Abstract
Study Objectives:
Pulmonary hypertension (PH) has been reported as a serious complication of obstructive sleep apnea (OSA) in children; however, estimated prevalence rates vary widely (zero to 85%). The purpose of this study is to determine the prevalence of PH in children with OSA and identify factors that may predict an increased PH risk in children with OSA.
Methods:
A retrospective review of all pediatric beneficiaries (88,058) in the San Antonio Military Health System with a diagnosis of OSA and a clinical evaluation by a pediatric cardiologist. OSA severity and nadir oxygen saturation were recorded from overnight polysomnography. Reason for referral, comorbid disorders, echocardiogram results, and cardiac diagnoses were obtained from cardiology records.
Results:
OSA was identified in 2,020 pediatric patients (2.3%). A pediatric cardiology consultation was reported for 296 patients with OSA. After excluding 95 patients for incorrect OSA diagnoses, incomplete data, or OSA treatment before cardiology evaluation, 163 patients were included in the final analysis. A diagnosis of PH was found in 3 patients with OSA (1.8%). Two of these patients had obesity, and all three had comorbid cardiac disorders.
Conclusions:
Prevalence of PH in pediatric patients with OSA is low and none of the patients with PH had severe OSA. Current guidelines recommend PH screening in patients with severe OSA, yet OSA severity may not accurately predict risk. Factors evaluated in this study did not demonstrate an increased PH risk; additional research is necessary to improve screening in pediatric patients with OSA.
Citation:
Burns AT, Hansen SL, Turner ZS, Aden JK, Black AB, Hsu DP. Prevalence of pulmonary hypertension in pediatric patients with obstructive sleep apnea and a cardiology evaluation: a retrospective analysis. J Clin Sleep Med. 2019;15(8):1081–1087.
Keywords: obstructive sleep apnea, pediatric, pulmonary hypertension
BRIEF SUMMARY
Current Knowledge/Study Rationale: Pulmonary hypertension is considered to be a significant consequence of pediatric obstructive sleep apnea, yet the prevalence and risk factors for its development remain unknown. This paucity of data makes it difficult for clinicians to predict which patients would benefit from screening echocardiograms.
Study Impact: This is the largest study to use consistent polysomnogram and echocardiogram data to determine the low prevalence of pulmonary hypertension in pediatric obstructive sleep apnea. These findings may be helpful to improve screening programs, provide evidence for future guideline development, and aid future research on factors that may increase the risk of pulmonary hypertension in patients with obstructive sleep apnea.
INTRODUCTION
Pediatric obstructive sleep apnea (OSA) is characterized by partial to complete upper airway obstructions during sleep.1 Pediatric OSA, which can contribute to growth and behavioral problems, is estimated to occur in approximately 1.2% to 5.7% of children.2–4 Children with underlying medical conditions, including obesity, craniofacial anomalies, neuromuscular disorders, and certain genetic syndromes, have higher rates of OSA.5–8 Clinical history is poorly predictive of the diagnosis, and polysomnography is required for the diagnosis of OSA.5,6
Pulmonary hypertension (PH), an abnormal elevation in pulmonary artery pressure, is commonly reported as a cardiovascular sequelae of OSA.5,9–14 PH is rare in pediatric patients, yet, despite available treatments, it contributes to significant morbidity and mortality in children.15,16 The pathogenesis of PH is multifactorial and includes genetic predisposition, environmental factors, hemodynamic stress, inflammation, and hypoxemia.16–18 The purported mechanism for the development of PH in patients with OSA is related to hypoxemia, which can occur as a result of partial to complete upper airway obstruction. Hypoxemia may lead to increased pulmonary vascular resistance with subsequent ventricular dysfunction and development of PH.9,10,19,20
PH caused by chronic upper airway obstruction was first reported in the pediatric literature more than 50 years ago.21–23 Since that time, prevalence rates of PH in children with OSA have been reported, with estimates ranging from zero to 85%.24–33 The wide range is most likely a result of methodological differences, such as small sample sizes, case reports, absence of polysomnogram, and varying diagnostic thresholds for OSA and PH. Consequently, there is limited evidence to support OSA as a significant risk factor for PH.
The objective of this retrospective analysis was to determine the prevalence of PH in a large cohort of pediatric patients with OSA. The secondary objective was to determine if there are predictive factors for PH in pediatric patients with OSA. Reliable and accurate prevalence estimates of PH in children with OSA will allow for improved resource allocation and health care planning.34
METHODS
This is a retrospective study of pediatric patients in the San Antonio Military Health System with a diagnosis of OSA and a consultation with a pediatric cardiologist between October 2011 and September 2016. The study was approved by Brooke Army Medical Center Regional Health Command-Central Institutional Review Board (IRB # C.2017.211d).
The Military Health System Management Analysis Reporting Tool (M2) was used to query the records of Department of Defense health care beneficiaries between birth and 18 years of age who sought care within and outside of the military treatment facilities in San Antonio, Texas. Patients with OSA were identified within the M2 system utilizing the International Classification of Diseases (ICD) diagnostic codes 32723 (ICD-9) and G47.33 (ICD-10). Detailed screening of individual electronic medical records ensued for patients with a diagnosis of OSA and a referral to a pediatric cardiologist.
Patients were included for review if their electronic medical record contained the results of a full-night polysomnography (PSG) meeting diagnostic criterion for OSA and at least one clinical encounter note from a cardiologist. Patient demographic, body mass index (BMI), PSG, and cardiology consultation data were recorded from electronic medical records.
BMI values were converted to z-scores and percentiles using the Centers for Disease Control and Prevention (CDC) growth charts and lambda-mu-sigma parameters.35,36 Percentiles were recorded as underweight (< 5%), normal weight (5% to 84%), overweight (85% to 94%), and obese (≥ 95%).37
PSG reports provided apnea-hypopnea index (AHI) and lowest oxygen saturation during sleep. AHI severity levels were classified as mild, moderate, or severe according to age at the time of PSG.1,9 Pediatric standards were used for patients younger than 13 years of age (AHI: mild = 1–4.9; moderate = 5–9.9; severe = 10 or higher). Adult standards were used for patients 13 years or older (AHI: mild = 5–14.9; moderate = 15–29.9; severe = 30 or higher).
Cardiology consultation reports identified the indication for referral, comorbid conditions, cardiac diagnoses, and results from electrocardiogram (ECG), echocardiogram, and cardiac catheterization, if performed. When patients had more than one cardiology encounter note, the results from the encounter closest to the date of the PSG were recorded.
Indications for cardiology referral were grouped into the following four categories: congenital heart disease, genetic disorders, OSA, and other health provider concerns. Other health provider concerns included referrals for cardiac symptoms, exposures, family history, and physical examination findings, as well as abnormal ECG findings.
The time between the cardiology consultation and PSG was calculated in days and months, and each patient was identified as having PSG prior to or after cardiology evaluation. Patient records with PSG prior to cardiology evaluation were further reviewed to identify evidence of OSA treatment. Patients were considered treated for OSA if they underwent upper airway surgery, such as tonsillectomy, or if the compliance data for positive airway pressure (PAP) indicated 4 or more hours of device use for at least 70% of nights in a 30-day period. Of note, universally accepted standards for optimal PAP adherence in children have not yet been established38; therefore, commonly accepted adult adherence standards were utilized,39 as described previously. Treatment was listed as possible if PAP adherence or surgical records were not available, despite medical records indicating they were prescribed or planned. Patients identified as treated or possibly treated for OSA were excluded from further analysis.
PH in children is defined as a mean pulmonary artery pressure greater than 25 mmHg as measured by right heart catheterization.15,40,41 Transthoracic echocardiography measurements can be used as a noninvasive screening test for PH; however, this method estimates the systolic pulmonary artery pressure through the tricuspid regurgitation peak velocity (TRV) and is considered a surrogate measure for PH.41,42 Because an echocardiogram estimate can vary with the skill of the interpreting physician,43 we had two pediatric cardiologists independently (and retrospectively) review each echocardiogram identified as having concern for left ventricular diastolic dysfunction, right ventricular dilation, atrioventricular septal defects, main pulmonary artery dilation, or PH. These echocardiograms included standard pediatric imaging with two-dimensional, Doppler, color Doppler, and M-mode measurements utilizing a Philips iE 33 or Epiq 7 (Koninklijke Philips N.V., Amsterdam, Netherlands) or a GE Vivid 7 or Vivid e95 (General Electric, Waukesha, Wisconsin, USA). The European Society of Cardiology guidelines were used to identity PH as possible (TRV of 2.9 to 3.4 m/sec) or likely (TRV > 3.4 m/sec).41 If a patient’s TRV was ≤ 2.8 m/sec the echocardiogram was recorded as normal. If the TRV was inadequate to assess the right ventricular pressure, indirect evidence of pulmonary hypertension was assessed utilizing septal roundness, right ventricular dilation, and velocity of the pulmonary regurgitant jet.
All statistical analyses were performed using JMP statistical software, v13.0, developed by Statistical Analysis Software (SAS Institute; Cary, North Carolina, USA). Descriptive statistics included means and standard deviations for normally distributed continuous variables or medians and interquartile ranges for continuous variables that were not normally distributed. Percentages were calculated for categorical variables. Prevalence of OSA and PH were reported as a percentage. Continuous variables were analyzed using the t test or Wilcoxon rank-sum test. Categorical data were analyzed using chi-square tests or Fisher exact test. Significance was defined as P < .05.
RESULTS
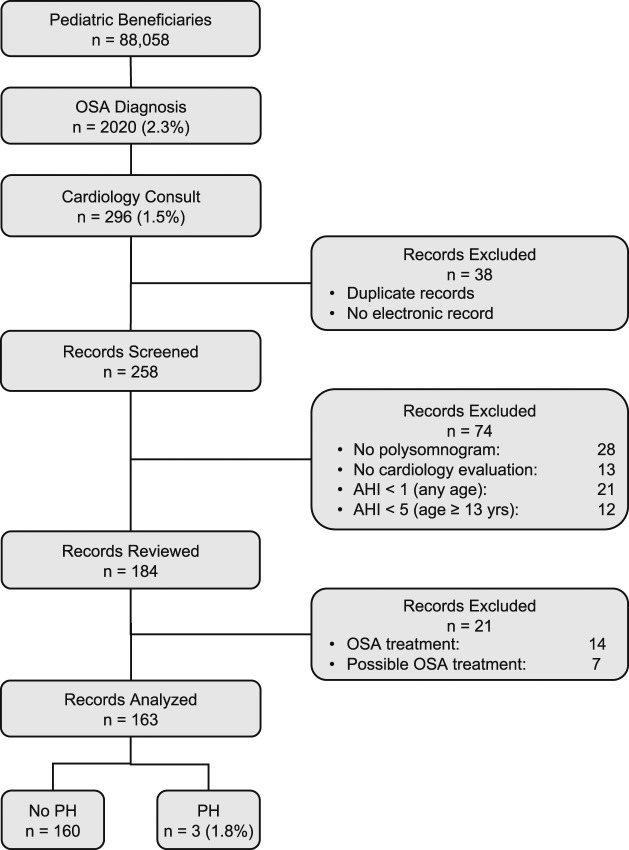
A total of 88,058 Department of Defense pediatric beneficiaries (age 18 years or younger) were enrolled in direct or private care in San Antonio, Texas between October 2011 and September 2016 (Figure 1). OSA was diagnosed in 2,020 patients (2.3%). Of the patients with OSA, 296 (1.5%) also had a referral to cardiology. Thirty-eight records were excluded because of duplications or nonsearchable electronic medical records. Electronic medical records were reviewed for 258 patients. Forty-one patients were excluded because of missing PSG and/or cardiology consult reports. Another thirty-three patients did not meet diagnostic criteria for OSA as defined previously. Twenty-one patients were excluded for known or suspected OSA treatment obtained before their cardiology evaluation.
Figure 1. Flow chart of study population.
AHI = apnea hypopnea index, OSA = obstructive sleep apnea, PH = pulmonary hypertension.
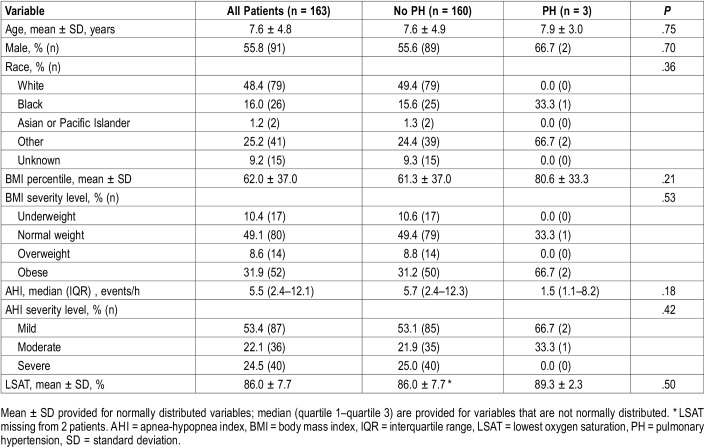
The final analysis included 163 pediatric patients. Their mean age was 7.7 ± 4.8 years (Table 1). Approximately half of the patients were male (n = 91; 56%) and white (n = 79; 48.4%). The mean BMI percentile was 62 ± 37 and nearly half of the patients were of normal weight (n = 80; 49.1%). The median AHI was 5.5 events/h with an interquartile range (IQR) of 2.4–12.1 events/h. Half of the patients had mild OSA (n = 87; 53%). The mean oxygen saturation nadir was 86 ± 7.7%.
Table 1.
Patient demographic and polysomnography data.
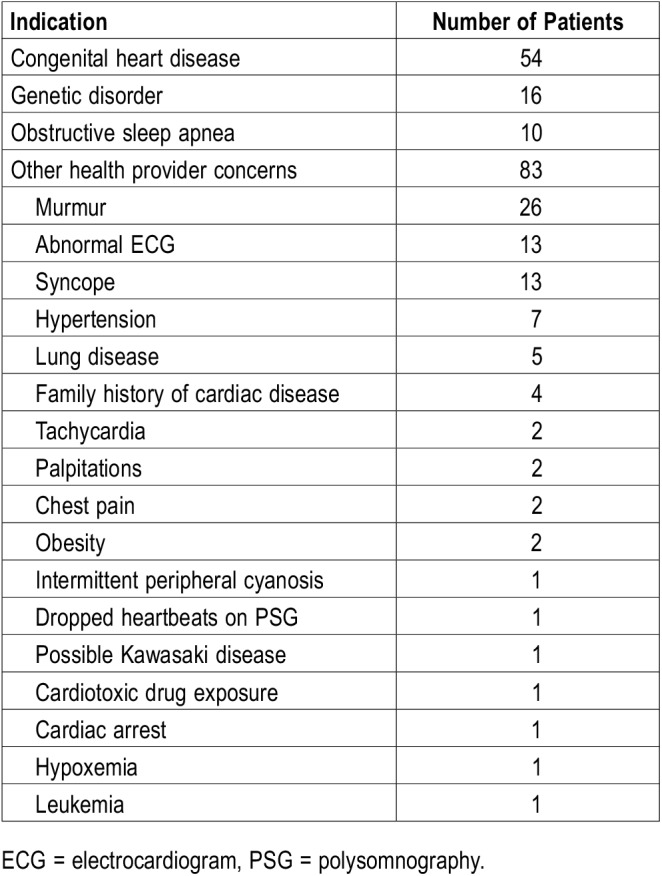
The indications for cardiology referral are detailed in Table 2. The largest category of indications was other health provider concerns (n = 83; 51%), and included murmurs (n = 26), abnormal ECG (n = 13), and syncope (n = 13), as well as several other concerning cardiac signs, symptoms, and medical history and examination findings. History of congenital heart disease was the indication for one-third of the patients (n = 54; 33%). Although more than one-tenth of the patients (n = 26; 16%) had Down syndrome, only 10% of the referrals to cardiology were for genetic disorders. The primary reason patients with Down syndrome were referred to cardiology was categorized as congenital heart disease. Half of the patients with Down syndrome had severe OSA (n = 13; 50%); moderate or mild OSA was diagnosed in eight and five patients, respectively. One patient with Down syndrome had PH. OSA was the indication for the remaining 6% of referrals. Of the 10 referrals for OSA, 7 patients had severe OSA, 2 had moderate, and 1 patient had mild OSA. None of the patients referred specifically for OSA were found to have PH. Just over 30% of the patients with OSA had obesity (n = 52; 31.9%) and almost 10% were overweight (n = 14; 8.6%). One-third of the patients with obesity (n = 17; 32.6%) were found to have severe OSA compared to one-fifth of the normal weight patients (n = 17; 21.3%).
Table 2.
Indications for cardiology referral.
More than half of the patients had a cardiology evaluation prior to PSG (n = 97; 60%), with a median time between events of 5.3 months (IQR 1.8–15.1). PSG was performed prior to cardiology evaluation in the remaining 66 patients, with a median time between events of 4.1 months (IQR 1.7–11.7). Eighteen patients had normal cardiology evaluations with an ECG only. Cardiac magnetic resonance imaging was normal in one patient. Normal echocardiograms were reported for 88 patients. Of the 56 remaining echocardiograms, the most common abnormalities included mitral valve regurgitation or prolapse (n = 7), patent foramen ovale (n = 7), and bicuspid aortic valves (n = 6).
Two pediatric cardiologists independently reviewed 23 echocardiograms, which were identified for review when the original report identified left ventricular diastolic dysfunction, right ventricular dilation, atrioventricular septal defects, main pulmonary artery dilation, or PH. This review was done retrospectively, and the cardiologists were not blinded to the OSA diagnoses of the patients. Fewer than 2% of patients had a diagnosis of PH (n = 3/163, 1.8%).
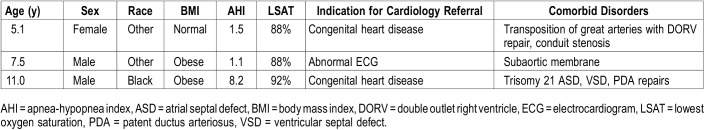
There were no statistically significant differences between patients with and without PH (Table 1). However, in the three patients with PH, none had severe OSA, two had obesity, and all three had cardiac pathology. The following cardiac abnormalities were identified: (1) evidence of repaired transposition of the great arteries with double outlet right ventricle and conduit stenosis, (2) subaortic membrane with stenosis, and (3) evidence of repaired patent ductus arteriosus and atrioventricular defects. Table 3 contains characteristics of the patients in whom PH was diagnosed.
Table 3.
Characteristics of patients with pulmonary hypertension.
DISCUSSION
The principal finding of this study is the low prevalence of PH in pediatric patients with OSA who were also referred to pediatric cardiology, occurring in only 1.8% of the study population. This result is similar to that of the only other study that used PSG to diagnose OSA in estimating the prevalence of PH.30 In that study, the sample size was smaller (n = 55) and the prevalence was determined to be zero. Other studies that report a prevalence of PH in pediatric patients with OSA used clinical judgement or did not report the methods used to diagnose OSA.24–29,31–33 The use of PSG to diagnose OSA in children is well established.5,6,44 Clinical evaluation alone does not have sufficient sensitivity or specificity to establish a diagnosis of OSA and should not be used in epidemiologic studies or as a basis for practice guidelines.
The current pediatric PH guidelines from the American Heart Association (AHA) and American Thoracic Society (ATS) recommend echocardiography in patients with severe OSA (class I, level of evidence B).15 However, the studies cited in support of this recommendation report right ventricular hypertrophy or reduced right ventricular ejection fraction instead of PH and did not consistently use PSG or echocardiography to diagnose OSA or identify PH, respectively.45–47 Although 25% of our analyzed population had severe OSA, none of these patients had PH. The three patients we identified with PH had mild or moderate OSA that would not have been detected using the AHA/ATS guidelines, which recommend a screening echocardiogram for severe OSA.
The strengths of our study include the use of widely accepted screening and diagnostic standards for PH and OSA. Also, patients with any reported or suspected treatment for OSA were excluded from analysis. All of the patients included in our final analysis had a diagnosis of OSA by PSG. The prevalence of OSA in our study population (2.3%) mirrors that of the general population (1.2% to 5.7%) and suggests that it may be a representative sample. Additionally, the European Society of Cardiology guidelines were followed to identify evidence of PH and the same two pediatric cardiologists reviewed echocardiograms. By studying patients with OSA and a cardiology referral for an indication other than OSA, we were able to analyze data from a much larger cohort. This study has the largest sample size of pediatric PH and OSA to date, and it supports the hypothesis that prevalence of PH is extremely low in pediatric patients with OSA, despite underlying medical conditions that may inherently increase risk for PH.
The retrospective nature of this study resulted in the following limitations and potential biases: (1) Not all of the patients identified with OSA (n = 2,020) had an echocardiogram. Cardiology evaluations were performed in a small number of these patients, and the indication for the referral was rarely due to OSA. Most of the referrals were for known or suspected cardiac disease, which could increase the risk for PH, independent of OSA. This may have falsely increased the prevalence rate. (2) Cardiologists were not blinded to the diagnosis of OSA. (3) Although echocardiograms are commonly used for noninvasive PH screening, cardiac catheterization is required to confirm the diagnosis of PH.15,42 Cardiac catheterization was not included for all patients in this study. (4) The recommended American Academy of Sleep Medicine scoring rules changed during the time period of the study.48 Many PSG reports did not indicate which rules were utilized to determine AHI. Therefore, it is possible that different rules would have produced different AHI severity levels for the same patient. (5) We were unable to obtain the time with oxygen saturation < 90%, desaturation index, arousal index, or degree of hypercarbia for each patient. Those additional items may play a role in the development of PH in patients with OSA. (6) The length of time necessary for PH to develop with untreated OSA remains undetermined. It was not possible to determine when initial development of OSA occurred in our patients. Although PH was not diagnosed during our study period, PH may develop at a later date.
The study was also limited by the low number of patients with PH (n = 3). This low prevalence limited the ability to draw any statistically significant conclusions regarding risk factors for PH in a pediatric patient with OSA. A power analysis with only 3 of 163 patients showed that any factor would need an effect size at least 1.7 times larger than the standard deviation in order to establish significance with a power of 80%. However, because all three of our patients with PH and OSA had underlying cardiac disease and two were obese, these findings may point to associations that necessitate further study.
Another interesting finding from our retrospective analysis of this large database was the mean age of 7.6 years in our population studied. This likely represents the wide array of patients referred to cardiology, which skews our study population. Typically, pediatric OSA is thought of in two distinct patient populations. Usually, children younger than school age with OSA are presumed to have tonsillar and/or adenoidal hypertrophy. This may be the case for postpubertal children; however, the common adult risk factor of overweight or obesity may contribute to the risk for the older pediatric patient. We noted approximately 40% of our study population was categorized as overweight or having obesity, which is an ongoing public health concern in pediatrics. Obesity is a growing problem that the American Academy of Pediatrics has identified as one of the current major public health threats in children.49 An increase in obesity rates in children may have an effect on the mean age of OSA diagnosis, as well as treatment considerations. Our study did not have a sufficient number of patients with PH to make any conclusion on the association of obesity with PH.
In conclusion, we found a low prevalence of PH in children with OSA, despite the large number of patients with underlying conditions that may independently increase risk for the disease. Although it is commonly assumed that PH is a complication of pediatric OSA, specifically severe OSA, our results do not support this assumption. The data used previously to identify PH as a serious complication of OSA in children was limited by small study populations and lacked recommended diagnostic and screening measures. Our findings include a large cohort with PSG and echocardiogram results and suggest that current screening guidelines, which recommend screening for severe OSA only, may not appropriately guide clinicians or assist in the identification of patients with PH.
Prospective studies are needed to identify risk factors in patients with OSA that lead to the development of PH, the time frame for development, and the best time to screen patients for PH. These additional data could improve the current AHA/ATS guideline recommendations for screening echocardiograms in pediatric patients with OSA and provide significant improvements in patient care and healthcare utilization.
DISCLOSURE STATEMENT
Work for this study was performed at Wilford Hall Ambulatory Services Center, Sleep Disorders Center, Lackland AFB, TX. All authors have seen and approved this manuscript. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Mr. John N. Harris, who was instrumental in the performance of this study.
ABBREVIATIONS
- AHA
American Heart Association
- AHI
apnea-hypopnea index
- ATS
American Thoracic Society
- BMI
body mass index
- CDC
Centers for Disease Control and Prevention
- ECG
electrocardiogram
- ICD
International Classification of Diseases
- IRB
International Review Board
- IQR
interquartile range
- M2
Military Health System Management Analysis Reporting Tool
- OSA
obstructive sleep apnea
- PH
pulmonary hypertension
- PSG
polysomnography
- TRV
tricuspid regurgitation peak velocity
REFERENCES
- 1.American Academy of Sleep Medicine . International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 2.Bixler EO, Vgontzas AN, Lin HM, et al. Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep. 2009;32(6):731–736. doi: 10.1093/sleep/32.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li AM, So HK, Au CT, et al. Epidemiology of obstructive sleep apnoea syndrome in Chinese children: a two-phase community study. Thorax. 2010;65(11):991–997. doi: 10.1136/thx.2010.134858. [DOI] [PubMed] [Google Scholar]
- 4.Ehsan Z, Ishman SL. Pediatric obstructive sleep apnea. Otolaryngol Clin North Am. 2016;49(6):1449–1464. doi: 10.1016/j.otc.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Marcus CL, Brooks LJ, Draper KA, et al. Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):576–584. doi: 10.1542/peds.2012-1671. [DOI] [PubMed] [Google Scholar]
- 6.Aurora RN, Zak RS, Karippot A, et al. Practice parameters for the respiratory indications for polysomnography in children. Sleep. 2011;34(3):379–388. doi: 10.1093/sleep/34.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bin-Hasan S, Katz S, Nugent Z, et al. Prevalence of obstructive sleep apnea among obese toddlers and preschool children. Sleep Breath. 2018;22(2):511–515. doi: 10.1007/s11325-017-1576-4. [DOI] [PubMed] [Google Scholar]
- 8.Kassim R, Harris MA, Leong GM, Heussler H. Obstructive sleep apnoea in children with obesity. J Paediatr Child Health. 2016;52(3):284–290. doi: 10.1111/jpc.13009. [DOI] [PubMed] [Google Scholar]
- 9.Sheldon SH, Kryger MH, Ferber R, Gozal D. Principles and Practice of Pediatric Sleep Medicine. Philadelphia, PA: Elsevier Health Sciences; 2014. [Google Scholar]
- 10.Mindell JA, Owens JA. A Clinical Guide to Pediatric Sleep: Diagnosis and Management of Sleep Problems. Philadelphia, PA: Wolters Kluwer; 2015. [Google Scholar]
- 11.Gozal D, O'Brien LM. Snoring and obstructive sleep apnoea in children: why should we treat? Paediatr Respir Rev. 2004;5(Suppl A):S371–S376. doi: 10.1016/s1526-0542(04)90066-8. [DOI] [PubMed] [Google Scholar]
- 12.Alsubie HS. BaHammam AS. Obstructive sleep apnoea: children are not little adults. Paediatr Respir Rev. 2017;21:72–79. doi: 10.1016/j.prrv.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Konstantinopoulou S, Tapia IE, Kim JY, et al. Relationship between obstructive sleep apnea cardiac complications and sleepiness in children with Down syndrome. Sleep Med. 2016;17:18–24. doi: 10.1016/j.sleep.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Weber SA, Pierri Carvalho R, Ridley G, Williams K, El Dib R. A systematic review and meta-analysis of cohort studies of echocardiographic findings in OSA children after adenotonsillectomy. Int J Pediatr. Otorhinolaryngol. 2014;78(10):1571–1578. doi: 10.1016/j.ijporl.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 15.Abman SH, Hansmann G, Archer SL, et al. Pediatric pulmonary hypertension: guidelines from the American Heart Association and American Thoracic Society. Circulation. 2015;132(21):2037–2099. doi: 10.1161/CIR.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 16.Nicolarsen J, Ivy D. Progress in the diagnosis and management of pulmonary hypertension in children. Curr Opin Pediatr. 2014;26(5):527–535. doi: 10.1097/MOP.0000000000000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abman SH, Ivy DD. Recent progress in understanding pediatric pulmonary hypertension. Curr Opin Pediatr. 2011;23(3):298–304. doi: 10.1097/MOP.0b013e3283464a52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25):D34–D41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 19.Grandner MA, Alfonso-Miller P, Fernandez-Mendoza J, Shetty S, Shenoy S, Combs D. Sleep: important considerations for the prevention of cardiovascular disease. Curr Opin Cardiol. 2016;31(5):551–565. doi: 10.1097/HCO.0000000000000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blum RH, McGowan FX., Jr Chronic upper airway obstruction and cardiac dysfunction: anatomy, pathophysiology and anesthetic implications. Paediatr Anaesth. 2004;14(1):75–83. doi: 10.1046/j.1460-9592.2003.01193.x. [DOI] [PubMed] [Google Scholar]
- 21.Menache VD, Farrehi C, Miller M. J Pediatr. 1965;67:198–203. [Google Scholar]
- 22.Forster CS. 50 years ago in the Journal of Pediatrics: hypoventilation and cor pulmonale due to chronic upper airway obstruction. J Pediatr. 2015;167(2):285. doi: 10.1016/j.jpeds.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 23.Bland JW, Jr, Edwards FK. Pulmonary hypertension and congestive heart failure in children with chronic upper airway obstruction. New concepts of etiologic factors. Am J Cardiol. 1969;23(6):830–837. doi: 10.1016/0002-9149(69)90378-6. [DOI] [PubMed] [Google Scholar]
- 24.Ingram DG, Singh AV, Ehsan Z, Birnbaum BF. Obstructive sleep apnea and pulmonary hypertension in children. Paediatr Respir Rev. 2017;23:33–39. doi: 10.1016/j.prrv.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Yilmaz MD, Onrat E, Altuna A, et al. The effects of tonsillectomy and adenoidectomy on pulmonary arterial pressure in children. Am J Otolaryngol. 2005;26(1):18–21. doi: 10.1016/j.amjoto.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 26.Duman D, Naiboglu B, Esen HS, Toros SZ, Demirtunic R. Impaired right ventricular function in adenotonsillar hypertrophy. Int J Cardiovasc Imaging. 2008;24(3):261–267. doi: 10.1007/s10554-007-9265-1. [DOI] [PubMed] [Google Scholar]
- 27.Naiboglu B, Deveci S, Duman D, et al. Effect of upper airway obstruction on pulmonary arterial pressure in children. Int J Pediatr Otolaryngol. 2008;72(9):1425–1429. doi: 10.1016/j.ijporl.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 28.van Loon RLE, Roofthooft MTR, van Osch-Gevers M, et al. Clinical characterization of pediatric pulmonary hypertension: complex presentation and diagnosis. J Pediatr. 2009;155(2):176–182.e1. doi: 10.1016/j.jpeds.2009.02.036. [DOI] [PubMed] [Google Scholar]
- 29.Granzotto EH, Aquino FV, Flores JA, Lubianca Neto JF. Tonsil size as a predictor of cardiac complications in children with sleep-disordered breathing. Laryngoscope. 2010;120:1246–1251. doi: 10.1002/lary.20870. [DOI] [PubMed] [Google Scholar]
- 30.Revenaugh PC, Chmielewski LJ, Edwards T, Krishna J, Krakovitz P, Anne S. Utility of preoperative cardiac evaluation in pediatric patients undergoing surgery for obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 2011;137(12):1269–1275. doi: 10.1001/archoto.2011.208. [DOI] [PubMed] [Google Scholar]
- 31.Berger RMF, Beghetti M, Humpl T, et al. Clinical features of paediatric pulmonary hypertension: a registry study. Lancet. 2012;379(9815):537–546. doi: 10.1016/S0140-6736(11)61621-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.del Cerro Marín MJ, Sabaté Rotés A, Rodriguez Ogando A, et al. Assessing pulmonary hypertensive vascular disease in childhood. Data from the Spanish registry. Am J Respir Crit Care Med. 2014;190(12):1421–1429. doi: 10.1164/rccm.201406-1052OC. [DOI] [PubMed] [Google Scholar]
- 33.Orji FT, Adiele DK, Umedum NG, Akpeh JO, Ofoegbu VC, Nwosu JN. The clinical and radiological predictors of pulmonary hypertension in children with adenotonsillar hypertrophy. Eur Arch Otorhinolaryngol. 2017;274(3):1237–1243. doi: 10.1007/s00405-016-4207-y. [DOI] [PubMed] [Google Scholar]
- 34.Araslanova R, Paradis J, Rotenberg BW. Publication trends in obstructive sleep apnea: Evidence of need for more evidence. World J Otorhinolaryngol. 2017;3(2):72–78. doi: 10.1016/j.wjorl.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flegal KM, Cole TJ. Construction of LMS Parameters for the Centers for Disease Control and Prevention 2000 Growth Charts. Natl Health Stat Rep. 2013;63:1–3. [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention National Center for Health Statistics. Data Tables. https://www.cdc.gov/growthcharts/data_tables.htm. Accessed February 6, 2018.
- 37.Centers for Disease Control and Prevention Overweight & Obesity. BMI for Children and Teens. https://www.cdc.gov/obesity/childhood/defining.html. Accessed February 6, 2018.
- 38.Kothare SV, Rosen CL, Lloyd RM, et al. Quality measures for the care of pediatric patients with obstructive sleep apnea. J Clin Sleep Med. 2015;11(3):385–404. doi: 10.5664/jcsm.4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1993;147:887–895. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- 40.Ivy D. Pulmonary hypertension in children. Cardiol Clin. 2016;34(3):451–472. doi: 10.1016/j.ccl.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bossone E, D’Andrea A, D’Alto M, et al. Echocardiography in pulmonary arterial hypertension: from diagnosis to prognosis. J Am Soc Echocardiogr. 2013;26(1):1–14. doi: 10.1016/j.echo.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 42.Koestenberger M, Friedberg MK, Nestaas E, Michel-Behnke I, Hansmann G. Transthoracic echocardiography in the evaluation of pediatric pulmonary hypertension and ventricular dysfunction. Pulm Circ. 2016;6(1):15–29. doi: 10.1086/685051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Groh GK, Levy PT, Holland MR, et al. Doppler echocardiography inaccurately estimates right ventricular pressure in children with elevated right heart pressure. J Am Soc Echocardiogr. 2014;27(2):163–171. doi: 10.1016/j.echo.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carroll JL, McColley SA, Marcus CL, Curtis S, Loughlin GM. Inability of clinical history to distinguish primary snoring from obstructive sleep apnea syndrome in children. Chest. 1995;108(3):610–618. doi: 10.1378/chest.108.3.610. [DOI] [PubMed] [Google Scholar]
- 45.Wilkinson AR, McCormick MS, Freeland AP, Pickering D. Electrocardiographic signs of pulmonary hypertension in children who snore. Br Med J (Clin Res Ed) 1981;282(6276):1579–1581. doi: 10.1136/bmj.282.6276.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tal A, Leiberman A, Margulis G, Sofer S. Ventricular dysfunction in children with obstructive sleep apnea: radio-nuclide assessment. Pediatr Pulmonol. 1988;4(3):139–143. doi: 10.1002/ppul.1950040304. [DOI] [PubMed] [Google Scholar]
- 47.Laurikainen E, Aitasalo K, Erkinjuntti M, Wanne O. Sleep apnea syndrome in children–secondary to adenotonsillar hypertrophy? Acta Otolaryngol Suppl. 1992;492:38–41. [PubMed] [Google Scholar]
- 48.Berry RB, Brooks R, Gamaldo CE, et al. for the American Academy of Sleep Medicine . The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.4. Darien, IL: American Academy of Sleep Medicine; 2017. [Google Scholar]
- 49.Kuo AA, Thomas PA, Chilton LA, et al. Pediatricians and public health: optimizing the health and well-being of the nation’s children. Pediatrics. 2018;141(2):e20173848. doi: 10.1542/peds.2017-3848. [DOI] [PubMed] [Google Scholar]