Abstract
Study Objectives:
To determine whether therapeutic positive airway pressure (PAP) level predicts response to hypoglossal nerve stimulation (HGNS) for obstructive sleep apnea using the coprimary outcomes of apnea-hypopnea index (AHI) and 4% oxygen desaturation index.
Methods:
Combined cohort study from two US sleep otolaryngology training programs. Subjects were adults with AHI > 15 events/h who underwent HGNS. Eligible subjects had diagnostic preoperative sleep studies, full-night efficacy postoperative studies and therapeutic PAP levels available for analysis. Low and high PAP groups were compared using the t test for continuous variables and chi-square test for categorical variables.
Results:
Fifty-six patients met all inclusion criteria. On average, patients were male, Caucasian, middle-aged, and overweight. Thirteen patients were in the low PAP group (< 8 cm H2O) and 43 patients in the high PAP group (≥ 8 cm H2O). Although both groups experienced improvement of polysomnographic measures with HGNS, the low PAP group achieved a significantly larger mean AHI reduction (36.7 ± 22.7 versus 18.4 ± 23.4, P = .02). Additionally, the low PAP group had a greater response rate (defined as AHI < 20 events/h and > 50% reduction of AHI) than the high PAP group (92% versus 44%, P < .01).
Conclusions:
Therapeutic PAP level may aid in the discernment of candidacy for HGNS, with a strong positive predictive value for PAP levels < 8 cm H2O. A larger prospective study is needed to confirm these findings.
Citation:
Lee CH, Seay EG, Walters BK, Scalzitti NJ, Dedhia RC. Therapeutic positive airway pressure level predicts response to hypoglossal nerve stimulation for obstructive sleep apnea. J Clin Sleep Med. 2019;15(8):1165–1172.
Keywords: hypoglossal nerve stimulation, obstructive sleep apnea, positive airway pressure, sleep surgery
BRIEF SUMMARY
Current Knowledge/Study Rationale: Hypoglossal nerve stimulation (HGNS) is a novel form of obstructive sleep apnea therapy, which may benefit patients with moderate-severe disease who do not tolerate positive airway pressure (PAP). However, the response rate remains suboptimal without clear predictors of success.
Study Impact: This is the first study to investigate therapeutic PAP level as a predictor of HGNS response. Although both low and high PAP groups experienced polysomnographic improvement with HGNS, the low PAP group achieved a larger apnea-hypopnea index reduction and higher response rate. Our findings suggest that therapeutic PAP levels may be used to identify optimal candidates for HGNS, with values less than 8 cm H2O predictive of success.
INTRODUCTION
Obstructive sleep apnea (OSA) is a disorder characterized by recurrent collapse of the upper airway during sleep. The resulting hypoxia and arousals place patients at risk for a variety of health consequences, including cardiovascular disease, insulin resistance, and neurocognitive dysfunction.1,2 First-line therapy for OSA is positive airway pressure (PAP). In patients with moderate to severe OSA, PAP has been shown to reduce cardiovascular risk, improve cognition, and increase quality of life.3–5 Despite its benefits, 46% to 83% of patients are nonadherent to PAP therapy6; thus, the need for PAP alternatives is evident.
One alternative to PAP is sleep surgery, which includes pharyngeal surgery, skeletal surgery (eg, maxillomandibular advancement), tracheostomy, and most recently, hypoglossal nerve stimulation (HGNS). Three elements comprise the Inspire HGNS system: a thoracic respiratory sensor, implanted pulse generator, and hypoglossal nerve stimulation electrode (Inspire Medical Systems, Maple Grove, Minnesota). With detection of inspiration, the pulse generator stimulates select branches of the hypoglossal nerve, stiffening and protruding the tongue to dilate the airway.7 Five-year data from the Stimulation Therapy for Apnea Reduction (STAR) trial show promising long-term improvements in objective and subjective measures of OSA. Yet, the response rate (defined as apnea-hypopnea index (AHI) < 20 events/h and > 50% reduction of AHI from full-night polysomnography) at 5 years appears to be approximately 60%, leaving many patients who have implants without adequate therapy.8
It is unclear why some patients respond poorly to HGNS while others succeed. Feasibility trials found that patients with body mass index (BMI) less than 32 kg/m2, AHI less than 50 events/h, and lack of complete concentric palatal collapse on drug-induced sleep endoscopy (DISE) were more likely to achieve surgical success.9 These findings have shaped current Food and Drug Administration recommendations for HGNS. The STAR trial examined various biomarkers as predictors of therapy response. Age, sex, neck size, and baseline AHI were not found to be predictive.8 A recent study by Schwab et al. utilized computed tomography (CT) to identify anatomical differences between patients with HGNS. Therapy responders were found to have smaller baseline soft palate volumes than nonresponders. In addition, responders were also noted to have more anterior tongue displacement, greater increase in retroglossal airway size, and increased shortening of the mandible-hyoid distance with therapy activation.10
Another important variable in OSA is airway collapsibility, defined as the critical pharyngeal closing pressure (Pcrit). In early models of HGNS, reductions in AHI were found to be associated with decreases in Pcrit.11 Although it is clear that reduction of airway collapsibility plays a role in the mechanism of HGNS, it is unknown whether baseline Pcrit can predict therapy response. Historically, studies of airway collapsibility have been limited by the difficulty of obtaining Pcrit measurements. However, a recent study by Landry et al. identified a positive predictive relationship between Pcrit and therapeutic PAP level, permitting the use of PAP levels as a proxy for airway collapsibility.12 This finding is interesting in the setting of three independent studies, which found that patients with low therapeutic PAP levels were more likely to succeed with oral appliances for OSA.13–15
In this study, we sought to determine whether therapeutic PAP level predicts outcomes following HGNS for OSA. Our primary aim was to determine whether there is a difference in reduction of AHI or 4% oxygen desaturation index (ODI4) between patients with low and high PAP levels. Second, we wanted to determine if there is a difference in improvement of subjective measures of OSA burden between the two groups. We hypothesized that patients with low PAP levels would achieve a larger reduction in key polysomnographic variables and greater subjective improvement, compared to patients with high PAP levels.
METHODS
Participants
Participants were recruited from two sites: Emory and San Antonio Military Medical Center (SAMMC). At the Emory site, a prospective cohort study was approved by the Emory University Institutional Review Board (IRB00088402). Individuals were recruited from May 2016 to October 2018 at the sleep surgery clinic of the senior author (R.C.D.). A retrospective chart review was subsequently added to include patients from September 2015 to May 2016 to maximize sample size. Inclusion criteria were age older than 18 years and HGNS for the treatment of OSA. Indications for HGNS were AHI > 15 events/h on most recent diagnostic sleep study, PAP intolerance, and lack of complete circumferential palatal collapse on preoperative DISE. Patients were excluded for the following reasons: no documented PAP trial; missing preoperative or postoperative sleep studies; diagnosis of trisomy 21. At the SAMMC site, the 59th Medical Wing (59 MDW) Institutional Review Board (FWH20180176H) approved a retrospective chart review of patients seen at the Otolaryngology and Sleep Medicine clinics between July 2015 and August 2018. Inclusion and exclusion criteria were identical to those of the Emory site.
Data Collection
Medical record extraction was performed by C.H.L, N.J.S., and B.K.W. Data were manually entered into an Excel spreadsheet (Microsoft Corporation, Redmond, Washington, USA) stored on a password-protected, institutional server at each institution. Following execution of a formal data transfer agreement between Emory and SAMMC, Emory electronically received the SAMMC data in a de-identified fashion. Variables extracted from the medical record were sex, race, ethnicity, age, BMI, past medical history, and past surgical history.
Responses to the following questionnaires were recorded: Epworth Sleepiness Scale (ESS) and Snoring Visual Analog Scale (VAS). The ESS is a validated measure of daytime sleepiness with possible scores ranging from 0 to 24, and scores higher than 10 implying pathologic sleepiness.16 A three-point decrease on the ESS represents a clinically meaningful improvement.17 At Emory, the Snoring VAS was administered by instructing patients to mark the degree to which snoring bothers their bed partner on a line labeled “no snoring” on the left and “extreme snoring noises causing the bed partner to leave room” on the right. The distance of the mark was divided by the length of the axis, and then normalized to 100. Postoperative values were obtained by averaging all responses after the efficacy study. At SAMMC, the Snoring VAS was administered by instructing patients to mark the severity of their snoring on a scale from 0 (none) to 10 (extremely loud). Values were normalized to 100 for analysis. Postoperative values were obtained at a visit at least 6 months after initiation of HGNS therapy.
At both sites, three methods were used to determine therapeutic PAP level. The preferred method was a download from an autotitrating positive airway pressure (APAP) device. In order to reflect physiologic airway requirements, the 90th or 95th percentile pressures were determined to be the therapeutic PAP level. If an APAP download was not available, a continuous positive airway pressure (CPAP) titration study was used. In this case, therapeutic PAP level was determined to be the pressure at which the AHI < 5 events/h for a minimum of 15 minutes, in accordance with the American Academy of Sleep Medicine guidelines.18 If neither of the aforementioned methods were available, a CPAP download was used, in which case therapeutic PAP level was determined to be the pressure at which the device was set.
Variables extracted from sleep studies were AHI and ODI4. Both in-laboratory polysomnography (PSG) and home sleep apnea testing (HSAT) were included. At Emory, preoperative values were obtained by averaging values from the most recent diagnostic sleep study and all studies obtained within 3 years prior to HGNS. Postoperative values were extracted from efficacy studies, which were obtained when the senior author (R.C.D.) deemed a patient to be optimized on therapy. Efficacy studies represent a full night of sleep at a single device setting. When possible, HGNS use was verified through Inspire Cloud, Model 6074, v2.2 software (Inspire Medical, Minneapolis, Minnesota, United States) that allows remote monitoring of nightly HGNS adherence. At SAMMC, preoperative values were extracted from the most recent sleep study prior to HGNS implantation. Postoperative values were obtained from efficacy studies performed at least 6 months after initiating HGNS. These studies also represent a full night of sleep at a single therapy setting. Usage data were obtained in the standard manner by connecting to the patients’ implanted pulse generators via telemetry.
Statistical Analysis
An a priori sample size calculation was performed for AHI reduction. Based on clinical experience, we estimated the mean reduction in AHI to be 20 events/h (standard deviation 15) in the low PAP group and 5 events/h (standard deviation 15) in the high PAP group. Based on a significance level (α = .05, two-tailed) and power (1 – β = .80), a sample of 32 patients was needed.
Analyses were performed with Microsoft Excel (Microsoft Corporation), Stata Statistical Software: Release 11 (Stata-Corp LP, College Station, Texas, USA) and SPSS (IBM, Armonk, New York, USA). Based on previous data collection and literature review, we hypothesized 8 cm H2O to be the threshold between low and high PAP groups.12 The t test was used to compare all continuous variables between SAMMC and Emory cohorts, preoperative and postoperative values, and low and high PAP groups. Chi-square testing was used to compare proportions of therapy responders. Response was defined under three different criteria: (1) AHI reduction > 50% and AHI < 20 events/h (Sher criteria); (2) AHI reduction > 50% and AHI < 10 events/h; (3) AHI < 5 events/h. Next, linear regression was performed for the coprimary outcomes. We intentionally did not adjust for age, sex, or BMI because some of these variables have previously been shown to correlate with therapeutic PAP level.19 Finally, receiver operating curve (ROC) analysis was used to identify the optimal cutoff value for PAP levels and compare its predictive value to known predictor variables.
RESULTS
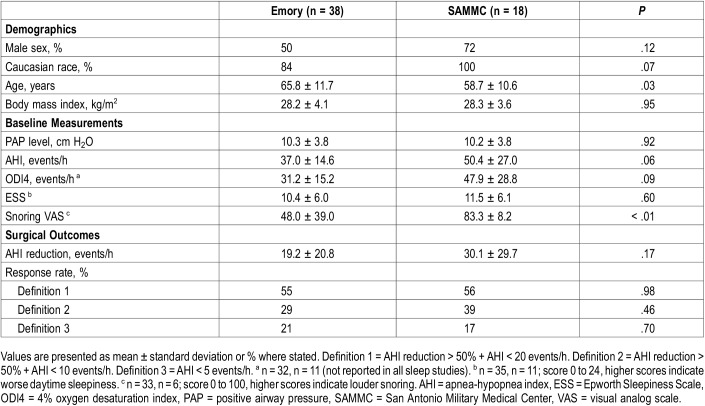
Fifty-six patients met all study criteria, including 38 patients from Emory and 18 patients from SAMMC. Patient demographics, baseline measurements and surgical outcomes for each cohort are described in Table 1. Compared to the SAMMC group, the Emory cohort included nonwhite subjects, more females and lower baseline AHI. Of statistical significance, the SAMMC cohort was older and reported greater snoring bother based on the visual analog scale. Following HGNS, both cohorts achieved similar mean AHI reductions and response rates.
Table 1.
Characteristics of Emory and SAMMC cohorts.
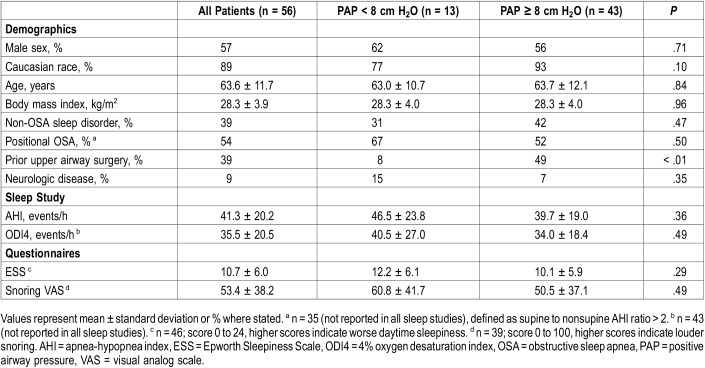
Therapeutic PAP levels ranged from 4 to 23 cm H2O. Thirteen patients had PAP levels < 8 cm H2O (low PAP group) and 43 patients had ≥ 8 cm H2O (high PAP group). Seven PAP levels were obtained through APAP downloads, while the remainder were extracted from titration studies and CPAP settings. Table 2 illustrates demographic, polysomnographic and symptomatic values for both the low and high PAP groups. Twenty-two patients had comorbid sleep disorders, most commonly insomnia (n = 18) and restless leg syndrome (n = 7). Twenty-two patients had prior airway surgery, including 14 tonsillectomies, 9 nasal surgeries and 7 palatal surgeries. Of note, a significantly greater proportion of patients in the high PAP group had prior airway surgery compared to the low PAP group. Of the five patients with neurological disease, two had prior strokes without residual effects, two had mild dementia and one had normal pressure hydrocephalus. Fifteen patients had moderate OSA (AHI 15–29.9 events/h) and 41 had severe OSA (AHI ≥ 30 events/h). Questionnaire responses indicated mild excessive daytime sleepiness and disturbances due to snoring.20
Table 2.
Comparison of low and high PAP groups.
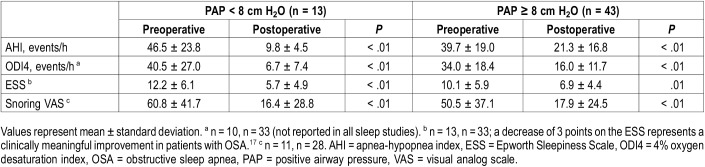
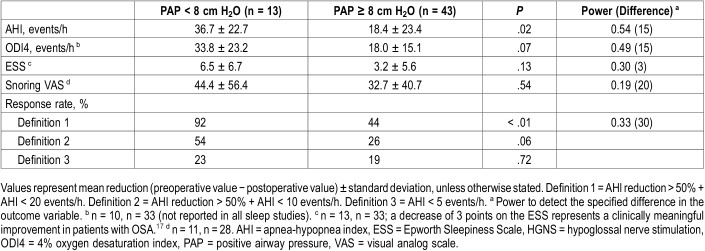
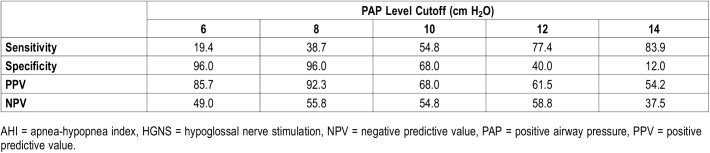
Table 3 uses the Student t test to compare preoperative and postoperative values of all key variables. Both the low and high PAP groups achieved significant improvements of AHI, ODI4, ESS and snoring VAS. Table 4 compares HGNS outcomes between low and high PAP groups. The low PAP group achieved a significantly larger AHI reduction than the high PAP group (36.7 ± 22.7 versus 18.4 ± 23.4, P = .02). The overall response rate was 55% according to criteria definition 1 (Sher criteria); the low PAP group had a significantly higher response rate than the high PAP group (92% versus 44%, P < .01). Response rates calculated by definitions 2 and 3 did not show significant differences between the low and high PAP groups. There were no significant differences in ODI4 reduction or improvement of subjective measures of OSA burden. Table 5 compares various PAP levels as cutoff values for predictors of HGNS response. Overall, higher cutoff values increase sensitivity but decrease specificity. Conversely, low PAP levels have greater specificity, with the highest positive predictive value (92%) for pressures less than 8 cm H2O.
Table 3.
Student t test comparing preoperative and postoperative values of polysomnographic and self-reported measures of OSA.
Table 4.
Comparison of HGNS outcomes between low and high PAP groups.
Table 5.
Comparison of PAP levels as cutoff values for prediction of HGNS response (according to definition 1: AHI < 20 events/h and > 50% reduction).
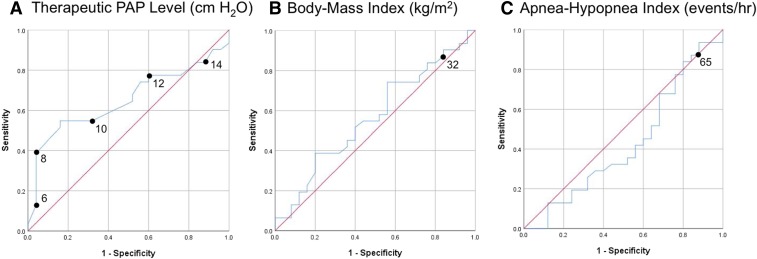
Figure 1A shows the ROC curve of therapeutic PAP level as a predictor of HGNS response. The area under curve (AUC) was 0.65 (95% confidence interval [CI] 0.51–0.80), demonstrating greater predictive value than BMI (Figure 1B, AUC 0.57, 95% CI 0.41–0.72) and AHI (Figure 1C, AUC 0.43, 95% CI 0.27–0.58). Linear regression analyses showed nonsignificant correlation between PAP level, AHI reduction and ODI4 reduction.
Figure 1. Predictors of HGNS response.
Blue line shows the receiver operating characteristic curve for (A) therapeutic PAP level, (B) body mass index, and (C) AHI as predictors of HGNS response. Response defined as AHI reduction > 50% and AHI < 20 events/h. Red line shows the null hypothesis. AHI = apnea-hypopnea index, HGNS = hypoglossal nerve stimulation, PAP = positive airway pressure.
DISCUSSION
This is the first study to examine therapeutic PAP level as a predictor of HGNS response. While both the low and high PAP groups experienced improvements in key polysomnographic variables following HGNS, the low PAP group (< 8 cm H2O) achieved a significantly larger AHI reduction. The low PAP group also had greater surgical success (93% versus 42%), defined according to Sher criteria (AHI reduction > 50% and AHI < 20 events/h). There was no difference in improvement of ESS or Snoring VAS between groups.
The current recommendations for HGNS patient selection stem from feasibility studies conducted by Van de Heyning et al9 In their study, twenty-two patients with moderate-severe OSA were implanted with HGNS. The cohort was entirely male with a mean age of 55.7 years, BMI of 29.8 kg/m2 and AHI of 43.6 events/h. Of the twenty-patients who underwent 6-month follow-up, six achieved surgical success according to Sher criteria. The combination of AHI < 50 events/hr and BMI < 32 kg/m2 was present in all responders and found to be significantly associated with success (P = .01). Additionally, a subset of patients underwent preoperative DISE. All patients with CCC (n = 4) were nonresponders, while those without CCC (n = 3) succeeded. In the next phase of the study, nine patients were implanted according to criteria of BMI < 32 kg/m2, AHI 20–50 events/h and lack of CCC. A higher success rate was observed, with only one nonresponder. However, a confounding factor was that the second cohort underwent implantation with selective stimulation of protruding branches of CN XII, while the first cohort received implants stimulating both protruding and retracting branches.
Despite the widespread use of BMI and AHI criteria, larger cohort studies have not supported the predictive value of these variables. In the STAR trial cohort of 53 responders and 18 nonresponders, neither BMI nor AHI were found to predict response at 60 months.8 A recent two-center study by Huntley et al compared outcomes between 113 patients with BMI < 32 kg/m2 and 40 patients with BMI > 32 kg/m2. There were no significant differences in postoperative AHI, ESS or surgical success between the low and high BMI groups.21 Additionally, no differences in outcomes have been identified between elderly (> 65 years) and nonelderly (< 65 years) cohorts, as well as those with and without prior palatal surgery.22,23
Our observed findings related to HGNS are in accordance with studies investigating the predictive value of therapeutic PAP level for oral appliance therapy (OAT) outcomes.13–15 Oral appliances are orthodontic retainers that advance the mandible to maintain a patent airway.24 In the first study by Tsuiki et al, a cohort of 35 CPAP-adherent Japanese men (mean age 55 years, BMI 26 kg/m2, baseline AHI 37 events/h) were asked to stop using CPAP prior to being fitted with an oral appliance. Therapeutic PAP levels were lower in responders, when defined as > 50% reduction in AHI and AHI < 5 events/h. In addition, PAP levels > 10.5 cm H2O were associated with therapy failure.13 In the second study by Sutherland et al, a cohort of 78 Australian adults with newly diagnosed OSA (mean age 49 years, BMI 29 kg/m2, baseline AHI 30 events/h) underwent a month-long crossover trial with CPAP and OAT. Therapeutic PAP levels were lower in responders, when defined as an AHI < 10 events/h; PAP levels > 13 cm H2O were associated with therapy failure.14 In the third study by Storesund et al, a cohort of 87 Norwegian adults nonadherent to CPAP (mean age 57 years, BMI 29 kg/m2, baseline AHI 24 events/h) underwent a trial of OAT. Therapeutic PAP level was lower in responders, defined as AHI < 5 events/h; PAP levels > 12 cm H2O were associated with therapy failure.15
While high PAP levels negatively predicted OAT response in the abovementioned studies, our HGNS findings do not demonstrate a similar pattern as greater than 40% in the high pressure group achieved success. This may be due to the heterogeneity of pathologies represented by patients with high PAP levels. From a physiologic standpoint, patients with cardiopulmonary disease often require high PAP levels to mitigate baseline hypoxemia, not for treatment of excessive airway collapsibility. Comorbid sleep disorders, neurological disease and prior surgical modifications may also contribute to high PAP levels. Less commonly, high PAP levels may represent epiglottic collapse caused by a retroflexed or abnormally lax epiglottis. This is often challenging to treat with PAP, as increased airway pressure may displace the epiglottis deeper into the airway and exacerbate obstruction.25 As a result, patients with epiglottic collapse may be prescribed high PAP levels yet are reasonable candidates for hypoglossal nerve stimulation. By understanding the basis for those patients with elevated PAP levels, we may better identify the patients most likely to succeed with HGNS.
A more accurate method of determining patient-specific PAP levels may be with the assistance of drug-induced sleep endoscopy (DISE). DISE utilizes flexible laryngoscopy to visualize the upper airway under various modes of pharmacologic sedation, providing information about the sites and patterns of airway collapse seen in OSA patients.26 While it is primarily used to identify treatment options for PAP-intolerant patients, a recent development has been the concurrent application of PAP during DISE.27 By directly observing PAP’s effect on the airway, the exact pressure at which the airway is patent can be determined.28 As oronasal masks require on average 1.5 cm H2O more pressure than nasal masks,29 our group has developed a standard “PAP DISE” protocol with a nasal mask to determine if the “PAP DISE” levels can better predict therapy response.
We acknowledge that our study has several limitations. As only 23% patients met criteria for the low PAP group, predictive factors for the majority patients remain at large. The retrospective nature of our data collection provided unwanted heterogeneity. We used three different sources to determine therapeutic PAP level, received sleep data from many different laboratories and did not account for various mask interfaces. Due to our having heterogenous referral centers, several preoperative sleep studies did not include ODI values, limiting meaningful analysis of this important sleep study variable. We excluded patients with Down syndrome due to their unique qualities of hypotonia and relative macroglossia in the setting of a tongue neurostimulation therapy. We included seven patients with minimally-impaired neurological disease (history of stroke, mild dementia and normal pressure hydrocephalus) as we felt these results remained generalizable. Finally, five patients did not achieve an AHI < 5 events/h on the therapy used to determine PAP level; this may have led to misrepresentation of therapeutic PAP levels.
Conversely, our study has several strengths. Chiefly, we utilized full-night efficacy studies at a single setting as our dependent variable, providing accurate HGNS results.30 When home sleep studies were performed, Inspire Cloud was used to verify HGNS use during these studies. Our subjects comprised two unique patient populations: a younger, predominantly male cohort with more severe OSA and an older, equally male and female civilian cohort with less severe OSA. Through inclusion of multiple sites and multiple surgeons, our results are generalizable. Finally, we chose to study therapeutic PAP level, a variable that is readily available in clinical settings, in contrast to Pcrit, which is obtained for research purposes.
In summary, our study indicates that therapeutic PAP levels below 8 cm H2O may be used to identify patients most likely to achieve success with HGNS. Larger, prospective studies are needed to confirm our findings and improve our success rates of this promising new paradigm in OSA therapy.
DISCLOSURE STATEMENT
The authors report no conflicts of interest.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- APAP
autotitrating positive airway pressure
- AUC
area under curve
- BMI
body mass index
- CCC
complete concentric collapse
- CPAP
continuous positive airway pressure
- CT
computed tomography
- DISE
drug-induced sleep endoscopy
- ESS
Epworth Sleepiness Scale
- HGNS
hypoglossal nerve stimulation
- HSAT
home sleep apnea test
- NPV
negative predictive value
- OAT
oral appliance therapy
- ODI4
4% oxygen desaturation index
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
- PPV
positive predictive value
- PSG
polysomnography
- ROC
receiver operating curve
- SAMMC
San Antonio Military Medical Center
- STAR
stimulation therapy for apnea reduction
- VAS
visual analog scale
REFERENCES
- 1.Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373(9657):82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- 2.Lal C, Strange C, Bachman D. Neurocognitive impairment in obstructive sleep apnea. Chest. 2012;141(6):1601–1610. doi: 10.1378/chest.11-2214. [DOI] [PubMed] [Google Scholar]
- 3.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 4.Kushida CA, Nichols DA, Holmes TH, et al. Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: The Apnea Positive Pressure Long-term Efficacy Study (APPLES) Sleep. 2012;35(12):1593–1602. doi: 10.5665/sleep.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batool-Anwar S, Goodwin JL, Kushida CA, et al. Impact of continuous positive airway pressure (CPAP) on quality of life in patients with obstructive sleep apnea (OSA) J Sleep Res. 2016;25(6):731–738. doi: 10.1111/jsr.12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thora Soc. 2008;5(2):173–178. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strollo PJ, Jr, Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139–149. doi: 10.1056/NEJMoa1308659. [DOI] [PubMed] [Google Scholar]
- 8.Woodson BT, Strohl KP, Soose RJ, et al. Upper airway stimulation for obstructive sleep apnea: 5-year outcomes. Otolaryngol Head Neck Surg. 2018;159(1):194–202. doi: 10.1177/0194599818762383. [DOI] [PubMed] [Google Scholar]
- 9.Van de Heyning PH, Badr MS, Baskin JZ, et al. Implanted upper airway stimulation device for obstructive sleep apnea. Laryngoscope. 2012;122(7):1626–1633. doi: 10.1002/lary.23301. [DOI] [PubMed] [Google Scholar]
- 10.Schwab RJ, Wang SH, Verbraecken J, et al. Anatomic predictors of response and mechanism of action of upper airway stimulation therapy in patients with obstructive sleep apnea. Sleep. 2018;41(4) doi: 10.1093/sleep/zsy021. [DOI] [PubMed] [Google Scholar]
- 11.Oliven A, O'Hearn DJ, Boudewyns A, et al. Upper airway response to electrical stimulation of the genioglossus in obstructive sleep apnea. J Appl Physiol (1985) 2003;95(5):2023–2029. doi: 10.1152/japplphysiol.00203.2003. [DOI] [PubMed] [Google Scholar]
- 12.Landry SA, Joosten SA, Eckert DJ, et al. Therapeutic CPAP level predicts upper airway collapsibility in patients with obstructive sleep apnea. Sleep. 2017;40(6) doi: 10.1093/sleep/zsx056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsuiki S, Kobayashi M, Namba K, et al. Optimal positive airway pressure predicts oral appliance response to sleep apnoea. Eur Respir J. 2010;35(5):1098–1105. doi: 10.1183/09031936.00121608. [DOI] [PubMed] [Google Scholar]
- 14.Sutherland K, Phillips CL, Davies A, et al. CPAP pressure for prediction of oral appliance treatment response in obstructive sleep apnea. J Clin Sleep Med. 2014;10(9):943–949. doi: 10.5664/jcsm.4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Storesund A, Johansson A, Bjorvatn B, Lehmann S. Oral appliance treatment outcome can be predicted by continuous positive airway pressure in moderate to severe obstructive sleep apnea. Sleep Breath. 2018;22(2):385–392. doi: 10.1007/s11325-017-1578-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 17.Patel S, Kon SSC, Nolan CM, et al. The Epworth Sleepiness Scale: minimum clinically important difference in obstructive sleep apnea. Am J Respir Crit Care Med. 2018;197(7):961–963. doi: 10.1164/rccm.201704-0672LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kushida CA, Chediak A, Berry RB, et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4(2):157–171. [PMC free article] [PubMed] [Google Scholar]
- 19.Lai CC, Friedman M, Lin HC, et al. Clinical predictors of effective continuous positive airway pressure in patients with obstructive sleep apnea/hypopnea syndrome. Laryngoscope. 2015;125(8):1983–1987. doi: 10.1002/lary.25125. [DOI] [PubMed] [Google Scholar]
- 20.Johns MW. The Epworth Sleepiness Scale website. http://epworthsleepinessscale.com/. Accessed April 1, 2019.
- 21.Huntley C, Steffen A, Doghramji K, Hofauer B, Heiser C, Boon M. Upper airway stimulation in patients with obstructive sleep apnea and an elevated body mass index: a multi-institutional review. Laryngoscope. 2018;128(10):2425–2428. doi: 10.1002/lary.27426. [DOI] [PubMed] [Google Scholar]
- 22.Zhu Z, Hofauer B, Wirth M, et al. Selective upper airway stimulation in older patients. Respir Med. 2018;140:77–81. doi: 10.1016/j.rmed.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Huntley C, Vasconcellos A, Doghramji K, Hofauer B, Heiser C, Boon M. Upper airway stimulation in patients who have undergone unsuccessful prior palate surgery: an initial evaluation. Otolaryngol Head Neck Surg. 2018;159(5):938–940. doi: 10.1177/0194599818792191. [DOI] [PubMed] [Google Scholar]
- 24.Ramar K, Dort LC, Katz SG, et al. Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015. J Clin Sleep Med. 2015;11(7):773–827. doi: 10.5664/jcsm.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torre C, Camacho M, Liu SY, Huon LK, Capasso R. Epiglottis collapse in adult obstructive sleep apnea: a systematic review. Laryngoscope. 2016;126(2):515–523. doi: 10.1002/lary.25589. [DOI] [PubMed] [Google Scholar]
- 26.Shteamer JW, Dedhia RC. Sedative choice in drug-induced sleep endoscopy: a neuropharmacology-based review. Laryngoscope. 2017;127(1):273–279. doi: 10.1002/lary.26132. [DOI] [PubMed] [Google Scholar]
- 27.De Vito A, Carrasco Llatas M, Ravensloot M, et al. European position paper on drug-induced sleep endoscopy (DISE): 2017 update. Clin Otolaryngol. 2018;43(6):1541–1552. doi: 10.1111/coa.13213. [DOI] [PubMed] [Google Scholar]
- 28.Civelek S, Emre IE, Dizdar D, et al. Comparison of conventional continuous positive airway pressure to continuous positive airway pressure titration performed with sleep endoscopy. Laryngoscope. 2012;122(3):691–695. doi: 10.1002/lary.22494. [DOI] [PubMed] [Google Scholar]
- 29.Andrade RGS, Viana FM, Nascimento JA, et al. Nasal vs oronasal CPAP for OSA treatment: a meta-analysis. Chest. 2018;153(3):665–674. doi: 10.1016/j.chest.2017.10.044. [DOI] [PubMed] [Google Scholar]
- 30.Dedhia RC, Woodson BT. Standardized reporting for hypoglossal nerve stimulation outcomes. J Clin Sleep Med. 2018;14(11):1835–1836. doi: 10.5664/jcsm.7470. [DOI] [PMC free article] [PubMed] [Google Scholar]