Abstract
Study Objectives:
To determine whether an oral iron supplement improves restless leg/restless sleep symptoms in a pediatric population.
Methods:
In a cohort study, 47 patients (age 5–18 years) exhibiting restless legs/restless sleep symptoms and low serum ferritin levels (< 50 ng/mL) were given a daily oral iron supplement (ferrous sulfate + vitamin C) and re-evaluated 8 weeks later. A diagnosis of definite Restless Legs Syndrome (RLS) was determined based on criteria established by the International RLS Study Group. Using Wilcoxon signed-rank tests and Spearman rho, the change and association between the measures of Pediatric Restless Legs Syndrome Severity Scale and serum ferritin levels were also examined.
Results:
Overall, the median change and distribution of ferritin was statistically significantly different after 8 weeks of treatment (40.0 versus 23.0 ng/mL, P < .0001). Median RLS score was also statistically significantly lower from baseline to follow-up (4.0 versus 6.0, P = .0283). Sixteen patients met criteria for definite RLS; however, the change in RLS score was not determined to be significant in our population (9.5 versus 7.0, P = .0558), despite significant change in ferritin (25.0 versus 42.5 ng/mL, P < .0001). In addition, no correlation was observed between change in RLS score and ferritin level (rho = −.39, P = .1362).
Conclusions:
In preliminary findings, we found a modest, yet nonsignificant improvement in children exhibiting restless sleep and RLS symptomatology, despite significant improvement in ferritin levels. Though not statistically significant, the findings can lend to the suggested benefit of iron supplementation in patients with RLS; however, clinical judgment and further research is necessary.
Citation:
Rosen GM, Morrissette S, Larson A, Stading P, Barnes TL. Does improvement of low serum ferritin improve symptoms of restless legs syndrome in a cohort of pediatric patients? J Clin Sleep Med. 2019;15(8):1149–1154.
Keywords: iron supplement, iron deficiency, pediatrics, restless legs syndrome, restless sleep, serum ferritin, sleep
BRIEF SUMMARY
Current Knowledge/Study Rationale: Iron deficiency is thought to be important in the pathophysiology of restless legs syndrome (RLS), but studies measuring the effects of iron supplementation on RLS symptoms have yielded mixed results. We assessed the relationship between RLS symptoms and serum ferritin in a cohort of pediatric patients and measured the effects of oral iron supplementation on ferritin levels and RLS symptoms.
Study Impact: We found that improving low serum ferritin levels modestly improved RLS symptoms in some children but did not lead to statistically significant improvement in children with definite RLS. Based on our findings, we recommend that treatment decisions regarding pharmacotherapy for RLS in children with iron deficiency and low serum ferritin levels should not be necessarily delayed, but warrant further study.
INTRODUCTION
Pediatric restless legs syndrome (RLS), a neurologic sleep disorder characterized by an uncomfortable urge to move the legs while at rest, is present in 1.9% of children in general pediatric population surveys1–5 and 5.9% of patients in pediatric sleep centers.5 In addition, restless legs are reported as a comorbid diagnosis in 12% to 35% of children with attention deficit hyperactivity disorder (ADHD).6 Beyond the urge to move, symptoms of RLS include tingliness and pain, difficulty staying still, and disrupted sleep and associated issues such as daytime sleepiness.2,7 To date, treatment for RLS in children has generally consisted of revised bedtime habits and medication.1,8,9 Iron supplementation also is a commonly cited approach for treatment, but reports of its effectiveness for RLS are mixed.9
Iron deficiency is believed to be important in the pathophysiology of RLS and ADHD,6,10 as well as periodic limb movement disorder (PLMD) (a condition similar to RLS but which occurs while a patient is sleeping versus awake as in RLS).1 All of these conditions are believed to be mediated through the brain dopamine production system, in which iron plays a key role.1,2 Iron is unique among the elements because of its ability to act as an electron donor and acceptor by readily interconverting between ferric (Fe3+) and ferrous (Fe2+) forms. This property allows iron to function as a key component for oxygen transport (hemoglobin), oxygen storage (myoglobin) and energy production (cytochromes), and as a catalyst for many enzymatic systems including tyrosine hydroxylase, the rate-limiting step in the production of dopamine. These same properties contribute to iron’s toxicity through the formation of oxygen free radicals if iron levels are too high. Consequently, iron homeostasis is tightly controlled through feedback loops, which control iron absorption, to maintain iron levels within an acceptable physiologic range. Hepcidin2,10 is recognized as a key regulator of iron homeostasis, controlling iron absorption, as well as efflux of iron into and out of the duodenum, liver, spleen, and blood cells. Hepcidin has a daily circadian rhythm,11 is decreased during iron deficiency, and increased by inflammation. Symptoms of ADHD have been shown to be increased in children with iron deficiency,8,12 and some studies have shown improvement with RLS and ADHD symptoms after treatment with supplemental iron.12 Iron deficiency is found more commonly in children with RLS compared to healthy control patients and some but not all studies have shown an improvement in both restless legs symptom severity and periodic limb movement index with successful treatment of iron deficiency.
The current recommendation for adults and children with RLS is to maintain a serum ferritin level above 50 ng/mL.2 This is much higher than defining iron deficiency as beginning at 12 ng/mL when iron stores are absent in the bone marrow,13 or 30 ng/mL when iron-deficient erythropoiesis begins.14 The difference is understood as reflecting different iron needs for the hematologic and neurologic systems. In the most recent national nutritional survey to include an assessment of iron status,15 the 50th percentile of ferritin in children was: for children ages 1 to 5 years, 26 ng/mL; for children ages 6 to 11 years, 30 ng/mL; and ages 12 to 19 years, 31 ng/mL. Because the serum ferritin threshold for beginning iron supplementation in children with RLS is so much higher than the threshold for children in a general pediatrics setting, most children presenting with a possible sleep disorder would be expected to fall below the 50 ng/mL threshold for defining a low serum ferritin. Thus, iron supplementation is often recommended for children presenting to a sleep center even though they may not be considered iron deficient in a different clinical setting.
Iron homeostasis is regulated by gastrointestinal (GI) absorption,16,17 and there is no mechanism for iron excretion. Only 2% to 20% of oral iron is absorbed, meaning that 80% to 98% of the iron ingested is not absorbed in the duodenum and passes into the colon, where it can cause inflammation and can adversely affect the gut microbiome.18 This is the likely cause of the common GI side effects of constipation, diarrhea, and abdominal pain that are seen in approximately 10% of children taking oral iron supplements. In developing countries oral iron supplementation has been associated with an increase in pathogenic gut bacteria, and increased morbidity from gastroenteritis,18 suggesting that oral iron supplements should not be considered an entirely benign treatment. A 2012 Cochrane Review on the treatment of RLS with iron in adults also found insufficient evidence for the benefits of iron therapy.19 Nonetheless, treatment with supplemental iron is currently recommended for children with RLS and PLMD before considering other pharmacologic treatments.1–5,8,9
With awareness of the conflicting evidence on using iron therapy for RLS and PLMD, we conducted a cohort study with two purposes: (1) to assess the relationship between RLS symptoms and iron deficiency in a cohort of pediatric patients seeking treatment for restless, disrupted sleep, and (2) to determine whether administering an oral iron supplement over 8 weeks improves iron deficiency and RLS symptoms. The hypothesis underpinning this study was that treatment with low-dose iron would be adequate for correcting both low ferritin levels and iron deficiency and RLS symptomology.
METHODS
Setting and Participants
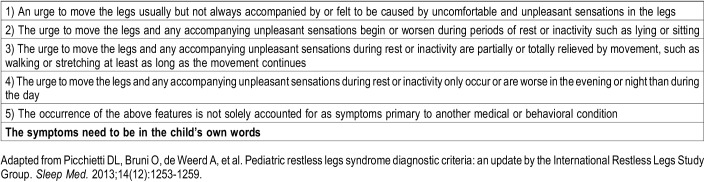
Children’s Minnesota Sleep Center is the largest pediatric sleep center in the state of Minnesota. Approximately 900 new children are evaluated each year, of whom approximately 200 complain of sleep-onset or sleep-maintenance insomnia. Children and adolescents 5 to 18 years of age with a complaint of restless, disrupted sleep who were seen in our clinic were recruited for this study between August 2012 and July 2015. As part of the evaluation for insomnia, children are generally screened for evidence of iron deficiency as part of their evaluation. Children who had biochemical evidence of iron deficiency or low serum ferritin (defined as serum ferritin < 50 ng/mL) and no evidence of inflammation (defined as C-reactive protein [CRP] < 0.3 mg/dL) and who had some symptoms of RLS were invited to participate in a study of iron and sleep. All children had a comprehensive medical history gathered and none presented with a history of inflammatory bowel disease, menorrhagia, known causes for iron malabsorption, or excess blood loss. Patients with a history suggestive of obstructive sleep apnea were not eligible. All patients were assessed at the initial consultation for restless sleep symptomology consistent with RLS. Using 2003 diagnosis criteria recommended and adapted by the International Restless Legs Syndrome Study Group (IRLSSG),20 we identified patients with definite RLS—based on the five criteria recommended by the IRLSSG20 (Table 1) and noted whether or not the symptoms were described in the “child’s own words.” We chose only to describe the children with definite RLS in this report to provide the clearest characterization of the relationship between iron treatment for low serum ferritin and iron deficiency and unequivocal RLS. A child was considered to have “definite” RLS if all criteria were met, including the symptoms expressed in the child’s own words and there was no evidence of mimics of RLS. Sixteen children met criteria for definite RLS based on these criteria.
Table 1.
International Restless Legs Syndrome Study Group consensus diagnostic criteria for restless legs syndrome.
After eligibility criteria were met and diagnosis documented, a signed consent for patients older than age 7 and/or assent to participate was also obtained. All enrolled patients were then prescribed iron supplements to be taken over an 8-week period in which their restless legs symptoms and serum ferritin levels would be assessed before and after the study period. Sleep hygiene counseling was also provided to all of the children and their parents as appropriate. This study was approved by the Institutional Review Board at Children’s Hospitals and Clinics of Minnesota.
Restless Legs Syndrome Severity Scale
During the baseline study visit, a version of the Pediatric Restless Legs Syndrome Severity Scale (P-RLS-SS)21 was administered to each patient by an experienced pediatric sleep nurse clinician (Table 1). The responses for each question were recorded on a 0-4 scale (0 = no symptoms, 1 = mild, 2 = moderate, 3 = severe, 4 = very severe) and were summed to give an RLS severity score. This classification method was used to define patients as meeting definite RLS criteria based solely on discrete criteria (yes or no) for diagnosis purposes. If a patient received a diagnosis of definite RLS, then their RLS score could range from 4 to 16 points, where 4 represents the minimum criteria for definite RLS, and 16 represents a maximum, severe case of RLS.
Iron Treatment
Consistent with current recommendations,13,14 oral iron treatment was 3 mg/kg/d of elemental iron up to a maximum daily dose of 65 mg with generic ferrous sulfate 325 mg (65 mg elemental iron), provided by Major Pharmaceuticals (Livona, Michigan, United States); ferrous sulfate 15 mg elemental iron/mL, provided by Akorn Inc. (Lake Forrest, Illinois, United States); or vitamin C, generic ascorbic acid 250 mg provided by Major Pharmaceuticals. The dose of iron was kept intentionally low to minimize adverse GI side effects. The parent was given all of the study medication and a drug log to self-report medication administration. Parents were instructed not to give their child milk or food within 2 hours of administering the medication. No recommendation was given regarding the timing of iron administration. Each family was queried weekly by phone about side effects. The descriptions and grading scales of side effects found in the revised NCI Common Terminology Criteria for Adverse Events version 4.0 were utilized for all adverse event reporting. The parents were instructed to bring leftover medication to their second follow-up study visit, which was scheduled 8 weeks after entrance into the study. At that visit the drug log was reviewed and the leftover medication was counted, to provide an estimate of the amount of medication the child was given. Also, at that visit, the restless legs questionnaire was re-administered and serum ferritin level and CRP were remeasured by blood draw.
Statistical Analysis
Basic descriptive procedures including median, interquartile range (IQR), and frequency (percent) were used to describe all demographic and clinical characteristics. Differences in median values including ferritin level and RLS score at baseline and follow-up also were compared using Wilcoxon signed-rank tests. Nonparametric analyses were selected because of the discrete, ordinal-like nature of the RLS score.
The association between RLS score and ferritin levels at baseline was estimated using a Spearman rank order correlation (Spearman rho). Spearman correlation was preferred to determine the strength and direction of the monotonic relationship between our two variables instead of focusing on a potential linear relationship as in a Pearson correlation, the parametric analog. Likewise, Spearman rank-order correlation was used to estimate the association between changes in RLS score from baseline to follow-up and changes in ferritin levels from baseline to follow-up. All analyses were conducted using SAS 9.4 (Cary, North Carolina, USA).
RESULTS
In total, 117 patients were screened for eligibility. Fifty-two participants either did not meet study criteria or declined to participate. Sixty-five participants enrolled into the study. Four patients were immediately dropped from the study because the patients never picked up the medication from the pharmacy. Fourteen other patients were dropped or removed from analyses because of the following reasons: elevated CRP at baseline or follow-up (n = 5); refused to take medication (n = 2); adverse GI side effect (n = 1); and missing data due to loss to follow-up (n = 6). In total, 47 patients were used in final analyses.
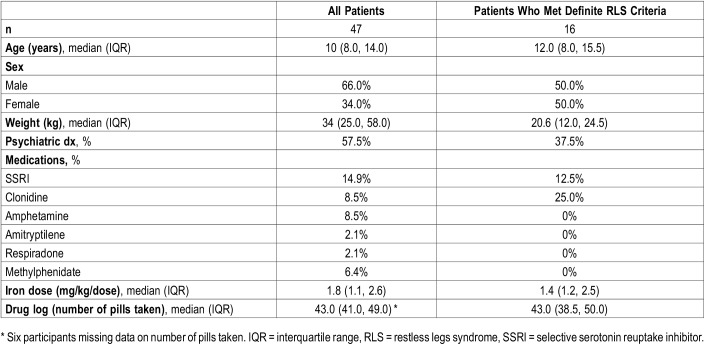
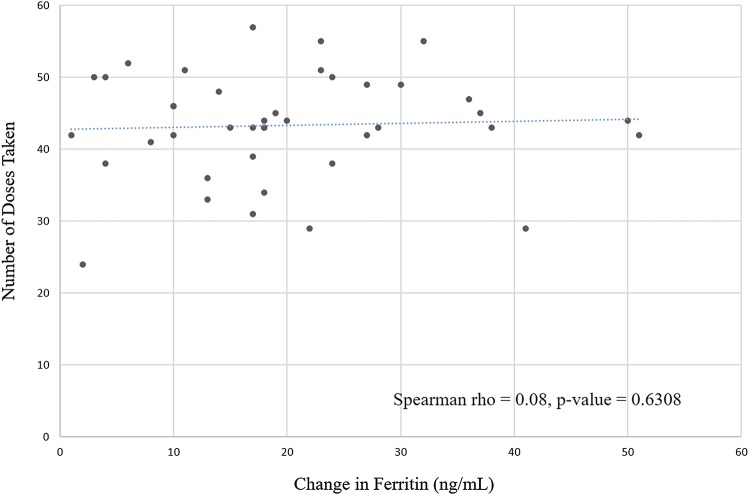
The median age of all participants was 10 years (IQR: 8 to 14 years) with a median weight of 34 kg (Table 2). Almost 58% of the total study group had a comorbid mental health or sleep diagnosis (ADHD 42.3%, mood disorder 15.4%, autism 1.9%, and narcolepsy 3.9%). After enrollment in the study, the median dose prescribed to participants was 1.8 mg/kg per dose. Over the course of the study, the median number of pills taken by patients was 43 (IQR: 41 to 49 pills). There was no significant correlation between the number of doses taken and the change in ferritin level (Spearman rho = .08, P = .6308; Figure 1).
Table 2.
Demographic and clinical characteristics of 47 pediatric patients presenting with restless sleep.
Figure 1. Change in ferritin and number of doses taken.
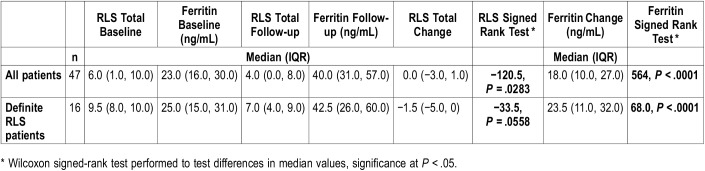
Using the RLS criteria listed in Table 1, 16 patients met the criteria to be classified as having definite RLS. Demographic and clinical characteristics for those patients with definite RLS are also presented in Table 2. The median ferritin level at baseline for all patients was 23 ng/mL (IQR: 16.0 to 30.0 ng/mL) and the median ferritin level at follow-up was 40.0 ng/mL (IQR: 31.0 to 57.0 ng/mL) (Table 3). Not surprisingly, the change and distribution of ferritin was statistically significantly different after 8 weeks of treatment (P < .0001). The median RLS score at baseline was 6 (IQR: 1.0 to 10.0) for all patients and 4 (IQR: 0.0 to 8.0) at follow-up. Like ferritin levels, this indicated a significant change in RLS score (P = .0283). A similar relationship was observed in the definite RLS patients; however, change in RLS score was not significant (P = .0558) (Table 3).
Table 3.
Change in serum ferritin and RLS scores from baseline to follow-up in 47 pediatric patients.
For the entire study group, at the beginning of treatment, there was no correlation between baseline RLS score and baseline ferritin level (rho = −.01, P = .9295; Table 4). When examining the correlation using the definite RLS criteria, there also were no significant correlations observed between baseline RLS score and baseline ferritin level. Change in RLS score from baseline to follow-up and change in ferritin level from baseline to follow-up also yielded a nonsignificant association across all patients (rho = −.03, P = .8594; Table 4). However, interestingly, the strength of the association increased when using only those patients who met the definite RLS criteria definition. Further, using the definite RLS criteria, a Spearman correlation coefficient of −.39 was observed between change in RLS score and change in ferritin level. This supports the notion that improving ferritin level will improve RLS symptomology; however, again this association was not found to be statistically significant in our analysis (P = .1362).
Table 4.
Correlations between serum ferritin and RLS scores from baseline to follow-up in 47 pediatric patients.
DISCUSSION
In this study, we demonstrated that iron deficiency or low serum ferritin level is common in children with restless sleep and with RLS. Treatment with low-dose iron 3 mg/kg/d up to a maximum dose of 65 mg elemental iron/d with vitamin C for 8 weeks was well tolerated and led to a median rise in serum ferritin level of 17.0 ng/mL. This corrected iron deficiency, raising the serum ferritin level to > 30 ng/mL for 75% of the children. The short duration of therapy and low dose (1.2–3 mg/kg/d) did result in very few adverse side effects, but proved both too short a duration and too low a dose to achieve the goal of raising serum ferritin levels > 50 ng/mL.
The rise in ferritin level did not correlate with the actual number of doses of iron taken between 24 to 60 doses. Though this result seems counterintuitive, it is consistent with the results of studies of iron absorption in adults,22 which showed that oral iron supplements led to increases in hepcidin (the key regulator of iron homeostasis) which in turn led to fractional decreases in iron absorption of oral doses of iron taken the same day or the next day. The recommendation of this study was that treatment every other day with supplemental iron was likely to be as effective as daily dosing in correcting iron deficiency. We found that improving iron deficiency did improve the symptoms of children with RLS who had definite RLS, but this improvement did not reach statistical significance. Based on the modest benefits found here, we recommend, in addition to correcting low serum ferritin levels in children with RLS symptomatology, that treatment decisions regarding pharmacotherapy for RLS in children with a low serum ferritin level or iron deficiency (ferritin < 50 ng/mL) should not necessarily be delayed until the low serum ferritin level is corrected, but rather should be based on the clinical severity of the symptoms and the effect of the symptoms on the child’s quality of life.
Our study addresses some of the limitations of previous work. For example, in a recent retrospective chart review, Dye et al23 found symptom improvements in patients with RLS or PLMD treated with oral iron for 2 years or more. However, that study was limited by its inclusion of patients with OSA, whose treatment for that condition may have contributed to improvements in RLS symptoms, and because only a third of the original study population contributed data for the 2-year analysis. Other chart reviews have found similar improvements in RLS symptoms with iron treatment, although limitations included not accounting for symptom severity24 and small sample size.25 These latter two studies documented the median or mean time to treatment effect, which was approximately 3 months, suggesting that our study was too short in duration to capture all of the improvement in symptoms that may result from iron supplementation.
This study is limited by a lack of a comparison group, an issue common to most of the other existing research on pediatric RLS and iron supplementation. Correction of low serum ferritin level is considered standard of care in children, so there was no control group that did not receive iron supplementation. The results of this study should be viewed as preliminary because of the small numbers of children in the study and the absence of polysomnography. Polysomnography would have allowed for the diagnosis of PLMD in some of the children with restless sleep but who did not meet the criteria for RLS. A larger sample size could potentially have driven our findings toward significance. The timing of when the iron supplement was given was not recorded, so any possible circadian effects of iron absorption could not be assessed. Dosing of iron every other day was not explicitly evaluated, so its effectiveness could not be evaluated. Finally, objective measures of sleep via actigraphy were not used for this study, so no data were gathered or reported on sleep duration or sleep efficiency. Future research should account for these considerations.
At the end of therapy, the median ferritin level was 40 ng/mL. This is above the current threshold for iron deficient erythropoiesis, but below the 50 ng/mL threshold recommended for treatment with supplemental iron in patients with RLS. It is possible that raising the ferritin level to 50 ng/mL or higher may have had a more significant effect on RLS symptoms.
Despite possible limitations of our study, findings indicate the need for more research on the effects of iron supplementation for pediatric RLS. Overall, our findings contribute to clinicians’ considerations with regard to management of pediatric patients who present with RLS symptoms.
DISCLOSURE STATEMENT
Work for this study was performed at Children’s Minnesota Sleep Center, Children’s Minnesota, St. Paul, MN, USA. All authors have seen and approved the manuscript. The authors report no conflicts of interest.
ABBREVIATIONS
- ADHD
attention deficit hyperactivity disorder
- CRP
C-reactive protein
- GI
gastrointestinal
- IQR
interquartile range
- IRLSSG
International Restless Legs Syndrome Study Group
- PLMD
periodic limb movement disorder
- P-RLS-SS
Pediatric Restless Legs Syndrome Severity Scale
- RLS
restless legs syndrome
REFERENCES
- 1.Picchietti MA, Picchietti DL. Advances in pediatric restless legs syndrome: Iron, genetics, diagnosis and treatment. Sleep Med. 2010;11(7):643–651. doi: 10.1016/j.sleep.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 2.Allen PA, Picchietti DL, Auerbach M, et al. Evidence-based and consensus clinical practice guidelines for the iron treatment of restless legs syndrome/Willis Ekbom disease in adults and children: an IRLSS task force report. Sleep Med. 2018;41:27–44. doi: 10.1016/j.sleep.2017.11.1126. [DOI] [PubMed] [Google Scholar]
- 3.Simakajornboon N, Kheirandish-Gozal L, Gozal D. Diagnosis and management of restless legs syndrome in children. Sleep Med Rev. 2009;13(2):149–156. doi: 10.1016/j.smrv.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khatwa U, Kothare SV. Restless legs syndrome and periodic limb movements disorder in the pediatric population. Curr Opin Pulm Med. 2010;16(6):559–567. doi: 10.1097/MCP.0b013e32833f11ae. [DOI] [PubMed] [Google Scholar]
- 5.Kotagal S, Silber MH. Childhood-onset restless legs syndrome. Ann Neurol. 2004;56(6):803–807. doi: 10.1002/ana.20292. [DOI] [PubMed] [Google Scholar]
- 6.Konofal E, Lecendreux M, Cortese S. Sleep and ADHD. Sleep Med. 2010;11(7):652–658. doi: 10.1016/j.sleep.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Picchietti D, Allen RP, Walters AS, Davidson JE, Myers A, Ferini-Strambi L. Restless legs syndrome: prevalence and impact in children and adolescents–the Peds REST study. Pediatrics. 2007;120(2):253–266. doi: 10.1542/peds.2006-2767. [DOI] [PubMed] [Google Scholar]
- 8.Frenette E. Restless legs syndrome in children: a review and update on pharmacological options. Curr Pharm Des. 2011;17(15):1436–1442. doi: 10.2174/138161211796197142. [DOI] [PubMed] [Google Scholar]
- 9.Dosman C, Witmans M, Zwaigenbaum L. Iron’s role in paediatric restless legs syndrome - a review. Paediatr Child Health. 2012;17(4):193–197. doi: 10.1093/pch/17.4.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hentze MW, Muckenthaler MU, Galy B, et al. Two to tango: regulation of Mammalian iron metabolism. Cell. 2010;142(1):24–38. doi: 10.1016/j.cell.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 11.Schaap C, Hendriks J, Kortman G, et al. Diurnal rhythm rather than dietary iron mediate hepcidin variations. Clin Chem. 2013;59(3):527–535. doi: 10.1373/clinchem.2012.194977. [DOI] [PubMed] [Google Scholar]
- 12.Konofal E, Cortese S, Marchand M, Mouren MC, Arnulf I, Lecendreux M. Impact of restless legs syndrome and iron deficiency on attention-deficit/hyperactivity disorder in children. Sleep Med. 2007;8(7-8):711–715. doi: 10.1016/j.sleep.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 13.Zimmermann MB, Hurrell RF. Nutritional iron deficiency. Lancet. 2007;370(9586):511–520. doi: 10.1016/S0140-6736(07)61235-5. [DOI] [PubMed] [Google Scholar]
- 14.Powers JM, Buchanan GR. Diagnosis and management of iron deficiency anemia. Hematol Oncol Clin North Am. 2014;28(4):729–745. doi: 10.1016/j.hoc.2014.04.007. vi-vii. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention Second National Report on Biochemical Indicators of Diet and Nutrition in the U.S. Population. 2012. https://www.cdc.gov/nutritionreport/pdf/Nutrition_Book_complete508_final.pdf. April 2012. Atlanta, GA: National Center for Environmental Health. Accessed October 24, 2017.
- 16.Goodnough LT, Nemeth E, Ganz T. Detection, evaluation, and management of iron-restricted erythropoiesis. Blood. 2010;116(23):4754–4761. doi: 10.1182/blood-2010-05-286260. [DOI] [PubMed] [Google Scholar]
- 17.Anderson GJ, Frazer DM, McLaren GD. Iron absorption and metabolism. Curr Opin Gastroenterol. 2009;25(2):129–135. doi: 10.1097/MOG.0b013e32831ef1f7. [DOI] [PubMed] [Google Scholar]
- 18.Jaeggi T, Kortman GA, Moretti D, et al. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut. 2015;64(5):731–742. doi: 10.1136/gutjnl-2014-307720. [DOI] [PubMed] [Google Scholar]
- 19.Trotti LM, Bhadriraju S, Becker LA. Iron for restless legs syndrome. Cochrane Database Syst Rev. 2012:Cd007834. doi: 10.1002/14651858.CD007834.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4(2):101–119. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 21.Arbuckle R, Abetz L, Durmer JS, et al. Development of the Pediatric Restless Legs Syndrome Severity Scale (P-RLS-SS): a patient-reported outcome measure of pediatric RLS symptoms and impact. Sleep Med. 2010;11(9):897–906. doi: 10.1016/j.sleep.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 22.Moretti D, Goede J, Zeder C, et al. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice daily doses in iron-depleted young women. Blood. 2015;126(17):1981–1989. doi: 10.1182/blood-2015-05-642223. [DOI] [PubMed] [Google Scholar]
- 23.Dye TJ, Jain SV, Simakajornboon N. Outcomes of long-term iron supplementation in pediatric restless legs syndrome/periodic limb movement disorder (RLS/PLMD) Sleep Med. 2017;32:213–219. doi: 10.1016/j.sleep.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Amos LB, Grekowicz ML, Kuhn EM, Olstad JD, Collins MM, Norins NA, et al. Treatment of pediatric restless legs syndrome. Clin Pediatr (Phila) 2014;53(4):331–336. doi: 10.1177/0009922813507997. [DOI] [PubMed] [Google Scholar]
- 25.Mohri I, Kato-Nishimura K, Kagitani-Shimono K, et al. Evaluation of oral iron treatment in pediatric restless legs syndrome (RLS) Sleep Med. 2012;13(4):429–432. doi: 10.1016/j.sleep.2011.12.009. [DOI] [PubMed] [Google Scholar]