomechanics have been proposed to contribute to the development of PFPS.[4] Patients with PFPS have altered trunk, hip, knee, and ankle kinematics during functional tasks. Females with PFPS display greater ipsilateral trunk lean and hip adduction,[5] in addition to excessive knee valgus,[6] and pronated foot posture.[7] These abnormal lower extremity mechanics result in abnormal stress distribution across the patellofemoral joint.[8]
It has been proposed that an imbalance of the vastus medialis oblique (VMO)/vastus lateralis (VL) muscle activities leads to excessive lateral tracking of the patella and rubbing of the lateral femoral condyle, which causes increased articular surface stress and induces pain.[9] The ideal VMO/VL electromyography (EMG) ratio for healthy individuals during knee extension is 1/1.[10] This ratio may change in patients with PFPS as 0.54/1; this is possibly as the imbalance of the VMO and VL muscle activities causes deficient in the medial patellar strength, producing patellar maltracking in PFPS.[11]
Elboim-Gabyzon, and Rotchild[12] showed that there was a significant reduction in gait speed, stride length, and cadence in backward walking (BW) versus forward walking (FW), and longer double limb support duration, both in shod and barefoot walking. In addition, examination of the spatiotemporal parameters of shod and barefoot walking during BW and FW can contribute to realize how elderly subjects adapt to changing walking conditions, and BW should be included during assessment of functional mobility of elderly subjects.
Yang et al.[13] recommended the use of BW for the improvement of the motor control during FW. Furthermore, Threlkeld et al.[14] reported that backward running reduced the joint stress and increased the quadriceps strength more than forward running. It has been also shown that BW training improves the quadriceps strength,[15] and hamstring flexibility in healthy individuals.[16] Moreover, it improves the body balance,[17] and gait spatiotemporal parameters in neurologically impaired patients.[18]
The onset of peak patellofemoral joint reaction force (JRF) occurs later in stance phase during BW compared to FW,[19] and the peak patellofemoral JRF is lower during backward running compared to forward running at the same speed.[20] There is also emerging evidence suggesting the valuable use of BW in the rehabilitation of patients with knee joint dysfunction and pain reduction, improved function, and increased knee extensor strength have been shown after the integration of BW into the rehabilitation protocol of patients with PFPS.[21]
In the literature, a few studies have investigated the differences in muscular activation patterns of the knee muscles during BW in mainly healthy populations. There is still a gap in the literature regarding the activation pattern of lower limb muscles in individuals with PFPS in BW versus FW. Therefore, in the present study, we aimed to investigate the myoelectric activity ratio of the VMO/VL in females with PFPS in two walking conditions.
Patients and Methods
This cross-sectional study included a total of 40 female participants (mean age 20.9±1.9 years; range, 19 to 26 years) were included in the study between September 2016 and December 2016. Inclusion criteria were as follows: (i) age between 19 and 26 years to reduce the possibility of osteoarthritis,[22] (ii) anterior or retropatellar knee pain after performing, at least, two of the following activities; prolonged sitting, stair climbing, squatting, running, kneeling, hopping/jumping and deep knee flexion,[23] (iii) a gradual onset of symptoms unrelated to previous trauma, (iv) pain score ranging from 2 to 5 on the 10-cm visual analog scale (VAS), and (v) at least two positive signs of the following were found during examination; pain and/or patellar crepitus following patellar compression test,[24] pain following resisted knee extension, Clarke’s sign; pain following isometric quadriceps contraction against suprapatellar resistance with the knee in slight flexion, tenderness upon palpation of the posterior surface of the patella or surrounding structures, and having negative findings on examination of the knee ligaments, menisci, and bursa. Those having had clinical evidence of meniscal or ligamentous injury, having a history of patellar subluxation or dislocation; having tenderness over the patellar tendon (patellar tendon pathology); and a recent history of knee or any lower limb surgery, or spinal referred pain were excluded.[22]
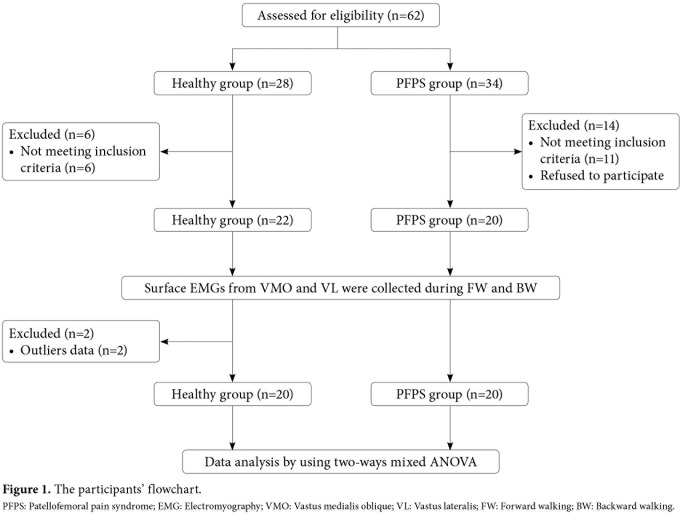
The participants were divided into two groups as those with unilateral PFPS (PFPS group, n=20) and healthy controls (Control group, n=20). The control group included healthy females selected to match the PFPS group in terms of age, weight, and height. None of the participants involved in either regular recreational or professional strength training. They had no musculoskeletal, or neurological problems. Also, they had no previous history of trauma or pain in the knee or surgery to the lower limb. In addition, they had a VAS score of 0 for the patellofemoral joint on the testing day and at least a week before. The study flow chart is shown in Figure 1.
Figure 1. The participants' flowchart. PFPS: Patellofemoral pain syndrome; EMG: Electromyography; VMO: Vastus medialis oblique; VL: Vastus lateralis; FW: Forward walking; BW: Backward walking.
A written informed consent was obtained from each participant. The study protocol was approved by the Faculty of Physical Therapy, Cairo University Institutional Review Board (Approval No. P.T.REC/012/00808). The study was conducted in accordance with the principles of the Declaration of Helsinki.
Procedure
All participants received a brief orientation about the nature and significance of the study, equipment, and the tasks to be performed. As BW is a strange task for most individuals, familiarization with BW was done prior to testing to minimize risks and to ensure that the participant would be comfortable during testing. All participants were instructed to hold the handrails and to report when the task was getting uncomfortable. The speed of the Biodex Gait trainer (Biodex Medical Systems Inc., Shirley, NY, USA) was, then, increased gradually according to the tolerance of the participant until 3 km/h.
The surface EMGs of VMO and VL were recorded for each participant during FW and BW using Myomonitor® IV EMG system (Delsys Inc., Boston, USA). For the VMO and VL muscles, a reference line connecting the anterior superior iliac spine to the center of the patella was identified to aid electrode placement. The participant assumed long sitting position with the tested knee slightly flexed. The VMO sensor was placed approximately 4-cm proximal and medial to the superomedial border of the patella at a 50 to 55° angle to the reference line. The VL sensor was placed at approximately 10-cm proximal to the superolateral border of the patella oriented at 15 to 20° to the reference line.[25] The maximum voluntary isometric contractions (MVICs) and EMG recordings during FW and BW were noted. Three trials were performed for each muscle: each was maintained for 6 sec and 30 sec rest period between trials was given to avoid possible fatigue. To ensure maximum effort, verbal encouragement was provided through the whole process. In addition, visual feedback was achieved through real-time visualizing of the recorded signal.
For the VMO and VL muscles, the participant was seated at the edge of the plinth with both hips and knees flexed to 90°. Both upper limbs were kept beside the trunk and were used for stabilization. The researcher’s hand was placed 2.5-cm above the medial malleolus to provide resistance to knee extension. The participant was, then, instructed to extend her knee and push against the applied resistance as hard as possible for six sec (Figure 2a).
Figure 2. Recording of the MVIC of VMO, and (a) VL muscles and (b) electromyography activities during walking. MVIC: Maximum voluntary isometric contraction; VMO: Vastus medialis oblique; VL: Vastus lateralis.
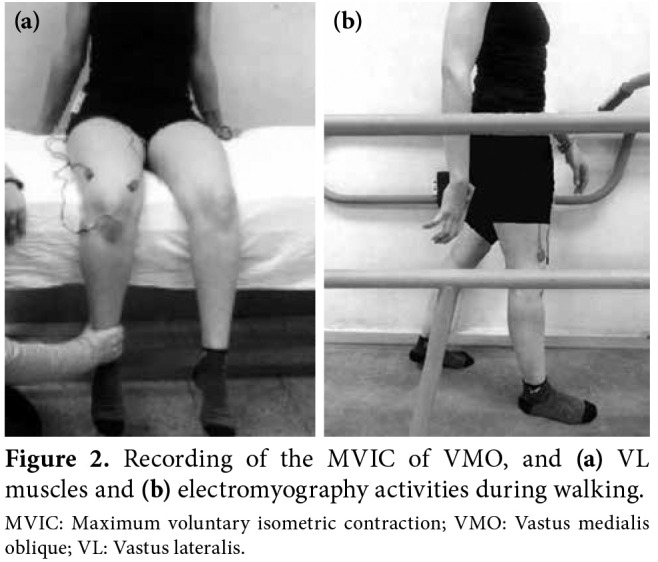
Electromyography recording during FW and BW, the participant was allowed to rest prior to performing the walking trials. Then, she was asked to step over the gait trainer to perform the FW trials. The speed was increased gradually until reaching the predetermined testing speed (3 km/h) and the EMG acquisition was, then, triggered from the host laptop. Three FW trials were performed each of 30 sec. The participant was given a rest period of one min and the belt direction was, then, reversed. The same protocol of FW was followed during BW (Figure 2b). The participant was instructed to report any dizziness, increased pain or discomfort.
Data were sampled at 1,000 Hz, filtered with 20 to 450 Hz band pass filter. The root mean square value (RMS) was calculated for the three trials of MVIC, FW, and BW for the tested muscles. The average RMS values of the three trials of MVIC, FW, and BW were calculated and normalized according to the following equation: Normalized RMS= ([Average 130 RMS during activity/Average RMS of MVIC ¥ 100]).
Statistical analysis
Statistical analysis was performed using the IBM SPSS version 20.0 software (IBM Corp., Armonk, NY, USA). Normally distributed data were indicated by histograms with normal curves. It was confirmed by non-significant results of the Shapiro-Wilks normality test (p>0.05). The Levene's test was performed to analyze significant differences between the variances of the tested groups for the tested dependent variables (p>0.05). Based on the previous findings of the normality and homogeneity assumptions, the parametric analysis was conducted. An independent t-test was used to identify any significant difference in the baseline characteristics between both groups. After excluding outliers, a two-way mixed analysis of variance (ANOVA) was performed to compare the normalized RMS values (%) of the VMO, VL and the VMO/VL ratio between both groups in both walking directions. A p value of <0.05 was considered statistically significant.
Results
There was no significant difference between the two groups in terms of age, weight, height, and body mass index (p>0.05) (Table 1).
Table 1. Baseline demographic characteristics of participants.
| PFPS group (n=20) | Healthy group (n=20) | ||
| Mean±SD | Mean±SD | p | |
| Age (year) | 21.4±2.3 | 20.3±1.3 | 0,106 |
| Weight (kg) | 65.1±10.6 | 63.7±6.9 | 0,66 |
| Height (m) | 1.6±0.1 | 1.6±0.1 | 0,624 |
| Body mass index (kg/m2) | 24.9±3.8 | 24.0±2.4 | 0,438 |
| PFPS: patellofemoral pain syndrome; SD: Standard deviation. | |||
Both PFPS and healthy groups showed a significant increase of the EMG activity of the VMO and VL muscles during BW compared to FW (p=0.001). In addition, there was no significant difference between the groups regarding the EMG activity of the VMO and VL muscles during FW (p=0.348 and 0.705) respectively, and the VL muscle during BW (p=0.916). However, during BW, the VMO muscle activity of the PFPS group was significantly higher than the control group (p=0.013) (Table 2).
Table 2. Normalized RMS values of VMO, and VL muscles during forward walking and backward walking in the PFPS and healthy groups.
| PFPS group (n=20) | Healthy group (n=20) | ||
| Mean±SD | Mean±SD | p | |
| Vastus medialis obliquus | |||
| Forward walking | 25.4±6.8 | 27.5±7.2 | 0,348 |
| Backward walking | 50.9±9.4 | 42.5±10.8 | 0,013 |
| p | 0,001 | 0,001 | |
| Vastus lateralis | |||
| Forward walking | 26.5±8.4 | 25.5±8.2 | 0,705 |
| Backward walking | 39.8±11.5 | 39.4±13.7 | 0,916 |
| p | 0,001 | 0,001 | |
| Vastus medialis obliquus /vastus lateralis | |||
| Forward walking | 1.0±0.2 | 1.1±0.2 | 0,1 |
| Backward walking | 1.3±0.3 | 1.1±0.2 | 0,016 |
| p | 0,001 | 0,841 | |
| PFPS: patellofemoral pain syndrome; RMS: Root mean square value; VMO: Vastus medialis oblique; VL: Vastus lateralis; SD: Standard deviation. | |||
The rate of increase of the VMO muscle normalized RMS mean value was 100.36% during BW versus FW. The rate of increase of the normalized EMG activity of the VL muscle during BW was 50.27% in the PFPS group. The VMO/VL ratio of the PFPS group during BW was significantly higher than the FW (p=0.001). However, there was no significant difference between the BW and FW in the control group (p=0.841) (Table 2). Inter-group comparison revealed that the VMO/VL ratio of the PFPS group was significantly higher than that of healthy group during BW (p=0.016). However, there was no significant difference between the groups during FW (p=0.100).
Discussion
The present study investigated the effect of BW and FW on the VMO/VL myoelectric activity ratio in PFPS patients compared to healthy individuals. Only females were included owing to the high incidence of developing PFPS, and sex-specific differences in the lower extremity mechanics during level walking.[23] The findings of the current study revealed a significant increase in the EMG activity of the VMO and VL muscles in BW versus FW in both PFPS and control groups.
Similarly, Chang et al.[26] reported that the exercises which activate the VMO muscle corrected the VMO/VL ratio and that it yielded beneficial effects on PFPS. The increased EMG activity of knee extensors found in this study may be associated with an increased cortical activation reported by Kurz et al.[27] during BW. These authors found increased oxygenated hemoglobin concentrations in the supplementary motor area, pre-central gyrus, and the superior parietal lobule during BW compared to FW. They argued that the greater activation of the medial sensorimotor cortices during BW reflects greater demand for controlling the lower extremity movement. This hypothesis can be also supported by the findings of Shibuya et al.[28] who found a direct relation between cortical activation as evidenced by the levels of oxygenated hemoglobin and the amplitude of the EMG activity.
The increased knee extensor EMG activity may be due to changing the type of activation during BW. It has been reported that BW is considered as a time reversed copy of FW. The FW stance phase starts with heel strike and ends with toe contact, while the BW stance starts with toe contact and ends with heel off,[29] which refers that the knee joint angular displacement curve is reversed during the BW stance phase. During FW, the knee undergoes flexion just after heel strike during the loading response sub-phase. In contrast, during BW, the knee joint is initially flexed at the toes contact and it continues to extend during most of the stance phase.[30]
This reversal of the knee joint kinematics alters the type of quadriceps muscle activation. The early knee flexion during FW is controlled by the eccentric activation of the knee extensors to provide shock absorption in response to ground impact.[31] However, during BW, the knee extension during the early stance is achieved by a concentric contraction of the quadriceps muscle to prevent the descent of the body center of gravity and propel the body backward.[29] The conversion of eccentric quadriceps activation during FW to concentric activation during BW may explain the increased VMO and VL electromyographic activities during BW. In addition, BW reduces compressive forces at the patellofemoral and, therefore, it is considered a secure closed kinetic chain exercise.[32] It reduces the quadriceps eccentric contraction, while it preserves the isometric and concentric quadriceps strength.[14]
In patients with PFPS, the increased EMG activity of the VMO and VL can be attributed to pain reduction during BW. Patients with anterior knee pain demonstrate greater quadriceps muscle inhibition, particularly VMO and VL, with higher pain levels of the patellofemoral joint.[33] Furthermore, Hassan et al.[34] found increased quadriceps maximum voluntary contraction after pain reduction in patients with knee osteoarthritis. Based on the findings of Kedia and Saurabh[21] which showed that pain levels significantly reduced in patients with PFPS after a four-week BW training program, one can assume that the increased quadriceps activation during BW may be due to pain-induced decrease in the quadriceps inhibition.
In the present study, the increased VMO and VL activity in the control group is consistent with previous studies. Han[35] reported an increased EMG activity level of the VMO and VL in BW compared to FW at all tested treadmill inclines. In addition, the VMO activity increased during underwater BW relative to underwater FW at slow, moderate, and fast walking speeds.[36] These results are consistent with the findings reported by Swati et al.[15] who found increased quadriceps strength after a six-week BW training in healthy college students.
The VMO/VL ratio represents the balance of the VMO and VL activation, which provides stable force to the patella. According to the results of the current study, the PFPS group showed a significant increase in the VMO/VL ratio during BW compared to FW. A probable explanation is that the percentage of increase in the EMG activity of VMO during BW was higher than that of the VL muscle. Consistent with our ratio, Souza and Gross[10] reported the ideal VMO/VL ratio to approximate one, indicating that the patella does not slide off the femoral groove. Consequently, it is anticipated that higher ratio would be beneficial for patients with PFPS and can be also used as a criterion to assess the effectiveness of exercise training.[37] This disagrees with Powers[11] who reported that, for patients with severe PFPS, the VMO/VL ratio was typically less than 0.54. Indeed, myoelectric activities of the VMO and VL were measured in the present study during functional closed kinetic chain walking activity, while the aforementioned authors assessed their participants during resisted open kinetic chain knee extension. Since it is confirmed that closed kinetic knee extension exercise elicits sport performance and strength better than open kinetic knee extension exercise,[38] the VMO/VL ratio, reported during the concentric contraction at an angle of 60o of knee flexion in closed kinetic knee extension exercise was closer to the ideal value than that observed in open kinetic knee extension exercise.[39]
Nonetheless, it is worth mentioning that surface EMG has a certain limitation regarding the dynamic nature of walking. Lower limb angular displacement results in possible displacement of the muscle in relation to the electrode which might have affected the recorded signal. Another limitation is the crosstalk from nearby muscles. This could not be avoided using surface electrodes due to the close anatomical proximity of the studied structures. Further researches are needed to examine the muscle imbalance around the hip joint in patients with PFPS and investigate the correlation between the activation patterns of knee and hip joint muscles during BW and FW. Also, as the addition of hip adduction increasing VMO contraction strengths is that it changes in the muscle length and tension relationship,[40] further studies evaluating the effects of the BW training on gait kinematic and kinetic variables of the trunk, upper, and lower extremities should be conducted to improve the gait abilities in patients with PFPS.
In conclusion, our study results show that BW is effective in increasing the VMO muscle activation and preservation of the ideal the VMO/VL ratio in PFPS patients. Of note, the VMO/VL ratio is higher during BW compared to FW. Therefore, addition of BW training to the rehabilitation program of female patients with PFPS may be useful in the clinical setting.
Acknowledgments.
We would like to thank Dr. Waleed Mohamed Aboelmeaty, Professor in Faculty of Education, Mansura University, Egypt, for helping in statistical analysis.
Footnotes
Conflict of Interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Financial Disclosure: The authors received no financial support for the research and/or authorship of this article.
References
- 1.Bizzini M, Childs JD, Piva SR, Delitto A. Systematic review of the quality of randomized controlled trials for patellofemoral pain syndrome. J Orthop Sports Phys Ther. 2003;33:4–20. doi: 10.2519/jospt.2003.33.1.4. [DOI] [PubMed] [Google Scholar]
- 2.Stathopulu E, Baildam E. Anterior knee pain: a long-term follow-up. Rheumatology (Oxford) 2003;42:380–382. doi: 10.1093/rheumatology/keg093. [DOI] [PubMed] [Google Scholar]
- 3.Crossley KM. Is patellofemoral osteoarthritis a common sequela of patellofemoral pain. Br J Sports Med. 2014;48:409–410. doi: 10.1136/bjsports-2014-093445. [DOI] [PubMed] [Google Scholar]
- 4.Dutton RA, Khadavi MJ, Fredericson M. Update on rehabilitation of patellofemoral pain. Curr Sports Med Rep. 2014;13:172–178. doi: 10.1249/JSR.0000000000000056. [DOI] [PubMed] [Google Scholar]
- 5.Noehren B, Pohl MB, Sanchez Z, Cunningham T, Lattermann C. Proximal and distal kinematics in female runners with patellofemoral pain. Clin Biomech (Bristol, Avon) 2012;27:366–371. doi: 10.1016/j.clinbiomech.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herrington L. Knee valgus angle during single leg squat and landing in patellofemoral pain patients and controls. Knee. 2014;21:514–517. doi: 10.1016/j.knee.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Barton CJ, Bonanno D, Levinger P, Menz HB. Foot and ankle characteristics in patellofemoral pain syndrome: a case control and reliability study. J Orthop Sports Phys Ther. 2010;40:286–296. doi: 10.2519/jospt.2010.3227. [DOI] [PubMed] [Google Scholar]
- 8.Feller JA, Amis AA, Andrish JT, Arendt EA, Erasmus PJ, Powers CM. Surgical biomechanics of the patellofemoral joint. Arthroscopy. 2007;23:542–553. doi: 10.1016/j.arthro.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Fagan V, Delahunt E. Patellofemoral pain syndrome: a review on the associated neuromuscular deficits and current treatment options. Br J Sports Med. 2008;42:789–795. doi: 10.1136/bjsm.2008.046623. [DOI] [PubMed] [Google Scholar]
- 10.Souza DR, Gross MT. Comparison of vastus medialis obliquus: vastus lateralis muscle integrated electromyographic ratios between healthy subjects and patients with patellofemoral pain. Phys Ther. 1991;71:310–316. doi: 10.1093/ptj/71.4.310. [DOI] [PubMed] [Google Scholar]
- 11.Powers CM. Patellar kinematics, part I: the influence of vastus muscle activity in subjects with and without patellofemoral pain. Phys Ther. 2000;80:956–964. [PubMed] [Google Scholar]
- 12.Elboim-Gabyzon M, Rotchild S. Spatial and temporal gait characteristics of elderly individuals during backward and forward walking with shoes and barefoot. Gait Posture. 2017;52:363–366. doi: 10.1016/j.gaitpost.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Yang YR, Yen JG, Wang RY, Yen LL, Lieu FK. Gait outcomes after additional backward walking training in patients with stroke: a randomized controlled trial. Clin Rehabil. 2005;19:264–273. doi: 10.1191/0269215505cr860oa. [DOI] [PubMed] [Google Scholar]
- 14.Threlkeld AJ, Horn TS, Wojtowicz G, Rooney JG, Shapiro R. Kinematics, ground reaction force, and muscle balance produced by backward running. J Orthop Sports Phys Ther. 1989;11:56–63. doi: 10.2519/jospt.1989.11.2.56. [DOI] [PubMed] [Google Scholar]
- 15.Swati K, Ashima C, Saurabh S. Efficacy of backward training on agility and quadriceps strength. Elixir Hum Physiol. 2012;53:11918–11921. [Google Scholar]
- 16.Whitley CR, Dufek JS. Effects of backward walking on hamstring flexibility and low back range of motion. Int J Exerc Sci. 2011;4:192–198. [Google Scholar]
- 17.El-Basatiny HM, Abdel-Aziem AA. Effect of backward walking training on postural balance in children with hemiparetic cerebral palsy: a randomized controlled study. Clin Rehabil. 2015;29:457–467. doi: 10.1177/0269215514547654. [DOI] [PubMed] [Google Scholar]
- 18.Abdel-Aziem AA, El-Basatiny HM. Effectiveness of backward walking training on walking ability in children with hemiparetic cerebral palsy: a randomized controlled trial. Clin Rehabil. 2017;31:790–797. doi: 10.1177/0269215516656468. [DOI] [PubMed] [Google Scholar]
- 19.Sussman D, Alrowayeh H, Walker M. Patellofemoral joint compressive forces during backward and forward running at the same speed. J Musculoskelet Res. 2000;4:107–118. [Google Scholar]
- 20.Roos PE, Barton N, van Deursen RW. Patellofemoral joint compression forces in backward and forward running. J Biomech. 2012;45:1656–1660. doi: 10.1016/j.jbiomech.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kedia S, Saurabh S. Effect of retro-treadmill ambulation training in patellofemoral pain patients. Physiother Occu Ther J. 2012;5:59–64. [Google Scholar]
- 22.Cowan SM, Bennell KL, Hodges PW, Crossley KM, McConnell J. Delayed onset of electromyographic activity of vastus medialis obliquus relative to vastus lateralis in subjects with patellofemoral pain syndrome. Arch Phys Med Rehabil. 2001;82:183–189. doi: 10.1053/apmr.2001.19022. [DOI] [PubMed] [Google Scholar]
- 23.Boling M, Padua D, Marshall S, Guskiewicz K, Pyne S, Beutler A. Gender differences in the incidence and prevalence of patellofemoral pain syndrome. Scand J Med Sci Sports. 2010;20:725–730. doi: 10.1111/j.1600-0838.2009.00996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrington L. Effect of a SERF strap on pain and knee- valgus angle during unilateral squat and step landing in patellofemoral patients. J Sport Rehabil. 2013;22:27–32. doi: 10.1123/jsr.22.1.27. [DOI] [PubMed] [Google Scholar]
- 25.Miao P, Xu Y, Pan C, Liu H, Wang C. Vastus medialis oblique and vastus lateralis activity during a double-leg semisquat with or without hip adduction in patients with patellofemoral pain syndrome. BMC Musculoskelet Disord. 2015;16:289–289. doi: 10.1186/s12891-015-0736-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang WD, Huang WS, Lai PT. Muscle activation of vastus medialis oblique and vastus lateralis in sling-based exercises in patients with patellofemoral pain syndrome: A cross-over study. Evid Based Complement Alternat Med. 2015;2015:740315–740315. doi: 10.1155/2015/740315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurz MJ, Wilson TW, Arpin DJ. Stride-time variability and sensorimotor cortical activation during walking. Neuroimage. 2012;59:1602–1607. doi: 10.1016/j.neuroimage.2011.08.084. [DOI] [PubMed] [Google Scholar]
- 28.Shibuya K, Kuboyama N, Tanaka J. Changes in ipsilateral motor cortex activity during a unilateral isometric finger task are dependent on the muscle contraction force. Physiol Meas. 2014;35:417–428. doi: 10.1088/0967-3334/35/3/417. [DOI] [PubMed] [Google Scholar]
- 29.Grasso R, Bianchi L, Lacquaniti F. Motor patterns for human gait: backward versus forward locomotion. J Neurophysiol. 1998;80:1868–1885. doi: 10.1152/jn.1998.80.4.1868. [DOI] [PubMed] [Google Scholar]
- 30.Lee M, Kim J, Son J, Kim Y. Kinematic and kinetic analysis during forward and backward walking. Gait Posture. 2013;38:674–678. doi: 10.1016/j.gaitpost.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 31.Neumann DA. Kinesiology of the Musculoskeletal System: Foundations for Physical Rehabilitation. 2. St. Louis: Mosby Elsevier; 2010. [Google Scholar]
- 32.Flynn TW, Soutas-Little RW. Patellofemoral joint compressive forces in forward and backward running. J Orthop Sports Phys Ther. 1995;21:277–282. doi: 10.2519/jospt.1995.21.5.277. [DOI] [PubMed] [Google Scholar]
- 33.Surer E, Herzog W, Souza KD, Bray R. Inhibition of the quadriceps muscles in patients with anterior knee pain. J Appl Biomech. 1998;14:360–373. [Google Scholar]
- 34.Hassan BS, Doherty SA, Mockett S, Doherty M. Effect of pain reduction on postural sway, proprioception, and quadriceps strength in subjects with knee osteoarthritis. Ann Rheum Dis. 2002;61:422–428. doi: 10.1136/ard.61.5.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han SW. The effect of forward walking and backward walking on quadriceps muscles with treadmill inclination: surface electromyographic analysis. Phys Ther Korea. 2005;12:63–70. [Google Scholar]
- 36.Masumoto K, Takasugi S, Hotta N, Fujishima K, Iwamoto Y. A comparison of muscle activity and heart rate response during backward and forward walking on an underwater treadmill. Gait Posture. 2007;25:222–228. doi: 10.1016/j.gaitpost.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 37.Paoloni M, Fratocchi G, Mangone M, Murgia M, Santilli V, Cacchio A. Long-term efficacy of a short period of taping followed by an exercise program in a cohort of patients with patellofemoral pain syndrome. Clin Rheumatol. 2012;31:535–539. doi: 10.1007/s10067-011-1883-2. [DOI] [PubMed] [Google Scholar]
- 38.Fehr GL, Junior AC, Cacho EWA, De Miranda JB. Effectiveness of the open and closed kinetic chain exercises in the treatment of the patellofemoral pain syndrome. Rev Bras Med Esporte. 2006;12:56–60. [Google Scholar]
- 39.Tang SF, Chen CK, Hsu R, Chou SW, Hong WH, Lew HL. Vastus medialis obliquus and vastus lateralis activity in open and closed kinetic chain exercises in patients with patellofemoral pain syndrome: an electromyographic study. Arch Phys Med Rehabil. 2001;82:1441–1445. doi: 10.1053/apmr.2001.26252. [DOI] [PubMed] [Google Scholar]
- 40.Peng HT, Kernozek TW, Song CY. Muscle activation of vastus medialis obliquus and vastus lateralis during a dynamic leg press exercise with and without isometric hip adduction. Phys Ther Sport. 2013;14:44–49. doi: 10.1016/j.ptsp.2012.02.006. [DOI] [PubMed] [Google Scholar]