Key Points
Question
Is administration of the oral protease inhibitor nelfinavir mesylate improve clinical outcomes with concurrent chemoradiotherapy assoicated with improved clinical outcomes in locally advanced non–small cell lung cancer?
Findings
This phase 1/2 clinical trial found that nelfinavir with concurrent chemoradiotherapy was well tolerated and had promising long-term local control and survival in 35 patients with locally advanced non–small cell lung cancer.
Meaning
The addition of the putative radiosensitizer nelfinavir with concurrent chemoradiotherapy in patients with locally advanced non–small cell lung cancer may improve clinical efficacy and outcomes.
Abstract
Importance
Local failure after chemoradiotherapy (CT-RT) significantly contributes to mortality in patients with locally advanced non–small cell lung cancer (LA-NSCLC). One approach to improve local control is through targeted radiosensitization of the tumor.
Objective
To evaluate the dose-limiting toxic effects, maximally tolerated dose, and recommended phase 2 dose of the protease inhibitor nelfinavir mesylate, administered concurrently with CT-RT in patients with LA-NSCLC, and, in the phase 2 portion of the study, to estimate the objective response rate, local and distant failure rates, and overall survival.
Design, Setting, and Participants
This prospective, open-label, single-group, single-institution phase 1/2 trial tested the oral protease inhibitor nelfinavir in combination with concurrent CT-RT in 35 patients aged 18 to 89 years with biopsy-confirmed unresectable stage IIIA/IIIB LA-NSCLC and a minimum Karnofsky performance status from June 29, 2007, to February 22, 2012, with an analysis date of May 9, 2017. Median follow-up for all patients was 6.8 years, with a minimum 5 years of follow-up for all survivors.
Interventions
Oral nelfinavir mesylate, 625 mg, twice daily or 1250 mg, twice daily was administered for 7 to 14 days before and during concurrent CT-RT.
Main Outcomes and Measures
Graded toxic effects, overall survival, local failure, distant failure, objective response rate, and progression-free survival as measured by Response Evaluation Criteria in Solid Tumors, version 1.1.
Results
Thirty-five patients (16 women and 19 men; median age, 60 years [range, 39-79 years]) enrolled and met protocol-specified criteria for adherence, with 5 at a dose of 625 mg twice daily and 30 at a dose of 1250 mg twice daily. No dose-limiting toxic effects were observed. No grade 4 or higher nonhematologic toxic effects were observed. Thirty-three of the 35 patients had evaluable posttreatment computed tomographic scans, with an objective response rate of 94% (31 of 33; 95% CI, 86%-100%). The cumulative incidence of local failure was 39% (95% CI, 30.5%-47.5%). Median progression-free survival was 11.7 months (95% CI, 6.2-17.1 months). Median overall survival for all patients was 41.1 months (95% CI, 19.0-63.1 months); the 5-year mean (SE) overall survival rate was 37.1% (8.2%).
Conclusions and Relevance
This study suggests that nelfinavir administered with concurrent CT-RT is associated with acceptable toxic effects and a promising objective response rate, local failure, progression-free survival, and overall survival in unresectable LA-NSCLC. These data suggest that nelfinavir may enhance the efficacy of standard CT-RT in this disease. Additional testing in the randomized phase 3 setting should be conducted to establish the improvement associated with nelfinavir with concurrent CT-RT.
Trial Registration
ClinicalTrials.gov identifier: NCT00589056
This phase 1/2 trial evaluates the toxic effects, maximally tolerated dose, and recommended phase 2 dose of the protease inhibitor nelfinavir administered concurrently with chemoradiotherapy for locally advanced non–small cell lung cancer, and estimates the objective response rate, local and distant failure rates, and overall and progression-free survival.
Introduction
Approximately 50 000 patients are diagnosed annually with stage III non–small cell lung cancer (NSCLC) in the United States. Median survival for these patients, despite advances in chemotherapy and radiotherapy delivery, remains poor at approximately 28 months.1 The current therapeutic approach for patients with unresectable stage IIIA disease is definitive radiotherapy to a dose of 60 Gy given concurrently with a platin-based regimen.2,3 One of the reasons for the poor cure rate in this disease is poor local control with definitive radiotherapy. Radiographic local failure rates range from 30% to 50% at 2 years.1 There is evidence to suggest an association between improved local control and better overall survival (OS). In a meta-analysis of patients receiving concurrent chemoradiotherapy (CT-RT), a 6% locoregional control benefit translated to a 5% improvement in OS with no change in the distant failure rate.2 Therefore, improving local control represents a central goal in designing new strategies to treat NSCLC.
One approach to improve local tumor control is through concomitant administration of a radiosensitizing drug during standard radiotherapy.4,5 Preclinical studies have shown that a class of protease inhibitors used to treat HIV can radiosensitize tumor cells both in vitro and in vivo.6,7 The mechanism for this radiosensitization appears to be mediated, in part, through inhibition of PI-3 kinase.6 Preclinical studies showed evidence of inhibition of Akt phosphorylation after 3 days of nelfinavir mesylate.6
Based on these preclinical data, our group initiated a novel phase 1/2 trial of the HIV protease inhibitor nelfinavir with concurrent CT-RT for unresectable stage IIIA/IIIB NSCLC. A 7- to 14-day lead-in period was chosen to ensure inhibition of Akt phosphorylation prior to initiation of CT-RT. The phase 1 trial has been previously reported.8 The primary objectives of the phase 2 portion of the trial were to determine the objective response rate, local and distant failure rates, and progression-free survival and OS and to further characterize the safety of nelfinavir when administered with concurrent CT-RT.
Methods
Eligibility
Patients aged 18 to 89 years with histologically proven, locally advanced NSCLC (LA-NSCLC) were enrolled in this prospective trial, conducted from June 29, 2007, to February 22, 2012. Patients had to have disease that was deemed unresectable at the multidisciplinary tumor board by the thoracic oncology team at the University of Pennsylvania and be eligible for definitive CT-RT. Patients were required to have a Karnofsky performance status of 80 to 100 and less than 10% unintended weight loss in the 6 months prior to enrollment. Patients were required to have sufficient renal function (serum creatinine level ≤1.2 mg/dL [to convert to millimoles per liter, multiply by 88.4]) to permit cisplatin-based chemotherapy. All patients underwent positron emission tomography/computed tomography and magnetic resonance imaging of the brain for staging within 6 weeks of study entry. Patients who had received prior thoracic radiotherapy were excluded. The University of Pennsylvania Institutional Review Board approved this study. All patients provided written informed consent.
Trial Design
This is a prospective, open-label, single-arm phase 1/2 trial of the oral HIV-protease inhibitor nelfinavir in combination with concurrent CT-RT in stage IIIA/IIIB LA-NSCLC. All patients began taking daily oral nelfinavir (either 625 mg twice daily or 1250 mg twice daily) 7 to 14 days prior to the start of CT-RT. A 3 + 3 trial design was used for the phase 1 portion of the trial, with expansion to include 30 patients at the maximally tolerated phase 2 dose (6 patients in the escalation phase and 24 evaluable patients in the expansion phase). Nelfinavir was continued during the complete course of CT-RT (eFigure in the Supplement). All patients underwent CT-based treatment planning. The gross tumor volume, clinical target volume, and planning target volume were defined according to ICRU (International Commission on Radiation Units and Measurements) 50.9 All patients were treated using involved field, 3-dimensional conformal radiotherapy or intensity-modulated radiotherapy to 66.6 Gy in 1.8 Gy per fraction with standard normal tissue constraints.8 Standard chemotherapy consisting of cisplatin and etoposide was administered concurrently with radiotherapy per the standard Southwest Oncology Group regimen.10,11
Assessment of Toxic Effects and Response
Toxic effects were graded by Common Terminology Criteria for Adverse Events, version 4.0.12 Dose-limiting toxic effects were defined as any treatment-related, grade 4 hematologic toxic effect requiring a break in therapy of more than 14 days or nonhematologic grade 3 or higher toxicity except esophagitis and pneumonitis.13 The maximally tolerated dose was defined as the highest dose associated with fewer than 2 dose-limiting toxic effects in 6 patients. All patients underwent computed tomographic imaging of the chest 3 months after treatment completion for assessment of response and computed tomographic imaging as per standard of care every 3 to 6 months thereafter until disease progression or death. Local failure was defined as radiographic evidence of relapse or progression within the primary tumor or nodal regions irradiated. Distant failure was defined as failure in a nonregional nodal or extrathoracic site. Progression-free survival was defined as the time from the start of treatment to disease progression or death. Patients who were progression free at the time of analysis were censored at the date of the most recent imaging that documented their progression-free status. Overall survival was defined as the time from the start of treatment to death due to any cause or last patient contact alive. Radiographic response was measured according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1.14
Statistical Analysis
Analysis was performed May 9, 2017. Patient characteristics were summarized by descriptive statistics; continuous variables were summarized by median and range, while categorical variables were summarized by frequency and percentage. Toxic effects were graded and tabled by dose level (DL). The objective response rate (ie, percentage of patients who achieved complete or partial response) and 95% CIs were estimated. Median potential follow-up was estimated by the reverse Kaplan-Meier method. Cumulative incidence of local failure and distant failure and their 95% CIs were analyzed using cumulative incidence analysis to account for competing risks. Median progression-free survival and OS were estimated using the Kaplan-Meier method with 95% CIs for medians based on the formula of Greenwood.15
The study was designed as a 3 + 3 escalation study with expansion to include 30 patients at the maximally tolerated phase 2 dose of nelfinavir (6 patients in the escalation phase and 24 evaluable patients in the expansion phase) to determine whether the median OS was increased to 30 months or more compared with the median OS of 17 months assumed from historical studies. With 30 patients accrued over 18 months and with 12 months of additional follow-up, there would be 80% power to detect this increase in median OS, with A 1-sided 10%, type I error rate. The study tested 2 DLs; since objective responses were seen at both DLs, the 2 groups were combined to estimate clinical outcomes with greater precision. All statistical analyses were performed using the software package SPSS (IBM SPSS), STATA (StataCorp LLC), or Microsoft Excel (Microsoft Corp). All statistics were descriptive and as such we did not prespecify P values or 1-sided or 2-sided tests as this was a single-group trial.
Results
Patient Characteristics
A total of 38 patients with biopsy-proven stage IIIA or IIIB NSCLC were enrolled from June 29, 2007, to February 22, 2012, of whom 35 received nelfinavir and initiated CT-RT and met the protocol adherence criteria. All 35 patients were followed up for acute toxic effects; 33 patients who received nelfinavir and initiated concurrent CT-RT had computed tomographic scans sufficient for RECIST, version 1.1, response assessment to therapy. The patient characteristics of the 35 patients followed up for response assessment are in Table 1. Median age was 60 years (range, 39-79 years), and there were 16 women and 19 men. Most patients (23 [66%]) had stage IIIA disease. T stage was T1 or T2 in 21 patients (60%), T3 in 7 patients (20%), T4 in 6 patients (17%), and TX in 1 patient (3%). N stage was N0 or N1 in 3 patients (9%), N2 in 24 patients (69%), and N3 in 8 patients (23%). The median Karnofsky performance status was 80 (range, 80-90). Histologic characteristics included adenocarcinoma in 10 patients (29%), squamous cell carcinoma in 16 patients (46%), and poorly differentiated NSCLC not otherwise specified in 9 patients (26%).
Table 1. Patient Characteristics.
| Characteristic | No. (%) (N = 35) |
|---|---|
| Age, median (range), y | 60 (39-79) |
| Sex | |
| Male | 19 (54) |
| Female | 16 (46) |
| Stage | |
| IIIA | 23 (66) |
| IIIB | 12 (34) |
| T stage | |
| T1 | 7 (20) |
| T2 | 14 (40) |
| T3 | 7 (20) |
| T4 | 6 (17) |
| TX | 1 (3) |
| N stage | |
| N0-N1 | 3 (9) |
| N2 | 24 (69) |
| N3 | 8 (23) |
| Karnofsky performance status score | |
| 80 | 27 (77) |
| 90 | 8 (23) |
| Histologic characteristics | |
| Adenocarcinoma | 10 (29) |
| Squamous cell | 16 (46) |
| Poorly differentiated NSCLC NOS | 9 (26) |
Abbreviations: NOS, not otherwise specified; NSCLC, non–small cell lung cancer.
Dose Escalation
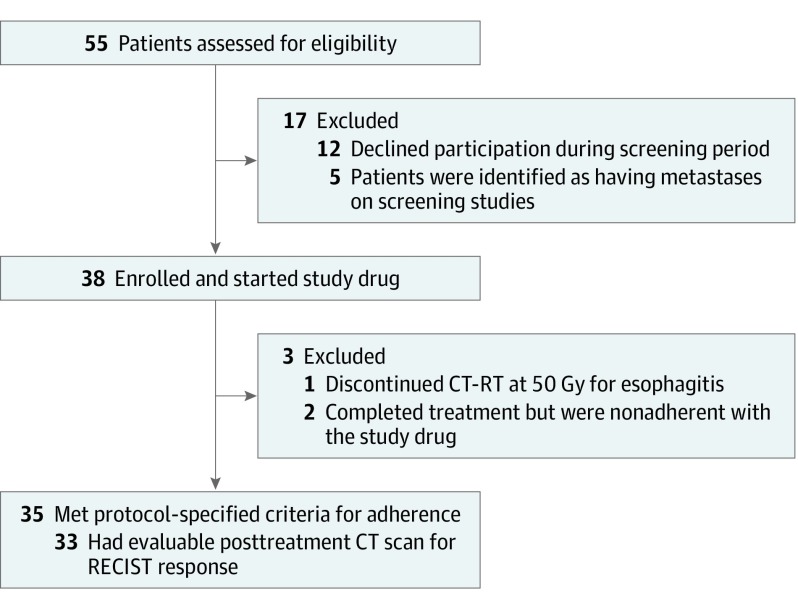
Five patients were enrolled at DL1 (625 mg orally twice daily). After the first 3 patients were scored as not having dose-limiting toxic effects, the next 2 patients in the dose cohort should have been escalated to 1250 mg twice daily (DL2), but they were found to have inadvertently taken the DL1 dose at the time of initial pill diary audit (14 days after initiation of drug). After discussion with the data safety monitoring committee, it was decided that these 2 patients should continue at the lower DL; thus, 5 patients were enrolled at DL1. Thirty patients, including the 6 patients treated in the phase 1 portion of the trial, were enrolled at DL2 (1250 mg orally twice daily). All 35 patients were followed up for survival and toxic effects. Figure 1 shows the CONSORT diagram of all 55 patients offered enrollment in the trial.
Figure 1. CONSORT Diagram.
CT indicates computed tomography; CT-RT, chemoradiotherapy; and RECIST, Response Evaluation Criteria in Solid Tumors.
Treatment Adherence
Protocol-specified adherence criteria included receiving 80% or more of the prescribed radiotherapy fractions and 70% or more of the prescribed nelfinavir doses. All but 1 patient received all radiotherapy treatments. The pill compliance rate ranged from 71% to 100%, with a median compliance rate of 97%. All 35 patients received 66.6 Gy in 37 fractions and received their prescribed chemotherapy without dose reductions or modifications.
Toxic Effects
The toxic effects associated with each DL of nelfinavir are detailed in Table 2 for the 35 patients analyzed. No dose-limiting toxic effects were observed. The rate of grade 3 or 4 toxicities appeared similar for the 2 DLs. Three patients initiated nelfinavir with concurrent CT-RT but then withdrew from the study: 1 patient at DL1 withdrew owing to anxiety (not drug related), and a second patient at DL1 elected to withdraw after 5 weeks of concurrent CT-RT and nelfinavir owing to treatment-related grade 3 esophagitis. A third patient at DL2 was nonadherent with nelfinavir despite weekly coaching. None of these 3 patients experienced grade 4 or 5 nonhematologic toxic effects. The 2 nonadherent patients did not experience any grade 2 or higher esophagitis or pneumonitis and completed all CT-RT as prescribed. All other patients completed nelfinavir with concurrent CT-RT per protocol.
Table 2. Toxic Effects Data for All 35 Patients Enrolleda.
| Effect | Grade | ||
|---|---|---|---|
| 1-2 | 3 | 4 | |
| Leukopenia | |||
| Total | 6 | 12 | 8 |
| DL1 | 1 | 1 | 1 |
| DL2 | 5 | 11 | 7 |
| Nausea | |||
| Total | 19 | 4 | 0 |
| DL1 | 2 | 0 | 0 |
| DL2 | 17 | 4 | 0 |
| Vomiting | |||
| Total | 10 | 0 | 0 |
| DL1 | 2 | 0 | 0 |
| DL2 | 8 | 0 | 0 |
| Dyspepsia | |||
| Total | 17 | 0 | 0 |
| DL1 | 0 | 0 | 0 |
| DL2 | 17 | 0 | 0 |
| Dysphagia | |||
| Total | 20 | 3 | 0 |
| DL1 | 2 | 0 | 0 |
| DL2 | 18 | 3 | 0 |
| Diarrhea | |||
| Total | 19 | 1 | 0 |
| DL1 | 1 | 0 | 0 |
| DL2 | 18 | 1 | 0 |
| Constipation | |||
| Total | 23 | 0 | 0 |
| DL1 | 3 | 0 | 0 |
| DL2 | 20 | 0 | 0 |
| Dehydration | |||
| Total | 15 | 3 | 0 |
| DL1 | 2 | 0 | 0 |
| DL2 | 13 | 3 | 0 |
| Anorexia | |||
| Total | 16 | 3 | 0 |
| DL1 | 0 | 1 | 0 |
| DL2 | 16 | 2 | 0 |
| Weight loss | |||
| Total | 19 | 0 | 0 |
| DL1 | 2 | 0 | 0 |
| DL2 | 17 | 0 | 0 |
| Cough | |||
| Total | 14 | 0 | 0 |
| DL 1 | 2 | 0 | 0 |
| DL 2 | 12 | 0 | 0 |
| Dyspnea | |||
| Total | 16 | 1 | 0 |
| DL1 | 0 | 0 | 0 |
| DL2 | 16 | 1 | 0 |
| Fatigue | |||
| Total | 23 | 3 | 0 |
| DL1 | 2 | 0 | 0 |
| DL2 | 21 | 3 | 0 |
| Headache | |||
| Total | 8 | 0 | 0 |
| DL1 | 2 | 0 | 0 |
| DL2 | 6 | 0 | 0 |
| Fever | |||
| Total | 6 | 0 | 0 |
| DL1 | 0 | 0 | 0 |
| DL2 | 6 | 0 | 0 |
| Hypotension | |||
| Total | 10 | 3 | 0 |
| DL1 | 2 | 0 | 0 |
| DL2 | 8 | 3 | 0 |
| Skin | |||
| Total | 30 | 0 | 0 |
| DL1 | 3 | 0 | 0 |
| DL2 | 27 | 0 | 0 |
| Esophagitis | |||
| Total | 25 | 4 | 0 |
| DL1 | 2 | 1 | 0 |
| DL2 | 23 | 3 | 0 |
| Anemia | |||
| Total | 20 | 5 | 0 |
| DL1 | 3 | 0 | 0 |
| DL2 | 17 | 5 | 0 |
| Hyperglycemia | |||
| Total | 20 | 4 | 0 |
| DL1 | 3 | 0 | 0 |
| DL2 | 17 | 4 | 0 |
| Hypoglycemia | |||
| Total | 7 | 0 | 0 |
| DL1 | 2 | 0 | 0 |
| DL2 | 5 | 0 | 0 |
| Hypoalbuminemia | |||
| Total | 24 | 0 | 0 |
| DL1 | 3 | 0 | 0 |
| DL2 | 21 | 0 | 0 |
| Hypocalcemia | |||
| Total | 20 | 0 | 0 |
| DL1 | 2 | 0 | 0 |
| DL2 | 18 | 0 | 0 |
| Hyperkalemia | |||
| Total | 3 | 0 | 0 |
| DL1 | 0 | 0 | 0 |
| DL2 | 3 | 0 | 0 |
| Hypokalemia | |||
| Total | 10 | 1 | 1 |
| DL1 | 0 | 0 | 0 |
| DL2 | 10 | 1 | 1 |
| Hyponatremia | |||
| Total | 22 | 3 | 0 |
| DL1 | 1 | 1 | 0 |
| DL2 | 21 | 2 | 0 |
| Hypomagnesemia | |||
| Total | 6 | 2 | 0 |
| DL1 | 1 | 0 | 0 |
| DL2 | 5 | 2 | 0 |
| Hyperbilirubinemia | |||
| Total | 3 | 0 | 0 |
| DL1 | 0 | 0 | 0 |
| DL2 | 3 | 0 | 0 |
| Alkaline phosphatase | |||
| Total | 7 | 0 | 0 |
| DL1 | 1 | 0 | 0 |
| DL2 | 6 | 0 | 0 |
| Creatinine | |||
| Total | 3 | 0 | 0 |
| DL1 | 0 | 0 | 0 |
| DL2 | 3 | 0 | 0 |
| Transaminases | |||
| Total | 8 | 1 | 0 |
| DL1 | 0 | 0 | 0 |
| DL2 | 8 | 1 | 0 |
| Thrombocytopenia and platelets | |||
| Total | 14 | 3 | 2 |
| DL1 | 1 | 0 | 0 |
| DL2 | 13 | 3 | 2 |
| Tinnitus | |||
| Total | 6 | 0 | 0 |
| DL1 | 0 | 0 | 0 |
| DL2 | 6 | 0 | 0 |
| Dizziness | |||
| Total | 10 | 0 | 1 |
| DL1 | 2 | 0 | 0 |
| DL2 | 8 | 0 | 1 |
| Hemoptysis | |||
| Total | 2 | 0 | 0 |
| DL1 | 0 | 0 | 0 |
| DL2 | 2 | 0 | 0 |
| Hiccoughs | |||
| Total | 5 | 0 | 0 |
| DL1 | 0 | 0 | 0 |
| DL2 | 5 | 0 | 0 |
| Voice alteration | |||
| Total | 12 | 0 | 0 |
| DL1 | 0 | 0 | 0 |
| DL2 | 12 | 0 | 0 |
| Abdominal pain | |||
| Totals | 5 | 1 | 0 |
| DL1 | 1 | 0 | 0 |
| DL2 | 4 | 1 | 0 |
| Pneumonitis | |||
| Total | 2 | 2 | 0 |
| DL1 | 0 | 0 | 0 |
| DL2 | 2 | 2 | 0 |
Abbreviation: DL, dose level.
Data are reported as number of patients, 5 patients with DL1 (625 mg orally twice daily) and 30 patients with DL2 (1250 mg twice daily).
Hematologic Toxic Effects
The primary grade 3 or 4 hematologic toxic effect observed was leukopenia. Two of 5 patients (40%) at DL1 and 18 of 30 patients at DL2 (60%) experienced grade 3 or 4 leukopenia. No patients required dose attenuation of chemotherapy or nelfinavir. There were no episodes of neutropenic fever.
Nonhematologic Toxic Effects
There were no nonhematologic grade 4 toxic effects. The primary nonhematologic grade 3 toxic effect was esophagitis, seen in 3 of 30 patients at DL 2 (10%) and 1 of 5 patients (20%) at DL1. Two of 30 patients (7%) experienced grade 3 or higher pneumonitis at DL2. All other toxic effects were grade 1 or 2.
Response and Follow-up
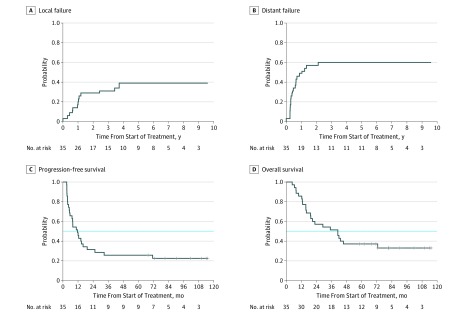
The median potential follow-up was 6.8 years. Figure 2A shows the cumulative incidence of local failure of 39% (95% CI, 30.5%-47.5%) based on all 35 patients. A total of 13 patients (37%) experienced local failure; 7 patients (20%) experienced local failure as the site of first failure. The 2-year local failure rate was 26%, and the 4-year local failure rate was 34%. The median time to local failure was not reached. All other patients are alive with local disease control or had local disease control at the time of death. Figure 2B shows the cumulative incidence of distant failure of 60% (95% CI, 51.7%-68.3%) based on all patients. A total of 21 patients (60%) experienced distant failure; 18 patients (51%) experienced distant failure as the site of first failure. The 1-year distant failure rate was 46%, and the 2-year distant failure rate was 54%. The median time to distant failure was 15.8 months. Figure 2C shows progression-free survival, with 27 patients (77%) experiencing local and/or distant disease progression during the follow-up period. Median progression-free survival was 11.7 months (95% CI, 6.2-17.1 months) for all patients. Figure 2D shows OS for all 35 patients. The median OS was 41.1 months (95% CI, 19.0-63.1 months). The lower bound of this 95% CI exceeds the 17-month median OS value assumed from a historical series. The mean (SE) OS rate was 57.1% (8.4%) at 2 years, 51.4% (8.4%) at 3 years, and 37.1% (8.2%) at 5 years. A total of 23 patients (66%) have died during the study follow-up period.
Figure 2. Cumulative Incidence Analysis of Outcomes.
A, The local failure rate at 2 years was 26% and at 4 years was 34%. B, The distant failure rate at 1 year was 46% and at 2 years was 54%. C, Median progression-free survival was 11.7 months. The horizontal dotted line indicates the median. D, Median overall survival was 41.1 months. The horizontal dotted line indicates the median.
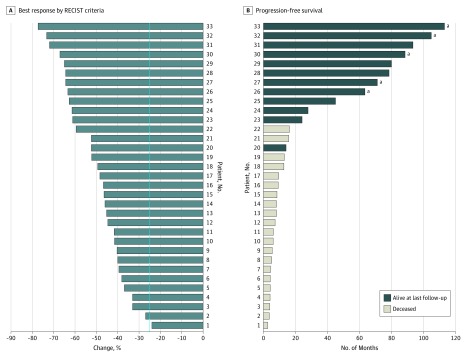
Figure 3A shows the waterfall plot of the best response rate of 33 patients who had RECIST-evaluable scans. Of these 33 patients, 31 (94%; 95% CI, 86%-100%) achieved a partial response, with the remaining 2 patients having stable disease (stable disease response rate, 6%). Figure 3B shows the swimmer plot of time to progression for all 33 patients with evaluable scans, with 8 patients remaining progression free at the time of last follow-up. No patient had a complete response on computed tomographic scan.
Figure 3. Response to Treatment.
A, Waterfall plot of best response by Response Evaluation Criteria in Solid Tumors (RECIST) criteria for 33 evaluable patients. The vertical dotted line indicates partial response. B, Swimmer plot of progression-free survival for 33 evaluable patients.
aNo recurrence or progression of disease at last follow-up.
Discussion
The benchmark for clinical outcome in unselected stage IIIA and IIIB NSCLC treated with concurrent CT-RT until 2017 was RTOG 0617, a multicenter randomized phase 3 trial of standard-dose (60 Gy) vs high-dose (74 Gy) CT-RT.1 The primary end point of RTOG 0617 was OS, with the governing hypothesis that dose escalation of radiotherapy would drive an improvement in local control, thereby improving OS. The study crossed a predefined futility boundary at interim analysis; therefore, the high-dose group was closed. The median OS for patients with LA-NSCLC receiving standard-dose CT-RT was 28.7 months (95% CI, 24.1-36.9 months) and was 20.3 months (95% CI, 17.7-25.0 months) for those who received high-dose radiotherapy. The 2-year local failure rate was 30.4% in the standard-dose group and 39.0% in the high-dose group.
A previous study reported the results of a phase 1 clinical trial of nelfinavir with concurrent CT-RT for patients with LA-NSCLC.8 Two DLs were tested in the phase 1 portion of the study: 625 mg orally twice daily and 1250 mg orally twice daily. Both DLs were well tolerated. The study proceeded to a phase 2 trial at the maximally tolerated dose of nelfinavir, 1250 mg orally twice daily. The clinical results of the combined phase 1/2 trial are the subject of this article.
Our study shows that administration of oral nelfinavir with concurrent CT-RT in patients with unresectable LA-NSCLC yields a promising 2-year local failure rate of 26%. In addition, we observed a median survival of 41.1 months and median progression-free survival of 11.7 months in this patient population. These numbers compare favorably with the historical benchmarks in the standard-dose group in RTOG 0617, although a randomized phase 3 trial is warranted to confirm our findings. Finally, we observed that this therapeutic approach was generally well tolerated, with no significant increase in grade 3 or 4 toxic effects beyond those expected with standard concurrent CT-RT.
One study has shown that nelfinavir, a protease inhibitor used in the treatment of HIV, inhibits PI-3 kinase and Akt signaling and sensitizes tumor cells to killing by ionizing radiation in vitro and in vivo.6 Another study has also demonstrated that nelfinavir in animal models improves tumor perfusion, suggesting that the observed enhancement of tumor oxygenation is due to increased blood flow to the tumor bed.16 In addition, PI-3 kinase inhibition has been demonstrated to have an independent antitumor immune-augmentation effect through the suppression of myeloid-derived suppressor cell activity in the tumor microenvironment and systemic circulation in preclinical metastatic tumor models.17 We hypothesize that it is these properties that drive the clinical results observed in this study.
There have been several prospective clinical trials examining the safety and efficacy of nelfinavir with concurrent radiotherapy in a variety of disease settings, including LA pancreatic cancer,18,19 LA rectal cancer,20,21 and glioblastoma multiforme,22 each of which documented good tolerability and promising clinical efficacy. The initial report of the phase 1 trial of nelfinavir with concurrent CT-RT in patients with LA-NSCLC8 similarly demonstrated excellent tolerability with promising clinical response rates. Brunner et al18 reported on 6 of 10 patients who were able to achieve complete surgical resection after induction CT-RT with nelfinavir in LA pancreatic cancer. In addition, 1 patient achieved complete tumor sterilization at the time of surgery. Five of 9 patients achieved a complete metabolic response as assessed by positron emission tomography/computed tomography. Alonso-Basanta et al22 reported a median OS of 13.7 months and median progression-free survival of 7.2 months with concurrent nelfinavir and temozolamide in glioblastoma multiforme. In addition, 3 of 18 patients in their study experienced out-of-field progression of disease as opposed to the more common in-field pattern of recurrence in glioblastoma multiforme after radiotherapy. These data suggest that nelfinavir may augment tumor response to radiotherapy in the setting of relatively radioresistant tumors such as pancreatic cancer, glioblastoma, and NSCLC.
Our overall objective response rate of 94% with a stable disease response rate of 6% is very promising. A retrospective series by Werner-Wasik et al23 examining best RECIST response to radiotherapy in nonoperative treatment of stage I to III NSCLC reported a 56% overall response rate and 35% stable disease rate, with 9% of patients having progressive disease. Some studies have previously reported that the tumor volume effect can be mitigated by significant escalation of radiotherapy dose to as high as 84 Gy in the setting of large-volume stage III NSCLC.24 These doses are not always achievable while meeting accepted dose-volume constraints in the stage III setting; the results of RTOG 0617 further call into question the use of dose escalation in LA-NSCLC. These observations underscore the clinical need for an alternative approach to improving tumor response to radiotherapy in LA-NSCLC and the potential utility of nelfinavir in this setting.
Limitations
This study is a nonrandomized phase 1/2 trial in patients with stage III NSCLC conducted in a single academic center, which introduces potential selection bias as well as imbalance of covariates when comparisons are made with historical controls. In addition, it is unclear whether this study approach will be generalizable to a larger, more heterogeneous patient population with variability in performance status. Finally, in 2018, the use of durvalumab in patients with LA-NSCLC after CT-RT has become the standard of practice in the United States; therefore, outcomes in this study need to be put in context. However, the use of durvalumab in the setting of consolidation treatment does not preclude further testing of nelfinavir concurrently with CT-RT either alone or in combination with checkpoint inhibition.25,26
Conclusions
This single-group phase 1/2 prospective trial of the HIV protease inhibitor nelfinavir administered with concurrent CT-RT in unresectable LA-NSCLC successfully met its enrollment target. This report delineates the long-term outcomes of the largest study to date with nelfinavir and concurrent CT-RT in any disease setting, to our knowledge, and demonstrated promising local control and OS in LA-NSCLC with no overt exacerbation of toxic effects. As nelfinavir is a US Food and Drug Administration–approved oral drug, this treatment approach is feasible and is potentially a readily exportable platform for daily clinical use. Additional testing in the randomized phase 3 setting should be conducted before this approach can be adopted more broadly for this study population.
eFigure. Phase I/II Trial of Protease Inhibitor Nelfinavir With Concurrent Chemoradiotherapy For Stage IIIA/IIIB Inoperable NSCLC
References
- 1.Bradley JD, Paulus R, Komaki R, et al. . Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16(2):187-199. doi: 10.1016/S1470-2045(14)71207-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aupérin A, Le Péchoux C, Rolland E, et al. . Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non–small-cell lung cancer. J Clin Oncol. 2010;28(13):2181-2190. doi: 10.1200/JCO.2009.26.2543 [DOI] [PubMed] [Google Scholar]
- 3.Ettinger DS, Bepler G, Bueno R, et al. ; National Comprehensive Cancer Network (NCCN) . Non-small cell lung cancer clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2006;4(6):548-582. doi: 10.6004/jnccn.2006.0046 [DOI] [PubMed] [Google Scholar]
- 4.McKenna WG, Muschel RJ. Targeting tumor cells by enhancing radiation sensitivity. Genes Chromosomes Cancer. 2003;38(4):330-338. doi: 10.1002/gcc.10296 [DOI] [PubMed] [Google Scholar]
- 5.Rengan R, Cengel KA, Hahn SM. Clinical target promiscuity: lessons from ras molecular trials. Cancer Metastasis Rev. 2008;27(3):403-414. doi: 10.1007/s10555-008-9133-z [DOI] [PubMed] [Google Scholar]
- 6.Gupta AK, Cerniglia GJ, Mick R, McKenna WG, Muschel RJ. HIV protease inhibitors block Akt signaling and radiosensitize tumor cells both in vitro and in vivo. Cancer Res. 2005;65(18):8256-8265. doi: 10.1158/0008-5472.CAN-05-1220 [DOI] [PubMed] [Google Scholar]
- 7.Pore N, Gupta AK, Cerniglia GJ, et al. . Nelfinavir down-regulates hypoxia-inducible factor 1α and VEGF expression and increases tumor oxygenation: implications for radiotherapy. Cancer Res. 2006;66(18):9252-9259. doi: 10.1158/0008-5472.CAN-06-1239 [DOI] [PubMed] [Google Scholar]
- 8.Rengan R, Mick R, Pryma D, et al. . A phase I trial of the HIV protease inhibitor nelfinavir with concurrent chemoradiotherapy for unresectable stage IIIA/IIIB non-small cell lung cancer: a report of toxicities and clinical response. J Thorac Oncol. 2012;7(4):709-715. doi: 10.1097/JTO.0b013e3182435aa6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monti AF, Ostinelli A, Frigerio M, et al. . An ICRU 50 radiotherapy treatment chart. Radiother Oncol. 1995;35(2):145-150. doi: 10.1016/0167-8140(95)01541-N [DOI] [PubMed] [Google Scholar]
- 10.Albain KS, Swann RS, Rusch VW, et al. . Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 2009;374(9687):379-386. doi: 10.1016/S0140-6736(09)60737-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rusch VW, Albain KS, Crowley JJ, et al. . Surgical resection of stage IIIA and stage IIIB non-small-cell lung cancer after concurrent induction chemoradiotherapy: a Southwest Oncology Group trial. J Thorac Cardiovasc Surg. 1993;105(1):97-104. [PubMed] [Google Scholar]
- 12.National Cancer Institute CTC v2.0 and Common Terminology Criteria for Adverse Events v4.0 (CTCAE). Bethesda, MD: National Cancer Institute; 2009. [Google Scholar]
- 13.O’Rourke N, Roqué I Figuls M, Farré Bernadó N, Macbeth F. Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Syst Rev. 2010;(6):CD002140. doi: 10.1002/14651858.CD002140.pub3 [DOI] [PubMed] [Google Scholar]
- 14.Eisenhauer EA, Therasse P, Bogaerts J, et al. . New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 15.Greenwood M. The natural duration of cancer. In: Reports on Public Health and Medical Subjects Vol. 33. London, UK: Her Majesty’s Stationery Office (HMSO); 1926: 1-26. [Google Scholar]
- 16.Qayum N, Muschel RJ, Im JH, et al. . Tumor vascular changes mediated by inhibition of oncogenic signaling. Cancer Res. 2009;69(15):6347-6354. doi: 10.1158/0008-5472.CAN-09-0657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim K, Skora AD, Li Z, et al. . Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of myeloid-derived cells. Proc Natl Acad Sci U S A. 2014;111(32):11774-11779. doi: 10.1073/pnas.1410626111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunner TB, Geiger M, Grabenbauer GG, et al. . Phase I trial of the human immunodeficiency virus protease inhibitor nelfinavir and chemoradiation for locally advanced pancreatic cancer. J Clin Oncol. 2008;26(16):2699-2706. doi: 10.1200/JCO.2007.15.2355 [DOI] [PubMed] [Google Scholar]
- 19.Wilson JM, Fokas E, Dutton SJ, et al. . ARCII: a phase II trial of the HIV protease inhibitor nelfinavir in combination with chemoradiation for locally advanced inoperable pancreatic cancer. Radiother Oncol. 2016;119(2):306-311. doi: 10.1016/j.radonc.2016.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buijsen J, Lammering G, Jansen RL, et al. . Phase I trial of the combination of the Akt inhibitor nelfinavir and chemoradiation for locally advanced rectal cancer. Radiother Oncol. 2013;107(2):184-188. doi: 10.1016/j.radonc.2013.03.023 [DOI] [PubMed] [Google Scholar]
- 21.Lenci R, Galante A, Borzi M, et al. . The effects of ticlopidine on platelet aggregation: study on 13 atherosclerotic patients [in Italian]. Clin Ter. 1983;104(3):205-209. [PubMed] [Google Scholar]
- 22.Alonso-Basanta M, Fang P, Maity A, Hahn SM, Lustig RA, Dorsey JF. A phase I study of nelfinavir concurrent with temozolomide and radiotherapy in patients with glioblastoma multiforme. J Neurooncol. 2014;116(2):365-372. doi: 10.1007/s11060-013-1303-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Werner-Wasik M, Xiao Y, Pequignot E, Curran WJ, Hauck W. Assessment of lung cancer response after nonoperative therapy: tumor diameter, bidimensional product, and volume: a serial CT scan–based study. Int J Radiat Oncol Biol Phys. 2001;51(1):56-61. doi: 10.1016/S0360-3016(01)01615-7 [DOI] [PubMed] [Google Scholar]
- 24.Rengan R, Rosenzweig KE, Venkatraman E, et al. . Improved local control with higher doses of radiation in large-volume stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;60(3):741-747. doi: 10.1016/j.ijrobp.2004.04.013 [DOI] [PubMed] [Google Scholar]
- 25.Antonia SJ, Özgüroğlu M. Durvalumab in stage III non-small-cell lung cancer. N Engl J Med. 2018;378(9):869-870. doi: 10.1056/NEJMc1716426 [DOI] [PubMed] [Google Scholar]
- 26.Antonia SJ, Villegas A, Daniel D, et al. ; PACIFIC Investigators . Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N Engl J Med. 2017;377(20):1919-1929. doi: 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Phase I/II Trial of Protease Inhibitor Nelfinavir With Concurrent Chemoradiotherapy For Stage IIIA/IIIB Inoperable NSCLC