Abstract
Study Objectives:
The herbal medicine Yokukansan (YKS; Yi-Gan San in Chinese) is reported to be effective for treating rapid eye movement sleep behavior disorder (RBD). However, the effectiveness and safety of YKS treatment have not been confirmed in a large sample. Thus, we retrospectively analyzed the outcomes of YKS treatment on patients with RBD using clinical records.
Methods:
Treatment outcomes were evaluated using the Clinical Global Impression of Illness Severity (CGI-S) and Improvement (CGI-I) scales. Patients with scores of 1 (very much improved) and 2 (much improved) on the CGI-I were classified as responders. After excluding patients with very mild RBD symptoms and those without detailed clinical information, 36 patients with idiopathic RBD including 17 receiving YKS monotherapy and 19 receiving YKS add-on therapy in addition to other medication were analyzed.
Results:
The patients’ mean age [standard deviation, SD] was 69.3 [6.8] years, and the mean duration of RBD morbidity [SD] was 5.7 [3.5] years at the start of YKS treatment. Importantly, 12 of 17 patients (70.6%) receiving YKS monotherapy were responders. However, among patients receiving YKS add-on therapy, the proportion of responders was substantially lower (4 of 19 patients; 21.1%). No adverse events were reported, other than mild gastric distress in one case.
Conclusions:
Considering the effectiveness of YKS and the low likelihood of adverse events, YKS should be considered as a potential treatment for patients with RBD.
Citation:
Matsui K, Sasai-Sakuma T, Ishigooka J, Nishimura K, Inoue Y. Effect of yokukansan for the treatment of idiopathic rapid eye movement sleep behavior disorder: a retrospective analysis of consecutive patients. J Clin Sleep Med. 2019;15(8):1173–1178.
Keywords: clonazepam, parasomnia, pramipexole, rapid eye movement sleep behavior disorder, treatment, Yokukansan
BRIEF SUMMARY
Current Knowledge/Study Rationale: The effects of Yokukansan (YKS) on rapid eye movement sleep behavior disorder (RBD) were described in a previous case report. However, the effectiveness and safety of YKS have not been confirmed in a large population. To examine the treatment outcomes of YKS, we retrospectively reviewed the clinical outcomes of patients with RBD receiving YKS treatment using clinical records.
Study Impact: Although the responder rate for YKS combination therapy with other medication was only 21.1%, the responder rate for YKS monotherapy was 70.6% and no adverse events were observed, other than mild gastric distress in one case. These results suggest that YKS could be considered as a treatment option for patients with RBD.
INTRODUCTION
Rapid eye movement sleep behavior disorder (RBD) is a type of parasomnia characterized by dream-enacting behaviors, unpleasant dreams, and the absence of rapid eye movement (REM) atonia on nocturnal polysomnogram (n-PSG).1 The oneiric behaviors of patients with RBD are typically violent or aggressive, sometimes leading to injuries of patients and/or their bed-partners.2–8 RBD is more common among older people, for whom injuries occasionally have serious consequences (eg, limb fractures or subdural hematoma).4 Thus, early and effective treatment interventions for suppressing dream-enacting behaviors are warranted.
RBD is reported to be strongly associated with alpha-synucleinopathies such as Parkinson disease, Lewy body disease, and multiple system atrophy. RBD symptoms frequently precede the onset of dementia or motor symptoms of these diseases.9,10 Regarding the treatment of RBD, clonazepam has been widely accepted as a first-line treatment for both idiopathic and symptomatic cases.2,4,11,12 However, considering the disease predilection for older people,1 the potential adverse effects of clonazepam (eg, motor incoordination, sedation particularly the next morning, memory dysfunction, and aggravation of obstructive sleep apnea [OSA]) in this age group should be considered at prescription.3,4,7,11,13,14 Melatonin has also been recommended as a second-line treatment for RBD,13,15–17 but the drug has not been approved for use in some countries, including Japan.
Yokukansan (YKS) is an herbal treatment (Yi-Gan San in Chinese) consisting of seven herbal ingredients (Japanese Angelica root, Uncaria hook, Cnidium rhizome, Atractylodes lancea rhizome, Poria sclerotium, Bupleurum root, and Glycyrrhiza). Previous studies have reported the effectiveness of YKS for the behavioral and psychological symptoms of dementia18,19 and psychophysiological insomnia.20 Regarding RBD, a case report of three patients demonstrated the effectiveness of YKS for treating the disorder.21 However, the effectiveness and safety of YKS for treating RBD have not been confirmed in a large sample. In the current study, we examined the effectiveness and adverse effects of YKS in patients with RBD using a retrospective review of patients’ clinical records.
METHODS
Participants and Procedures
The medical records of all patients who received YKS for the treatment of idiopathic RBD at Yoyogi Sleep Disorder Center from January 2010 to December 2013 were retrospectively reviewed. These patients had received the diagnosis of idiopathic RBD based on both nocturnal polysomnographic (n-PSG) findings and clinical interviews by board-certified sleep-disorder expert neuropsychiatrists or neurologists. All patients met diagnostic criteria for RBD in the second edition of International Classification of Sleep Disorders,1 including the presence of REM sleep without atonia (RSWA) on polysomnographic findings, a history of injurious or potentially injurious disruptive dream-enactment behaviors and/or abnormal REM sleep behaviors during polysomnography, absence of REM-related epileptiform activity, and absence of another sleep disorder that provided a better explanation for their sleep disturbance.1
The following data were collected from patients’ clinical records and analyzed: demographic information, age of onset of RBD reported by patients or their bed partners, frequency of dream-enacting behaviors, dose of YKS used, presence/absence of adverse events possibly due to the drug, and duration of YKS treatment. Patients’ clinical symptoms, including the frequency and intensity of RBD symptoms, were evaluated based on patients’ or their bed partners’ reports up to the last visit or the end of YKS administration before December 2014. To evaluate the treatment response more clearly, we set the inclusion criteria as the presence of dream-enacting behaviors once or more per week.13 Patients with remaining dream-enacting behaviors once or more per week even during the preceding treatment period with drugs other than YKS (ie, clonazepam and/or pramipexole) were included. However, cases without sufficient information, such as the frequency or severity of RBD symptoms before treatment, were excluded. Ninety-one consecutive patients with idiopathic RBD had been treated with YKS during the investigation period. Of these, 39 patients whose dream-enacting behaviors occurred less than once a week, 12 patients whose medical records did not provide sufficient information regarding the frequency or severity of RBD symptoms, and four patients in whom parkinsonism developed during the follow-up period were excluded. Finally, 36 patients with idiopathic RBD treated with YKS were analyzed. For the analysis of treatment outcome, we used two treatment categories; a group in which YKS alone was received (monotherapy group), and a group in which YKS was added to patients’ current drug treatment (add-on therapy group).
Treatment response was determined using the Clinical Global Impression of Illness Severity (CGI-S) and Improvement (CGI-I) scales.12,22 CGI-S was rated at the first visit, before starting and at the end of YKS treatment, or the last observation (endpoint). CGI-I was rated at the endpoint, considering the changes in RBD symptom frequency and/or severity in comparison with that before the treatment period. Patients with scores of 1 (very much improved) and 2 (much improved) on the CGI-I were classified as responders, whereas those with scores ranging from 3 (minimally improved) to 7 (very much worse) were classified as nonresponders.
The Ethical Committee of the Neuropsychiatric Research Institute approved this study protocol, and written informed consent was obtained from all participants.
Sourcing and Standardization of YKS
All YKS in this study was “Tsumura Yokukansan Extract Granules for prescription” (Product code; TJ-54, Tsumura & Co. Lot Number E43021, Tokyo, Japan), the quality of which is standardized based on appropriate manufacturing standards stipulated by the Ministry of Health, Labour and Welfare in Japan. It is composed of seven dried medicinal herbs: Japanese Angelica root (3.0 g, root of Angelica acutiloba Kitagawa, Umbelliferae), Uncaria hook (3.0 g, thorn of Uncaria rhynchophylla Miquel, Rubiaceae), Cnidium rhizome (3.0 g, rhizome of Cnidium officinale Makino, Umbelliferae), Atractylodes lancea rhizome (4.0 g, rhizome of Atractylodes lancea De Candolle, Compositae), Poria sclerotium (4.0 g, sclerotium of Poria cocos Wolf, Polyporaceae), Bupleurum root (2.0 g, root of Bupleurum falcatum Linné, Umbelliferae), and Glycyrrhiza (1.5 g, root and stolon of Glycyrrhiza uralensis Fisher, Leguminosae). Identification of the plant material and procedure for producing the dried YKS extract powder have been described previously.23
Statistical Analyses
Demographic variables (age and sex), length of disease morbidity, duration of preceding treatment for RBD prior to administration of YKS, dose of YKS (per day), duration of YKS treatment, and the rate of the number of patients with comorbidities with OSA (apnea-hypopnea index [AHI] > 5 events/h) were compared between the monotherapy and add-on therapy groups using chi-square tests for categorical variables, or Mann-Whitney U tests for continuous variables. CGI-S scores, CGI-I scores, and responder rate were also compared between the two groups using one-way repeated analysis of variance with the Bonferroni multiple comparison test, Mann-Whitney U test, and chi-square test, respectively. These statistical analyses were conducted using a software package (Statistical Package for the Social Sciences [SPSS], version 22.0J, SPSS Inc., Tokyo, Japan), with the significance level set at a two-tailed α level of 5%.
RESULTS
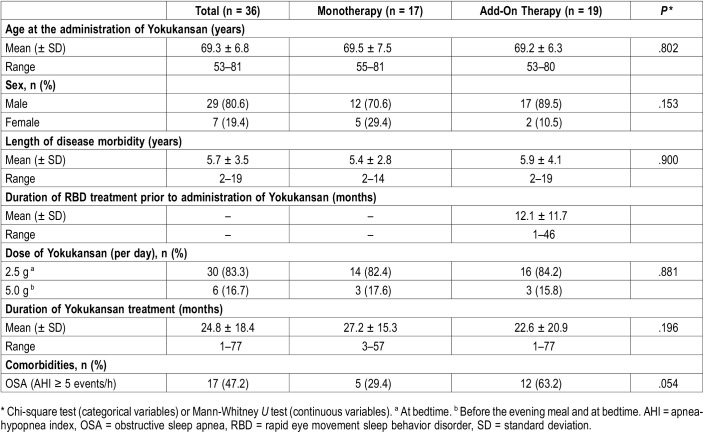
The patients’ mean age (standard deviation [SD]) was 69.3 (6.8) years, with a mean duration of RBD morbidity (SD) of 5.7 (3.5) years at the start of YKS treatment. Mean duration of YKS treatment (SD) was 24.8 (18.4) months (range 1–77 months). Seventeen patients exhibited AHI scores above 5 on n-PSG, including three patients who had already started to receive treatment for OSA; one with continuous positive airway pressure and two with oral appliance before the initiation of YKS medication. No patient had a history of alcohol abuse or other substance use disorders, narcolepsy, Alzheimer disease, or antidepressants/antipsychotics use. The dose of YKS was 2.5 g/d (at bedtime) in 30 cases, and 5.0 g/d (before the evening meal and at bedtime) in six cases. YKS was used as monotherapy in 17 cases and added on in 19 cases. Demographic and clinical features of each group are shown in Table 1.
Table 1.
Demographic and clinical data.
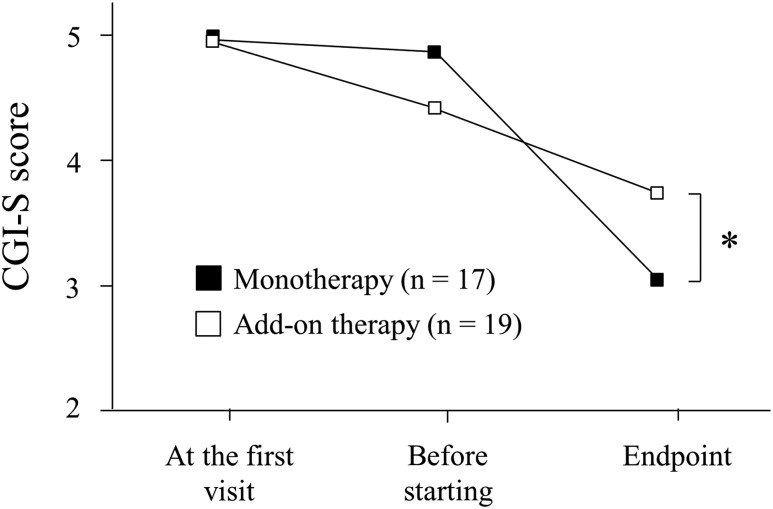
Changes in CGI-S scores are shown in Figure 1. Mean (SD) CGI-S scores at the first visit, before starting, and at the endpoint of YKS treatment, were 5.0 (0.9), 4.9 (1.0), and 3.1 (1.4) in the monotherapy group, and 5.0 (0.8), 4.4 (0.8), and 3.7 (1.0) in the add-on therapy group, respectively. There was an interaction between groups and time points (F1,58 = 9.40, P < .01). A comparison of CGI-S scores between the two groups revealed no significant difference at the first visit and before starting (P = .87 and P = .08, respectively). At the endpoint, the CGI-S score was significantly lower in the monotherapy group than in the add-on therapy group (P < .05).
Figure 1. Changes in CGI-S score.
CGI-S score at the first visit, before starting and at the end of treatment or the last observation (endpoint). One-way repeated-measures analysis of variance with the Bonferroni multiple comparison test was used. * P < .05. CGI-S = Clinical Global Impression of Illness Severity.
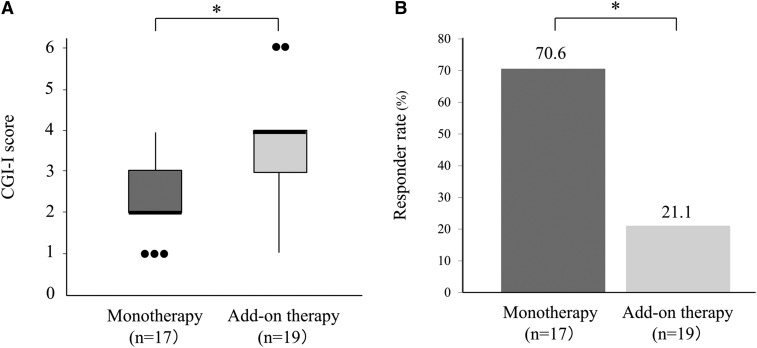
The mean (SD) CGI-I score of the monotherapy group at the endpoint was 2.3 (1.0), which was significantly lower than that of the add-on therapy group; 3.5 (1.3) (P < .01). Twelve of 17 patients (70.6%) were classified as responders in the monotherapy group, and 4 of 19 patients (21.1%) were classified as responders in the add-on therapy group (Figure 2). In the monotherapy group, 9 of 14 patients who received 2.5 g/d of YKS and 3 of 3 patients who received 5.0 g/d of YKS were classified as responders. In the add-on therapy group, 3 of 16 patients who received 2.5 g/d and 1 of 3 patients who received 5.0 g/d were classified as responders. Among patients who received YKS as an add-on to clonazepam (n = 8), none showed much or very much improvement. Regarding adverse effects, mild gastric distress was reported in only in one patient, who was receiving 2.5 g/d of YKS, but the adverse effects were not sufficiently severe to discontinue YKS treatment.
Figure 2. CGI-I score and responder rate.
(a) CGI-I score of Yokukansan treatment between the monotherapy group and the add-on therapy group. The edges of the box plots denote 25 and 75% tiles, whereas the solid black horizontal line denotes the cohort median. Upper and lower whiskers denote the limits of the nominal range of the data inferred from the upper and lower quartiles, and plotted points are outliers from these ranges. * P < .01, Mann-Whitney U test. (b) Comparison of Yokukansan treatment responders (defined as “very much” or “much” improved) between monotherapy group and add-on therapy group. * P < .01, chi-square test. CGI-I = Clinical Global Impression of Illness Improvement.
DISCUSSION
In total, 16 of 36 patients in the current study (44.4%) were classified as responders to YKS treatment. Importantly, 12 of 17 patients (70.6%) who received YKS monotherapy were responders, which is a comparable response rate to that reported in previous open-labeled, uncontrolled trials of clonazepam, melatonin, and pramipexole.4,11,12,16,24,25 The effectiveness and low frequency of adverse events of YKS18,19 suggest that it should be considered for treatment of RBD, particularly among older people who are at an increased risk of falling or exacerbation of OSA with clonazepam treatment.
At the endpoint, CGI-S scores in the add-on therapy group were higher than scores in the monotherapy group, resulting in a low responder rate in this group. Thus, unlike melatonin, which was reported to be effective in cases in which clonazepam did not work,13 YKS appeared to be ineffective in cases in which other drugs did not improve RBD symptoms. The maximum treatment dose of YKS received by the patients in the current study was 5.0 g/d, which was lower than the dose used in previously reported cases.21 All three patients who received 5.0 g/d in the monotherapy group were responders, suggesting that a dose of 5.0 g/d or more may have a greater therapeutic effect than lower doses. Future research should examine the optimal dosage of YKS for RBD treatment.
The detailed mechanisms of action of YKS are currently unclear. Reportedly, at least 25 ingredients in the methanol fraction of YKS extract have been identified in three-dimensional high-performance liquid chromatographic analysis.23 Thus, a mixture of various ingredients derived from seven medicinal herbs in YKS makes it more difficult to identify the specific positive mechanism of action for RBD symptoms. Descending glutamatergic projection from the sublaterodorsal tegmental nucleus to spinal and cranial motoneurons induces the phasic motor activation leading to the vigorous movements in patients with RBDs.26 The glutamate uptake function of YKS27 suggests that the drug possibly reduces oneiric behavior through the suppression of phasic muscle activity. Future studies should be conducted to clarify the effects of YKS on RSWA.
The current study involved several limitations that should be considered. First, the treatment outcomes of YKS were only evaluated using the CGI, by retrospectively reviewing patients’ clinical records. Moreover, the detailed content of dreams, and frequency and severity of injury caused by dream-enacting behaviors were based solely on the reports of the attending physician. It would be useful for future studies to use validated severity rating scales such as the rapid eye movement sleep behavior disorder questionnaire28,29 together with posttreatment n-PSG. Similarly, the possibility of missing minor adverse effects is a further potential limitation. Second, the patients of this study were from a single institution, and cannot be considered representative of patients with RBD. Future prospective multicenter randomized controlled trials are needed. Third, there was a lack of information about clinical factors that could potentially affect RBD manifestation, such as the precise amount of daily alcohol ingestion, detailed neurocognitive measurement, and brain imaging to rule out neurodegenerative comorbidities, particularly α-synucleinopathies. The effects of YKS on secondary RBD caused by neurodegenerative diseases should be investigated in future studies.
In conclusion, YKS treatment may be effective for RBD symptoms, with a low frequency of adverse events. However, YKS is less effective for patients who are refractory to other drugs. This finding suggests that YKS should be considered as a treatment option for patients with RBD who are at an increased risk of the side effects associated with other drug treatments.
DISCLOSURE STATEMENT
All authors have read and approved this manuscript. Work for this study was performed at Japan Somnology Center. Kentaro Matsui has received speaker’s honoraria from MSD, Otsuka Pharmaceutical, Meiji Seika Pharma, Eisai, Yoshitomi Pharmaceutical and Mochida Pharmaceutical; he has received research funding from Eisai. Jun Ishigooka has received support for consultancy from Otsuka Pharmaceutical, Sumitomo Dainippon Pharma, Novartis Pharma, MSD and Takeda Pharmaceutical; he has received speaker’s honoraria from Otsuka Pharmaceutical, Sumitomo Dainippon Pharma, Astellas, MSD and Eli Lilly. Katsuji Nishimura has received speaker’s honoraria from Meiji Seika Pharma, Mochida Pharmaceutical, Takeda Pharmaceutical, Eli Lilly, MSD, Shionogi, Janssen Pharmaceutical, Eisai, Otsuka Pharmaceutical, Novartis, Mitsubishi Tanabe Pharma, and Chugai Pharmaceutical; he has received research/grant support from Mochida Pharmaceutical, Takeda Pharmaceutical, Otsuka Pharmaceutical, Novartis, Mitsubishi Tanabe Pharma, Sumitomo Dainippon Pharma, MSD, Eisai, Tsumura, and Mebix. Yuichi Inoue has received speaker’s honoraria from Eisai, MSD and Takeda Pharmaceutical; he has received research/grant support from MSD, Takeda Pharmaceutical, Philips Respironics GK and Koike Medical. Taeko Sasai-Sakuma reports no conflicts of interest.
ACKNOWLEDGMENTS
This study was funded by a MEXT/JSPS KAKENHI Grant-in-Aid for Young Scientists (No. 15K19499 and 18K07512) to Dr. Sasai-Sakuma. The authors thank Benjamin Knight, MSc. from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- CGI-I
Clinical Global Impression of Illness Improvement
- CGI-S
Clinical Global Impression of Illness Severity
- n-PSG
nocturnal polysomnogram
- OSA
obstructive sleep apnea
- RBD
rapid eye movement sleep behavior disorder
- REM
rapid eye movement
- RSWA
rapid eye movement sleep without atonia
- SD
standard deviation
- YKS
Yokukansan
REFERENCES
- 1.American Academy of Sleep Medicine . International Classification of Sleep Disorders: Diagnostic and Coding Manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 2.Schenck CH, Bundlie SR, Ettinger MG, Mahowald MW. Chronic behavioral disorders of human REM sleep: a new category of parasomnia. Sleep. 1986;9(2):293–308. doi: 10.1093/sleep/9.2.293. [DOI] [PubMed] [Google Scholar]
- 3.Iranzo A, Santamaria J, Tolosa E. The clinical and pathophysiological relevance of REM sleep behavior disorder in neurodegenerative diseases. Sleep Med Rev. 2009;13(6):385–401. doi: 10.1016/j.smrv.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Olson EJ, Boeve BF, Silber MH. Rapid eye movement sleep behaviour disorder: demographic, clinical and laboratory findings in 93 cases. Brain. 2000;123(Pt 2):331–339. doi: 10.1093/brain/123.2.331. [DOI] [PubMed] [Google Scholar]
- 5.Frauscher B, Gschliesser V, Brandauer E, et al. REM sleep behavior disorder in 703 sleep-disorder patients: the importance of eliciting a comprehensive sleep history. Sleep Med. 2010;11(2):167–171. doi: 10.1016/j.sleep.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Montplaisir J, Gagnon JF, Fantini ML, et al. Polysomnographic diagnosis of idiopathic REM sleep behavior disorder. Mov Disord. 2010;25(13):2044–2051. doi: 10.1002/mds.23257. [DOI] [PubMed] [Google Scholar]
- 7.McCarter SJ, St Louis EK, Boeve BF. REM sleep behavior disorder and REM sleep without atonia as an early manifestation of degenerative neurological disease. Curr Neurol Neurosci Rep. 2012;12(2):182–192. doi: 10.1007/s11910-012-0253-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohayon MM, Schenck CH. Violent behavior during sleep: prevalence, comorbidity and consequences. Sleep Med. 2010;11(9):941–946. doi: 10.1016/j.sleep.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schenck CH, Bundlie SR, Mahowald MW. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder. Neurology. 1996;46(2):388–393. doi: 10.1212/wnl.46.2.388. [DOI] [PubMed] [Google Scholar]
- 10.Iranzo A, Molinuevo JL, Santamaría J, et al. Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. Lancet Neurol. 2006;5(7):572–577. doi: 10.1016/S1474-4422(06)70476-8. [DOI] [PubMed] [Google Scholar]
- 11.Schenck CH, Mahowald MW. Polysomnographic, neurologic, psychiatric, and clinical outcome report on 70 consecutive cases with REM sleep behavior disorder (RBD): sustained clonazepam efficacy in 89.5% of 57 treated patients. Cleve Clin J Med. 1990;57(Supplement):S-9–S-23. [Google Scholar]
- 12.Ferri R, Marelli S, Ferini-Strambi L, et al. An observational clinical and video-polysomnographic study of the effects of clonazepam in REM sleep behavior disorder. Sleep Med. 2013;14(1):24–29. doi: 10.1016/j.sleep.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Boeve BF, Silber MH, Ferman TJ. Melatonin for treatment of REM sleep behavior disorder in neurologic disorders: results in 14 patients. Sleep Med. 2003;4(4):281–284. doi: 10.1016/s1389-9457(03)00072-8. [DOI] [PubMed] [Google Scholar]
- 14.Schenck CH, Mahowald MW. Long-term, nightly benzodiazepine treatment of injurious parasomnias and other disorders of disrupted nocturnal sleep in 170 adults. Am J Med. 1996;100(3):333–337. doi: 10.1016/S0002-9343(97)89493-4. [DOI] [PubMed] [Google Scholar]
- 15.Anderson KN, Shneerson JM. Drug treatment of REM sleep behavior disorder: the use of drug therapies other than clonazepam. J Clin Sleep Med. 2009;5(3):235–239. [PMC free article] [PubMed] [Google Scholar]
- 16.Takeuchi N, Uchimura N, Hashizume Y, et al. Melatonin therapy for REM sleep behavior disorder. Psychiatry Clin Neurosci. 2001;55(3):267–269. doi: 10.1046/j.1440-1819.2001.00854.x. [DOI] [PubMed] [Google Scholar]
- 17.Kunz D, Mahlberg R. A two-part, double-blind, placebo-controlled trial of exogenous melatonin in REM sleep behaviour disorder. J Sleep Res. 2010;19(4):591–596. doi: 10.1111/j.1365-2869.2010.00848.x. [DOI] [PubMed] [Google Scholar]
- 18.Mizukami K, Asada T, Kinoshita T, et al. A randomized cross-over study of a traditional Japanese medicine (kampo), yokukansan, in the treatment of the behavioural and psychological symptoms of dementia. Int J Neuropsychopharmacol. 2009;12(2):191–199. doi: 10.1017/S146114570800970X. [DOI] [PubMed] [Google Scholar]
- 19.Iwasaki K, Satoh-Nakagawa T, Maruyama M, et al. A randomized, observer-blind, controlled trial of the traditional Chinese medicine Yi-Gan San for improvement of behavioral and psychological symptoms and activities of daily living in dementia patients. J Clin Psychiatry. 2005;66(2):248–252. doi: 10.4088/jcp.v66n0214. [DOI] [PubMed] [Google Scholar]
- 20.Ozone M, Yagi T, Chiba S, et al. Effect of yokukansan on psychophysiological insomnia evaluated using cyclic alternating pattern as an objective marker of sleep instability. Sleep Biol. Rhythms. 2012;10(2):157–160. [Google Scholar]
- 21.Shinno H, Kamei M, Nakamura Y, Inami Y, Horiguchi J. Successful treatment with Yi-Gan San for rapid eye movement sleep behavior disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(7):1749–1751. doi: 10.1016/j.pnpbp.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 22.Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976. [Google Scholar]
- 23.Ikarashi Y, Mizoguchi K. Neuropharmacological efficacy of the traditional Japanese Kampo medicine yokukansan and its active ingredients. Pharmacol Ther. 2016;166:84–95. doi: 10.1016/j.pharmthera.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt MH, Koshal VB, Schmidt HS. Use of pramipexole in REM sleep behavior disorder: results from a case series. Sleep Med. 2006;7(5):418–423. doi: 10.1016/j.sleep.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 25.Sasai T, Inoue Y, Matsuura M. Effectiveness of pramipexole, a dopamine agonist, on rapid eye movement sleep behavior disorder. Tohoku J Exp Med. 2012;226(3):177–181. doi: 10.1620/tjem.226.177. [DOI] [PubMed] [Google Scholar]
- 26.Luppi PH, Clement O, Valencia Garcia S, Brischoux F, Fort P. New aspects in the pathophysiology of rapid eye movement sleep behavior disorder: the potential role of glutamate, gamma-aminobutyric acid, and glycine. Sleep Med. 2013;14(8):714–718. doi: 10.1016/j.sleep.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Kawakami Z, Kanno H, Ueki T, et al. Neuroprotective effects of yokukansan, a traditional Japanese medicine, on glutamate-mediated excitotoxicity in cultured cells. Neuroscience. 2009;159(4):1397–1407. doi: 10.1016/j.neuroscience.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Li SX, Wing YK, Lam SP, et al. Validation of a new REM sleep behavior disorder questionnaire (RBDQ-HK) Sleep Med. 2010;11(1):43–48. doi: 10.1016/j.sleep.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 29.Sasai T, Matsuura M, Wing YK, Inoue Y. Validation of the Japanese version of the REM sleep behavior disorder questionnaire (RBDQ-JP) Sleep Med. 2012;13(7):913–918. doi: 10.1016/j.sleep.2012.04.011. [DOI] [PubMed] [Google Scholar]