Abstract
Study Objectives:
Mandibular advancement device (MAD) outcome varies between patients. We hypothesized that upper airway collapse sites, patterns, and degrees assessed during baseline drug-induced sleep endoscopy (DISE) affect MAD outcome.
Methods:
One hundred patients with obstructive sleep apnea (OSA) were included and underwent baseline type 1 polysomnography. MAD was fitted intraorally at fixed 75% maximal protrusion. A total of 72 patients completed 3-month follow-up polysomnography and baseline DISE. Response was defined as apnea-hypopnea index (AHI) reduction ≥ 50%, deterioration as AHI increases during MAD treatment compared to baseline.
Results:
Adjusting for baseline AHI and body mass index, patients with tongue base collapse showed 3.69 higher odds (1.27–10.73; P = .0128) for response. Complete concentric collapse at the level of the palate (5.32 [1.21–23.28]; P = .0234) and complete laterolateral oropharyngeal collapse (6.62 [1.14–38.34]; P = .0330) related to deterioration. Results for tongue base collapse and complete concentric collapse at the level of the palate were confirmed in the moderate to severe OSA subgroup. Applying these results to this selected subgroup increased response rate with 54% and decreased deterioration rate with 53%. These results were confirmed using other response and deterioration definitions.
Conclusions:
Three baseline DISE phenotypes identified during drug-induced sleep were significantly related to MAD treatment outcome: one beneficial, tongue base collapse, and two adverse, complete concentric collapse at the level of the palate and complete laterolateral oropharyngeal collapse. If confirmed in future prospective studies, these results could guide patient selection for MAD outcome.
Clinical Trial Registration:
This prospective clinical trial (PROMAD) was registered on Clinicaltrials.gov with identifier: NCT01532050.
Citation:
Op de Beeck S, Dieltjens M, Verbruggen AE, Vroegop AV, Wouters K, Hamans E, Willemen M, Verbraecken J, De Backer WA, Van de Heyning PH, Braem MJ, Vanderveken OM. Phenotypic labelling using drug-induced sleep endoscopy improves patient selection for mandibular advancement device outcome: a prospective study. J Clin Sleep Med. 2019;15(8):1089–1099.
Keywords: DISE, MAD, obstructive sleep apnea, OSA, personalized medicine
BRIEF SUMMARY
Current Knowledge/Study Rationale: Mandibular advancement device (MAD) treatment is a noninvasive therapy for obstructive sleep apnea (OSA) showing high patient adherence. Because treatment outcome is patient specific, improvement of upfront therapeutic response prediction would be advantageous. This study assessed the effect of drug-induced sleep endoscopy (DISE) phenotyping as identified during induced sleep, on MAD treatment outcome.
Study Impact: Our findings suggest that DISE is a promising tool to identify beneficial and adverse DISE phenotypes for upfront patient selection for MAD treatment. The results correspond to the next step in the development and application of a DISE phenotype-specific management of OSA.
INTRODUCTION
Obstructive sleep apnea (OSA) is a highly prevalent disorder affecting up to 9% and 17% of middle-aged women and men, respectively.1 OSA is characterized by repetitive upper airway narrowing (hypopnea) or complete collapse (apnea) during at least 10 seconds. OSA severity is quantified using the apnea-hypopnea index (AHI), representing the number of apneas and hypopneas per hour of sleep.2
The standard treatment for moderate (15 ≤ AHI < 30 events/h) to severe (AHI ≥ 30 events/h) OSA is continuous positive airway pressure (CPAP), applying pressurized air to keep the upper airway patent.3 Although CPAP treatment efficacy is high, adherence to this therapy is often rather low,4 supporting the need for other treatments such as oral appliance therapy, sleep position trainers, and surgical solutions.5–7
The most frequently prescribed oral appliances for OSA treatment are mandibular advancement devices (MAD), which protrude the lower jaw and reopen the upper airway.8 By protruding the lower jaw, the tongue moves forward. Because of palatoglossus coupling, as shown for upper airway stimulation therapy,9,10 moving the tongue forward with an MAD leads to opening the soft palate. Custom-made, titratable MADs are preferred, as they allow individual treatment optimization by titration and optimal retention.7,11,12 However, response to non-CPAP therapies such as MAD is patient dependent. MAD response rates range from 30% to 85%, resulting in 15% to 70% of the patients remaining undertreated.13 Upfront patient selection should thus be beneficial. Previous studies showed increased MAD response for patients who are young and female with supine-dependent OSA and who have lower AHI, mild airway collapsibility, and low loop gain, referring to a more stable ventilatory control system.13,14
Upper airway collapsibility, in terms of the level(s), the degree, and the configuration of collapse, can be studied using drug-induced sleep endoscopy (DISE).15 Our research group has shown an adverse effect of complete concentric collapse at the level of the palate (CCCp), assessed during DISE, on upper airway stimulation (UAS) response.16 However, little research has been done on effects of site(s) and patterns of collapse during DISE on MAD treatment response. Therefore, the current study aims to assess the effects of site(s), degree(s), and pattern(s) of upper airway collapse assessed during DISE at baseline on the observed MAD treatment outcome.
METHODS
Study Population
This prospective clinical trial was registered on Clinicaltrials.gov with identifier NCT01532050 and approved by the local ethical committee at University of Antwerp and Antwerp University Hospital. Written informed consent was obtained from all patients.
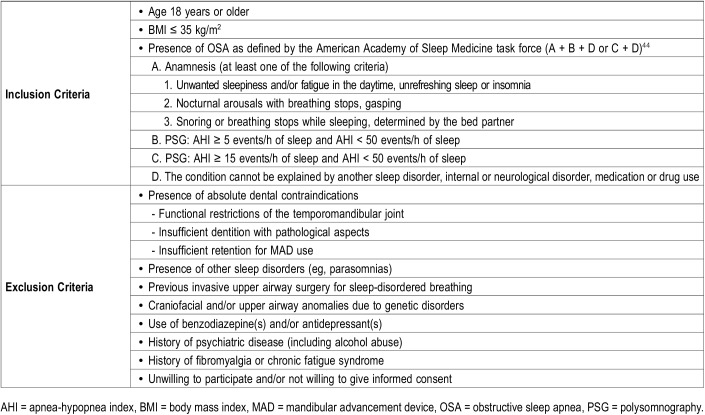
One hundred patients were prospectively recruited by the multidisciplinary team including a dental sleep professional, an ear, nose, and throat (ENT) surgeon and a board-certified sleep specialist responsible for patient care. Table 1 shows inclusion and exclusion criteria.
Table 1.
Inclusion and exclusion criteria.
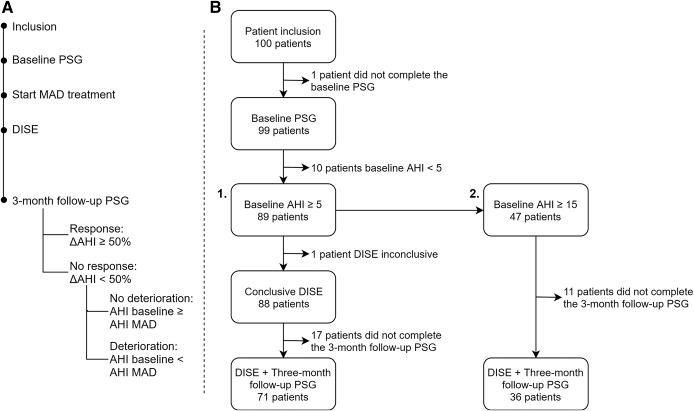
The study protocol (Figure 1A) was previously published in detail by Verbruggen et al17 At inclusion, all patients underwent new baseline type 1 polysomnography (PSG) to confirm inclusion criteria (AHI 5 to < 50 events/h). If eligible, custom-made MAD treatment (RespiDent Butterfly MAD, Orthodontic Clinics NV, Antwerp, Belgium), was started.18
Figure 1. Timeline study protocol (A) and patient flow (B).
The patient flow for all study patients (baseline AHI ≥ 5 events/h) is shown in arm 1, the patient flow for patients with moderate to severe obstructive sleep apnea (baseline AHI ≥ 15 events/h) is shown in arm 2. Reasons for dropout are given in the supplemental material. AHI = apnea-hypopnea index, DISE = drug-induced sleep endoscopy, MAD = mandibular advancement device, PSG = polysomnography.
The maximum protrusion was measured three times in each individual patient and averaged. To allow standardization, MAD was then placed at 75% of this individual patient’s maximal protrusion without further titration, in which 100% is measured as the distance between maximal protrusion and maximal retrusion, more specifically the centric relation position. By doing so, the individual protrusion range of each patient was respected.
All patients underwent DISE. The multidisciplinary team members were blinded to the DISE results throughout the study. After 3 months, a follow-up type 1 PSG with MAD was scheduled. Response was defined as ΔAHI ≥ 50%, deterioration as AHI with MAD > baseline AHI. To take into account the effect of different response definitions, the analysis for the patient subgroup with AHI ≥ 15 events/h was repeated for 6 other response definitions reported in literature19,20: AHI < 5, AHI < 5 and ΔAHI ≥ 50%, AHI < 5 or ΔAHI ≥ 50%, AHI < 10, AHI < 10 and ΔAHI ≥ 50%, AHI < 10 or ΔAHI ≥ 50%. Furthermore, an additional deterioration definition with a cutoff of 10% deterioration was applied to the dataset.
Drug-Induced Sleep Endoscopy (DISE)
DISE was performed by an ENT surgeon experienced in DISE in a semidark and silent room. Patients lay on a hospital bed in the supine position. Electrocardiography, blood pressure, and oximetry were monitored. A flexible fiberoptic nasopharyngoscope (Olympus END-GP, 3.7 mm diameter, Olympus Europe GmbH, Hamburg, Germany) was inserted through one of the nostrils, without decongestion, to visualize the collapsible segment of the patient’s upper airway. Subsequently, sedation was induced using 1.5 mg bolus midazolam injection and intravenous propofol administration using target-controlled infusion (2.0–3.0 μg). Sedation level was continuously monitored using four bispectral index (BIS) monitoring sensor electrodes (BIS VISTA monitor and BIS Quatro; Aspect Medical Systems Inc., Norwood, USA) attached to the forehead. BIS values range from 0 to 100, with 0 representing no brain activity and 100 representing a fully awake patient. During our DISE procedure, values between 50 and 70 were targeted during at least 5 minutes.
During DISE, the sites of upper airway collapse were assessed using a standard scoring system (Figure 2). In each patient, collapse level(s), degree(s), and pattern(s) were registered: collapse at the level of the palate, oropharynx, tongue base, pharyngeal lateral walls and/or epiglottis, being complete or partial with anteroposterior, laterolateral, or concentric pattern. During an expert meeting, the video-recorded DISE procedures were analyzed and discussed by four experienced ENT surgeons. The results of this central, independent core scoring were used in all analyses.
Figure 2. Standard scoring system for DISE.
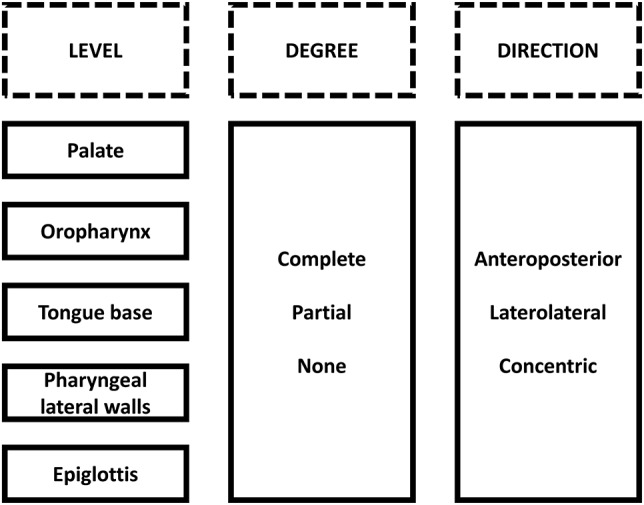
Rows represent the site of upper airway collapse, columns represent level, degree and direction. Originally published in Verbruggen et al.17 Reprinted with permission from the American Academy of Dental Sleep Medicine. Available from: http://dx.doi.org/10.15331/jdsm.6250. DISE = drug-induced sleep endoscopy.
Statistical Analysis
Statistical analysis was performed using statistical software packages JMP (JMP Pro 13.0.0. SAS Institute Inc., Cary, North Carolina, USA) and R (R Core Team, R Foundation for Statistical Computing, Vienna, Austria). Descriptive statistics were reported as mean ± standard deviation, median (quartile 1–quartile 2), or number and percentages. Normality was tested using the Shapiro-Wilk test. Normally distributed continuous variables were compared using paired or unpaired t tests, nonnormally distributed variables using the Wilcoxon signed-rank or Mann-Whitney U test.
Collapse patterns were compared between responders/nonresponders and deterioration/no deterioration with Fisher exact tests. Separate logistic regression models, explaining response or deterioration, were built for each collapse level with and without correction for AHI and body mass index (BMI). All analyses were repeated for the patient subgroup with moderate to severe OSA (baseline AHI ≥ 15 events/h). Accuracy was assessed using positive and negative predictive values (PPV and NPV) and receiver operating characteristic analyses. Reported P values are two-sided, statistical significance was considered if P < .05.
RESULTS
Data Characteristics and Descriptive Statistics
One hundred patients with OSA (83% male; age 47.6 ± 10.0 years; BMI 26.9 ± 3.3 kg/m2; AHI 21.0 ± 11.2 events/h sleep) were included in the study protocol and underwent a new baseline PSG. One patient did not complete this PSG and 10 patients showed AHI < 5 events/h, confirming OSA in 89/100 patients. Overall, 72/100 patients underwent both DISE and PSG with MAD. DISE was inconclusive in 1 patient due to agitation, resulting in a final dataset of 71 patients with both conclusive DISE and PSG results (Figure 1B). No significant differences in baseline parameters were found between patients lost to follow-up (n = 17) and patients completing 3-month follow-up (n = 72; see supplemental material for dropout reasons and Table S1). No permanent adverse effects were noted related to the 75% protrusion, only showing mild, temporary side effects as is usual during the start-up of any MAD treatment. No one reported treatment for temporomandibular joint complaints after treatment start-up.
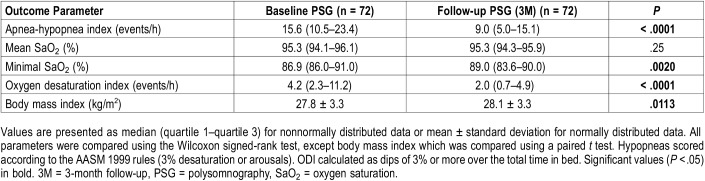
Thirty-six patients (50%) experienced mild (5 ≤ AHI < 15 events/h), 27 (37.5%) moderate (15 ≤ AHI < 30 events/h), and 9 (12.5%) severe (AHI ≥ 30 events/h) OSA. Significant differences between baseline and follow-up with MAD were present for AHI, minimal O2 saturation, oxygen desaturation index (ODI) and BMI (Table 2). Results on all parameters are shown in Table S2 (supplemental material).
Table 2.
Patient characteristics at baseline and 3-month follow-up.
In total, 33 patients (46%) were responder (ΔAHI ≥ 50%), 39 patients (54%) were nonresponders, of which 17 (24% of total study population, 44% of nonresponders) deteriorated under MAD treatment in 75% of the maximal protrusion. Responders and nonresponders at 3-month follow-up did not significantly differ regarding baseline characteristics (Table S3, supplemental material).
In 71 patients, conclusive DISE results could be obtained. Palatal collapse was most commonly present (n = 68/71; 96%), followed by tongue base (n = 37/71; 52%), oropharyngeal (n = 22/71; 31%), pharyngeal lateral wall (n = 16/71; 22%) and epiglottic (n = 13/71; 18%) collapse. Most patients (n = 53/71; 75%) showed multilevel collapse (Figure S1, supplemental material).
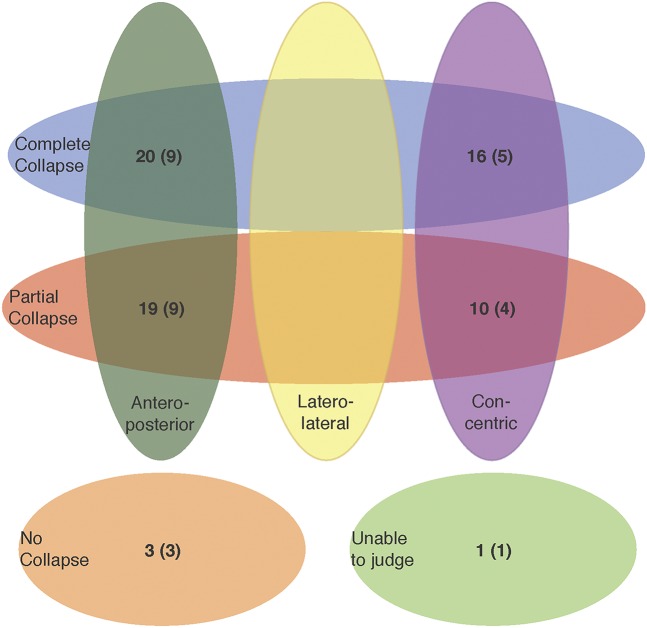
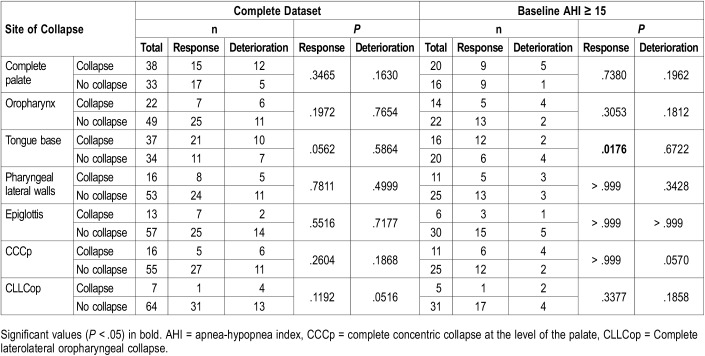
Palatal collapse (Figure 3 and Figure S1 in the supplemental material) was present in both anteroposterior (57%) and concentric (38%) patterns. Oropharyngeal and hypopharyngeal collapse only occurred in laterolateral configuration, and tongue base collapse only in anteroposterior configuration. Epiglottic collapse was present in both anteroposterior (92%) and concentric direction (8%). No significant difference in proportion was present between responders and nonresponders for any collapse site (Table 3 and Figure S2 in the supplemental material).
Figure 3. Distribution for degree and direction of collapse in patients with palatal collapse.
Number of responders shown in brackets. Three patients could not be classified in this Venn diagram as they showed doubtful complete concentric collapse at the level of the palate (CCCp) (2 complete collapse, of which 1 responder and 1 partial collapse, of which 1 responder). Venn diagrams for the other collapse sites are shown in the supplemental material (Figure S2).
Table 3.
Number of patients per collapse site and results of Fisher exact tests (2-sided).
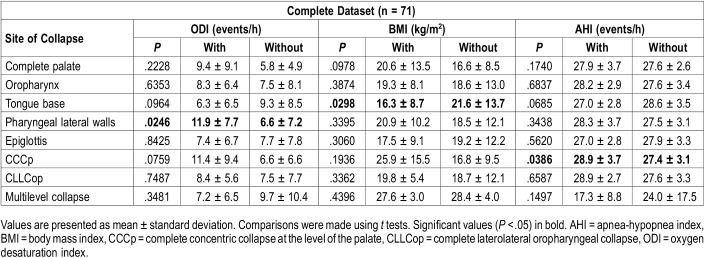
Correlation analyses between baseline AHI, BMI, and ODI and the collapse patterns showed that patients with tongue base collapse had significantly lower BMI. Pharyngeal lateral wall collapse was related to significantly higher ODI. CCCp was associated with significantly higher AHI (Table 4).
Table 4.
Comparing mean baseline parameters in patients with and without collapse at the different collapse sites.
Logistic Modeling
Next, each collapse site’s relation to response or deterioration was tested using logistic modeling. Partial and complete collapse were pooled: “collapse” at a given level was compared to “no collapse.” However, palatal collapse was highly prevalent (94.2%). Therefore, patients with complete palatal collapse (38/71, 54%) were compared to patients without (4/71, 6%) or with partial palatal collapse (29/71, 41%) (Figure 3). Apart from the five potential levels of upper airway collapse (palate, oropharynx, tongue base, pharyngeal lateral walls, and epiglottis), analyses were also performed for complete concentric collapse at the level of the palate (CCCp), related to UAS therapy response and CPAP pressure,16,21 and complete laterolateral oropharyngeal collapse (CLLCop), affecting CPAP pressure and OSA surgery outcome.21,22
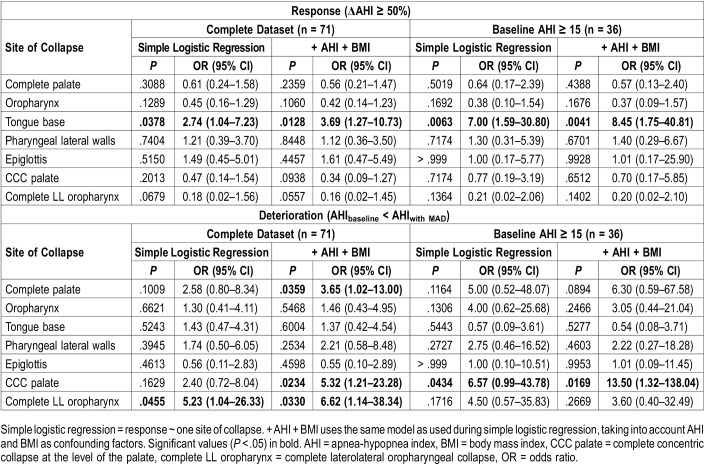
Simple logistic regression showed a significant relationship between response and tongue base collapse, increasing the odds for response with 2.74 (95% confidence interval [CI] 1.04–7.23; P = .0378). This significant relationship was preserved when corrected for AHI and BMI (P = .0128, odds ratio [OR] 3.69, 95% CI 1.27–10.73) (Table 5, Figure 4A). If corrected for baseline AHI and BMI, a PPV of 57.14% and NPV of 62.79% could be obtained with an area under the curve (AUC) of 0.68.
Table 5.
Logistic regression relating sites of collapse to response (top) and deterioration (bottom) in the complete dataset and in patients with baseline AHI ≥ 15 events/h.
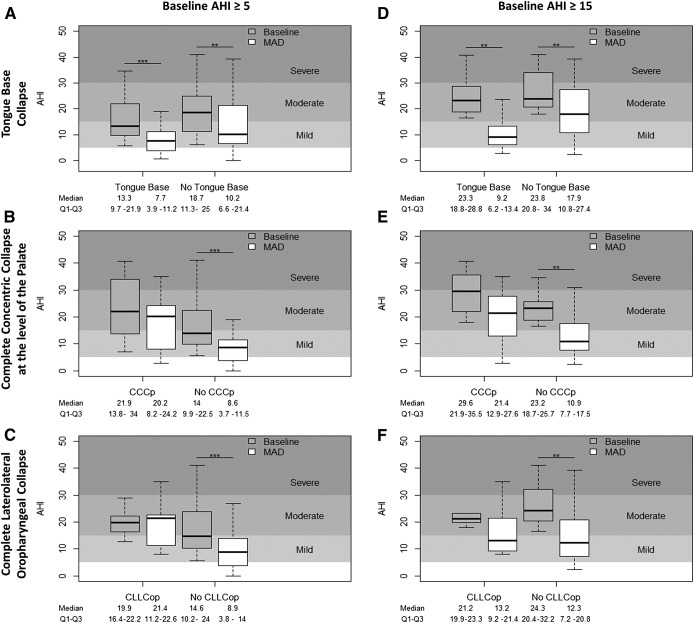
Figure 4. Change in AHI for each participant.
Change in AHI for each participant with (left) and without (right) tongue base collapse, complete concentric collapse at the level of the palate and complete laterolateral oropharyngeal collapse, taken into account all patients (left column) or only patients with baseline AHI ≥ 15 (right column). Outliers were omitted in this graphical representation. * P < .05, ** P < .01, *** P < .0001. AHI = apnea-hypopnea index, CCCp = complete concentric collapse at the level of the palate, CLLCop = complete laterolateral oropharyngeal collapse, MAD = mandibular advancement device.
Regarding deterioration, an identical analysis strategy was followed. Significant relationships were found for CLLCop using simple logistic regression, and for CCCp and CLLCop using logistic regression corrected for baseline AHI and BMI (Table 5). Concerning ORs, the strongest effect was present for CLLCop (6.62, 95% CI 1.14–38.34; P = .0330, PPV = 60%, NPV = 78.79%, AUC = 0.73), followed by CCCp (5.32, 95% CI 1.21-23.28; P = .0234, PPV = 60%, NPV = 76.12%, AUC = 0.74), resulting in a sixfold and fivefold increased likelihood for deterioration, respectively (Figure 4B and Figure 4C). A complete palatal collapse, regardless of collapse direction, resulted in a 3.65-fold increased probability for deterioration (95% CI 1.02–13.00; P = .0359, PPV = 66.67%, NPV = 80%, AUC = 0.73). If CCCp and CLLCop were combined in one model and corrected for AHI and BMI, a PPV of 60%, NPV of 78.79%, and AUC of 0.73 was obtained. The significant effects of complete palatal collapse (P = .0215, OR 5.58, 95% CI 1.08–28.85), CCCp (P = .0202, OR 6.13, 95% CI 1.32–28.45) and CLLCop (P = .0483, OR 6.25, 95% CI 1.08–36.00) were maintained using the more stringent deterioration definition of an increase in AHI ≥ 10%, corrected for baseline AHI and BMI.
If these results would have been used as exclusion parameters in our initial dataset with fixed protrusion, the 46% response rate would have increased to 57% (21/37) if only patients with tongue base collapse were included and even further increased to 60% (18/30) if also patients with CCCp and/or CLLCop were excluded. If patients with CCCp would have been excluded, the deterioration rate would have decreased from 24% (45% of nonresponders) to 20% (11/55, 39% [11/28] of nonresponders), and to 21% (11/52, 42% [11/26] of nonresponders), if patients with CCCp and/or CLLCop were excluded. Among the patients with CCCp (n = 16), a nonresponder rate of 69% (11/16) and a deterioration rate of 38% (6/16) was noted. Patients with CLLCop (n = 7), showed a nonresponder rate of 86% (6/7) and a deterioration rate of 57% (4/7).
Results for Patients With Moderate to Severe OSA (Baseline AHI ≥ 15 events/h)
All analyses were repeated for the moderate to severe OSA subgroup. In total, 36 of the 47 patients with moderate to severe OSA completed 3-month follow-up (Figure 1B). Eighteen patients (50%) were responders whereas 6 (17%; 33% of nonresponders) deteriorated. Applying the Sher criteria23 to the patients with a baseline AHI ≥ 20 events/h resulted in 12/26 patients (46%) who responded, whereas 14/26 patients (54%) did not respond.
The results obtained for patients with moderate to severe OSA were largely equivalent to those for all patients (Table 3 and Table 5). Fisher exact tests showed a significant difference in proportion between responders and nonresponders for patients with tongue base obstruction (Table 3). Simple logistic modeling (Table 5) showed a significant relation between tongue base collapse and response (P = .0063, OR 7.00, 95% CI 1.59–30.80) (Figure 4D). This relationship was sustained if corrected for AHI and BMI at baseline (P = .0041, OR 8.45, 95% CI 1.75–40.81, PPV = 81.25%, NPV = 75.00%, AUC = 0.82). The significant relationship between response and tongue base collapse was retained in four out of seven different response definitions. No further significant relationships were found for other collapse levels.
Regarding deterioration (Table 5), a significant relationship was found for CCCp using simple logistic modeling (P = .0434, OR 6.57, 95% CI 0.99–43.78) and if adjusted for baseline AHI and BMI (P = .0169, OR 13.50, 95% CI 1.32–138.04, PPV = 50.00%, NPV = 87.50%, AUC = 0.87) (Figure 4E). In this subgroup, no relationship was found between response or deterioration, and CLLCop (Table 5 and Figure 4F). This result was maintained using the more stringent definition of AHI increase ≥ 10% (P = .0472, OR 10.98, 95% CI 0.83–145.18, if adjusted for baseline AHI and BMI).
If these results would have been used as exclusion parameters in our initial dataset, the 50% response rate would have increased to 75% (12/16) if only patients with tongue base collapse were included, and further increased to 77% (10/13) if also patients with CCCp were excluded. The deterioration rate would decrease from 17% (33% of nonresponders) to 8% (2/25, 17% (2/12) of nonresponders) if patients with CCCp would have been excluded. Among our patients with CCCp (n = 11), a nonresponder rate of 55% (6/11) and deterioration rate of 36% (4/11) was noted. Patients with CLLCop (n = 5), showed a nonresponder rate of 80% (4/5) and deterioration rate of 40% (2/5).
DISCUSSION
The key finding of this study is that presence of tongue base collapse during baseline DISE examination is strongly correlated to favorable MAD response in patients with OSA. A second finding is that patients with complete concentric collapse at the level of the palate (CCCp) and/or complete laterolateral oropharyngeal collapse (CLLCop) during DISE tend to deteriorate under MAD treatment. Considering DISE findings are often correlated with baseline parameters24 and, as shown in Table 4, significant differences in baseline parameters were present in those with and without tongue base collapse, pharyngeal lateral wall collapse, and complete concentric collapse at the level of the palate (CCCp), the logistic models built in this study were adjusted for both baseline AHI and BMI. Because a significant correlation of 0.81 (P < .0001) was present between ODI and AHI, no additional correction was done for ODI. Despite this correction, the significant effects of tongue base collapse in favor, and, CCCp and CLLCop at disadvantage, were preserved for response and deterioration, respectively, clearly adding to the power of our findings. The results of this prospective study emphasize MAD treatment response in OSA patients is specifically related to the site, degree and pattern of upper airway collapse as assessed during DISE. Based on the results of this prospective trial, the authors introduce the concept of DISE phenotypic labeling, referring to the specific pharyngeal collapse during DISE that is a combination of a certain degree and pattern at a particular level of the upper airway. According to the current results, tongue base collapse is a beneficial DISE phenotype and both CCCp and CLLCop are adverse DISE phenotypes toward MAD treatment outcome.
Compared to CPAP, MAD treatment shows, on average, lower treatment efficacy in unselected patients. However, because of higher MAD adherence relative to CPAP, similar overall clinical mean disease alleviation can be obtained.4,25,26 Previous research suggested increased MAD efficacy can be realized by improved patient selection.7,27–29 The current findings suggest DISE as a clinical procedure for upfront patient selection toward MAD treatment, including patients with tongue base collapse for MAD treatment and counseling patients with CCCp and/or CLLCop of the probability toward MAD treatment failure.
In our study population, about half of the patients (52%; Figure S1) demonstrated tongue base collapse, whereas 23% showed CCCp and 10% CLLCop. If the current concept of beneficial and adverse DISE phenotypes would have been applied as inclusion and exclusion parameters for patients with moderate to severe OSA (AHI ≥ 15 events/h) in our study population, the response rate (ΔAHI ≥ 50%) would have increased from 50% to 77% if only patients with tongue base collapse without CCCp would have been included. An increase of 54% in response rate would have been obtained according to this conceptual approach of DISE phenotype-specific OSA management. If patients with CCCp were excluded, the deterioration rate under MAD treatment would decrease to 53%, from 17% to 8%. Furthermore, a PPV of 81.25%, NPV of 75% and AUC of 0.82 could be obtained for the logistic model explaining response including tongue base collapse and corrected for baseline AHI and BMI. A PPV of 50%, NPV of 87.5%, and AUC of 0.87 could be obtained for the model including CCCp, corrected for baseline AHI and BMI, explaining deterioration. These selection parameters would thus avoid patients being treated with MAD who tend to have lower likelihood for response and even high probability of deterioration.
Previous research using both awake endoscopy, DISE, and MRI, showed an increase in velopharyngeal cross-sectional area is associated with beneficial MAD treatment outcome.8,27,30–32 Regarding collapsibility, lower passive anatomical compromise predicts MAD treatment success,14 which might relate to the increased odds for deterioration we found in patients with CCCp and/or CLLCop.
DISE already showed its potential for upfront upper airway stimulation response prediction, in which CCCp was found to be a negative DISE predictor.16 In our study, a significant relationship was present between deterioration to MAD treatment and the presence of this CCCp DISE phenotype. Parallel to UAS findings, patients with CCCp as observed during DISE were no suitable candidates for MAD treatment. The finding that patients with OSA with anteroposterior tongue base collapse have significantly higher odds for being a responder under MAD treatment, might be explained by the longitudinal muscle direction at the level of the tongue base. UAS acts on these longitudinal muscles by increasing both the retrolingual and retropalatal region in an anteroposterior direction, suggesting mechanical palatoglossus coupling between these areas.9,10 As in our study tongue base collapse is found to be positively associated with MAD response, MAD and UAS treatment might act in a similar, longitudinal way, being optimal treatment options for patients with anteroposterior collapse patterns and suboptimal for nonanteroposterior collapse pattern resolution.
Increased BMI and AHI is associated with increased collapsibility of the upper airway.33 Furthermore, CCCp is associated with increased BMI and AHI as well.24 Therefore, based on the current result showing that CCCp is correlated with deterioration after MAD treatment, we hypothesize that deterioration to MAD treatment is correlated to high upper airway collapsibility and as such is associated with this OSA phenotype. Regarding CLLCop, we hypothesize that this is an anatomical phenotype, correlated to tonsil hypertrophy, which plays a role in MAD treatment response. Future research, aiming at these specific outcome variables is needed to address both hypotheses.
In the absence of a gold standard titration protocol for MAD treatment,34 the mandibular advancement was fixed at 75% of the maximal protrusion, whereas other studies19,27 performed a personalized titration to find optimal protrusion. Titration in our study population could therefore even have enhanced treatment response as in clinical practice in our hospital; most patients end up at their maximal comfortable protrusive position. However, the authors argue that the current approach was imperative to allow for a more objective and prospective comparison between baseline and MAD treatment outcomes.
Often, OSA study results are response definition dependent.19,20 Therefore, we assessed six additional response definitions. In four out of seven definitions, tongue base collapse significantly related to response. These results highlight our finding of tongue base collapse being a beneficial DISE phenotype for MAD treatment outcome. A more stringent deterioration definition (increase in AHI ≥ 10%) also yielded similar results.
During this study, multiple hypotheses were tested. However, we choose not to apply any correction as this study was intended as a first exploration of relating DISE phenotyping with MAD treatment outcome. The authors stress that future prospective studies with the current results as primary outcome are needed to further quantify and confirm the current results.
A strength of our study is the prospective study design: patients started MAD therapy and underwent a DISE evaluation. However, the results of the DISE evaluation were blinded for both the patient and the clinical multidisciplinary team throughout the study. Therefore, DISE outcomes had no effect on the offered MAD treatment.
In addition, regarding the prevalence of the different collapse sites (Figure S1 and Figure S2 in the supplemental material), our study results are in line with previous reports in literature.16,24,27,35 Palatal collapse is the most prevalent site (94%), followed by tongue base (52%), oropharyngeal (31%), hypopharyngeal (23%) and epiglottic (18%) collapse. Multilevel collapse was present in 75% of the study patients. In previous studies, the prevalence of CCCp ranged from 10% to 32% whereas complete oropharyngeal collapse occurred in 1% to 7%.24,35–37 In our study, 23% of the patients showed CCCp, whereas 10% showed CLLCop.
A potential drawback of DISE is its so-called subjective nature depending on experience and the used scoring system.24,38–40 In our research setting, this point of criticism on DISE was tackled to a large extent by using a consensus scoring relying on four ENT surgeons with broad experience in DISE. For each level, degree, and direction, a consensus was made, limiting possible intra-rater and interrater variability. In clinical practice, the authors would like to stress the importance to have an ENT surgeon experienced in DISE performing the DISE evaluation in order to reduce intra-rater and interrater variability.
Another possible limitation of DISE is its accessibility in clinical practice. DISE should be performed in an operating theater involving multiple health care professionals. Recent studies41–43 suggest the possibility of upper airway collapse site prediction during natural sleep using polysomnographic signals. If the predictive value of this technique can be confirmed, this may reduce the need for DISE and make the results of this study potentially generally applicable to other techniques allowing assessment of the upper airway dynamics in patients with OSA.
In conclusion, DISE is a promising tool for upfront MAD patient selection. We defined three DISE phenotypes: one beneficial DISE phenotype associated with MAD response: tongue base collapse, and two adverse DISE phenotypes: complete concentric collapse at the level of the palate (CCCp) and complete laterolateral oropharyngeal collapse (CLLCop). Patients with a tongue base collapse DISE phenotype showed significantly higher odds for being responders whereas patients with a CCCp and/or CLLCop DISE phenotype tended to show deterioration to MAD treatment. To the best of the authors’ knowledge, the association between the CLLCop DISE phenotype and MAD outcome is a new finding and not reported previously in the literature. Based on these results, the authors advocate the need for future prospective studies with the current results as primary outcome to further explore development and application of DISE phenotype-specific OSA management when prescribing non-CPAP therapies.
DISCLOSURE STATEMENT
This study was funded by a 3-year grant of the Flemish government agency for Innovation by Science and Technology (IWT-090864). SO, AEV, AVV, KW, MW, WAD, PHV report no competing interests. MD holds a Postdoctoral fellowship at Research Foundation Flanders (FWO)—12H4516N. EH reports grants from Philips outside the submitted work. JV reports grants from SomnoMed outside the submitted work. MJB reports grants from SomnoMed outside the submitted work and sits on the advisory boards of ResMed and SomnoMed. OMV reports grants from Philips, grants from Somnomed, personal fees from Somnomed, other from Inspire Medical Systems, personal fees from Inspire Medical Systems, other from Zephyr, other from NightBalance, other from Galvani, outside the submitted work. OV holds a Senior Clinical Investigator Fellowship from the Research Foundation Flanders (FWO)—2016-2021.
ACKNOWLEDGMENTS
The authors are grateful for the administrative and organizational support of the secretarial staff of the Special Dentistry Care and the Ear-Nose-Throat department at the Antwerp University Hospital. Furthermore, we thank the co-workers of the Sleep Laboratory of the Antwerp University Hospital for conducting the polysomnographic examination. Author contributions: conception and design of the work: OMV, MJB, PHV; data collection, analysis, and interpretation: SO, MD, AEV, AVV, KW, OMV, MJB; drafting the article: SO, MD, OMV, MJB; critical revision of the article: SO, MD, AEV, AVV, KW, EH, MW, JV, WAD, PHV, MJB, OMV; final approval of the version to be published: SO, MD, AEV, AVV, KW, EH, MW, JV, WAD, PHV, MJB, OMV.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- CCCp
complete concentric collapse at the level of the palate
- CLLCop
complete laterolateral collapse at the level of the oropharynx
- CPAP
continuous positive airway pressure
- DISE
drug-induced sleep endoscopy
- MAD
mandibular advancement device
- ODI
oxygen desaturation index
- OSA
obstructive sleep apnea
- PSG
polysomnography
REFERENCES
- 1.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eckert DJ, Malhotra A. Pathophysiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):144–153. doi: 10.1513/pats.200707-114MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1(8225):862–865. doi: 10.1016/s0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- 4.Phillips CL, Grunstein RR, Darendeliler MA, et al. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2013;187(8):879–887. doi: 10.1164/rccm.201212-2223OC. [DOI] [PubMed] [Google Scholar]
- 5.Epstein LJ, Kristo D, Strollo PJ, Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263–276. [PMC free article] [PubMed] [Google Scholar]
- 6.Randerath WJ, Verbraecken J, Andreas S, et al. Non-CPAP therapies in obstructive sleep apnoea. Eur Respir J. 2011;37(5):1000–1028. doi: 10.1183/09031936.00099710. [DOI] [PubMed] [Google Scholar]
- 7.Marklund M, Verbraecken J, Randerath W. Non-CPAP therapies in obstructive sleep apnoea: mandibular advancement device therapy. Eur Respir J. 2012;39(5):1241–1247. doi: 10.1183/09031936.00144711. [DOI] [PubMed] [Google Scholar]
- 8.Chan AS, Sutherland K, Schwab RJ, et al. The effect of mandibular advancement on upper airway structure in obstructive sleep apnoea. Thorax. 2010;65(8):726–732. doi: 10.1136/thx.2009.131094. [DOI] [PubMed] [Google Scholar]
- 9.Heiser C, Edenharter G, Bas M, Wirth M, Hofauer B. Palatoglossus coupling in selective upper airway stimulation. Laryngoscope. 2017;127(10):E378–E383. doi: 10.1002/lary.26487. [DOI] [PubMed] [Google Scholar]
- 10.Safiruddin F, Vanderveken OM, de Vries N, et al. Effect of upper-airway stimulation for obstructive sleep apnoea on airway dimensions. Eur Respir J. 2015;45(1):129–138. doi: 10.1183/09031936.00059414. [DOI] [PubMed] [Google Scholar]
- 11.Vanderveken OM, Devolder A, Marklund M, et al. Comparison of a custom-made and a thermoplastic oral appliance for the treatment of mild sleep apnea. Am J Respir Crit Care Med. 2008;178(2):197–202. doi: 10.1164/rccm.200701-114OC. [DOI] [PubMed] [Google Scholar]
- 12.Johal A, Haria P, Manek S, Joury E, Riha R. Ready-made versus custom-made mandibular repositioning devices in sleep apnea: a randomized clinical trial. J Clin Sleep Med. 2017;13(2):175–182. doi: 10.5664/jcsm.6440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sutherland K, Vanderveken OM, Tsuda H, et al. Oral appliance treatment for obstructive sleep apnea: an update. J Clin Sleep Med. 2014;10(2):215–227. doi: 10.5664/jcsm.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards BA, Andara C, Landry S, et al. Upper-airway collapsibility and loop gain predict the response to oral appliance therapy in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2016;194(11):1413–1422. doi: 10.1164/rccm.201601-0099OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Vito A, Carrasco Llatas M, Vanni A, et al. European position paper on drug-induced sedation endoscopy (DISE) Sleep Breath. 2014;18(3):453–465. doi: 10.1007/s11325-014-0989-6. [DOI] [PubMed] [Google Scholar]
- 16.Vanderveken OM, Maurer JT, Hohenhorst W, et al. Evaluation of drug-induced sleep endoscopy as a patient selection tool for implanted upper airway stimulation for obstructive sleep apnea. J Clin Sleep Med. 2013;9(5):433–438. doi: 10.5664/jcsm.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verbruggen AER, Vroegop AVMT, Dieltjens M, et al. Predicting therapeutic outcome of mandibular advancement device treatment in obstructive sleep apnoea (PROMAD): study design and baseline characteristics. J Dent Sleep Med. 2016;3(4):119–138. [Google Scholar]
- 18.Dieltjens M, Vanderveken OM, Hamans E, et al. Treatment of obstructive sleep apnea using a custom-made titratable duobloc oral appliance: a prospective clinical study. Sleep Breath. 2013;17(2):565–572. doi: 10.1007/s11325-012-0721-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukuda T, Tsuiki S, Kobayashi M, Nakayama H, Inoue Y. Selection of response criteria affects the success rate of oral appliance treatment for obstructive sleep apnea. Sleep Med. 2014;15(3):367–370. doi: 10.1016/j.sleep.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Sutherland K, Phillips CL, Davies A, et al. CPAP pressure for prediction of oral appliance treatment response in obstructive sleep apnea. J Clin Sleep Med. 2014;10(9):943–949. doi: 10.5664/jcsm.4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lan MC, Hsu YB, Lan MY, et al. The predictive value of drug-induced sleep endoscopy for CPAP titration in OSA patients. Sleep Breath. 2018;22(4):949–954. doi: 10.1007/s11325-017-1600-8. [DOI] [PubMed] [Google Scholar]
- 22.Liu SY, Huon LK, Powell NB, et al. Lateral pharyngeal wall tension after maxillomandibular advancement for obstructive sleep apnea is a marker for surgical success: observations from drug-induced sleep endoscopy. J Oral Maxillofac Surg. 2015;73(8):1575–1582. doi: 10.1016/j.joms.2015.01.028. [DOI] [PubMed] [Google Scholar]
- 23.Sher AE, Schechtman KB, Piccirillo JF. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep. 1996;19(2):156–177. doi: 10.1093/sleep/19.2.156. [DOI] [PubMed] [Google Scholar]
- 24.Vroegop AV, Vanderveken OM, Boudewyns AN, et al. Drug-induced sleep endoscopy in sleep-disordered breathing: report on 1,249 cases. Laryngoscope. 2014;124(3):797–802. doi: 10.1002/lary.24479. [DOI] [PubMed] [Google Scholar]
- 25.Gagnadoux F, Fleury B, Vielle B, et al. Titrated mandibular advancement versus positive airway pressure for sleep apnoea. Eur Respir J. 2009;34(4):914–920. doi: 10.1183/09031936.00148208. [DOI] [PubMed] [Google Scholar]
- 26.Vanderveken OM, Dieltjens M, Wouters K, De Backer WA, Van de Heyning PH, Braem MJ. Objective measurement of compliance during oral appliance therapy for sleep-disordered breathing. Thorax. 2013;68(1):91–96. doi: 10.1136/thoraxjnl-2012-201900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vroegop AV, Vanderveken OM, Dieltjens M, et al. Sleep endoscopy with simulation bite for prediction of oral appliance treatment outcome. J Sleep Res. 2013;22(3):348–355. doi: 10.1111/jsr.12008. [DOI] [PubMed] [Google Scholar]
- 28.Ng AT, Qian J, Cistulli PA. Oropharyngeal collapse predicts treatment response with oral appliance therapy in obstructive sleep apnea. Sleep. 2006;29:666–671. [PubMed] [Google Scholar]
- 29.Dort LC, Hadjuk E, Remmers JE. Mandibular advancement and obstructive sleep apnoea: A method for determining effective mandibular protrusion. Eur Respir J. 2006;27(5):1003–1009. doi: 10.1183/09031936.06.00077804. [DOI] [PubMed] [Google Scholar]
- 30.Chan AS, Lee RW, Srinivasan VK, Darendeliler MA, Grunstein RR, Cistulli PA. Nasopharyngoscopic evaluation of oral appliance therapy for obstructive sleep apnoea. Eur Respir J. 2010;35(4):836–842. doi: 10.1183/09031936.00077409. [DOI] [PubMed] [Google Scholar]
- 31.Okuno K, Sasao Y, Nohara K, et al. Endoscopy evaluation to predict oral appliance outcomes in obstructive sleep apnoea. Eur Respir J. 2016;47(5):1410–1419. doi: 10.1183/13993003.01088-2015. [DOI] [PubMed] [Google Scholar]
- 32.Vanderveken OM, Van de Heyring PH, Braem MJ. Nasopharyngoscopy during wakefulness for predicting treatment outcome of OSA with mandibular advancement splints. Breathe (Sheff) 2010;7(1):94–95. [Google Scholar]
- 33.Isono S. Obesity and obstructive sleep apnoea: mechanisms for increased collapsibility of the passive pharyngeal airway. Respirology. 2012;17(1):32–42. doi: 10.1111/j.1440-1843.2011.02093.x. [DOI] [PubMed] [Google Scholar]
- 34.Dieltjens M, Vanderveken OM, Heyning PH, Braem MJ. Current opinions and clinical practice in the titration of oral appliances in the treatment of sleep-disordered breathing. Sleep Med Rev. 2012;16(2):177–185. doi: 10.1016/j.smrv.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Ong AA, Murphey AW, Nguyen SA, et al. Efficacy of upper airway stimulation on collapse patterns observed during drug-induced sedation endoscopy. Otolaryngol Head Neck Surg. 2016;154(5):970–977. doi: 10.1177/0194599816636835. [DOI] [PubMed] [Google Scholar]
- 36.Ravesloot MJ, de Vries N. One hundred consecutive patients undergoing drug-induced sleep endoscopy: results and evaluation. Laryngoscope. 2011;121(12):2710–2716. doi: 10.1002/lary.22369. [DOI] [PubMed] [Google Scholar]
- 37.Steffen A, Frenzel H, Wollenberg B, Konig IR. Patient selection for upper airway stimulation: is concentric collapse in sleep endoscopy predictable? Sleep Breath. 2015;19(4):1373–1376. doi: 10.1007/s11325-015-1277-9. [DOI] [PubMed] [Google Scholar]
- 38.Kezirian EJ, White DP, Malhotra A, Ma W, McCulloch CE, Goldberg AN. Interrater reliability of drug-induced sleep endoscopy. Arch Otolaryngol Head Neck Surg. 2010;136(4):393–397. doi: 10.1001/archoto.2010.26. [DOI] [PubMed] [Google Scholar]
- 39.Vroegop AV, Vanderveken OM, Wouters K, et al. Observer variation in drug-induced sleep endoscopy: experienced versus nonexperienced ear, nose, and throat surgeons. Sleep. 2013;36(6):947–953. doi: 10.5665/sleep.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.da Cunha Viana A, Jr, Mendes DL, de Andrade Lemes LN, Thuler LC, Neves DD, de Araujo-Melo MH. Drug-induced sleep endoscopy in the obstructive sleep apnea: comparison between NOHL and VOTE classifications. Eur Arch Otorhinolaryngol. 2016;274(2):627–635. doi: 10.1007/s00405-016-4081-7. [DOI] [PubMed] [Google Scholar]
- 41.Azarbarzin A, Marques M, Sands SA, et al. Predicting epiglottic collapse in patients with obstructive sleep apnoea. Eur Respir J. 2017;50(3):1700345. doi: 10.1183/13993003.00345-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Azarbarzin A, Sands SA, Marques M, et al. Palatal prolapse as a signature of expiratory flow limitation and inspiratory palatal collapse in patients with obstructive sleep apnoea. Eur Respir J. 2018;51(2):1701419. doi: 10.1183/13993003.01419-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Genta PR, Sands SA, Butler JP, et al. Airflow shape is associated with the pharyngeal structure causing OSA. Chest. 2017;152(3):537–546. doi: 10.1016/j.chest.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.American Academy of Sleep Medicine . International Classification of Sleep Disorders: Diagnostic and Coding Manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.