Abstract
Toca 511 (vocimagene amiretrorepvec) is an investigational non-lytic, retroviral replicating vector (RRV) that delivers a yeast cytosine deaminase, which converts subsequently administered courses of investigational prodrug Toca FC (extended-release 5-fluorocytosine,) into the antimetabolite 5-fluorouracil. Forty-five subjects with recurrent or progressive high grade glioma were treated. The endpoints of this phase 1, open-label, ascending dose, multicenter trial included safety, efficacy, and molecular profiling; survival was compared to a matching subgroup from an external control. Overall survival for recurrent high grade glioma was 13.6 months (95% CI 10.8, 20.0) and was statistically improved relative to an external control (HR 0.45, p=0.003). Tumor samples from subjects surviving greater than 52 weeks after Toca 511 delivery disproportionately displayed a survival-related mRNA expression signature, identifying a potential molecular signature that may correlate with treatment-related survival rather than being prognostic. Toca 511 and Toca FC show excellent tolerability with RRV persisting in the tumor and RRV control systemically. The favorable assessment of Toca 511 and Toca FC support confirmation in an ongoing randomized phase 2/3 trial (NCT02414165).
One sentence Summary
Treatment of recurrent high grade glioma with Toca 511 (vocimagene amiretrorepvec) and Toca FC (extended-release 5-fluorocytosine) showed promising survival, excellent tolerability and a molecular signature that may correlate with treatment-related survival.
Introduction
High grade gliomas (HGG), including glioblastomas and anaplastic astrocytomas, are the most aggressive malignant brain tumors (1). Treatments for recurrent glioblastoma are associated with an overall survival (OS) of 7.1 to 9.8 months (2–6). Patients generally suffer considerable morbidity from the underlying disease, including seizures, peritumoral edema, venous thromboembolism, fatigue, cognitive dysfunction and depression. There are few therapeutic options for recurrent HGG, and the improvement in care over the last several decades has lagged behind nearly all other malignant tumors.
Toca 511 (vocimagene amiretrorepvec) is an investigational non-lytic, retroviral replicating vector (RRV) (7). As a RRV based on a gamma retrovirus with an amphotropic envelope, Toca 511 infects human cells with selectivity for cancer cells because genome integration is dependent on cell division and viral replication is normally restricted by innate and adaptive immune responses that are defective in malignant tissues (8–10). Toca 511 spreads through cancer cells and stably delivers a codon-optimized yeast cytosine deaminase (CD) gene whose protein product converts courses of the prodrug Toca FC (an investigational extended-release version of 5-fluorocytosine, 5-FC) into 5-fluorouracil (5-FU). 5-FU is a canonical chemotherapeutic that has a poor therapeutic index for the treatment of brain tumors unlike 5-FC, which is commonly used to treat fungal infections of the brain and more efficiently crosses the blood brain barrier (7, 11, 12). In addition to direct killing of cells by production of intracellular 5-FU, Toca 511 and Toca FC therapy also operates through metabolic cooperation, in which non-infected but dividing neighboring cells are killed through the transfer of the antimetabolite, 5-FU, from nearby CD-expressing cells. This type of bystander effect has been associated with CD and 5-FC because 5-FU is a small molecule capable of diffusion through cellular membranes (13, 14). Further, 5-FU has direct cytotoxic effects on myeloid derived suppressor cells located in glioblastoma tumors (15). As 5-FU is generated directly within infected tumors and its half-life is quite short, adverse effects of systemic chemotherapy, such as myelotoxicity, are avoided. This also enables the immune system to remain intact, preserving the capacity to develop anti-tumor immune responses. In in vivo studies, Toca 511 and 5-FC treatment stimulates a local and systemic immune response against the tumor (8, 16).
This phase 1 trial (NCT01470794) includes subjects with HGG who have recurred after treatment with at least subtotal resection, postoperative radiation and temozolomide. This trial evaluated combination therapy of surgical resection followed by ascending doses of Toca 511 administered under direct visualization by multiple injections using a blunt-tipped needle into the walls of the resection cavity. This method of administration has been utilized in prior gene transfer studies and has generally been well tolerated (17). Approximately 6 weeks after Toca 511 injection and consequent viral replication, just prior to Toca FC administration, subjects underwent evaluation, including radiological assessment using MRI and neurological exam. Subjects were subsequently treated with Toca FC administered for 7 days every 4–8 weeks in repeat cycles until radiological tumor progression or clinical progression. Some subjects received Toca FC beyond tumor progression. Here we report the results of the phase 1 study including safety, OS, objective response rate by independent radiology review, progression-free survival (PFS), and biomarker identification. To provide context to the results observed, the OS and safety profile of subjects with glioblastoma in first or second recurrence who were treated with Toca 511 and Toca FC were compared to an external control of matched subjects who received standard therapy with lomustine (6).
Results
Subjects
Between February 2012 and May 2015 across seven medical centers, 45 subjects were enrolled and treated with Toca 511. As of September 18, 2015, 43 subjects, considered the efficacy evaluable population, received at least one planned course of Toca FC (Fig. S1 and Table S1). The majority of subjects were diagnosed with glioblastoma (82.2%). Their median age was 56 years, with first (51.1%), second (22.3%), or >2 (26.6%) recurrences and a Karnofsky Performance Status (KPS) of 70–80 (24.5%) or 90–100 (75.5%). All subjects had prior radiotherapy and chemotherapy with temozolomide (Table S2). The majority of these recurrent high grade gliomas were located in the cerebral hemispheres, including the frontal, temporal, parietal or occipital lobes. While not confirmed by a comparison of the pre- and immediate post-operative MRI scans, the estimated percentage of tumor resection in the majority of cases was 80–100%.
Subjects were treated with Toca 511 from 1.4 × 107 to 4.8 × 109 Transducing Units (TU) and with Toca FC from 130 mg/kg/day to 220 mg/kg/day (Table S3); generating concentrations known to be effective in cultured cells and in vivo (8, 18).
Efficacy
Treatment with Toca 511 led to viral transduction of tumor cells as demonstrated by detection of Toca 511 DNA and expression of Toca 511 RNA and CD protein in resected recurred tumor samples after Toca 511 delivery and several cycles of Toca FC (Table S4 and Fig. S2).
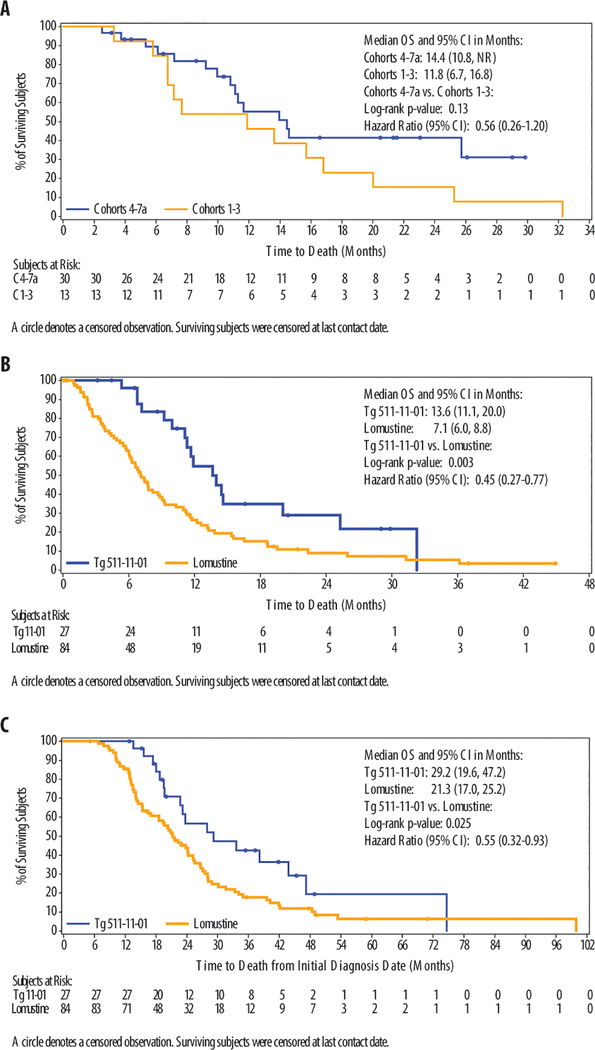
In the efficacy evaluable population, OS for recurrent HGG subjects (n=43) was 13.6 months (95% confidence interval (CI) 10.8, 20.0) and for HGG subjects at 1st or 2nd recurrence was 14.4 months (95% CI 11.3, 32.3) (Table 1). OS for glioblastoma subjects was 11.6 months (95% CI 9.2, 14.6) and for glioblastoma subjects at 1st or 2nd recurrence was 13.6 months (95% CI 11.1, 20.0). In the efficacy evaluable population, landmark survival data included OS6 (87.9%), OS9 (72.4%), OS12 (52.5%), OS24 (29.1%). In an analysis comparing the higher dose cohorts (Cohorts 4–7a) with the lower dose cohorts (Cohorts 1–3), there was a trend for dose response in survival (hazard ratio (HR) 0.56; 95% CI 0.26, 1.20) with median survival of 14.4 months (95% CI 10.8, not reached (NR)) for the higher doses versus the lower dose cohort with a median survival of 11.8 months (95% CI 6.7, 16.8) (Fig. 1A). Progression free survival was 3.2 months (95% CI 3.0, 3.4) and progression-free survival at 6 months was 16.3%.
Table 1.
Toca 511 and Toca FC Overall Survival
| Population | N | Median Survival, 95% CI (Months) |
|---|---|---|
| Efficacy evaluable – HGG | 43 | 13.6 (10.8, 20.0) |
| Efficacy evaluable – HGG, 1st and 2nd recurrence | 32 | 14.4 (11.3, 32.3) |
| Glioblastoma efficacy evaluable | 35 | 11.6 (9.2, 14.6) |
| Glioblastoma efficacy evaluable – 1st and 2nd recurrence | 27 | 13.6 (11.1, 20.0) |
| Landmark OS for Efficacy Evaluable – HGG | 43 | K-M Survival Rate |
| OS6 | 87.9% | |
| OS9 | 72.4% | |
| OS12 | 52.5% | |
| OS24 | 31.6% |
Fig. 1. Overall Survival and Comparison of Toca 511 and Toca FC to Lomustine External Control.
(A) The overall survival Kaplan-Meier plot of subjects who have received higher vs. lower doses Toca 511 and Toca FC. (B) The overall survival Kaplan-Meier plot of subjects with glioblastoma at first or second recurrence who received Toca 511 and Toca FC vs. the lomustine external control is shown. (C) The overall survival Kaplan-Meier plot from initial diagnosis of subjects with glioblastoma at first or second recurrence treated with Toca 511 and Toca FC vs. lomustine external control is shown.
Evaluating tumor response in the post-operative setting requires careful analysis as subjects may have evaluable but not measurable disease. Based on the independent radiology review, best overall response included complete response (CR) (4.7%, in two subjects with anaplastic astrocytoma), partial response (PR) (4.7%, in two subjects with glioblastoma), an objective response rate of 9.3%, stable disease (SD) (18.6%), and a clinical benefit rate (CR+PR+SD) of 27.9% (Table S5). This objective response rate (13.3%), stable disease (23.3%)ORR and clinical benefit rate (36.7%) were seen in the higher dose cohorts. Two subjects with anaplastic astrocytoma are reported by independent radiology review to have a complete response with a duration of response of more than one year. Concerted efforts were made to approach 6 sponsors of randomized phase 2 and 3 trials, including trials with surgical resection of recurrent glioblastoma. One sponsor of a phase 3 trial agreed to share data from subjects in the control arm who were treated with lomustine. Efficacy evaluable subjects with glioblastoma at first and second recurrence were compared to the external control lomustine-treated subjects who met eligibility criteria of a phase 3 trial, potentially identifying a less ill population than typical historical controls (Table 2). Both the study subjects and the external control lomustine studies were fairly contemporaneous (study subjects: 2012–2015; lomustine: 2006–2010).
Table 2.
Demographics GBM at First or Second Recurrence: Toca 511 and Toca FC vs. Lomustine External Control
| Treatment Group | Toca 511&Toca FC (N=27) |
Lomustine (N=84) |
|---|---|---|
| Characteristics | n (%) | n (%) |
| Sex | ||
| Female | 4 (14.8) | 33 (39.3) |
| Male | 23 (85.2) | 51 (60.7) |
| Age, years | ||
| Median | 61 | 54 |
| Range | 41–71 | 18–75 |
| Age, years | ||
| < 50 | 5 (18.5) | 27 (32.1) |
| ≥ 50 | 22 (81.5) | 57 (67.9) |
| Karnofsky performance status | ||
| 70–80 | 7 (25.9) | 41 (48.8) |
| 90–100 | 20 (74.1) | 43 (51.2) |
| Time from diagnosis (months) | ||
| Median | 11.6 | 11.5 |
| Range | 5.1–49.4 | 4.4–92.1 |
| Recurrence | ||
| First | 19 (70.4) | 65 (77.4) |
| Second | 8 (29.6) | 19 (22.6) |
| Baseline Steroid Administration | ||
| Yes | 23 (85.2) | 46 (54.8) |
| No | 4 (14.8) | 38 (45.2) |
Demographics of the study subjects with glioblastoma at first and second recurrence and lomustine control were comparable, although data were not available to match the subjects for enhancing tumor volume at the start of treatment with lomustine. The lomustine control had a slightly younger population, more subjects in first recurrence, and fewer subjects requiring corticosteroids at baseline while the Toca 511 and Toca FC trial had a higher percentage of subjects with a KPS of 90–100. Median OS for study subjects was 13.6 months (95% CI 11.1, 20) compared to 7.1 months (95% CI 6.01, 8.80) for the lomustine control. The OS hazard ratio was 0.45 (95% CI 0.27, 0.77; p=0.003). The separation of the curves occurred initially with OS6 of 96.0% vs. 61.8% (p<0.001) and continued through 30 months (Fig. 1B; Table 3). Analysis of survival comparing time from initial diagnosis shows a median OS for study subjects at 29.2 months (95% CI 19.6, 47.2) compared to 21.3 months (95% CI 17.0, 25.2) for lomustine control. The OS hazard ratio was 0.55 (95% CI 0.32, 0.93; p=0.025) (Fig. 1C). A Forest plot for subgroups showed improved survival for study subjects in all subgroups (Fig. S3).
Table 3.
Landmark Overall Survival GBM at First or Second Recurrence Toca 511 and Toca FC vs. Lomustine External Control
| Treatment Group | Toca 511&Toca FC (N=27) |
Lomustine (N=84) |
p-value |
|---|---|---|---|
| Landmark OS Rate | |||
| OS6 | 96.0% | 61.8% | <0.001 |
| OS9 | 83.5% | 38.4% | <0.001 |
| OS12 | 54.8% | 26.4% | 0.017 |
| OS24 | 29.1% | 9.1% | 0.065 |
During the trial, increases were observed in total circulating CD4-positive T cell counts over the course of treatment (Fig. S4). The increase in CD4-positive T cells greater than 21 weeks after delivery of Toca 511 was significant (p= 0.019) compared to the start of therapy using Wilcoxon signed rank test.
Adverse events
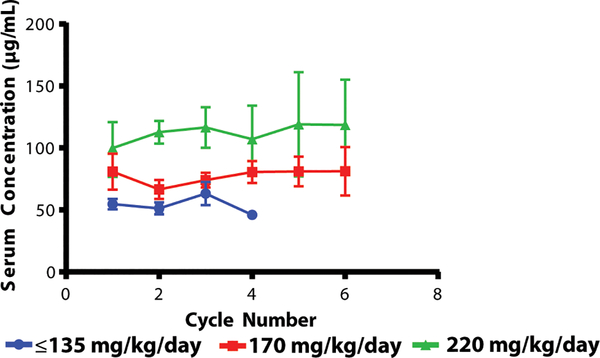
The median duration of treatment with Toca FC was 2 cycles (range of 1 to 7 cycles). Approximately 35% of the subjects on this trial received Toca FC on a continuation protocol of cycles of 7 days every 6 weeks (range 1 to 14 cycles). Toca FC serum concentrations increased in a dose dependent fashion (Fig. 2). Toca 511 and Toca FC treatment led to few related adverse events and serious adverse events (Table S6). Two dose limiting toxicities were observed: one in Cohort 3 of Grade 3 asthenia, which was possibly related to Toca 511, and one in Cohort 5a of Grade 3 normal pressure hydrocephalus, which was considered unrelated. A maximum tolerated dose was not reached. There were no treatment related deaths. Additionally, safety analyses were conducted comparing adverse event profiles in glioblastoma study subjects in the first and second recurrence subgroup to the lomustine external control. Subjects treated with Toca 511 and Toca FC had fewer related treatment-emergent Grade ≥ 3 adverse events (3.7% vs 36.9%) (Table 4). There were far fewer Grade ≥ 3 adverse events and a virtual absence of hematologic toxicity for study subjects relative to the lomustine control where Grade ≥ 3 thrombocytopenia occurred in 23.8% of patients (Table S7). Given that Toca 511 is surgically delivered, the treatment-emergent adverse events regardless of attribution were also reported, including Grade 2 incision site pain (25.9%) and procedural pain (18.5%) (Table S8). These results demonstrate a more favorable safety profile with fewer severe toxicities for Toca 511 and Toca FC compared to lomustine.
Fig. 2.
Toca FC Concentrations
The Toca FC serum concentrations (μg/mL) over time at increasing Toca FC doses (error bars are standard error). Cycles are approximately 1 week long and 4–8 weeks apart; measurements were normally on day 4 or 5 of the cycle.
Table 4.
Summary of adverse and serious adverse events
| All Grades | Grade ≥ 3 | |||
|---|---|---|---|---|
| Toca 511&Toca FC N=27 n (%) |
Lomustine N=84 n (%) |
Toca 511&Toca FC N=27 n (%) |
Lomustine N=84 n (%) |
|
| Any Treatment-Emergent Adverse Event | 27 (100.0) | 70 (83.3) | 17 (63.0) | 45 (53.6) |
| Related Treatment-Emergent Adverse Event | 11 (40.7) | 52 (61.9) | 1 (3.7) | 31 (36.9) |
| Leading to Study discontinuation Treatment-Emergent Adverse Event | 0 | 4 (4.8) | 0 | 4 (4.8) |
| Treatment-Emergent Serious Adverse Event | 9 (33.3) | 24 (28.6) | 7 (25.9) | 21 (25.0) |
| Related Treatment-Emergent Serious Adverse Event | 2 (7.4) | 6 (7.1) | 1 (3.7) | 5 (6.0) |
To date, there has been no persistent viremia observed; Toca 511 virus in blood appears to be well controlled and cleared quickly (Fig. S5).
mRNA expression profiles associated with survival after Toca 511 and Toca FC therapy
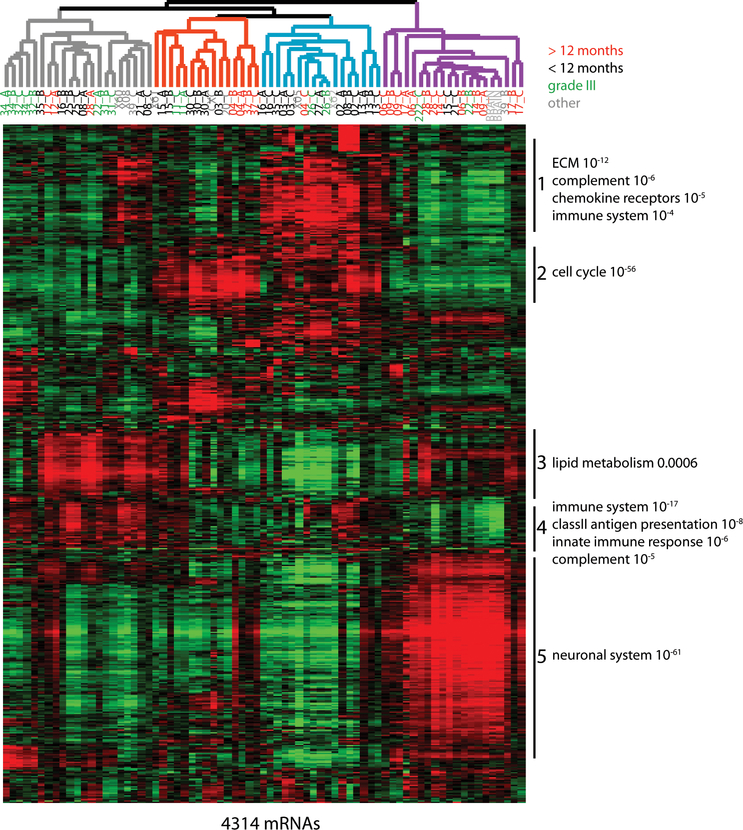
HGG is molecularly heterogeneous, with several subtypes classically defined by histology and more recently through mRNA expression and genomic mutation profiles (19). Variability in the underlying tumor molecular features leading to differences in virus-host interactions, 5-FU-induced cell death, and immune activation are expected to contribute to the differential benefit derived from Toca 511 and Toca FC, as measured by OS. Therefore, we systematically profiled tumor RNA expression from frozen tissue biopsies, taken at the time of tumor resection and immediately prior to Toca 511 administration, by next-generation sequencing. Available RNA from two or three spatially-distinct pieces was isolated from biopsies in order to gauge intra-tumor heterogeneity. A total of 64 available tumor samples were tested from 26 efficacy evaluable subjects. In order to gauge variation in mRNA expression across tumors, the 4314 mRNAs with the greatest variation in expression (standard deviation > 0.8) were identified and their relative expression was used as a metric of similarity for unsupervised hierarchical clustering (Fig. 3). In this analysis, subject samples clustered into four groups (color-coded) based on expression of functionally related sets of mRNAs (sets 1–5). As expected, the majority of grade III samples clustered together (grey). In the purple-coded group, 76% of tumor samples (13/17) came from five of the eight glioblastoma subjects who lived greater than one year after Toca 511 and Toca FC therapy. Tumor samples preferentially expressed mRNAs encoding proteins involved in neuronal functions, which we term survival-related neuronal subtype (SRNS).
Fig. 3. Tumor mRNA expression profiles correlate with survival.
Unsupervised hierarchical cluster of mRNA expression across sixty-four efficacy evaluable study tumor samples from twenty-six biopsies. The heatmap includes the 4313 mRNAs with the greatest variation in expression (Standard deviation > 0.8) with red bars representing increased and green bars decreased gene expression compared to normal human brain. Samples segregated into four main groups, which are color-coded in the dendrogram. Sample numbers subjects with glioblastoma that survived greater than 12 months after Toca 511 delivery are red, from subjects with glioblastoma that survived for less than 12 months are black, from subjects with grade III tumors are green, and other sample numbers are grey. Many of these 4313 mRNAs fell into one of five distinct clusters, characterized by common functional themes. Functional themes associated with the proteins encoded by mRNAs in specific clusters were identified using Reactome gene sets obtained from the Molecular Signatures Database. Examples of gene sets whose members are overrepresented in specific clusters are listed to the right of the heatmap along with associated p-values (hypergeometric distribution function).
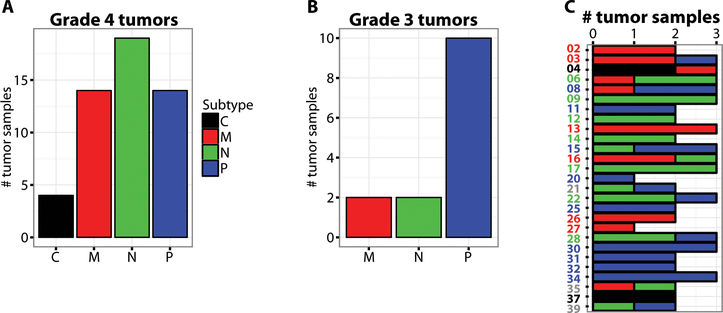
Previous studies by The Cancer Genome Atlas (TCGA) consortium have shown that HGG RNA expression profiles from newly diagnosed tumors reproducibly segregate samples into four molecular classes, coinciding with specific genomic alterations and cell-type of origin: “classical (C)” –astrocytes, “mesenchymal (M)” – microglia, “proneural (P) ” – oligodendrocytes and “neural (N)” – neuron-like (19, 20). To determine if this classification was applicable to recurrent HGG, for which there is a paucity of published genomic datasets, study samples were hierarchically clustered using a molecular classification gene set provided by TCGA consortium for newly diagnosed HGG (Fig. S6). Samples clustered into the four groups, coinciding with the defined molecular classes; neural (n=19), mesenchymal (n=14), proneural (n=14), and classical (n=4) (Fig. 4A). Most grade III samples were proneural (n=10/14) consistent with published results on newly diagnosed tumors (Fig. 4B) (21). Samples showed differing ranges of intra-tumor heterogeneity (Fig. S7) (22), which was not attributable to RNA quality or sequencing coverage (Fig. S8). While in 23 of 26 efficacy evaluable subjects a majority of samples corresponded to a specific subtype, 8 of these 23 subjects displayed multiple subtypes within their tumor (Fig. 4C), underscoring the heterogeneity maintained in recurrent HGG. A multivariate Cox regression analysis showed that the hazard ratio of death of the neural signature is significantly reduced compared to other subtypes (HR=0.11; p=0.01) after controlling for number of recurrences and HGG grade (Table S9). However, the TCGA neural signature is not a prognostic marker for survival in newly diagnosed HGG (Fig. S9).
Fig. 4. Molecular classification of tumor samples from study subjects based on mRNA expression.
(A) Bar plot representation of the number of glioblastoma samples in each molecular subtype: Classical (C) is black, Mesenchymal (M) is red, Neural (N) is green, Proneural (P) is blue. (B) Same as in (A), except for grade III samples. (C) Bar plot representation of molecular subtype for each subject, with the consensus subtype indicated by the color of subject identifier. Grey indicates no consensus was reached, i.e., the two samples profiled had different subtypes.
The SRNS identified in recurrent glioblastoma tumors via unsupervised hierarchical clustering (Fig. 3) bears functional similarities to the TCGA neural subtype identified in newly diagnosed HGG tumors. To more precisely classify subjects via the SRNS and its relationship to the neural subtype signature, samples were hierarchically clustered based on the expression of the 890 mRNAs in cluster 5, from Figure 3 (Fig. S10A). Tumors from subjects who survived at least one year post Toca 511 treatment disproportionately displayed the SRNS (19/21) (Fig. S10B). Using the first bifurcation of the dendrogram to separate SRNS from not SRNS, 28 of 50 samples from efficacy evaluable glioblastoma subjects were SRNS (Fig. S10A) including 18/19 tumors defined as neural and 4/4 tumors defined as classical (Fig. S10C).. The hazard ratio of death of those with SRNS is significantly reduced compared to not SRNS (HR=0.11, p=0.003) after controlling for number of recurrences and HGG grade using a multivariate Cox regression analysis (Table S10). Like the TCGA neural signature, the SRNS signature is not associated with survival in newly diagnosed glioblastoma (Fig. S11).
A recent study compared expression profiles of glioblastoma tumors from the contrast-enhancing tumor core (CE) with the leading edge non-enhancing (NE) margins of the tumors (23). Samples from CE resembled classical, mesenchymal and proneural subtypes whereas samples from NE largely resembled neural. The raw sequencing data from this study was retrieved and processed identically to the samples from the Toca 511 and Toca FC study and combined. Neural tumors segregated with NE samples and normal brain, whereas classical, mesenchymal, proneural samples were intermixed with CE samples (Fig. S12A). Neural tumor samples were distinct from normal brain, including alterations in proliferation-related mRNA expression (Fig. S12B).
Separate from the SRNS signature, there were numerous mRNAs whose expression correlated with survival time. For instance, expression of SPOC1/PHF13 negatively correlates (Pearson correlation −0.71) with survival after Toca 511 therapy in glioblastoma subjects (Fig. S13). SPOC1 modulates chromatin structure, acts as an adenovirus host-restriction factor and likely interferes with retroviral life-cycle (24, 25).
O-6-methylguanine-DNA methyltransferase (MGMT) encodes a DNA repair protein that repairs alkylation at the O6 position of guanine (26). MGMT promoter hypermethylation results in reduced expression and an impaired ability for cells to repair damage induced by chemotherapeutic agents and radiation (27, 28). Accordingly, MGMT promoter hypermethylation is associated with improved outcome in newly diagnosed HGG (29). MGMT promoter methylation was measured using a bisulfite-free DNA methylation detection assay (30). Most study samples were MGMT methylation negative and there were 3 instances where MGMT methylation status differed between two distinct samples from the same tumor (Fig. S14A). Unlike SRNS, MGMT methylation status did not correlate with survival (Fig. S15B).
Discussion
Recurrent HGG is associated with dismal clinical outcomes and patients are in need of safe and more efficacious therapy. The non-lytic, retroviral replicating vector Toca 511 and an extended release 5-FC have the potential to fill this medical need. Detection of viral transduction in resected tumors post-Toca 511 treatment, while being cleared from blood, suggests that a reservoir of Toca 511 may exist selectively in tumors to continually kill tumor cells and activate the immune system over multiple cycles of Toca FC (8). Cancer selectivity for Toca 511 is supported by the lack of virus staining in normal brain from resected tumors after Toca 511 delivery and has been well established in preclinical tumor models (8, 10).
To provide context to the results observed, comparisons to the only available contemporaneous randomized Phase 2/3 external controls were conducted. Subjects with glioblastoma at first and second recurrence treated with Toca 511 and Toca FC were compared to those treated with lomustine. While such a comparison does not overcome the lack of an internal control and randomized data, it is considered robust, reflecting a Phase 3 clinical trial patient population and establishing comparability to the subjects receiving Toca 511 and Toca FC. Efficacy is strongly suggested in the Toca 511 and Toca FC treated recurrent HGG population by the almost 2-fold improvement in OS compared to this external control, and by a median OS of 13.6 months that approaches the OS observed in newly diagnosed glioblastoma (31, 32). While Toca 511 and Toca FC are administered following tumor resection and lomustine is not, a similar effect was seen in another phase I Toca 511 trial which did not require surgical resection (33). The improvement in OS is not reflected by an improvement in progression-free survival, which may be due to radiological changes of pseudoprogression as has been observed in immunotherapies for HGG (Fig. S15) (34). Despite the challenge in evaluating tumor response in the post-operative setting and in the context of a potentially immune activating therapy, with longer follow up of 30 evaluable subjects in the higher dose cohorts, 2 had complete responses, 2 had partial responses, and 8 had stable disease, which provides additional supportive evidence of efficacy. While the safety profile appears manageable, further attribution of the adverse events observed as complications of surgery, Toca 511 and/or Toca FC treatment can be assessed in a randomized trial. No spontaneous cases of autoimmune disorders, such as vitiligo, thyroiditis, or hypophysitis, which have been observed with the immune-checkpoint inhibitors anti-CTLA-4 and anti PD-1, have been reported.
Remarkable improvement in OS and a favorable safety profile of Toca 511 and Toca FC support moving forward with this treatment regimen. In the external control comparison, there was a separation of the OS curves that occurred initially and continued through 30 months with an OS24 of 31.6%. This is consistent with an initial cytoreductive effect from intratumorally-generated 5-FU followed by a sustained effect from augmented anti-tumor immunity due to the combination of cancer and immune-suppressor cell killing by 5-FU in the tumor microenvironment (8, 35). A maximum tolerated dose has not been established due to infrequent dose limiting toxicities. A randomized trial is being conducted at a maximum feasible dose of 4mL using 40 injections into the wall of the resection cavity. Based on immunohistochemistry and analysis of viral RNA and DNA, Toca 511 successfully transduced recurrent HGG with selectivity for tumor cells, and was readily cleared from subjects’ blood. Being the first reported RRV in humans, the documentation of tumor virus persistence with systemic clearance supports the importance of further study of RRVs in humans for cancer treatment.
Toca 511 and Toca FC therapy increased levels of CD4+ T cells in the blood suggesting that immunomodulatory activity may be part of this therapy. In addition to the adjuvant properties of a replicating virus, like Toca 511, and cancer cell killing by 5-FU, myeloid derived suppressor cells are known to be sensitive to 5-FU and the changes in immune cell populations in the blood may be due to induction of an antitumor response generated by Toca 511 and Toca FC (15).
Our molecular analyses reinforce the heterogeneity of HGG tumors including MGMT methylation, a prognostic factor that may sensitize cells to 5-FU (36, 37). The data presented stress the importance for obtaining measurements of multiple spatially distinct tumor biopsy samples when prognostic determinations can potentially affect treatment decisions.
The underlying prognostic and predictive molecular features that contribute to survival in this study may identify subjects most likely to benefit from Toca 511 and Toca FC therapy and suggest pathways to further improve treatment. MGMT promoter methylation did not explain variation in survival among treated subjects with glioblastoma at first or second recurrence. Subjects whose tumors displayed the SRNS (and TCGA neural subtype) survived significantly longer than subjects whose tumors displayed other subtypes (SRNS p= 0.003 and TCGA p= 0.01). In principle, subjects with SRNS could have a less aggressive, more differentiated tumor or a more successful surgery and thus live longer. However, neither the SRNS nor the related TCGA neural subtype correlate with survival in newly diagnosed glioblastoma, suggesting SRNS tumor phenotype predicts response to Toca 511 and Toca FC therapy (21, 38). Our results are consistent with the SRNS signature being associated with the non-enhancing region of advancing edge of the tumor (23). This environment may be more favorable for Toca 511 persistence and spread as the advancing edges are associated with increased cell division which is important for Toca 511 infection and 5-FU killing (39). A recent clinical trial reported encouraging survival in recurrent high grade glioma transcranially injected with Toca 511 into the non-enhancing edge of a glioma using a biopsy needle compared to infusion into the center of the tumor (33). Expression of a known virus-host interaction viral restriction factor, SPOC1, has a strong negative correlation with survival after treatment with Toca 511 and Toca FC, supporting the importance of Toca 511 tumor spread for survival improvement.
Two meta-analyses have demonstrated that surgical resection of recurrent tumor does not affect OS (40, 41). Adverse events will be understood more clearly from a randomized trial in combination with surgical resection, which is currently ongoing.
Use of Toca 511 and Toca FC following glioma resection may be an optimal setting for testing this immunotherapy treatment. Typically, neurosurgeons try to resect enhancing tumor, leaving behind non-enhancing tumor in the resection cavity wall. Toca 511 can then be injected into the non-enhancing region left behind in the advancing edge of the tumor where the SRNS cells likely reside.
A randomized Phase 2/3 trial (NCT02414165) in subjects with recurrent glioblastoma and anaplastic astrocytoma is underway. This trial will compare the overall survival of subjects treated with Toca 511 combined with Toca FC to subjects treated according to standard of care after tumor resection. This design provides an opportunity to validate the promising OS, tolerability, immune cell changes and predictive mRNA expression signature observed in this Phase 1 study and to quantify cytotoxic and immune mediated effects in an effort to further confirm the mechanism of action of Toca 511 and Toca FC. It is conceivable that treatment with Toca 511 at the time of resection of newly diagnosed HGG may provide better efficiency of treatment and will be further explored in a Phase 1 trial (NCT02598011).
Materials and Methods
Study Design Section
Eligible patients had histologically proven HGG with recurrence or progression following initial definitive therapy or therapy for recurrence. Other key eligibility criteria included age between 18 and 80 years, Karnofsky performance score ≥ 70, single recurrent HGG tumor ≤ 6 cm, preoperative evaluation that ≥ 80% of tumor was resectable, and adequate organ function.
The protocol was approved by the institutional review board at each site and complied with the International Ethical Guidelines for Biomedical Research Involving Human Subjects, Good Clinical Practice guidelines, the Declaration of Helsinki, and local laws. All subjects provided written informed consent.
This phase 1, open-label, ascending dose, multicenter trial was designed to evaluate the safety and tolerability of Toca 511 and Toca FC. The primary endpoint was to identify the highest, safe and well tolerated dose of Toca 511. Secondary objectives were to evaluate safety and tolerability of repeated treatment with Toca FC at various doses and schedules following administration of Toca 511. Efficacy was to be assessed by OS, OS at 6 (OS6), 9 (OS9), 12 (OS12) and 24 (OS24) months. Using an independent radiology review, objective response rate, progression-free survival and progression-free survival at 6 months (PFS6) was determined. Exploratory objectives included developing a predictive diagnostic factor based on RNA expression and assessing potential clinical utility. This was done in accordance within the SPIRIT 2013 Guidelines.
Subjects underwent gadolinium enhanced MRI scan of the brain approximately every 8 weeks with the baseline MRI scan obtained after surgical resection and just prior to Toca FC. Tumor response was assessed by an independent radiology review using the Macdonald criteria, which require measurable contrast-enhancing disease of at least 10 mm in both dimensions and confirmation at least 4 weeks after the initial response (42).
All adverse events were classified and graded with the use of the Common Terminology Criteria for Adverse Events, version 4.0 (43). If any subject experienced a related, dose limiting toxicity (DLT) attributed to Toca 511, Toca FC, or the combination, then another 3 subjects were studied at that dose level. If 2 of 6 subjects experience a related DLT at any dose level, then no further dose escalation was planned. The highest dose at which 6 subjects were studied with < 2 DLTs was considered the maximum tolerated dose. A Data Monitoring Committee periodically reviewed the safety data. Subjects were assessed for adverse events from the time of Toca 511 administration and will be followed for up to 15 years, as stipulated by FDA guidance through a continuation study.(44)
Testing for the presence of viral RNA by qRT-PCR, viral DNA by qPCR, antibodies to Toca 511, and immunologic parameters was performed in blood. Urine and saliva were monitored for viral shedding.
Molecular analyses
Genomic DNA and total RNA from spatially distinct bulk tumor pieces were isolated and analyzed further. Additional methodologic details are provided in the Supplemental Methods.
Study Oversight
The study was designed jointly by the investigators and representatives of the sponsor, Tocagen. The sponsor collected and analyzed the data. All the authors were involved in the data analysis and manuscript preparation and vouch for the completeness and accuracy of the data and analyses and for the adherence of the study to the protocol. No one who is not listed as an author contributed to the writing of the manuscript.
External Control
Tocagen approached 6 sponsors with recently published phase 2 or 3 data in recurrent HGG or glioblastoma, including a study in which subjects underwent resection of recurrent glioblastoma. One sponsor (Denova Biopharma LLC) agreed to share lomustine data from a phase 3 randomized, open label study evaluating enzastaurin in subjects with recurrent glioblastoma who did not undergo resection of recurrent tumor. The study enrolled subjects: ≥ 18 years of age; Karnofsky performance status ≥ 70; histologically confirmed glioblastoma at first or second recurrence; ≤ 2 prior chemotherapy regimens; and adequate organ function. While 92 subjects were randomized to lomustine, 84 received lomustine treatment. The study was discontinued early for futility after an interim analysis of the primary endpoint of progression-free survival.(6)
A subset of subjects with recurrent glioblastoma at first or second recurrence treated with Toca 511 and Toca FC were compared to these lomustine treated subjects.
Statistical Analysis
The efficacy evaluable population included subjects who received at least one dose of Toca 511 and one planned course of Toca FC. The safety population included all subjects who received Toca 511. Continuous variables were summarized with means, standard deviations, medians, minimums, and maximums. Categorical variables were summarized by counts and by percentage of subjects in corresponding categories. OS and progression-free survival were analyzed using Kaplan-Meier method and plots were generated and the median OS and progression-free survival and 95% CI are summarized. The log-rank test was used for between group comparisons and the hazard ratios with 95% CI were presented. In addition, Z-test was used for between group comparison in landmark survival rate at 6 months (OS6), 9 months (OS9), 12 months (OS12) and 24 months (OS24). All analyses were performed using SAS® version 9.4 (SAS Institute Inc.). Molecular characteristics of neural vs. other subtypes and SRNS vs other subtypes were investigated using a multivariate Cox regression model for prediction of survival. For this ongoing trial, these analyses were performed based on a data transfer date of September 18, 2015. Additionally, an independent radiology review occurred based on a data transfer date of January 22, 2016.
Supplementary Material
Fig. S1. Simplified Schematic for the Phase 1 Trial of Vocimagene Amiretrorepvec and 5-Fluorocytosine for Recurrent High Grade Glioma (NCT01470794)
Fig. S2. Tumor specific Toca 511 staining in re-resected tumors after multiple cycles of Toca FC
Fig. S3. Forest Plot of Subgroups comparing the subjects with glioblastoma at first or second recurrence treated with Toca 511 and Toca FC to the lomustine external control
Fig. S4. Significant peripheral blood CD4+ T cell modulation after Toca 511 and Toca FC dosing
Fig. S5. Tracking of Toca 511 DNA and RNA signal in whole blood and plasma over time
Fig. S6. Molecular classification of tumor samples from study subjects based on mRNA expression
Fig. S7. Intra- vs inter-tumor heterogeneity in RNA expression profiles
Fig. S8. Variation in mRNA expression is not due to confounding technical factors
Fig S9. The neural subtype is not a prognostic of newly diagnosed glioblastoma
Fig. S10. Identification of a mRNA profile (SRNS) associated with subjects who survived for more than one year post-Toca 511 administration
Fig S11. Survival-related neuronal subtype (SRNS) is not prognostic in newly diagnosed glioblastoma
Fig. S12. Study neural subtype samples likely derive from non-enhancing regions of tumors
Fig. S13. SPOC1 expression negatively correlates to subject survival time with Toca 511 and Toca FC therapy.
Fig. S14. MGMT promoter methylation
Fig. S15. Evidence of potential pseudoprogression in resected tumors after Toca 511 and Toca FC treatment
Table S1. Toca 511 and Toca FC: Baseline Demographic and Clinical Characteristics
Table S2. Additional Baseline Demographic and Clinical Characteristics
Table S3. Dosing Cohorts
Table S4. Detection of Toca 511 in re-resected tumors
Table S5. Best Overall Response in the Efficacy Evaluable Population Using Macdonald Criteria by Independent Radiology Review
Table S6. Adverse Events and Serious Adverse Events Related to Toca 511 and Toca FC
Table S7. Related Adverse Events: Toca 511 and Toca FC Compared to Lomustine External Control
Table S8. Adverse Events Regardless of Attribution: Toca 511 and Toca FC Compared to Lomustine External Control
Table S9. Summary of Multivariate Analysis for Survival (TCGA Neural Signature)
Table S10. Summary of Multivariate Analysis for Survival (SRNS Signature)
Acknowledgments
Funding: The authors thank the ABC2 Foundation (Washington, DC), the National Brain Tumor Society (Watertown, MA), the American Brain Tumor Association (Chicago, IL), the Musella Foundation (Hewlett, NY), and Voices Against Brain Cancer (New York, NY) for their support and collaborations. NK was also supported in part by U01NS059821 from the National Institute of Neurological Diseases and Stroke (to NK).
Competing interests: D.J.H., A.C., A.D., D.G., H.E.G. D.J.J. D.O., L.M., M.R., O.R.D., and J.M.R. are employees and/or shareholders of Tocagen. N.K. is a consultant, has ownership interest in, and is the recipient of a research grant from Tocagen.
References
- 1.Chamberlain MC, Treatment options for glioblastoma. Neurosurgical focus 20, E19 (2006). [DOI] [PubMed] [Google Scholar]
- 2.Taal W, Oosterkamp HM, Walenkamp AM, Dubbink HJ, Beerepoot LV, Hanse MC, Buter J, Honkoop AH, Boerman D, de Vos FY, Dinjens WN, Enting RH, Taphoorn MJ, van den Berkmortel FW, Jansen RL, Brandsma D, Bromberg JE, van Heuvel I, Vernhout RM, van der Holt B, van den Bent MJ, Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol 15, 943–953 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T, Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 27, 4733–4740 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Kunwar S, Chang S, Westphal M, Vogelbaum M, Sampson J, Barnett G, Shaffrey M, Ram Z, Piepmeier J, Prados M, Croteau D, Pedain C, Leland P, Husain SR, Joshi BH, Puri RK, Group PS, Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro-oncology 12, 871–881 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batchelor TT, Mulholland P, Neyns B, Nabors LB, Campone M, Wick A, Mason W, Mikkelsen T, Phuphanich S, Ashby LS, Degroot J, Gattamaneni R, Cher L, Rosenthal M, Payer F, Jurgensmeier JM, Jain RK, Sorensen AG, Xu J, Liu Q, van den Bent M, Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 31, 3212–3218 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wick W, Puduvalli VK, Chamberlain MC, van den Bent MJ, Carpentier AF, Cher LM, Mason W, Weller M, Hong S, Musib L, Liepa AM, Thornton DE, Fine HA, Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 28, 1168–1174 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez OD, Logg CR, Hiraoka K, Diago O, Burnett R, Inagaki A, Jolson D, Amundson K, Buckley T, Lohse D, Lin A, Burrascano C, Ibanez C, Kasahara N, Gruber HE, Jolly DJ, Design and selection of Toca 511 for clinical use: modified retroviral replicating vector with improved stability and gene expression. Mol Ther 20, 1689–1698 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ostertag D, Amundson KK, Lopez Espinoza F, Martin B, Buckley T, Galvao da Silva AP, Lin AH, Valenta DT, Perez OD, Ibanez CE, Chen CI, Pettersson PL, Burnett R, Daublebsky V, Hlavaty J, Gunzburg W, Kasahara N, Gruber HE, Jolly DJ, Robbins JM, Brain tumor eradication and prolonged survival from intratumoral conversion of 5-fluorocytosine to 5-fluorouracil using a nonlytic retroviral replicating vector. Neuro-oncology 14, 145–159 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalba C, Klatzmann D, Logg CR, Kasahara N, Beyond oncolytic virotherapy: replication-competent retrovirus vectors for selective and stable transduction of tumors. Curr Gene Ther 5, 655–667 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Hiraoka K, Kimura T, Logg CR, Kasahara N, Tumor-selective gene expression in a hepatic metastasis model after locoregional delivery of a replication-competent retrovirus vector. Clin Cancer Res 12, 7108–7116 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson CO, Beale JM, Block JH, Wilson and Gisvold’s textbook of organic medicinal and pharmaceutical chemistry. (Lippincott Williams & Wilkins, Baltimore, MD, ed. 12th, 2011), pp. x, 1010 p. [Google Scholar]
- 12.Formica V, Leary A, Cunningham D, Chua YJ, 5-Fluorouracil can cross brain-blood barrier and cause encephalopathy: should we expect the same from capecitabine? A case report on capecitabine-induced central neurotoxicity progressing to coma. Cancer chemotherapy and pharmacology 58, 276–278 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Kuriyama S, Masui K, Sakamoto T, Nakatani T, Kikukawa M, Tsujinoue H, Mitoro A, Yamazaki M, Yoshiji H, Fukui H, Ikenaka K, Mullen CA, Tsujii T, Bystander effect caused by cytosine deaminase gene and 5-fluorocytosine in vitro is substantially mediated by generated 5-fluorouracil. Anticancer Res 18, 3399–3406 (1998). [PubMed] [Google Scholar]
- 14.Huber BE, Austin EA, Richards CA, Davis ST, Good SS, Metabolism of 5-fluorocytosine to 5-fluorouracil in human colorectal tumor cells transduced with the cytosine deaminase gene: significant antitumor effects when only a small percentage of tumor cells express cytosine deaminase. Proc Natl Acad Sci U S A 91, 8302–8306 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rebe C, Ghiringhelli F, 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res 70, 3052–3061 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Huang TT, Parab S, Burnett R, Diago O, Ostertag D, Hofman FM, Espinoza FL, Martin B, Ibanez CE, Kasahara N, Gruber HE, Pertschuk D, Jolly DJ, Robbins JM, Intravenous administration of retroviral replicating vector, Toca 511, demonstrates therapeutic efficacy in orthotopic immune-competent mouse glioma model. Hum Gene Ther 26, 82–93 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rainov NG, A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Ther 11, 2389–2401 (2000). [DOI] [PubMed] [Google Scholar]
- 18.Twitty CG, Diago O, Hogan D, Burrascano C, Ibanez CE, Jolly D, Ostertag D, Retroviral Replicating Vectors Deliver Cytosine Deaminase Leading to Targeted 5-FU-Mediated Cytotoxicity in Multiple Human Cancer Types. Human gene therapy methods, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, Alexe G, Lawrence M, O’Kelly M, Tamayo P, Weir BA, Gabriel S, Winckler W, Gupta S, Jakkula L, Feiler HS, Hodgson JG, James CD, Sarkaria JN, Brennan C, Kahn A, Spellman PT, Wilson RK, Speed TP, Gray JW, Meyerson M, Getz G, Perou CM, Hayes DN, N. Cancer Genome Atlas Research, Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17, 98–110 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ, Berman SH, Beroukhim R, Bernard B, Wu CJ, Genovese G, Shmulevich I, Barnholtz-Sloan J, Zou L, Vegesna R, Shukla SA, Ciriello G, Yung WK, Zhang W, Sougnez C, Mikkelsen T, Aldape K, Bigner DD, Van Meir EG, Prados M, Sloan A, Black KL, Eschbacher J, Finocchiaro G, Friedman W, Andrews DW, Guha A, Iacocca M, O’Neill BP, Foltz G, Myers J, Weisenberger DJ, Penny R, Kucherlapati R, Perou CM, Hayes DN, Gibbs R, Marra M, Mills GB, Lander E, Spellman P, Wilson R, Sander C, Weinstein J, Meyerson M, Gabriel S, Laird PW, Haussler D, Getz G, Chin L, Network TR, The somatic genomic landscape of glioblastoma. Cell 155, 462–477 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooper LA, Gutman DA, Long Q, Johnson BA, Cholleti SR, Kurc T, Saltz JH, Brat DJ, Moreno CS, The proneural molecular signature is enriched in oligodendrogliomas and predicts improved survival among diffuse gliomas. PLoS One 5, e12548 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parker NR, Khong P, Parkinson JF, Howell VM, Wheeler HR, Molecular heterogeneity in glioblastoma: potential clinical implications. Frontiers in oncology 5, 55 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gill BJ, Pisapia DJ, Malone HR, Goldstein H, Lei L, Sonabend A, Yun J, Samanamud J, Sims JS, Banu M, Dovas A, Teich AF, Sheth SA, McKhann GM, Sisti MB, Bruce JN, Sims PA, Canoll P, MRI-localized biopsies reveal subtype-specific differences in molecular and cellular composition at the margins of glioblastoma. Proc Natl Acad Sci U S A, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schreiner S, Kinkley S, Burck C, Mund A, Wimmer P, Schubert T, Groitl P, Will H, Dobner T, SPOC1-mediated antiviral host cell response is antagonized early in human adenovirus type 5 infection. PLoS pathogens 9, e1003775 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinkley S, Staege H, Mohrmann G, Rohaly G, Schaub T, Kremmer E, Winterpacht A, Will H, SPOC1: a novel PHD-containing protein modulating chromatin structure and mitotic chromosome condensation. Journal of cell science 122, 2946–2956 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Pegg AE, Mammalian O6-alkylguanine-DNA alkyltransferase: regulation and importance in response to alkylating carcinogenic and therapeutic agents. Cancer Res 50, 6119–6129 (1990). [PubMed] [Google Scholar]
- 27.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R, MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352, 997–1003 (2005). [DOI] [PubMed] [Google Scholar]
- 28.Esteller M, Garcia-Foncillas J, Andion E, Goodman SN, Hidalgo OF, Vanaclocha V, Baylin SB, Herman JG, Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med 343, 1350–1354 (2000). [DOI] [PubMed] [Google Scholar]
- 29.Thuy MN, Kam JK, Lee GC, Tao PL, Ling DQ, Cheng M, Goh SK, Papachristos AJ, Shukla L, Wall KL, Smoll NR, Jones JJ, Gikenye N, Soh B, Moffat B, Johnson N, Drummond KJ, A novel literature-based approach to identify genetic and molecular predictors of survival in glioblastoma multiforme: Analysis of 14,678 patients using systematic review and meta-analytical tools. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia 22, 785–799 (2015). [DOI] [PubMed] [Google Scholar]
- 30.McCarthy DR, Hanna MM, Cotter PD, MethylMeter(r): A Quantitative, Sensitive, and Bisulfite-Free Method for Analysis of DNA Methylation. (INTECH Open Access Publisher, 2012). [Google Scholar]
- 31.Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea D, Brandes AA, Hilton M, Abrey L, Cloughesy T, Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 370, 709–722 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, R. European Organisation for, T. Treatment of Cancer Brain, G. Radiotherapy, G. National Cancer Institute of Canada Clinical Trials, Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352, 987–996 (2005). [DOI] [PubMed] [Google Scholar]
- 33.Timothy Cloughesy JL, Vogelbaum Michael A., Kesari Santosh, Kalkanis Steven, Portnow Jana, Mikkelsen Tom, Elder J Bradley, Baskin David, Chu Alice, Robbins Joan, Gruber Harry, Cobb Charles, Foltz Greg, Kaptain George, Aghi Manish K., in Society of Neurooncology 2015 Annual Conference (San Antonio, Tx, 2015). [Google Scholar]
- 34.Okada H, Weller M, Huang R, Finocchiaro G, Gilbert MR, Wick W, Ellingson BM, Hashimoto N, Pollack IF, Brandes AA, Franceschi E, Herold-Mende C, Nayak L, Panigrahy A, Pope WB, Prins R, Sampson JH, Wen PY, Reardon DA, Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol 16, e534–542 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang W, Tai CK, Kershaw AD, Solly SK, Klatzmann D, Kasahara N, Chen TC, Use of replication-competent retroviral vectors in an immunocompetent intracranial glioma model. Neurosurgical focus 20, E25 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murakami J, Lee YJ, Kokeguchi S, Tsujigiwa H, Asaumi J, Nagatsuka H, Fukui K, Kuroda M, Tanaka N, Matsubara N, Depletion of O6-methylguanine-DNA methyltransferase by O6-benzylguanine enhances 5-FU cytotoxicity in colon and oral cancer cell lines. Oncology reports 17, 1461–1467 (2007). [PubMed] [Google Scholar]
- 37.Oliver JA, Ortiz R, Melguizo C, Alvarez PJ, Gomez-Millan J, Prados J, Prognostic impact of MGMT promoter methylation and MGMT and CD133 expression in colorectal adenocarcinoma. BMC Cancer 14, 511 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guan X, Vengoechea J, Zheng S, Sloan AE, Chen Y, Brat DJ, O’Neill BP, de Groot J, Yust-Katz S, Yung WK, Cohen ML, Aldape KD, Rosenfeld S, Verhaak RG, Barnholtz-Sloan JS, Molecular subtypes of glioblastoma are relevant to lower grade glioma. PLoS One 9, e91216 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mazor T, Pankov A, Johnson BE, Hong C, Hamilton EG, Bell RJ, Smirnov IV, Reis GF, Phillips JJ, Barnes MJ, Idbaih A, Alentorn A, Kloezeman JJ, Lamfers ML, Bollen AW, Taylor BS, Molinaro AM, Olshen AB, Chang SM, Song JS, Costello JF, DNA Methylation and Somatic Mutations Converge on the Cell Cycle and Define Similar Evolutionary Histories in Brain Tumors. Cancer Cell 28, 307–317 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gorlia T, Stupp R, Brandes AA, Rampling RR, Fumoleau P, Dittrich C, Campone MM, Twelves CC, Raymond E, Hegi ME, Lacombe D, van den Bent MJ, New prognostic factors and calculators for outcome prediction in patients with recurrent glioblastoma: a pooled analysis of EORTC Brain Tumour Group phase I and II clinical trials. Eur J Cancer 48, 1176–1184 (2012). [DOI] [PubMed] [Google Scholar]
- 41.Clarke JL, Ennis MM, Yung WK, Chang SM, Wen PY, Cloughesy TF, Deangelis LM, Robins HI, Lieberman FS, Fine HA, Abrey L, Gilbert MR, Mehta M, Kuhn JG, Aldape KD, Lamborn KR, Prados MD, North C American Brain Tumor, Is surgery at progression a prognostic marker for improved 6-month progression-free survival or overall survival for patients with recurrent glioblastoma? Neuro-oncology 13, 1118–1124 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Macdonald DR, Cascino TL, Schold SC Jr., Cairncross JG, Response criteria for phase II studies of supratentorial malignant glioma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 8, 1277–1280 (1990). [DOI] [PubMed] [Google Scholar]
- 43.Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0 (May 28 2009). [Google Scholar]
- 44.FDA Guidance for Industry Considerationsfor the Design of Early-Phase Clinical Trials of Cellular and Gene Therapy Products.
- 45.Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL, TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome biology 14, R36 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gill BJ, Pisapia DJ, Malone HR, Goldstein H, Lei L, Sonabend A, Yun J, Samanamud J, Sims JS, Banu M, Dovas A, Teich AF, Sheth SA, McKhann GM, Sisti MB, Bruce JN, Sims PA, Canoll P, MRI-localized biopsies reveal subtype-specific differences in molecular and cellular composition at the margins of glioblastoma. Proc Natl Acad Sci U S A 111, 12550–12555 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sandberg R, Neilson JR, Sarma A, Sharp PA, Burge CB, Proliferating cells express mRNAs with shortened 3’ untranslated regions and fewer microRNA target sites. Science 320, 1643–1647 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Simplified Schematic for the Phase 1 Trial of Vocimagene Amiretrorepvec and 5-Fluorocytosine for Recurrent High Grade Glioma (NCT01470794)
Fig. S2. Tumor specific Toca 511 staining in re-resected tumors after multiple cycles of Toca FC
Fig. S3. Forest Plot of Subgroups comparing the subjects with glioblastoma at first or second recurrence treated with Toca 511 and Toca FC to the lomustine external control
Fig. S4. Significant peripheral blood CD4+ T cell modulation after Toca 511 and Toca FC dosing
Fig. S5. Tracking of Toca 511 DNA and RNA signal in whole blood and plasma over time
Fig. S6. Molecular classification of tumor samples from study subjects based on mRNA expression
Fig. S7. Intra- vs inter-tumor heterogeneity in RNA expression profiles
Fig. S8. Variation in mRNA expression is not due to confounding technical factors
Fig S9. The neural subtype is not a prognostic of newly diagnosed glioblastoma
Fig. S10. Identification of a mRNA profile (SRNS) associated with subjects who survived for more than one year post-Toca 511 administration
Fig S11. Survival-related neuronal subtype (SRNS) is not prognostic in newly diagnosed glioblastoma
Fig. S12. Study neural subtype samples likely derive from non-enhancing regions of tumors
Fig. S13. SPOC1 expression negatively correlates to subject survival time with Toca 511 and Toca FC therapy.
Fig. S14. MGMT promoter methylation
Fig. S15. Evidence of potential pseudoprogression in resected tumors after Toca 511 and Toca FC treatment
Table S1. Toca 511 and Toca FC: Baseline Demographic and Clinical Characteristics
Table S2. Additional Baseline Demographic and Clinical Characteristics
Table S3. Dosing Cohorts
Table S4. Detection of Toca 511 in re-resected tumors
Table S5. Best Overall Response in the Efficacy Evaluable Population Using Macdonald Criteria by Independent Radiology Review
Table S6. Adverse Events and Serious Adverse Events Related to Toca 511 and Toca FC
Table S7. Related Adverse Events: Toca 511 and Toca FC Compared to Lomustine External Control
Table S8. Adverse Events Regardless of Attribution: Toca 511 and Toca FC Compared to Lomustine External Control
Table S9. Summary of Multivariate Analysis for Survival (TCGA Neural Signature)
Table S10. Summary of Multivariate Analysis for Survival (SRNS Signature)