Abstract
Study Objectives:
The aim was to investigate how the severity of apneas, hypopneas, and related desaturations is associated with obstructive sleep apnea (OSA)-related daytime sleepiness.
Methods:
Multiple Sleep Latency Tests and polysomnographic recordings of 362 patients with OSA were retrospectively analyzed and novel diagnostic parameters (eg, obstruction severity and desaturation severity), incorporating severity of apneas, hypopneas, and desaturations, were computed. Conventional statistical analysis and multivariate analyses were utilized to investigate connection of apnea-hypopnea index (AHI), oxygen desaturation index (ODI), conventional hypoxemia parameters, and novel diagnostic parameters with mean daytime sleep latency (MSL).
Results:
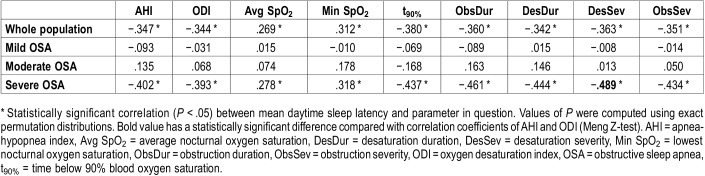
In the whole population, 10% increase in values of desaturation severity (risk ratio = 2.01, P < .001), obstruction severity (risk ratio = 2.18, P < .001) and time below 90% saturation (t90%) (risk ratio = 2.05, P < .001) induced significantly higher risk of having mean daytime sleep latency ≤ 5 minutes compared to 10% increase in AHI (risk ratio = 1.63, P < .05). In severe OSA, desaturation severity had significantly (P < .02) stronger negative correlation (ρ = −.489, P < .001) with mean daytime sleep latency compared to AHI (ρ = −.402, P < 0.001) and ODI (ρ = −.393, P < .001). Based on general regression model, desaturation severity and male sex were the most significant factors predicting daytime sleep latency.
Conclusions:
Severity of sleep-related breathing cessations and desaturations is a stronger contributor to daytime sleepiness than AHI or ODI and therefore should be included in the diagnostics and severity assessment of OSA.
Citation:
Kainulainen S, Töyräs J, Oksenberg A, Korkalainen H, Sefa S, Kulkas A, Leppänen T. Severity of desaturations reflects OSA-related daytime sleepiness better than AHI. J Clin Sleep Med. 2019;15(8):1135–1142.
Keywords: daytime sleepiness, desaturation severity, diagnostics, MSLT, obstructive sleep apnea, sleep latency
BRIEF SUMMARY
Current Knowledge/Study Rationale: Previous studies, where the effect of sleep apnea on daytime sleepiness has been investigated, have mainly focused on evaluating the effect of the number of arousals, apneas, hypopneas, or desaturations on daytime sleepiness. No previous studies have assessed the effect of the severity of individual apneas, hypopneas, and related desaturations on objectively measured daytime sleepiness.
Study Impact: The current findings indicate that the severity of apneas, hypopneas, and related desaturations has a stronger effect on daytime sleepiness than their number. This highlights the need for characterizing the severity of individual obstructive events and related desaturations when assessing the severity of obstructive sleep apnea.
INTRODUCTION
Obstructive sleep apnea (OSA) causes multiple nocturnal and daytime symptoms that have substantial effect on quality of life.1 Excessive daytime sleepiness (EDS) is one of the most important daytime symptoms.2 Depending on its severity, it can significantly decrease working ability and cognitive performance.1 Furthermore, EDS is a factor used in diagnostics of OSA and a criterion for continuous positive airway pressure (CPAP) treatment in mild OSA.3
EDS can be evaluated based on questionnaires, such as the Epworth Sleepiness Scale (ESS) and objective tests such as Multiple Sleep Latency Test (MSLT). Daytime sleepiness caused by OSA is suggested to be related to inefficient and fragmented sleep, caused by apnea- and hypopnea-related arousals.4,5 Previous studies have shown that OSA decreases arousal threshold and an increase in the disease severity leads to even more fragmented sleep.6 However, the connection between arousal index and daytime sleepiness is reported to be weak in patients with OSA.7 Desaturations caused by apneas or hypopneas result in repetitive nocturnal activation of the sympathetic nervous system, increasing physiological stress during sleep even without related arousals.8 In addition, an increase in apnea-hypopnea index (AHI) (regardless of presence of arousals or desaturations) decreases mean daytime sleep latency (MSL) in the MSLT,9 and snoring can independently cause EDS.10,11 Furthermore, it has been shown that patients with severe OSA have decreased MSL compared with patients having mild or moderate OSA and similar ESS scores.7 In addition, in severe OSA, subjectively sleepy patients (ESS score > 16) have higher AHI, apnea index, and arousal index compared to nonsleepy patients (ESS score < 10).5
Controversially, AHI and oxygen desaturation index (ODI) have only a weak correlation with different daytime sleepiness measures.9,12–14 This suggests that albeit an increase in AHI is slightly correlated with decreased MSL, the underlying physiological mechanisms causing EDS in patients with OSA remains partially unresolved. This might be because AHI and ODI estimate OSA severity in a very simplistic manner, taking into account only the number of events. We have previously introduced novel diagnostic parameters called desaturation severity and obstruction severity, which also consider durations of apneas and hypopneas and the severity of related desaturation events.15,16 Furthermore, we have shown that these parameters are more strongly related to increased risk of severe health consequences and thus estimate the severity of OSA better than AHI.17,18 Therefore, we hypothesize that the severity of breathing cessations is also more strongly correlated with daytime sleepiness than AHI or ODI.
In the current study, the aim was to investigate how the severity of individual apneas, hypopneas, and related desaturations affect the MSL. In addition, we compare the capability of AHI and ODI with the capability of novel diagnostic parameters to explain daytime sleepiness.
METHODS
A total of 533 consecutive pairs of polysomnography (PSG) and MSLT recordings, conducted at the Sleep Disorders Unit, Loewenstein Hospital – Rehabilitation Center (Raanana, Israel) during the years 2001–2003 were included in this study. Based on a clinical protocol at Loewenstein Hospital, MSLTs were conducted for all patients with complaints of sleepiness during clinical interview before PSG. The PSG recordings were conducted the night preceding the day of MSLT. Both PSG and MSLT recordings were conducted with REMbrandt Manager System-setup (Medcare, Amsterdam, Netherlands). MSLTs were conducted in conformity with a four-nap protocol according to American Academy of Sleep Medicine (AASM) guidelines.19 Afterward, all PSG and MSLT recordings were reanalyzed for research purposes at the Department of Clinical Neurophysiology, Kuopio University Hospital, in conformity with The AASM Manual for Scoring Sleep and Associated Events: Rules, Terminology and Technical Specifications (2007 recommended criteria).3 All hypopneas (rule 4A) and apneas fulfilling the AASM 2007 recommended criteria were scored and considered in the analyses regardless of their type (ie, obstructive, central, mixed). Ethical permission was obtained from the Ethical Committee of Loewenstein Hospital (0006-17-LOE).
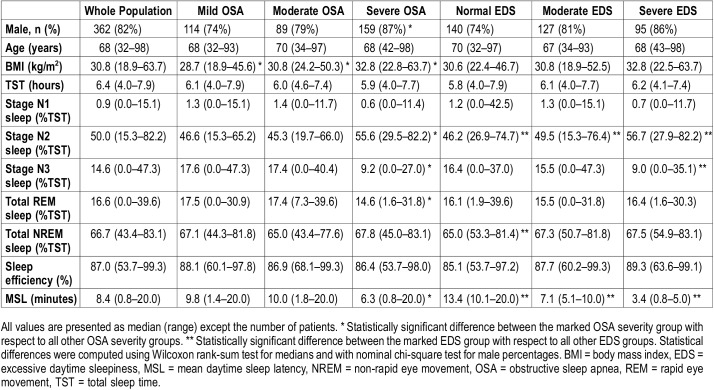
Total sleep time (TST) was computed based on manual sleep staging. Patients having TST < 4 hours (n = 58) or AHI < 5 events/h (n = 113) were excluded, leaving 362 patients for further analyses. Patients’ demographic data are presented in Table 1. Raw signals and information on scored events were exported from REMbrandt. The conventional diagnostic parameters (AHI, ODI, average nocturnal oxygen saturation [Avg SpO2], minimum nocturnal oxygen saturation [min SpO2], time below 90% blood oxygen saturation [t90%]), novel diagnostic parameters (obstruction duration [ObsDur], desaturation duration [DesDur], desaturation severity [DesSev], and obstruction severity [ObsSev]) and MSL were computed using custom-made MatLab (MathWorks, Natick, MA, USA) functions. ObsDur and DesDur are defined as the sum of durations of all breathing cessation events (ie, apneas and hypopneas) and desaturation events, respectively, normalized by TST. DesSev represents the sum of areas of all desaturation events (DesArea, Figure S1 in the supplemental material) normalized by TST. Furthermore, ObsSev is calculated as the TST-normalized sum of the multiplication of the duration of each individual breathing cessation event with the area of the related desaturation event. Computation of the novel parameters has been described with details in the supplemental material and in our previous studies.15,16
Table 1.
Demographic data from patients included in statistical analyses.
Patients were grouped by sex, age, the severity of OSA, and severity of EDS. Age groups were determined based on the age distribution to achieve equal sized groups: younger than 59 years (75 patients), 59–68 years (88 patients), 68–73 years (90 patients) and older than 73 years (98 patients). Patients were classified to EDS groups based on their MSL: normal (MSL > 10 minutes), moderate EDS (MSL > 5 to 10 minutes), and severe EDS (MSL ≤ 5 minutes). Correlations of conventional and novel diagnostic parameters with MSL were determined using Spearman ρ-rank correlation. The statistical significance of differences between correlation coefficients was evaluated by Meng Z-test. Statistical analyses were performed in MatLab using custom-made functions and Statistics and Machine Learning Toolbox (MathWorks, Natick, Massachusetts, United States).
Multinomial hierarchical logistic regression adjusted for sex, age, and body mass index (BMI) was utilized to examine the probability of belonging to a certain EDS group compared with the probability of belonging to a normal group (ie, MSL > 10 minutes). Furthermore, probabilities of belonging to a clinically sleepy group (MSL < 8 minutes) were computed for different OSA groups. Prior to multinomial logistic regression, parameter values were normalized by the maximum value of each parameter in question. An increase of one unit in normalized parameter values were further scaled to equal 10% relative change in parameter values. This was done in order to provide more convenient interpretation of the results and to enable the comparison of relative risks between parameters. Sex was considered as a categorical variable and relative normalization and scaling was not applied for age and BMI.
General regression model was obtained for the whole population by using stepwise multivariate logistic regression. The regression analysis utilized logarithmic link function and Bayesian Information Criterion as an excluding factor to avoid overfitting of nonsignificant variables to the model. In addition, quantile regression analyses with quantiles of 10%, 50%, and 90% were performed between diagnostic parameters and MSLT. In general and quantile regression, parameter values were normalized similarly as in multinomial analysis.
RESULTS
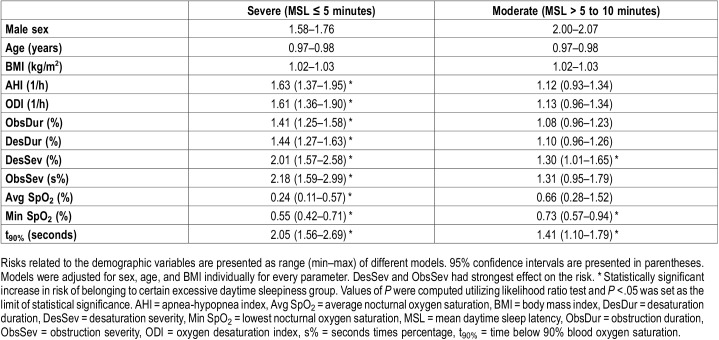
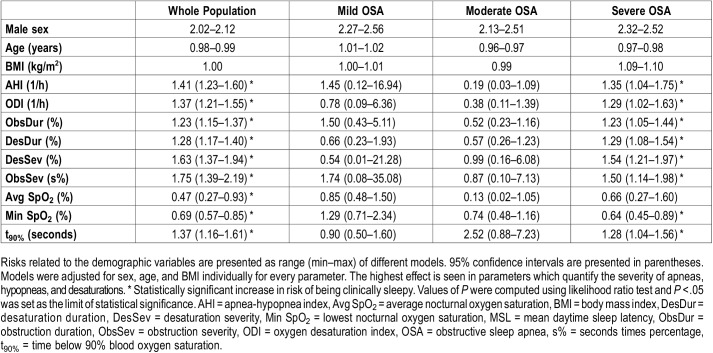
Multinomial analysis indicated that increase in the severity of sleep-related breathing cessations and related desaturations has a stronger connection with the severity of daytime sleepiness than a similar increase in AHI or ODI (Table 2 and Table 3). A relative 10% increase in Desaturation Severity (risk ratio = 2.01, P < .001), Obstruction Severity (risk ratio = 2.18, P < .001) and t90% (risk ratio = 2.05, P < .001) resulted in over twofold increase in risk of suffering from severe daytime sleepiness in the whole population (Table 2). 10% relative increase in all values of diagnostic parameters resulted in statistically significant risk ratios of having MSL < 8 minutes only in the whole population and severe OSA group (Table 3).
Table 2.
The effect of one-unit increase in values of demographic parameters (sex, age, and BMI) and relative 10% increase in values of diagnostic parameters on the risk of belonging to the severe or moderate excessive daytime sleepiness groups compared to that of belonging to the normal group.
Table 3.
The effect of one-unit increase in demographic parameters (sex, age, and BMI) and relative 10% increase in diagnostic parameter values to probability of being clinically sleepy (mean daytime sleep latency < 8 minutes).
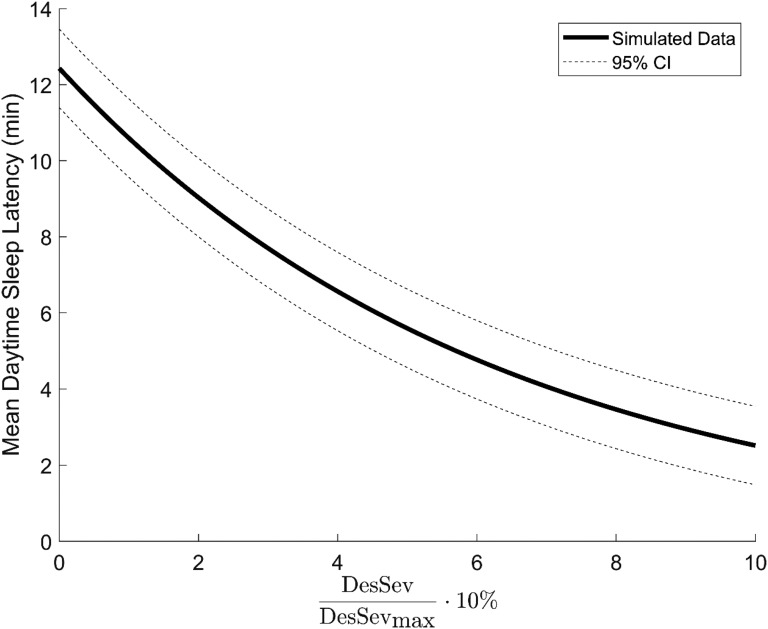
Based on stepwise logistic regression, only two variables were selected to the general regression model (Figure 1): sex (β = .192, P < .001, standard error = .069) and Desaturation Severity (β = .155, P < .001, standard error = .015). Other statistically significant parameters, such as Obstruction Severity (β < .001, P < .001), AHI (β < .0001, P < .001), and t90% (β < .0001, P < .05) were discarded because they did not explain decreased mean daytime sleep latency as effectively as Desaturation Severity in Bayesian sense. Figure 1 illustrates the simulated decrease in MSL with increasing Desaturation Severity based on the general regression model.
Figure 1. Simulated relationship between DesSev and mean daytime sleep latency.
General regression model-based visualization of the effect of normalized and scaled DesSev on mean daytime sleep latency in the studied patient population. DesSev = desaturation severity.
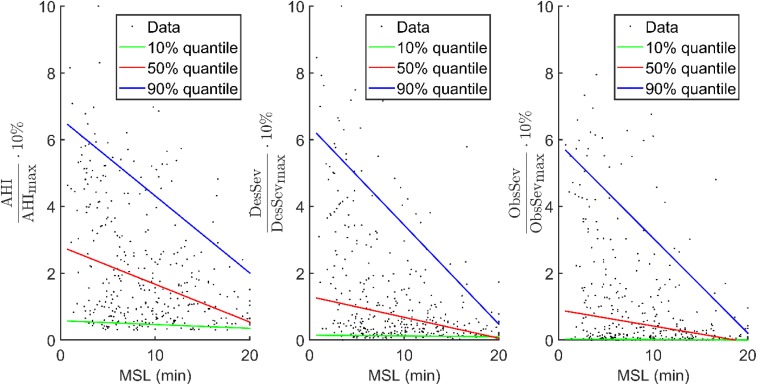
In the severe OSA group, all novel parameters incorporating the severity of individual apneas, hypopneas, and desaturations showed slightly stronger correlation with MSL than AHI and ODI (Table 4). The correlation between Desaturation Severity and mean daytime sleep latency (ρ = −.489, P < .001) was stronger (P < .02) than correlations between conventional parameters and MSL (AHI: ρ = −.402, P < .001 and ODI: ρ = −.393, P < .001). Based on correlation coefficients, Desaturation Severity was able to explain 23.9% of the variance in sleep latencies in patients with severe OSA whereas AHI and ODI explain 16.1% and 15.4% of this variance, respectively. All novel and conventional diagnostic parameters had statistically significant (P < .05) correlation with MSL in the severe OSA group. In the whole population, the correlations of novel and conventional diagnostic parameters with MSL were very similar. In mild and moderate OSA severity groups, correlations diminished and were not statistically significant. Results of severity-grouped correlation analyses were confirmed with quantile regression analysis (Figure 2).
Table 4.
Spearman rank correlation coefficients indicates that ObsDur, DesDur, DesSev, and ObsSev are more strongly connected to mean daytime sleep latency than AHI and ODI in severe OSA group.
Figure 2. Quantile regressions of 10%, 50%, and 90% quantiles for AHI, DesSev, and ObsSev.
Quantile regression-based simulations of the connection between increasing number and severity of breathing cessations and related desaturations. AHI = apnea-hypopnea index, DesSev = desaturation severity, MSL = mean daytime sleep latency, ObsSev = obstruction severity.
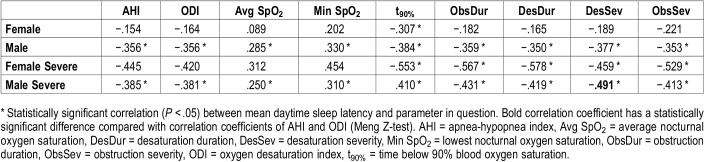
In males having severe OSA, among all diagnostic parameters Desaturation Severity had the strongest (P = .03) correlation (ρ = −.491, P < .001) with MSL (Table 5). Although AHI and ODI explained approximately 14.8% of the variance in MSL, Desaturation Severity was able to explain 24.1% of this variance. Furthermore, in the group of females with severe OSA, correlations between MSL and novel diagnostic parameters were all statistically significant (ObsDur: ρ = −.567, P = .01; DesDur: ρ = −.578, P = .01; DesSev: ρ = −.459, P = .04, and ObsSev: ρ = −.529, P = .02) whereas AHI (ρ = −.445, P = .06) and ODI (ρ = −.420, P = .07) were not. Obstruction Duration, Desaturation Duration, and t90% had strongest correlations with MSL and each of them independently explains almost 33% of the variance in sleep latencies (Table 5). However, in the whole female population all correlations diminished and were not statistically significant.
Table 5.
Spearman rank correlation coefficients between mean daytime sleep latency and diagnostic parameters determined separately for males and females.
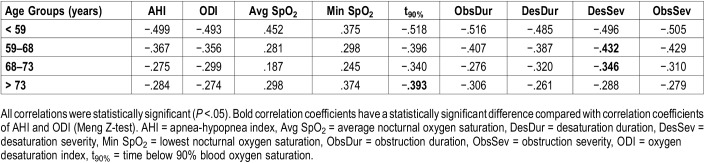
In age group analysis, all correlations between diagnostic parameters and MSL were statistically significant (Table 6). The correlation of Desaturation Severity with MSL was statistically significantly higher compared to the correlations of AHI and ODI with MSL in age groups 59–68 years and 68–73 years. Furthermore, the correlation of t90% with MSL was statistically significantly higher compared to the correlations of AHI and ODI with MSL in patients older than 73 years. In general, the strength of correlations decreased with increasing age.
Table 6.
Spearman rank correlation coefficients between mean daytime sleep latency and diagnostic parameters determined separately for different age groups.
DISCUSSION
In this study, we evaluated the connection of AHI, ODI, hypoxemia parameters, and novel diagnostic parameters to objectively measured daytime sleepiness in 362 patients with OSA who had undergone full-night PSG and consecutive MSLT. We found that the novel parameters that quantify the severity of apneas, hypopneas, and desaturations had stronger correlation to MSL compared to AHI and ODI. Furthermore, increase in the values of novel parameters resulted in a higher risk of severe daytime sleepiness (MSL ≤ 5 minutes) compared to similar increase in the values of AHI or ODI. This is consistent with our previous findings that the severity of individual apneas, hypopneas, and desaturations is more strongly connected to severe health consequences than the number of events.16–18,20 This highlights the clinical importance of considering also the severity of breathing cessations and related desaturations in the assessment of the OSA severity and risk of related outcomes. However, connection between hypoxemic periods, quantified by desaturation severity and t90%, and MSL cannot be interpreted as implying that arousals and poor sleep quality are not significant factors explaining daytime sleepiness in patients with OSA. Rather, these implications can be captured from the SpO2 signal.
Present results indicate that the contribution of the severity of the desaturations to EDS increases along increasing OSA severity. A relative 10% increase in Desaturation Severity and Obstruction Severity parameters as well as in t90% caused over twofold risk of severe EDS (MSL ≤ 5 minutes) outperforming the capabilities of AHI and ODI to explain decreased daytime sleep latency (Table 2). These findings are in line with previous studies,7,9 which reported that MSL is lowered on average while the severity of OSA increases, but AHI and ODI have only a weak connection to MSL. In addition, general regression models obtained from the stepwise regression did not even include AHI, ODI, or other conventional hypoxemia parameters in the model, suggesting that their value in explaining decreased MSL is minor in comparison with that of desaturation severity (Figure 1). Our previous findings suggest that the detection of patients having the highest risk of the most detrimental outcomes of OSA can be enhanced by applying the novel parameters to the diagnostics of OSA.17,18 Based on the current results, utilizing desaturation severity or obstruction severity in the evaluation of OSA severity would be beneficial because the detection of those patients with the highest risk of being excessively sleepy is enhanced as well.
Desaturations cause repetitive nocturnal activation of the sympathetic nervous system, leading to increase in physiological stress during sleep.8 Based on the current results, it seems that longer and deeper desaturations may increase physiological stress more than shorter and shallower events, as the increase in the values of Desaturation Severity increased the risk of having EDS. Furthermore, it has been suggested that repetitive nocturnal hypoxemias together with fragmented sleep could be the primary reason for EDS, at least in patients with severe OSA.21 Based on a previous study, OSA phenotype differs between sleepy and nonsleepy patients with severe OSA, suggesting that sleepy patients with severe OSA experience the more severe type of OSA (eg lower minimum SpO2 and higher apnea index and arousal index).5 The current results are in line with the aforementioned findings, as an increase in severity of OSA increased correlation between novel diagnostic parameters and EDS (Table 4 and Table 5). No statistically significant correlations between any of the diagnostic parameters and daytime sleepiness were seen in mild and moderate OSA groups. This effect is also observed in the quantile regression analyses (Figure 2). At lower extreme quantile and median quantile, a weak trend was observed between values of diagnostic parameters and decreased MSL. These quantiles, however, are unable to explain the decreased MSL at high parameter values, whereas the upper extreme quantile (0.9) of DesSev and ObsSev presents clearly that novel parameter values increase while the MSL decreases. This may be explained by the fact that in mild and moderate OSA, apneas and hypopneas are shorter and less frequent compared to severe OSA, resulting in less severe nocturnal hypoxemias.20 As it has been shown that the relationship between AHI and MSLT is weak,7,9 it could be speculated that presence of EDS in patients with OSA is more related to severity than the number of the events.
Desaturation severity, obstruction severity, and t90% were the best predictors for sleepiness in all analyses and populations except the severe OSA group consisting only of females (Table 5). In that group, the highest correlations were found between obstruction duration and MSL and between desaturation duration and MSL. This may result from the fact that OSA differs between sexes: OSA in females is more hypopnea weighted and thus desaturation events are less severe in comparison with males.22 Therefore, it is reasonable to interpret that as OSA characteristics vary between sexes, also the physiological mechanism causing EDS varies. In addition, age group analyses confirmed the observations seen in the multivariate analysis (Table 2 and Table 6). Age-grouped correlation analyses indicate that younger age, combined with more severe hypoxemic periods, increases the risk of being severely sleepy. This difference is most notable between hypoxemia-related parameters and conventional AHI and ODI. This further emphasizes the importance of patient specific quantification of the severity of apneas, hypopneas, and desaturations in the diagnostics of OSA.
It is acknowledged that the relatively low number of female patients (n = 67) can affect the significance of results obtained for females in this study. Especially in the multinomial analyses, the male sex-induced risk of being excessively sleepy may be partially explained by the difference in the number of patients in the male and female groups. This might also explain the large differences between sexes in the correlation analyses. However, it has been shown that the severity of apneas, hypopneas, and related desaturations is sex dependent.22 Therefore, we believe that the physiological mechanisms causing EDS are also sex dependent and therefore the difference between sexes was expected. Furthermore, it is acknowledged that the studied patient population was relatively old. We have previously reported that the severity of breathing cessations and related desaturations increases as age increases.23 However, no statistically significant correlation between age and MSL was found and the results of multivariate analyses indicated that age had only a minor contribution to daytime sleepiness as an independent factor in this patient population. In addition, it is also noted that BMI and diagnostic parameters are correlated to some extent. To overcome this bias, a hierarchical model was utilized in multinomial analysis. Because of the retrospective nature of the study, no information on studied patients’ medication or comorbidities at the time of the analysis was available. It is acknowledged that the presence of medication or comorbidities (eg, chronic obstructive pulmonary disease) may affect the degree of sleepiness. However, the aim of this study was to investigate how the severity of breathing cessations and related desaturations is connected with daytime sleepiness and therefore we believe that the absence of aforementioned information does not diminish the obtained results.
It is acknowledged that the termination time of 20 minutes in MSLT affects the numerical value of MSL and using different modeling techniques instead of mean of four latencies would be more demonstrative. However, using the exact termination time and mean is the current clinical protocol for evaluating sleepiness and was therefore utilized in this study. In addition, patients included in the current study (also those in the control group) reported sleepiness in the clinical interview and were therefore scheduled for MSLT. It is acknowledged that this results in a selection bias related to subjective sleepiness, because this study lacks subjectively nonsleepy patients. However, the aim of this study was to investigate objective sleepiness. As seen from the data (Table 1), most of the patients in this study were not objectively sleepy, and therefore we believe that this selection bias does not affect the connection between parameters and MSLT results. Furthermore, all analyses were performed without considering hypopneas, which were related to arousals only. It is acknowledged that this decreases the value of AHI. However, the correlation between arousal index and MSL and between AHI (which incorporates arousal-related hypopneas) and MSL is also reported to be low.7 Therefore, we believe that ignoring hypopneas related to arousals does not jeopardize the conclusions of the current study, as the aim was to investigate the effect of OSA-related physiological stress on sleepiness. As it has been suggested that arousals and sleep fragmentation could be key components determining subjective daytime sleepiness in patients with OSA,5 further studies are warranted to investigate the combined effect of number and duration of arousals, and severity of apneas, hypopneas, and desaturations on objectively measured daytime sleepiness.
In conclusion, parameters quantifying the severity of individual apneas, hypopneas, and desaturations are more strongly connected to objectively measured daytime sleepiness compared to conventional AHI and ODI. These parameters have also been shown to be more strongly linked to the most detrimental outcomes of OSA compared to AHI.15–18,20 Therefore, we suggest that desaturation severity or obstruction severity should be used in the diagnostics of OSA at least alongside AHI. This could enhance the detection of patients with EDS and having the highest risk of the most severe OSA-related health consequences.
DISCLOSURE STATEMENT
This study was financially supported by the Research Committee of the Kuopio University Hospital Catchment Area for the State Research Funding (projects 5041767 and 5041768), by Seinäjoki Central Hospital (grants 6020 and 6047), the Competitive State Research Financing of Expert Responsibility Area of Tampere University Hospital (grant numbers VTR3221, VTR3228, and EVO2089), by the Academy of Finland (decision number 313697), by the Respiratory Foundation of Kuopio Region, Päivikki & Sakari Sohlberg Foundation and by Finnish-Norwegian Medical Foundation. The authors report no conflicts of interest.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- DesDur
desaturation duration
- DesSev
desaturation severity
- EEG
electroencephalography
- ESS
Epworth Sleepiness Scale
- EDS
excessive daytime sleepiness
- MSL
mean daytime sleep latency
- MSLT
Multiple Sleep Latency Test
- ObsDur
obstruction duration
- ObsSev
obstruction severity
- OSA
obstructive sleep apnea
- ODI
oxygen desaturation index
- PSG
polysomnography
- t90%
total sleep time under 90% saturation
REFERENCES
- 1.Engleman HM, Douglas NJ. Sleep. 4: Sleepiness, cognitive function, and quality of life in obstructive sleep apnoea/hypopnoea syndrome. Thorax. 2004;59(7):618–622. doi: 10.1136/thx.2003.015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kapur VK, Auckley DH, Chowdhuri S, et al. clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479–504. doi: 10.5664/jcsm.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iber C, Ancoli-Israel S, Chesson AL Jr, Quan SF, for the American Academy of Sleep Medicine, editors. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 4.Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90(1):47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oksenberg A, Arons E, Nasser K, Shneor O, Radwan H, Silverberg DS. Severe obstructive sleep apnea: sleepy versus nonsleepy patients. Laryngoscope. 2010;120(3):643–648. doi: 10.1002/lary.20758. [DOI] [PubMed] [Google Scholar]
- 6.Jordan AS, Eckert DJ, Catcheside PG, McEvoy RD. Ventilatory response to brief arousal from non-rapid eye movement sleep is greater in men than in women. Am J Respir Crit Care Med. 2003;168(12):1512–1519. doi: 10.1164/rccm.200302-150OC. [DOI] [PubMed] [Google Scholar]
- 7.Fong S, Ho C, Wing Y. Comparing MSLT and ESS in the measurement of excessive daytime sleepiness in obstructive sleep apnoea syndrome. J Psychosom Res. 2005;58(1):55–60. doi: 10.1016/j.jpsychores.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Foldvary-Schaefer N, Waters T. Sleep-disordered breathing. Continuum (Minneap Minn) 2017;23(4, Sleep Neurology):1093–1116. doi: 10.1212/01.CON.0000522245.13784.f6. [DOI] [PubMed] [Google Scholar]
- 9.Koch H, Schneider LD, Finn LA, et al. Breathing disturbances without hypoxia are associated with objective sleepiness in sleep apnea. Sleep. 2017;40(11) doi: 10.1093/sleep/zsx152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neau J, Paquereau J, Bailbe M, Meurice J, Ingrand P, Gil R. Relationship between sleep apnoea syndrome, snoring and headaches. Cephalalgia. 2002;22(5):333–339. doi: 10.1046/j.1468-2982.2002.00303.x. [DOI] [PubMed] [Google Scholar]
- 11.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 12.Sagaspe P, Taillard J, Chaumet G, et al. Maintenance of wakefulness test as a predictor of driving performance in patients with untreated obstructive sleep apnea. Sleep. 2007;30(3):327–330. [PubMed] [Google Scholar]
- 13.Sun Y, Ning Y, Huang L, et al. Polysomnographic characteristics of daytime sleepiness in obstructive sleep apnea syndrome. Sleep Breath. 2012;16(2):375–381. doi: 10.1007/s11325-011-0515-z. [DOI] [PubMed] [Google Scholar]
- 14.Dündar Y, Saylam G, Tatar EÇ, et al. Does AHI value enough for evaluating the obstructive sleep apnea severity? Indian J Otolaryngol Head Neck Surg. 2015;67(Suppl 1):16–20. doi: 10.1007/s12070-014-0722-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kulkas A, Tiihonen P, Eskola K, Julkunen P, Mervaala E, Töyräs J. Novel parameters for evaluating severity of sleep disordered breathing and for supporting diagnosis of sleep apnea-hypopnea syndrome. J Med Eng Technol. 2013;37(2):135–143. doi: 10.3109/03091902.2012.754509. [DOI] [PubMed] [Google Scholar]
- 16.Kulkas A, Tiihonen P, Julkunen P, Mervaala E, Töyräs J. Novel parameters indicate significant differences in severity of obstructive sleep apnea with patients having similar apnea–hypopnea index. Med. Biol Eng Comput. 2013;51(6):697–708. doi: 10.1007/s11517-013-1039-4. [DOI] [PubMed] [Google Scholar]
- 17.Leppänen T, Särkkä M, Kulkas A, et al. RemLogic plug-in enables clinical application of apnea-hypopnea index adjusted for severity of individual obstruction events. J Med Eng Technol. 2016;40(3):119–126. doi: 10.3109/03091902.2016.1148791. [DOI] [PubMed] [Google Scholar]
- 18.Muraja-Murro A, Kulkas A, Hiltunen M, et al. Adjustment of apnea-hypopnea index with severity of obstruction events enhances detection of sleep apnea patients with the highest risk of severe health consequences. Sleep Breath. 2014;18(3):641–647. doi: 10.1007/s11325-013-0927-z. [DOI] [PubMed] [Google Scholar]
- 19.Littner MR, Kushida C, Wise M, et al. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005;28(1):113–121. doi: 10.1093/sleep/28.1.113. [DOI] [PubMed] [Google Scholar]
- 20.Muraja-Murro A, Nurkkala J, Tiihonen P, et al. Total duration of apnea and hypopnea events and average desaturation show significant variation in patients with a similar apnea-hypopnea index. J Med Eng Technol. 2012;36(8):393–398. doi: 10.3109/03091902.2012.712201. [DOI] [PubMed] [Google Scholar]
- 21.Garbarino S, Scoditti E, Lanteri P, Conte L, Magnavita N, Toraldo DM. Obstructive sleep apnea with or without excessive daytime sleepiness: clinical and experimental data-driven phenotyping. Front Neurol. 2018;9:505. doi: 10.3389/fneur.2018.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leppänen T, Kulkas A, Duce B, Mervaala E, Töyräs J. Severity of individual obstruction events is gender dependent in sleep apnea. Sleep Breath. 2017;21(2):397–404. doi: 10.1007/s11325-016-1430-0. [DOI] [PubMed] [Google Scholar]
- 23.Leppänen T, Töyräs J, Mervaala E, Penzel T, Kulkas A. Severity of individual obstruction events increases with age in patients with obstructive sleep apnea. Sleep Med. 2017;37:32–37. doi: 10.1016/j.sleep.2017.06.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.