Abstract
Hallucinations, delusions, and functional neurological manifestations (conversion and somatic symptom disorders) of Parkinson Disease (PD) and Dementia with Lewy Bodies (DLB) increase in frequency with disease progression, predict the onset of cognitive decline, and eventually blend with and are concealed by dementia. These symptoms share the absence of reality constraints and can be considered comparable elements of the PD-DLB psychosis. We propose that PD-DLB psychotic disorders depend on thalamic dysfunction promoting a theta burst mode and subsequent thalamo-cortical dysrhythmia (TCD) with focal cortical coherence to Theta EEG rhythms. This Theta EEG activity, also called fast-theta or pre-alpha, has been shown to predict cognitive decline and fluctuations in Parkinson Disease with Dementia (PDD) and DLB. These EEG alterations are now considered a predictive marker for progression to dementia. The resulting TCD inhibits the frontal attentional network and favors the decoupling of the Default Mode Network (DMN). As the DMN is involved in integration of self-referential information into conscious perception, unconstrained DMN activity, as revealed by recent imaging studies, leads to random formation of connections that link strong autobiographical correlates to trivial stimuli, thereby producing hallucinations, delusions, and functional neurological disorders. The TCD-DMN decoupling hypothesis provides the rationale for design and testing of novel therapeutic pharmacological and non-pharmacological interventions in the context of PD, PDD, and DLB.
Keywords: Hallucinations, Somatic symptoms, Functional Disorders, Parkinson’s Disease, Default Mode Network, Thalamus
Introduction
Hallucinations, usually in visual or, more rarely, in somatic-haptic, acoustic, or olfactory [1] modality are well-recognized manifestations of Parkinson’s Disease (PD), Parkinson’s Disease with Dementia (PDD), and Dementia with Lewy Bodies (DLB) [2–6]. Hallucinations are strong clinical predictors of impending cognitive decline in PD and a core criterion for the diagnosis of DLB [2–6]. They have also been shown to correlate with aggregation of Lewy bodies [7].
Somatic Symptom Disorder (in other words, bodily symptoms that are more than motor) and functional disorders (such as distractible motor symptoms), hereafter referred to as Somatic Symptom and Functional Disorders (SFD), were only recently reconsidered in PD and DLB by systematic studies that highlighted their critical impact on therapeutic management and their role as predictors of cognitive decline [8–15]. In this review, we propose that SFDs, which lack reality constraints and are “experiences that depart from consensual reality”, as from the last broad definition of psychosis [16], should be considered, along with hallucinations and delusions, as one of the three elements of the psychosis complex associated with synucleinopathies. Hallucinations and SFD share similar associations with cognitive decline [8, 13, 14], have similar prevalence [11, 15, 17], and share a similar course of progression [8, 15].
Finally, delusions are the hallmarks of the psychotic narratives that precede or associate with onset of motor symptoms or concomitantly appear with dementia [4, 18, 19].
We will discuss a set of consequential hypotheses, which are supported by numerous studies that we believe can promote further research and help in re-addressing the treatment of the PD-DLB psychosis spectrum. We propose a common underlying mechanism for these phenomena that involves thalamic-driven decoupling of the Default Mode Network (DMN) from fronto-parietal control and attention networks [20, 21]. The model posits that, as recently indicated by neuroimaging studies [20, 22], the DMN, the source of the internal narratives [23, 24] and the final hub for top-down processing of perceptions [25], when decoupled, as in psychedelic states and REM sleep [26], produces connection motifs which form and fragment across time, thereby generating the psychosis signs found in synucleinopathies. We propose that DMN decoupling is functionally associated with generation of thalamic-driven theta EEG rhythms, the electrical biomarkers of PDD and DLB [6, 27] that also predict onset of cognitive decline in PD [27–29]. The theta EEG activity of PDD-DLB is generated by thalamo-cortical dysrhythmia (TCD) [30, 31]. Upon Theta TCD, cortical areas become dissociated from cortical networks and get locked in dissociative states that are found in sleep phases (e.g., Theta rhythms of N1 and REM sleep stages). The TCD hypothesis reconciles several hallucination-related theories based on the disinhibition of the DMN with theories based on REM sleep intrusions [2, 32].
Our model posits a causative link between the increased frequency of TCD-driven decoupling of the DMN and progression of the clinical manifestations. The model also highlights a central role for the thalamus as the main pathological hub for development of PD-DLB psychosis.
Hallucinations
The occurrence of hallucinations invariably portends an unfavorable prognostic outcome [4, 33], as they predict the onset of cognitive decline. However, in some patients hallucinations may appear, mostly those with DLB, during the early stages of the disease before or at the onset of treatment [1, 4, 6, 34]. Early visual hallucinations may consist of simple distortions of perceived images that are often characterized by perception of anthropomorphic traits (eidol-idol) of visual elements [4, 35]. This hallucination type, properly defined as “illusions”, is also known as pareidolia and can be assessed with specific ambiguous-unambiguous imaging tests (Pareidolia tests) [36]. The description of visual hallucinations in early PD and DLB also includes the sudden appearance of complex images, animals, miniature figures, etc., which generally disappear when the patient, employing a coping strategy, re-focuses his/her attention [2–4, 37] and regains insight into the reality of the percepts. Based on insight preservation, these early hallucinations have also been identified as “pseudohallucinations”. However, the term is usually better employed to indicate a type of intentionally controlled mental image that occurs in schizophrenia and does not originate from perceptions [38, 39]. With disease progression and the appearance of cognitive decline, early, simple hallucinations, with preserved insight, are supplanted by the appearance of complex hallucinations. At this stage, a complex structural narrative emerges in which hallucinatory images interact, move across the visual field with kinetic properties [4, 40], and are perceived along with delusional contents. These complex hallucinations, properly identified as paraphrenic hallucinations [35, 41], represent an unmet clinical and therapeutic problem [35]. Complex hallucinations may appear either randomly and last for a short time or present themselves as a constant hallucinatory state, similar to crepuscular status, that lasts for days [6, 42, 41].
Somatic symptom and Functional Disorders (SFD)
The occurence of bodily sensations in the absence of external or objective internal stimuli encompasses disorders which have had a different taxonomy over time. Earlier terms employed to describe the phenomenon were hysteria, hypochondria, conversion disorders, somatization, somatoform, facticious disorders, or psychogenic disorders [8, 43]. The Diagnostic and Statistical Manual of Mental Disorders-5 (DSM-5) eventually categorized the phenomena under the SFD term as “somatic symptom disorder, conversion, illness anxiety disorder, factitious disorder” as well as a generic “other” category or “psychological factors affecting other medical conditions” [44]. The last category has been likely added to finally acknowledge the presence of functional disorders in patients affected by recognizable medical disorders and is suitable for SFD that occur in synucleinopathies. As symptoms may express themselves in conjunction with motor signs, movement disorder experts have debated for years as to whether “psychogenic” or “functional” is the most suitable term to describe them [10, 43, 45, 46]. The term “functional” eventually prevailed, to include conversion and somatic symptom disorders, and a Functional Movement Disorder Study Group was established [47].
The description of PD-related SFD symptoms goes back to the beginning of the L-dopa era [48–50], and they were also a core feature of post-encephalitic parkinsonism [51–56]. Indeed, post-encephalitic parkinsonism was the first and unexpected evidence that SFD-related manifestations were rooted in a pathologically confirmed disease and not the mere result of psychodynamic processes. Today, high scores for SFD-related items within several neuropsychological scales are found in 29 −67% of PD patients [11, 15, 17, 57, 58]. We suggest that SFD symptoms, identified by neuropsychological scales, and described as transient and tolerable phenomena, can be considered equivalent to early simple hallucinations.
The presence of PD-DLB-related SFD was first reconsidered by a systematic study [8], confirmed by others [9–15], that showed that SFD: 1) might precede or go along for several years with the appearance of motor and non-motor features of the disease [8, 15]; 2) may also have a variable pattern of presentation [9–15]; 3) may occur with far higher incidence in PD and DLB compared to other neurodegenerative disorders associated with parkinsonism and/or dementia [8]; or 4) may correlate with and predict onset of cognitive decline [8, 13, 15].
SFD symptoms in PD-DLB include the disproportionate concern exhibited by the patient for underlying diseases or side effects of drugs (hypochondria), inconsistent issues of drug intolerance so severe as to lead to drug discontinuation, occurrence of globus sensation when taking oral medications, inconsistent pain complaints (like in fibromyalgia or reflex sympathetic dystrophy), inconsistent gait patterns, or motor symptoms or tremor that are suppressed by distracting the patient. Interestingly, several reports have now described patients who displayed signs of functional parkinsonism years before a confirmed PD diagnosis by dopamine transporter scans or L-DOPA binding [8, 12, 15]. In PD patients who exhibit motor symptoms along with cognitive decline, somatic complaints become more complex and are characterized by the presence of psychotic elements that should be properly classified as Somatic Delusions [8, 17]. These delusions include ritualized avoidance behavior, perceptions of body deformation, the idea of being physically influenced from external sources, overwhelming allergies or parasitic infestation, or necrotic or liquified internal organs (the last two also known as Ekbom and Cotard delusions) [4, 8].
The clinical overlap
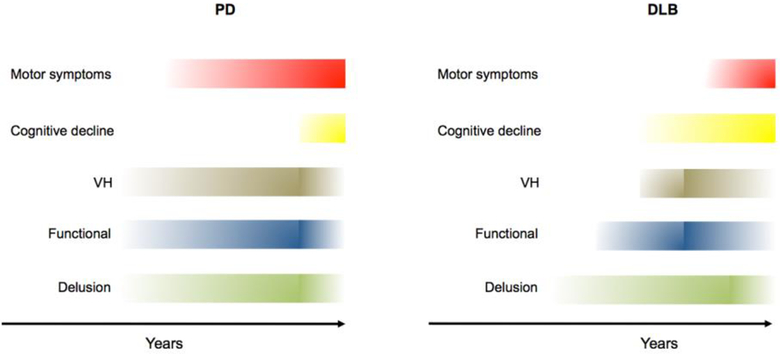
Hallucinations and SFD may present as an early or late disturbance characterized by delusional contents and correlate with the occurrence of cognitive decline [4, 8, 15, 33] (Fig. 1). Delusional thoughts may appear before the appearance of cognitive decline or motor symptoms as shown by epidemiological evidence increased access of PD patients to psychiatric facilities years before a definite diagnosis of the disease [19, 59–61]. The patterns of appearance of the three disorders, their variable association with motor disorders, increased frequency with disease progression, increased severity as the loss of insight progresses, and their concealment within the clinical signs of full-blown dementia suggest a common etiologic mechanism that we propose is to be found in defective functioning of specific networks devoted to the control of attention and management of narrative productions.
Figure 1. Progression of clinical symptoms in PD and DLB.
The panel depicts a schematic representation of the time course of hallucinations, functional-somatoform disorders, delusions, motor symptoms, and dementia in PD and DLB. Color hues indicate the severity and frequency of PD-DLB-related disorders in the patient population. Abbreviations: PD: Parkinson’s disease; DLB: Dementia with Lewy Bodies; VH=visual hallucinations.
Anatomical, pathophysiological overlaps, and mechanisms
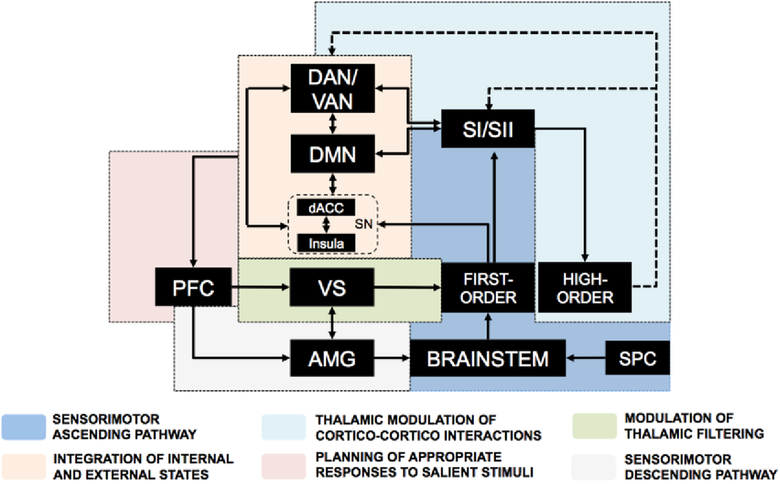
The hypotheses concerning the mechanisms involved in PD-DLB hallucinations have been based on models that integrate bottom-up and top-down processing, and, specifically, the dynamic interplay between attention, perceptual afference from the sensory system (bottom-up), and (top-down) comparisons with preconscious perceptual templates, defined as “priors”, that are stored in autobiographical memory and predict the outcome of sensory evidence [16, 20, 21]. The recruitment of prior visual images depends on top-down control of perception pathways that converge in the activation of the DMN through combined engagement of networks that include the primary, associative cortices, the thalamus, amygdala, insula, and hippocampus (Fig. 2).
Figure 2. Network interplay involved in visual processing.
This diagram depicts the physiological interplay of networks that control visual processing. Upon physiological stimulation, the visual inputs from the retina reach the primary visual cortex through activation of the first-order thalamic nuclei. The dorsal (DAN) and ventral (VAN) networks integrate visual signals from the primary cortex and, in concert with salience network (SN), select the more significant peripheral stimuli. The DMN integrates inputs from the hippocampus and amygdala and contributes to shaping visual processing by integrating emotion- or memory-enriched introspective information. The high-order thalamic nuclei modulate cortical network activity via a negative feedback mechanism. The overall net input of these activities converges on the prefrontal cortex (PFC) that manages cognitive representation of salient stimuli. The model envisions that the activity of the SN is complementary to the DMN. The SN is a key checkpoint for anticorrelating activities of the DMN and the task-positive fronto-parietal network, thereby controlling the appropriateness of the responses given to salient stimuli. Altered engagement of the SN promotes decoupling of the DMN from the fronto-parietal network and offers a functional substrate for the onset of psychosis.
Abbreviations: dorsal anterior cingulate cortex, dACC; Default Mode Network, DMN; Dorsal attention network, DAN; prefrontal cortex, PFC; hippocampus, HP; inferior temporal, IT; Ventral attention network, VAN; salience network, SN.
Recent models for visual hallucinations suggest combined impairment in perceptual and attentional networks that leads to altered balance between top-down and bottom-up processes [20, 62–64]. Perception and Attention Deficit (PAD) [21], Integrative [2], and Attentional [20] models suggest that production of unrestrained perceptual activity occurs when the attentional focuses are impaired, and the recruited prior perception of a scene is not disconfirmed or blocked by discrepant visual inputs.
In the case of SFD, earlier psychodynamic explanations have been replaced by models of somatic perceptions. As with vision, somatic sensations are integrated into top-down/bottom-up systems that predict the outcome of perceptions [43, 65–68]. The monitoring systems, which are actively modulated by memory, compare afferent perceptions with prior images or preconscious templates of somatic sensations and make use of networks that, as described above for hallucinations, encompass activation of the primary associative cortices, thalamus, amygdala, insula, and hippocampus (Fig. 3).
Figure 3. Network interplay involved in somatosensory processing.
This diagram depicts the physiological interplay of networks that control somatosensory processing. The somatosensory inputs reach the thalamus through the spinal cord (SPC) and the brainstem. The first-order thalamic nuclei then filter and transmit peripheral stimuli to the somatosensory cortices The SN participates by integrating ascending signals to somatosensory cortices through modulation of the DAN and VAN networks that, in turn, discriminate and select the more significant peripheral inputs. For its part, the DMN receives inputs from the hippocampus and amygdala and contributes to shaping sensory processing by integrating emotion- or memory-enriched introspective information. These network dynamics are regulated by activity of high-order thalamic nuclei that receive inputs from somatosensory cortices and modulate network activity via negative feedback mechanisms. The overall net input of these activities converges on the PFC that, throughout its dorsal region, plans appropriate responses to salient triggers and, via its medial portion and, in conjunction with the ventro-striatal region (VS), modulates thalamic filtering as well as the limbic response.
Abbreviations: amygdala, AMG; dorsal anterior cingulate cortex, dACC; Default Mode Network, DMN; Dorsal attention network, DAN; Salience network, SN; spinal cord, SPC; somatosensory cortices, SI/SII, ventral striatum, VS.
As for visual hallucinations, recent models of SFD [43, 65] suggest that dysfunction of top-down attentional processes may lead to abnormal prediction (prior) of the outcome of bodily perceptions and the consequent production of false perceptions. These false perceptions may (in the case of conversion-functional disorders) or may not (in the case of somatic symptoms) be followed by induction of compensatory motor outputs, thereby generating subcortical patterns of motor activity or motor suppression that range from conversion paresis to catatonia [8, 10]. Although these models have been employed for visual hallucinations and SFD, given the higher prevalence of these symptoms in PD-DLB, they also are a conceptual framework for less frequent psychotic phenomena like auditory, tactile (haptic), olfactory, passage, and presence hallucinations [69].
These models find a neuroanatomical site of action in regions that belong to the Dorsal Attention Network (DAN)/Ventral Attention Network (VAN) or the frontoparietal control network and Salience Network (SN) as well as the DMN [20]. The DMN includes connectivity nodes in the Posterior Cingulate Cortex (PCC), the ventromedial prefrontal cortex, the lateral inferior parietal lobes, and the medial temporal structures. On a cytoarchitectonic standpoint, the PCC belongs to the paralimbic cortex, exhibits a transitional cell architecture that is between the isocortex and the allocortex (limbic system), and is densely connected to the thalamus [25, 70, 71]. Functionally, the PCC is considered the main DMN hub and is involved in modulation of internally directed thoughts [25]. The DMN controls imagery production and incorporation of self-referential information into conscious perception [23, 24]. Attentional and control networks modulate the DMN hubs to compare perceptual sensations with preconscious prior sensations [72]. The coupling of the DMN with attention and fronto-parietal control networks provides the ground for a complex connectivity interplay also defined as metastable interaction [73, 74]. The metastable concept indicates that networks oscillate from stable and ordered activities in which frontal attention and control networks monitor and constrain parts of the DMN and disordered, high-entropy states in which the DMN is decoupled. During physiological functioning, the DMN rapidly oscillates toward stable motifs and rapidly escapes in entropic states in conditions like daydreaming, transliminal states, internal narratives, or REM sleep. During these entropic states, self-referential information, the identity, is preserved, but reality checking is missing, thereby generating a primitive, infantile or primary state of consciousness. On the contrary, the, ordered, stable features of network interaction generate consciousness states that include reality checking and social awareness [26, 75]. The collapse of this ordered activity generated by DMN decoupling leads to production of dream-like, dissociative, and consciousness-clouding states. The highly entropic state generates connectivity motifs that form and fragment in an unpredictable fashion, thereby producing the absence of logic, the lack of physical constraints, and the metamorphoses that are part of dreams, delirium, and psychedelic states [26, 75, 76].
Imaging studies performed during sleep and REM sleep show potent inhibition (reaching suppression) of the activity of the frontal control networks [76]. The same decoupling is observed in the different sedation states that lead to anesthesia (whereas anesthesia is associated with activity suppression of the DMN) [77]. DMN decoupling is also described to occur in psychotic states [78] and after the administration of psychedelic drugs [75, 79]. Most psychedelic drugs (e.g., lysergic acid diethylamide, mescaline) are active on a subset of serotonin receptors (5-HT2A), and induce production of complex multimodal hallucinations, delirium, and residual psychosis, whereas the K-opioid agonist, salvinorin, has been described to induce mind-body dissociation that breaks the borders of the somatic self and induce a dysperceptive state where bodily sensations are undifferentiated from external stimuli or autobiographic memories [80]. Drugs acting on these receptors represent potential new targets for SFD-related research.
The conceptual link between DMN decoupling and altered states found in psychotic conditions and dreaming supports the possibility of similar involvement of the network in the psychosis associated with synucleinopathies. Taking into account the clinical presentation characterized by early vanishing symptoms suppressed by attention that eventually worsen in paraphrenic states, DMN decoupling may first occur as a random phenomenon, still managed and suppressed most of the time by activation of control systems, but that eventually fails in a chronic high entropy state.
Hyperactivity of the DMN is functionally supported by the cingulate island sign [81–85],which is now a supportive element for diagnosis of DLB and PDD [6]. The positron emission tomography sign refers to the presence of a restricted area of preserved activity, localized in the PCC, that is embedded in hypoactive cortical regions. Further evidence is provided by several functional magnetic resonance imaging (fMRI) studies performed at rest and during tasks that show altered DMN activity and/or connectivity in PD and DLB patients [22, 86–97] that exhibit simple [95, 97] or complex hallucinations [22, 89, 90] (Table 1). Moreover, structural MRI studies have lent support to the idea that altered activity/connectivity and decoupling of the DMN in PD-DLB is of a functional rather than an anatomical character [90]. While the role of the DMN in PD-DLB visual hallucinations is supported by many studies (Table 1), these mechanisms have not been investigated in SFD associated with PD-DLB. However, some fMRI studies of SFD in patients without associated synucleinopathies have shown increased activity or connectivity in the cingulate cortex and the PCC [43, 98–102], thereby providing preliminary functional support for the idea that the same mechanisms can play a role in PD-DLB associated SFD.
Table 1.
Neuroimaging findings related to DMN regions obtained from previous studies on synucleinopathies.
| Authors | Results | Method |
|---|---|---|
| Bejr-Kasem H et al, 2019 | DMN decoupling in simple visual hallucinations, more severe in complex hallucinations ° | rs-fMRI (Seed) |
| Graff-Radford et al, 2014 | Preserved activity of the PCC in DLB patients * | 18F-FDG-PET |
| Iizuka et al, 2016 | CIS ratio significantly correlates with RAVLT score and hallucinations ° | 18F-FDG-PET |
| Imabayashi et al, 2017 | Preserved perfusion of the PCC in DLB patients * | Perfusion SPECT ([99mTc]-ethyl cysteinate dimer) |
| Lim et al, 2009 | Preserved metabolism of the MCC and PCC in DLB patients * | 18F-FDG-PET |
| Whitwell et al, 2017 | Symmetric preservation of PCC metabolism in DLB patients * | 18F-FDG-PET |
| Baggio et al, 2015 | Increased FC between the DMN and lateral occipito-parietal regions in PD patients with MCI vs PD patients without MCI | rs-fMRI (ICA) |
| Fang et al, 2017 | Higher nodal degree, global efficiency, and local efficiency; lower path length in PD patients ^ | rs-fMRI |
| Franciotti et al, 2013 | Increased activity of the PCC in DLB patients * | rs-fMRI (ICA) |
| Franciotti et al, 2015 | Higher FC between the IPL and PCC in PD patients with VH§ | rs-fMRI (ICA) |
| Galvin et al, 2011 | Increased FC between the PCC, DAN, and putamen in PD patients* | rs-fMRI (Seed) |
| Kenny et al, 2012 | Increased FC between the right PCC and cerebellar tonsils, culmen, ACC, GP in DLB patients* | rs-fMRI (Seed) |
| Krajcovicova et al, 2012 | Increased FC between the PCC and DMN regions after levodopa administration ^ | rs-fMRI (ICA) |
| Lee et al, 2018 | Increased FC of the PCC in PD patients with higher volumes of SIas compared with PD patients with lower volumes of SI | rs-fMRI (Seed) |
| Rektorova et al, 2014 | Increased FC in DAN-related regions of PDD patients ^ | rs-fMRI (ICA) |
| Shine et al, 2015 | Increased FC between the DMN and primary visual system in PD patients ° | rs-fMRI (ICA) |
| Schumacher et al, 2017 | Increased FC in DMN areas in DLB patients * | rs-fMRI (ICA) |
| Yao et al, 2014 | Increased FC between the DMN-related regions in PD with VH vs PD without VH | rs-fMRI (ICA) |
Abbreviations: DMN, Default mode network; PCC, Posterior cingulate cortex; DLB, Dementia with Lewy Bodies; 18F-FDG-PET, 18-fluorodeoxyglucose positron emission tomography; CIS, Cingulate Island Sign; RAVLT, Rey Auditory Verbal Learning Test; SPECT, Single-photon emission computed tomography; MCC, Mid cingulate cortex; rs-fMRI, Resting state - functional magnetic resonance imaging; FC, Functional connectivity; IPL, Inferior parietal lobule; PD, Parkinson’s Disease; VH, Visual hallucinations; DAN, Dorsal attentional network; ACC, Anterior cingulate cortex; GP, Globus pallidus; MPFC, Medial Prefrontal Cortex; SI, substantia innominata; MCI, Mild cognitive impairment; PDD, Parkinson’s disease with dementia.
Symbols:
No Control group.
Control group: Alzheimer disease.
Control group: Multiple system atrophy.
Control group: Healthy subjects.
Thalamo-Cortical Dysrhythmia as the driver of DMN decoupling
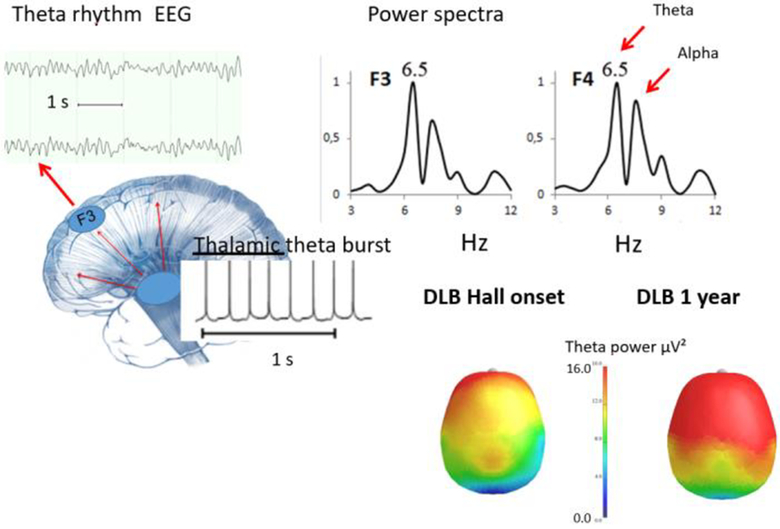
DMN decoupling should be linked with the (now) uncontroversial evidence of EEG abnormalities in PDD and DLB, consisting of progressive appearance of theta rhythms [6, 27–29]. These rhythms are the core features of the Thalamo-Cortical Dysrhythmia (TCD) theory which postulates that, in several disorders including PD, thalamocortical neurons enter into a state of theta-burst mode that drives cortical EEG theta and dysfunctional cortical activity [30, 31, 103]. These theta rhythms, now used as a diagnostic biomarker, are the hallmark of Thalamo-Cortical dysfunction, as EEG rhythmic activity needs a thalamic pacer [30] only arrhythmic activities depend on focal cortical alterations [104]. The presence of theta rhythm, also called pre-alpha or fast theta (McKeith 2017), predicts the occurrence of cognitive decline or progression of mild cognitive impairment to dementia in PDD and correlates with the severity of cognitive fluctuations in DLB [27, 28, 105, 106]. Theta-pre-alpha activity appears in PD-DLB, first pseudoperiodic activity superimposed on the physiological resting state background activity that takes place mainly in the frontal regions, but with time these EEG alterations become present on all the scalp derivations [27, 28] (Fig. 4). The pattern is rhythmic, typical of thalamocortical spindling activity [30], and is classified in progressive scores ranging from random presence to stable presence or presence intermingled with the slower rhythmic or arrhythmic theta-delta activity due to cortical disconnection [6, 27, 28]. Diffuse theta activity has been recorded during hallucinations and hallucinatory delusional states [32, 89, 90, 107, 108].
Figure 4. EEG correlates of Theta inscription.
The top right panel depicts frequency analysis with power spectra exhibiting theta bands. The bottom left panel shows theta burst frequencies that originate in thalamic neurons to produce thalamocortical dysrhythmia (TCD). The bottom right panel shows the topography of the theta activity in a DLB patient recorded at the onset of hallucinations and one year later.
In physiologic conditions, theta bursts are recorded from thalamic nuclei during the switch from waking states to sleep [31], and theta appears, with a predominant frontal distribution, at the onset of sleep, corresponding to the N1 sleep phase, and often at the beginning of REM sleep, replacing desynchronization [109]. Instead, continuous theta activity is observed during REM sleep in PD patients and REM Sleep Behavior Disorder of PD-RBD [110]. Detection of the theta-pre-alpha rhythm in PD is now a core feature of machine learning projects developed for assessment of PD [103, 111] and RBD [112].
The TCD theory [30] originally postulated that portions of the thalamocortical system become locked in spindle-like, waking to sleep status, theta activity [31, 113] whereas other parts of the brain remain in a waking state, a condition similar to parasomnia. The original TCD [113] study suggested that the Theta burst mode shown by thalamic neurons, replacing alpha and gamma frequencies, is due to activation of low threshold calcium spike bursts that are induced by long-lasting hyperpolarization generated on altered projections from the globus pallidus or the pedunculopontine nuclei (PPN) to the thalamoreticular nuclei (TRN). Contrary to earlier studies [30, 103] that have proposed TCD as the mechanism for PD motor symptoms, more recent evidence indicates that PD-related theta-pre-alpha EEG activity only correlates with ongoing cognitive decline and not with motor symptoms [27–29]. We, therefore, suggest that the TCD theory should be repositioned from being the conceptual framework of PD motor symptoms to a central role in driving DMN decoupling and ensuing disconnections from contextual and consensual reality, i.e.hallucinations, SFD, delusions, and fluctuating cognition.
The role of thalamic structures in the origin of psychotic (unconstrained to reality) features is not only inferred by the presence of theta EEG activity but is supported by modeling neuropathological and imaging studies. Recent studies [114–117] provided theoretical, connectional, functional, and modeling evidence to support the notion of a “cognitive thalamus”, thereby indicating the critical role exerted by the region in control of cognition. The “cognitive thalamus” hypothesis postulates that the region is a key modulating hub for cortico-cortical interactions [118–120]. According to the hypothesis, high-order thalamic nuclei (i.e., associative and posterior nuclei) exhibit critical functional and structural connections with the DMN [71], thereby affecting network activity and, ultimately, cognition. The activity of high-order thalamic nuclei is under the control of cholinergic neurons that project from the PPN to TRN [121, 122]. Cholinergic inhibition of the TRN promotes increased activity of high-order thalamic nuclei, typically working in a tonic mode, driving high frequency cortical oscillations [121]. In the presence of synuclein-related pathology, the deregulated PPN cholinergic neurons [123] unlock the TRN, which prominently consist of gabaergic inhibitory neurons, inducing hyperpolarization of high-order thalamic nuclei. These nuclei lose their tonic mode and enter the burst firing mode, promoting TCD Theta rhythms. The low-frequency cortical oscillations disentangle the DMN from frontoparietal control systems (Suppl. Fig. 1).
Supporting the model, several studies have shown the presence of neuropathological thalamic alterations in DLB and PDD along with altered GABAergic and cholinergic receptors and structural and cellular abnormalities of the thalamic nuclei [124–132]. Interestingly, a recent review extensively analyzed the role of α7 nicotinic receptor activity of TRN neurons in production of DLB-hallucinations [133]. Of note, the presence of DMN decoupling along with signs of primary thalamic degeneration has also been observed in frontotemporal dementia patients carrying the C9orf72 mutation, a condition characterized by severe psychosis [134]. Also, the occurrence of isolated vascular lesions within the anteromedial thalamic nuclei that project to the frontal cortex are known to induce disconnection from consensual reality [135].
The central role of TCD driven DMN decoupling also allows us to reconsider other hypotheses based on the similarity between hallucinations and REM sleep conditions, suggesting that Hall phenomena are due to REM sleep intrusions into conscious wakefulness [32]. In this study, the presence of EEG theta activity was considered to be an indicator of REM sleep intrusions, rather than the sudden onset of desynchronization and muscle atonia (e.g., as it happens in narcolepsy); it was the presence of theta activity that drove the categorization of possible REM intrusions [32]. However, the presence of continuous theta activity in PDD and DLB argues against any possible categorization of REM sleep phases different from a healthy population where transient theta is observed in coincidence with REM sleep onset and desynchronization [110, 112].
The TCD driven DMN decoupling model suggests that occurrence of psychosis is due to entry into a condition where DMN is decoupled such as during dreaming, yet that is not a transient, triggering event, like in narcolepsy. Instead it is a status which could be initially overruled by re-entering frontal control networks, to later evolve, with disease progression, into the dominant status.
Implications for future studies and therapeutic efforts
The arguments provided in this review are derived from previous studies that have been re-analyzed in light of the “TCD-DMN decoupling” hypothesis for psychosis and cognitive fluctuations [27, 28, 89, 90, 106]. The hypothesis warrants further experimental validation as well as the investigation of additional features and mechanisms. Further studies should focus on the distinct mechanisms underlying the production of specific psychotic phenotypes, the differentiation of the phenomena upon the stage of the disease as well as their distinct EEG signatures. Interventional studies should also be envisioned in which monitoring of EEG activity can be employed to evaluate, in real-time, pharmacological modulation of theta activity exerted by anticholinergic drugs or by secondary anticholinergic activity of some neuroleptics. PD and DLB patients showing psychosis also need to be better assessed by taking advantage of dedicated scales that precisely quantify the frequency and severity of hallucinations, SFD, delusions, and cognitive fluctuations. Moreover, studies employing simultaneous EEG and fMRI recordings will be needed to detect distinct pathological patterns in subsets of patients. The circadian variations of TCD should also be investigated with longer EEG recordings. Finally, the use of MR spectroscopy will open a window on neurotransmitter correlates of the PD-DLB psychosis by allowing a detailed analysis of the regional changes in GABA and glutamate concentrations taking place in the DMN and control networks of patients [136].
The TCD-DMN decoupling model also allows the possibility to envisage novel treatments. To date, only two 5-HT2A antagonist drugs, primavanserin and clozapine, are employed for treatment of PD psychosis. K opioid receptors have been shown to modulate the DMN, and their pharmacological blockade may offer new therapeutic options. The known modulation of GABA transmission by 5-HT2A receptors [136] is a potential target. Furthermore, further evidence should be collected on the use of cholinesterase inhibitors as these compounds are known to reduce theta activity in DLB [137] and have some effects on visual hallucinations and cognitive fluctuations in PD-DLB [138]. The recent data that support the role of the α7 nicotinic receptors in modulation of TRN [133] neurons should be the basis for a renewed attention on cholinergic drugs. Additional options are offered by the re-evaluation of drugs that affect spiking and bursting activities. In that regard, antiepileptics that act on calcium channel-dependent spiking may offer a way to modulate the fast-theta or pre-alpha activity of PDD-DLB patients. Ethosuximide, an old antiepileptic drug with inhibitory activity on the thalamic 3-Hz spike-and-wave diffuse complexes of patients suffering from absence [139] should be tested to counteract TCD. In that respect, a recent study indicated that another antiepileptic drug, zonisamide, employed at low doses, promotes some improvement in DLB patients [140], an effect that may be ascribed to inhibition of thalamic bursts. In addition, the TCD-DMN decoupling model may be a target for deep brain stimulation.
We believe that a significant limitation of our model concerns the conceptualization of SFD. While decoupling of DMN to form random connections seems to many [20, 22, 86–94, 96] to be a reasonable account of hallucinations, it is less convincing as an account of SFD or Delusions, which, according to main objections, remain fixed over a long period of time, or are seen in younger patients, or are considered a negligible feature of PD, or are considered indistinguishable from somatic hallucinations. The nosologic entity of SFD is still unresolved and a matter of lively debate between movement disorder specialists and psychiatrists.
Undoubtedly SFD are not ranked as a psychosis [44], and should not be, therefore, included in discussions of psychosis mechanisms. Our counter objection is that SFD show commonalities with other manifestations of the PD-DLB psychosis, as they are the result of perceptions that lack consensual reality checking and that may be a distinct manifestation of a phenomenological continuum that encompasses different perceptive domains like the viscero-motor, ideational and visual modalities. In that conceptual framework, SFD (with or without motor signs) represents a transient or stable break from reality, like what occurs with hallucinations and delusions. In the case of visual hallucinations, the notion that simple (eidolic) and complex (paraphrenic) hallucinations in PD-DLB are part of a continuum is supported scientifically [1, 97]. We here propose that increased somatization scores on neuropsychological testing and complex somatic delusions are part of an SFD continuum occurring in PD-DLB. Along that line of thinking, it defies logic why a positive pareidolic test [36] should be interpreted as a valid sign of impending psychosis and a positive somatic symptom test cannot predict the delusional progression of SFD. We would also like to point out that the model is the first to posit a significant emphasis on the dysfunction of key anatomical regions like the thalamus and the PCC. This notion is in line with recent evidence indicating a weak correlation between the severity of the clinical phenotype and the pathological load found in the brain of PD patients, thereby suggesting that strategic alterations of critical regions plays a significant role in shaping the disease course [141]. Finally, we intend our model to be a work-in-progress hypothesis set to foster further research that will provide the necessary experimental confirmation and/or refinement of the proposed mechanisms and pathways.
Supplementary Material
Supplementary Figure 1. High-order thalamic modulation of cortical activity. The scheme depicts the modulation exerted by the high-order thalamic nuclei on the DMN upon physiological and pathological conditions. The red boxes indicate overactivation, the blue boxes inhibition. Abbreviations: Default Mode Network, DMN; pedunculopontine nucleus, PPN; thalamic reticular nucleus, TNR.
Acknowledgments:
We are in debt to Profs. L Ferini-Strambi and M. Tinazzi for reading and commenting on earlier versions of the manuscript, to Drs. V. Di Stefano, M. Russo, and F. Dono for helping with bibliography and figures in an earlier version of the manuscript.
Disclosures: Prof. Marco Onofrj has served on the scientific advisory boards of GlaxoSmithKline, Novartis, Lundbeck, Eisai, Valeant, Medtronic, and Newron; has received speaker honoraria from Zambon, the World Parkinson Congress, the Movement Disorder Society, and the Atypical Dementias congress; publishing royalties from Springer; was an invited guest and lecturer for the Mental Disorders in Parkinson Disease Congress; serves on the editorial board of Medicine (Baltimore) and Frontiers in Neurology; has been employed as a speaker for Boehringer Ingelheim, GlaxoSmithKline, UCB, and Zambon; and has received research support from the Italian Ministry of Health and the Italian Ministry of Education. Prof. Alberto Espay has received grant support from the NIH, Great Lakes Neurotechnologies and the Michael J Fox Foundation; personal compensation as a consultant/scientific advisory board member for Abbvie, Adamas, Acadia, Acorda, Neuroderm, TEVA, Impax, Sunovion, Lundbeck, Osmotica Pharmaceutical, and US World Meds; publishing royalties from Lippincott Williams & Wilkins, Cambridge University Press, and Springer; and honoraria from Abbvie, UCB, USWorldMeds, Lundbeck, Acadia, Sunovion, the American Academy of Neurology, and the Movement Disorders Society. Dr. Laura Bonanni has received research support from the Italian Ministry of Health and the European Community,serves as an editorial board member for Journal of Alzheimer’s disease. Dr. Stefano Delli Pizzi serves as accademic editor of Scientific Reports, Behavioral and Brain Functions, and Medicine. Prof. Stefano Sensi serves as associate editor of Frontiers in Neuroscience, Frontiers in Psychiatry, PlosOne, and Scientific Reports and is supported by non-profit agencies [the Italian Ministry of Health, the AIRAlzh Onlus (ANCC-COOP), the Alzheimer’s Association - Part the Cloud: Translational Research Funding for Alzheimer’s Disease (18PTC-19–602325) and the Alzheimer’s Association - GAAIN Exploration to Evaluate Novel Alzheimer’s Queries (GEENA-Q-19–596282)].
This study is not industry-sponsored. Study Funding: no targeted funding reported.
References
- 1.Goetz CG, Stebbins GT, Ouyang B. Visual plus nonvisual hallucinations in Parkinson’s disease: development and evolution over 10 years. Mov Disord 2011;26(12):2196–2200. [DOI] [PubMed] [Google Scholar]
- 2.Diederich NJ, Fenelon G, Stebbins G, Goetz CG. Hallucinations in Parkinson disease. Nat Rev Neurol 2009;5(6):331–342. [DOI] [PubMed] [Google Scholar]
- 3.Fenelon G, Mahieux F, Huon R, Ziegler M. Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain 2000;123 (Pt 4):733–745. [DOI] [PubMed] [Google Scholar]
- 4.Ffytche DH, Creese B, Politis M, et al. The psychosis spectrum in Parkinson disease. Nat Rev Neurol 2017;13(2):81–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goetz CG, Leurgans S, Pappert EJ, Raman R, Stemer AB. Prospective longitudinal assessment of hallucinations in Parkinson’s disease. Neurology 2001;57(11):2078–2082. [DOI] [PubMed] [Google Scholar]
- 6.McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 2017;89(1):88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams DR, Lees AJ. Visual hallucinations in the diagnosis of idiopathic Parkinson’s disease: a retrospective autopsy study. Lancet Neurol 2005;4(10):605–610. [DOI] [PubMed] [Google Scholar]
- 8.Onofrj M, Bonanni L, Manzoli L, Thomas A. Cohort study on somatoform disorders in Parkinson disease and dementia with Lewy bodies. Neurology 2010;74(20):1598–1606. [DOI] [PubMed] [Google Scholar]
- 9.Stone J, Carson A, Duncan R, et al. Which neurological diseases are most likely to be associated with “symptoms unexplained by organic disease”. J Neurol 2012;259(1):33–38. [DOI] [PubMed] [Google Scholar]
- 10.Edwards MJ, Bhatia KP. Functional (psychogenic) movement disorders: merging mind and brain. Lancet Neurol 2012;11(3):250–260. [DOI] [PubMed] [Google Scholar]
- 11.Hallett M Patients with Parkinson disease are prone to functional neurological disorders. J Neurol Neurosurg Psychiatry 2018;89(6):557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parees I, Saifee TA, Kojovic M, et al. Functional (psychogenic) symptoms in Parkinson’s disease. Mov Disord 2013;28(12):1622–1627. [DOI] [PubMed] [Google Scholar]
- 13.Frasca Polara G, Fleury V, Stone J, et al. Prevalence of functional (psychogenic) parkinsonism in two Swiss movement disorders clinics and review of the literature. J Neurol Sci 2018;387:37–45. [DOI] [PubMed] [Google Scholar]
- 14.Aprahamian I, Yassuda MS, Martinelli JE. Somatoform and Conversion Disorder Preceding Lewy Body Dementia: A Newly Described Phenomenological Manifestation of the Disease. J Am Geriatr Soc 2015;63(9):1967–1969. [DOI] [PubMed] [Google Scholar]
- 15.Wissel BD, Dwivedi AK, Merola A, et al. Functional neurological disorders in Parkinson disease. J Neurol Neurosurg Psychiatry 2018;89(6):566–571. [DOI] [PubMed] [Google Scholar]
- 16.Corlett PR, Horga G, Fletcher PC, Alderson-Day B, Schmack K, Powers AR 3rd., Hallucinations and Strong Priors. Trends Cogn Sci 2019;23(2):114–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carrozzino D, Bech P, Patierno C, et al. Somatization in Parkinson’s Disease: A systematic review. Prog Neuropsychopharmacol Biol Psychiatry 2017;78:18–26. [DOI] [PubMed] [Google Scholar]
- 18.Factor SA, Steenland NK, Higgins DS, et al. Disease-related and genetic correlates of psychotic symptoms in Parkinson’s disease. Mov Disord 2011;26(12):2190–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X, Sundquist J, Hwang H, Sundquist K. Impact of psychiatric disorders on Parkinson’s disease : a nationwide follow-up study from Sweden. J Neurol 2008;255(1):31–36. [DOI] [PubMed] [Google Scholar]
- 20.Shine JM, O’Callaghan C, Halliday GM, Lewis SJ. Tricks of the mind: Visual hallucinations as disorders of attention. Prog Neurobiol 2014;116:58–65. [DOI] [PubMed] [Google Scholar]
- 21.Collerton D, Perry E, McKeith I. Why people see things that are not there: a novel Perception and Attention Deficit model for recurrent complex visual hallucinations. Behav Brain Sci 2005;28(6):737–757; discussion 757–794. [DOI] [PubMed] [Google Scholar]
- 22.Yao N, Shek-Kwan Chang R, Cheung C, et al. The default mode network is disrupted in Parkinson’s disease with visual hallucinations. Hum Brain Mapp 2014;35(11):5658–5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A 2003;100(1):253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raichle ME. The brain’s default mode network. Annu Rev Neurosci 2015;38:433–447. [DOI] [PubMed] [Google Scholar]
- 25.Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain 2014;137(Pt 1):12–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carhart-Harris RL, Leech R, Hellyer PJ, et al. The entropic brain: a theory of conscious states informed by neuroimaging research with psychedelic drugs. Front Hum Neurosci 2014;8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonanni L, Thomas A, Tiraboschi P, Perfetti B, Varanese S, Onofrj M. EEG comparisons in early Alzheimer’s disease, dementia with Lewy bodies and Parkinson’s disease with dementia patients with a 2-year follow-up. Brain 2008;131(Pt 3):690–705. [DOI] [PubMed] [Google Scholar]
- 28.Bonanni L, Perfetti B, Bifolchetti S, et al. Quantitative electroencephalogram utility in predicting conversion of mild cognitive impairment to dementia with Lewy bodies. Neurobiol Aging 2015;36(1):434–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geraedts VJ, Boon LI, Marinus J, et al. Clinical correlates of quantitative EEG in Parkinson disease: A systematic review. Neurology 2018;91(19):871–883. [DOI] [PubMed] [Google Scholar]
- 30.Llinas RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: A neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci U S A 1999;96(26):15222–15227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magnin M, Rey M, Bastuji H, Guillemant P, Mauguiere F, Garcia-Larrea L. Thalamic deactivation at sleep onset precedes that of the cerebral cortex in humans. Proc Natl Acad Sci U S A 2010;107(8):3829–3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arnulf I, Bonnet AM, Damier P, et al. Hallucinations, REM sleep, and Parkinson’s disease: a medical hypothesis. Neurology 2000;55(2):281–288. [DOI] [PubMed] [Google Scholar]
- 33.Goetz CG, Fan W, Leurgans S, Bernard B, Stebbins GT. The malignant course of “benign hallucinations” in Parkinson disease. Arch Neurol 2006;63(5):713–716. [DOI] [PubMed] [Google Scholar]
- 34.Barnes J, David AS. Visual hallucinations in Parkinson’s disease: a review and phenomenological survey. J Neurol Neurosurg Psychiatry 2001;70(6):727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Onofrj M, Gilbert GJ. GABA and hallucinations in Parkinson disease: Who is that sitting on my chair? Neurology 2018;91(7):293–294. [DOI] [PubMed] [Google Scholar]
- 36.Uchiyama M, Nishio Y, Yokoi K, Hosokai Y, Takeda A, Mori E. Pareidolia in Parkinson’s disease without dementia: A positron emission tomography study. Parkinsonism Relat Disord 2015;21(6):603–609. [DOI] [PubMed] [Google Scholar]
- 37.Diederich NJ, Goetz CG, Stebbins GT. Repeated visual hallucinations in Parkinson’s disease as disturbed external/internal perceptions: focused review and a new integrative model. Mov Disord 2005;20(2):130–140. [DOI] [PubMed] [Google Scholar]
- 38.Hamada H Pseudo-hallucination in schizophrenia. Annales Médico-psychologiques revue psychiatrique 1998;156(4):236–243. [Google Scholar]
- 39.Onofrj M, Thomas A, Martinotti G, Anzellotti F, DiGiannantonio M, Ciccocioppo F, Bonanni L The clinical associations of visual hallucinations In: Collerton D, Mosiman UP, Perry E The neuroscience of visual hallucinations. Oxford: Wiley Blackwell; 2015; p 91–12. [Google Scholar]
- 40.Fenelon G, Goetz CG, Karenberg A. Hallucinations in Parkinson disease in the prelevodopa era. Neurology 2006;66(1):93–98. [DOI] [PubMed] [Google Scholar]
- 41.Ey H, editor. Traite des Hallucinations, Paris, Masson and Co., 1973, pp 1–1451. [Google Scholar]
- 42.Onofrj M, Taylor JP,Monaco D,Franciotti R,Anzellotti F,Bonanni l,Onofrj V,Thomas A. Visual Hallucinations in PD and Lewy body dementias:old and new hypotheses. Behav Neurol. 2013;27(4) :479–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baizabal-Carvallo JF, Hallett M, Jankovic J. Pathogenesis and pathophysiology of functional (psychogenic) movement disorders. Neurobiol Dis 2019;127:32–44. [DOI] [PubMed] [Google Scholar]
- 44.American Psychiatric Association, editor. Diagnostic and statistical manual of mental disorders (5th ed.), Arlington, VA, 2013. [Google Scholar]
- 45.Edwards MJ, Stone J, Lang AE. Functional/psychogenic movement disorders: do we know what they are? Mov Disord 2014;29(13):1696–1697; discussion 1699–1701. [DOI] [PubMed] [Google Scholar]
- 46.Fahn S, Olanow CW. “Psychogenic movement disorders”: they are what they are. Mov Disord 2014;29(7):853–856. [DOI] [PubMed] [Google Scholar]
- 47. https://www.movementdisorders.org/MDS/About/Committees--Other-Groups/Study-Groups/FunctionalMD-Study-Group.htm.
- 48.Marsh GG, Markham CH. Does levodopa alter depression and psychopathology in Parkinsonism patients? J Neurol Neurosurg Psychiatry 1973;36(6):925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martin WE, Loewenson RB, Resch JA, Baker AB. Parkinson’s disease. Clinical analysis of 100 patients. Neurology 1973;23(8):783–790. [DOI] [PubMed] [Google Scholar]
- 50.Morel-Maroger A, editor. Maladie de Parkinson et syndrome parkinsonien Encyclopédie Médico-Chirurgicale sur Le Systeme Nerveux. EMC, 1975. [Google Scholar]
- 51.Bergouignan M, Loiseau P. Encéphalite épidémique: maladie d’Economo-Cruchet. Encyclopédie Medicale Sur Le Systéme Nerveux 1964:1–12. [Google Scholar]
- 52.Claude H, Ey H, editors. Troubles psychosensoriels et etats onirique dans l’encephalite epidemique chronique. Presse Medicale, 1933;256–270 [Google Scholar]
- 53.Ey H, Bernard P, Brisset C. Troubles Mentaux dans l’Encephalite Epidemique In: Ey H, Benard P, Brisset C. Eds. Manuel de Psychiatrie. Paris: Masson et c.ie. 1973; p853–856 [Google Scholar]
- 54.Neville F, editor. Les complications et les sequelles mentales de l’encephalite epidemique. Annales MedicoPsychologiques, 1941, pp 312–321. [Google Scholar]
- 55.van Bogaert L, editor. L’hystérie et les fonctions diencéphaliques, étude neurologique Comptes rendus: Congrès des médecins aliénistes et neurologistes de France et des pays de langue française. Bruxelles, Masson et Cie., 1935, pp 169–277. [Google Scholar]
- 56.Van Bogaert L, editor. Encéphalite léthargique Hb. Spez. Path. Anat Berlin, Springer, 1958, pp 121–143. [Google Scholar]
- 57.Siri C, Cilia R, De Gaspari D, et al. Psychiatric symptoms in Parkinson’s disease assessed with the SCL-90R self-reported questionnaire. Neurol Sci 2010;31(1):35–40. [DOI] [PubMed] [Google Scholar]
- 58.Costa A, Peppe A, Carlesimo GA, Salamone G, Caltagirone C. Neuropsychological correlates of alexithymia in Parkinson’s disease. J Int Neuropsychol Soc 2007;13(6):980–992. [DOI] [PubMed] [Google Scholar]
- 59.Lin HL, Lin HC, Chen YH. Psychiatric diseases predated the occurrence of Parkinson disease: a retrospective cohort study. Ann Epidemiol 2014;24(3):206–213. [DOI] [PubMed] [Google Scholar]
- 60.Lubomski M, Rushworth RL, Tisch S. Hospitalisation and comorbidities in Parkinson’s disease: a large Australian retrospective study. J Neurol Neurosurg Psychiatry 2015;86(3):324–330. [DOI] [PubMed] [Google Scholar]
- 61.Nilsson FM, Kessing LV, Bolwig TG. Increased risk of developing Parkinson’s disease for patients with major affective disorder: a register study. Acta Psychiatr Scand 2001;104(5):380–386. [DOI] [PubMed] [Google Scholar]
- 62.Barrell K, Bureau B, Turcano P, et al. High-Order Visual Processing, Visual Symptoms, and Visual Hallucinations: A Possible Symptomatic Progression of Parkinson’s Disease. Front Neurol 2018;9:999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pagonabarraga J, Soriano-Mas C, Llebaria G, Lopez-Sola M, Pujol J, Kulisevsky J. Neural correlates of minor hallucinations in non-demented patients with Parkinson’s disease. Parkinsonism Relat Disord 2014;20(3):290–296. [DOI] [PubMed] [Google Scholar]
- 64.Radziunas A, Deltuva VP, Tamasauskas A, et al. Brain MRI morphometric analysis in Parkinson’s disease patients with sleep disturbances. BMC Neurol 2018;18(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Edwards MJ, Adams RA, Brown H, Parees I, Friston KJ. A Bayesian account of ‘hysteria’. Brain 2012;135(Pt 11):3495–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 2013;14(7):502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rauschecker JP. Where, When, and How: Are they all sensorimotor? Towards a unified view of the dorsal pathway in vision and audition. Cortex 2018;98:262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vuilleumier P Brain circuits implicated in psychogenic paralysis in conversion disorders and hypnosis. Neurophysiol Clin 2014;44(4):323–337. [DOI] [PubMed] [Google Scholar]
- 69.Perez-Perez J, Pagonabarraga J, Fernandez-Bobadilla R, Kulisevsky J. “String Hallucinations”: Multimodal Tactile and Visual Hallucinations in Parkinson’s Disease. Mov Disord Clin Pract 2016;3(2):180–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain 2006;129(Pt 3):564–583. [DOI] [PubMed] [Google Scholar]
- 71.Cunningham SI, Tomasi D, Volkow ND. Structural and functional connectivity of the precuneus and thalamus to the default mode network. Hum Brain Mapp 2017;38(2):938–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Callard F, Margulies DS. What we talk about when we talk about the default mode network. Front Hum Neurosci 2014;8:619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hellyer PJ, Scott G, Shanahan M, Sharp DJ, Leech R. Cognitive Flexibility through Metastable Neural Dynamics Is Disrupted by Damage to the Structural Connectome. J Neurosci 2015;35(24):9050–9063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fingelkurts AA, Fingelkurts AA. Persistent operational synchrony within brain default-mode network and self-processing operations in healthy subjects. Brain Cogn 2011;75(2):79–90. [DOI] [PubMed] [Google Scholar]
- 75.Preller KH, Razi A, Zeidman P, Stampfli P, Friston KJ, Vollenweider FX. Effective connectivity changes in LSD-induced altered states of consciousness in humans. Proc Natl Acad Sci U S A 2019;116(7):2743–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hobson JA, Pace-Schott EF. The cognitive neuroscience of sleep: neuronal systems, consciousness and learning. Nat Rev Neurosci 2002;3(9):679–93. [DOI] [PubMed] [Google Scholar]
- 77.Amico E, Gomez F, Di Perri C, et al. Posterior cingulate cortex-related co-activation patterns: a resting state FMRI study in propofol-induced loss of consciousness. PLoS One 2014;9(6):e100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Manoliu A, Riedl V, Zherdin A, et al. Aberrant dependence of default mode/central executive network interactions on anterior insular salience network activity in schizophrenia. Schizophr Bull 2014;40(2):428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carhart-Harris RL, Muthukumaraswamy S, Roseman L, et al. Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proc Natl Acad Sci U S A 2016;113(17):4853–4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maqueda AE, Valle M, Addy PH, et al. Salvinorin-A Induces Intense Dissociative Effects, Blocking External Sensory Perception and Modulating Interoception and Sense of Body Ownership in Humans. Int J Neuropsychopharmacol 2015;18(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Graff-Radford J, Murray ME, Lowe VJ, et al. Dementia with Lewy bodies: basis of cingulate island sign. Neurology 2014;83(9):801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Imabayashi E, Soma T, Sone D, et al. Validation of the cingulate island sign with optimized ratios for discriminating dementia with Lewy bodies from Alzheimer’s disease using brain perfusion SPECT. Ann Nucl Med 2017;31(7):536–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Iizuka T, Kameyama M. Cingulate island sign on FDG-PET is associated with medial temporal lobe atrophy in dementia with Lewy bodies. Ann Nucl Med 2016;30(6):421–429. [DOI] [PubMed] [Google Scholar]
- 84.Lim SM, Katsifis A, Villemagne VL, et al. The 18F-FDG PET cingulate island sign and comparison to 123I-beta-CIT SPECT for diagnosis of dementia with Lewy bodies. J Nucl Med 2009;50(10):1638–1645. [DOI] [PubMed] [Google Scholar]
- 85.Whitwell JL, Graff-Radford J, Singh TD, et al. (18)F-FDG PET in Posterior Cortical Atrophy and Dementia with Lewy Bodies. J Nucl Med 2017;58(4):632–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee Y, Ham JH, Cha J, et al. The cholinergic contribution to the resting-state functional network in non-demented Parkinson’s disease. Sci Rep 2018;8(1):7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baggio HC, Segura B, Sala-Llonch R, et al. Cognitive impairment and resting-state network connectivity in Parkinson’s disease. Hum Brain Mapp 2015;36(1):199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fang J, Chen H, Cao Z, et al. Impaired brain network architecture in newly diagnosed Parkinson’s disease based on graph theoretical analysis. Neurosci Lett 2017;657:151–158. [DOI] [PubMed] [Google Scholar]
- 89.Franciotti R, Falasca NW, Bonanni L, et al. Default network is not hypoactive in dementia with fluctuating cognition: an Alzheimer disease/dementia with Lewy bodies comparison. Neurobiol Aging 2013;34(4):1148–1158. [DOI] [PubMed] [Google Scholar]
- 90.Franciotti R, Delli Pizzi S, Perfetti B, et al. Default mode network links to visual hallucinations: A comparison between Parkinson’s disease and multiple system atrophy. Mov Disord 2015;30(9):1237–1247. [DOI] [PubMed] [Google Scholar]
- 91.Galvin JE, Price JL, Yan Z, Morris JC, Sheline YI. Resting bold fMRI differentiates dementia with Lewy bodies vs Alzheimer disease. Neurology 2011;76(21):1797–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kenny ER, Blamire AM, Firbank MJ, O’Brien JT. Functional connectivity in cortical regions in dementia with Lewy bodies and Alzheimer’s disease. Brain 2012;135(Pt 2):569–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Krajcovicova L, Mikl M, Marecek R, Rektorova I. The default mode network integrity in patients with Parkinson’s disease is levodopa equivalent dose-dependent. J Neural Transm (Vienna) 2012;119(4):443–454. [DOI] [PubMed] [Google Scholar]
- 94.Rektorova I, Krajcovicova L, Marecek R, Novakova M, Mikl M. Default mode network connectivity patterns associated with visual processing at different stages of Parkinson’s disease. J Alzheimers Dis 2014;42 Suppl 3:S217–228. [DOI] [PubMed] [Google Scholar]
- 95.Shine JM, Muller AJ, O’Callaghan C, Hornberger M, Halliday GM, Lewis SJ. Abnormal connectivity between the default mode and the visual system underlies the manifestation of visual hallucinations in Parkinson’s disease: a task-based fMRI study. NPJ Parkinsons Dis 2015;1:15003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schumacher J, Peraza LR, Firbank M, et al. Functional connectivity in dementia with Lewy bodies: A within- and between-network analysis. Hum Brain Mapp 2018;39(3):1118–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bejr-Kasem H, Pagonabarraga J, Martinez-Horta S, et al. Disruption of the default mode network and its intrinsic functional connectivity underlies minor hallucinations in Parkinson’s disease. Mov Disord 2019;34(1):78–86. [DOI] [PubMed] [Google Scholar]
- 98.Blakemore RL, Sinanaj I, Galli S, Aybek S, Vuilleumier P. Aversive stimuli exacerbate defensive motor behaviour in motor conversion disorder. Neuropsychologia 2016;93(Pt A):229–241. [DOI] [PubMed] [Google Scholar]
- 99.Boeckle M, Liegl G, Jank R, Pieh C. Neural correlates of conversion disorder: overview and meta-analysis of neuroimaging studies on motor conversion disorder. BMC Psychiatry 2016;16:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Diez I, Ortiz-Teran L, Williams B, et al. Corticolimbic fast-tracking: enhanced multimodal integration in functional neurological disorder. J Neurol Neurosurg Psychiatry 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hedera P Metabolic hyperactivity of the medial posterior parietal lobes in psychogenic tremor. Tremor Other Hyperkinet Mov (N Y) 2012; 2:tre-02-50-441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Otti A, Guendel H, Henningsen P, Zimmer C, Wohlschlaeger AM, Noll-Hussong M. Functional network connectivity of pain-related resting state networks in somatoform pain disorder: an exploratory fMRI study. J Psychiatry Neurosci 2013;38(1):57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vanneste S, Song JJ, De Ridder D. Thalamocortical dysrhythmia detected by machine learning. Nat Commun 2018;9(1):1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Andersen PA, Andersen SA. Thalamic origin of cortical rhythmic activity in: Handbook of EEG and Clinical Neurophysiology. In: Remond A, ed. Handbook of EEG and Clinical Neurophysiology. Amsterdam: Elsevier, 1974:91–118. [Google Scholar]
- 105.Walker MP, Ayre GA, Cummings JL, et al. Quantifying fluctuation in dementia with Lewy bodies, Alzheimer’s disease, and vascular dementia. Neurology 2000;54(8):1616–1625. [DOI] [PubMed] [Google Scholar]
- 106.Franciotti R, Iacono D, Della Penna S, et al. Cortical rhythms reactivity in AD, LBD and normal subjects: a quantitative MEG study. Neurobiol Aging 2006;27(8):1100–1109. [DOI] [PubMed] [Google Scholar]
- 107.Dauwan M, Hoff JI, Vriens EM, Hillebrand A, Stam CJ, Sommer IE. Aberrant resting-state oscillatory brain activity in Parkinson’s disease patients with visual hallucinations: An MEG source-space study. Neuroimage Clin 2019;22:101752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Manni R, Mazzarello P. Hallucinations, REM sleep, and Parkinson’s disease: a medical hypothesis. Neurology 2001;57(7):1350–1351. [DOI] [PubMed] [Google Scholar]
- 109.Meir K Atlas of Clinical Sleep Medicine. Elsevier; 2009. [Google Scholar]
- 110.Fantini ML, Ferini-Strambi L, Montplaisir J. Idiopathic REM sleep behavior disorder: toward a better nosologic definition. Neurology 2005;64(5):780–786. [DOI] [PubMed] [Google Scholar]
- 111.Betrouni N, Delval A, Chaton L, et al. Electroencephalography-based machine learning for cognitive profiling in Parkinson’s disease: Preliminary results. Mov Disord 2019;34(2):210–217. [DOI] [PubMed] [Google Scholar]
- 112.Ruffini G, Ibanez D, Kroupi E, et al. Algorithmic Complexity of EEG for Prognosis of Neurodegeneration in Idiopathic Rapid Eye Movement Behavior Disorder (RBD). Ann Biomed Eng 2019;47(1):282–296. [DOI] [PubMed] [Google Scholar]
- 113.Llinas R, Urbano FJ, Leznik E, Ramirez RR, van Marle HJ. Rhythmic and dysrhythmic thalamocortical dynamics: GABA systems and the edge effect. Trends Neurosci 2005;28(6):325–333. [DOI] [PubMed] [Google Scholar]
- 114.Ferrarelli F, Tononi G. Reduced sleep spindle activity point to a TRN-MD thalamus-PFC circuit dysfunction in schizophrenia. Schizophr Res 2017;180:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.John YJ, Zikopoulos B, Bullock D, Barbas H. Visual Attention Deficits in Schizophrenia Can Arise From Inhibitory Dysfunction in Thalamus or Cortex. Comput Psychiatr 2018;2:223–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zikopoulos B, Barbas H. Prefrontal projections to the thalamic reticular nucleus form a unique circuit for attentional mechanisms. J Neurosci 2006;26(28):7348–7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pratt JA, Morris BJ. The thalamic reticular nucleus: a functional hub for thalamocortical network dysfunction in schizophrenia and a target for drug discovery. J Psychopharmacol 2015;29(2):127–137. [DOI] [PubMed] [Google Scholar]
- 118.Nakajima M, Halassa MM. Thalamic control of functional cortical connectivity. Curr Opin Neurobiol 2017;44:127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jaramillo J, Mejias JF, Wang XJ. Engagement of Pulvino-cortical Feedforward and Feedback Pathways in Cognitive Computations. Neuron 2019;101(2):321–336 e329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Saalmann YB, Kastner S. The cognitive thalamus. Front Syst Neurosci 2015;9:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Benarroch EE. Pedunculopontine nucleus: functional organization and clinical implications. Neurology 2013;80(12):1148–1155. [DOI] [PubMed] [Google Scholar]
- 122.Varela C Thalamic neuromodulation and its implications for executive networks. Front Neural Circuits 2014;8:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Janzen J, van ‘t Ent D, Lemstra AW, Berendse HW, Barkhof F, Foncke EM. The pedunculopontine nucleus is related to visual hallucinations in Parkinson’s disease: preliminary results of a voxel-based morphometry study. J Neurol 2012;259(1):147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Erskine D, Thomas AJ, Attems J, et al. Specific patterns of neuronal loss in the pulvinar nucleus in dementia with lewy bodies. Mov Disord 2017;32(3):414–422. [DOI] [PubMed] [Google Scholar]
- 125.Erskine D, Ding J, Thomas AJ, et al. Molecular changes in the absence of severe pathology in the pulvinar in dementia with Lewy bodies. Mov Disord 2018;33(6):982–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Perry EK, Morris CM, Court JA, et al. Alteration in nicotine binding sites in Parkinson’s disease, Lewy body dementia and Alzheimer’s disease: possible index of early neuropathology. Neuroscience 1995;64(2):385–395. [DOI] [PubMed] [Google Scholar]
- 127.Delli Pizzi S, Franciotti R, Taylor JP, et al. Thalamic Involvement in Fluctuating Cognition in Dementia with Lewy Bodies: Magnetic Resonance Evidences. Cereb Cortex 2015;25(10):3682–3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Delli Pizzi S, Franciotti R, Taylor JP, et al. Structural Connectivity is Differently Altered in Dementia with Lewy Body and Alzheimer’s Disease. Front Aging Neurosci 2015;7:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lisman JE. Bursts as a unit of neural information: making unreliable synapses reliable. Trends Neurosci 1997;20(1):38–43. [DOI] [PubMed] [Google Scholar]
- 130.Miners JS, Renfrew R, Swirski M, Love S. Accumulation of alpha-synuclein in dementia with Lewy bodies is associated with decline in the alpha-synuclein-degrading enzymes kallikrein-6 and calpain-1. Acta Neuropathol Commun 2014;2:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pinault D N-methyl d-aspartate receptor antagonists ketamine and MK-801 induce wake-related aberrant gamma oscillations in the rat neocortex. Biol Psychiatry 2008;63(8):730–735. [DOI] [PubMed] [Google Scholar]
- 132.Swirski M, Miners JS, de Silva R, et al. Evaluating the relationship between amyloid-beta and alpha-synuclein phosphorylated at Ser129 in dementia with Lewy bodies and Parkinson’s disease. Alzheimers Res Ther 2014;6(5–8):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Esmaeeli S, Murphy K, Swords GM, Ibrahim BA, Brown JB, Llano DA Visual hallucinations, thalamocortical physiology and lewy body disease: a review. Neuroscience & Biobehavioral Reviews In press. [DOI] [PubMed] [Google Scholar]
- 134.Lee SE, Khazenzon AM, Trujillo AJ, et al. Altered network connectivity in frontotemporal dementia with C9orf72 hexanucleotide repeat expansion. Brain 2014;137(Pt 11):3047–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Onofrj V, Delli Pizzi S, Franciotti R, et al. Medio-dorsal thalamus and confabulations: Evidence from a clinical case and combined MRI/DTI study. Neuroimage Clin 2016;12:776–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Firbank MJ, Parikh J, Murphy N, et al. Reduced occipital GABA in Parkinson disease with visual hallucinations. Neurology 2018;91(7):675–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Onofrj M, Thomas A, Iacono D, Luciano AL, Di Iorio A. The effects of a cholinesterase inhibitor are prominent in patients with fluctuating cognition: a part 3 study of the main mechanism of cholinesterase inhibitors in dementia. Clin Neuropharmacol 2003;26(5):239–251. [DOI] [PubMed] [Google Scholar]
- 138.Thomas AJ, Burn DJ, Rowan EN, et al. A comparison of the efficacy of donepezil in Parkinson’s disease with dementia and dementia with Lewy bodies. Int J Geriatr Psychiatry 2005;20(10):938–944. [DOI] [PubMed] [Google Scholar]
- 139.Coulter DA, Huguenard JR, Prince DA. Characterization of ethosuximide reduction of low-threshold calcium current in thalamic neurons. Ann Neurol 1989;25(6):582–593. [DOI] [PubMed] [Google Scholar]
- 140.Murata M, Odawara T, Hasegawa K, et al. Adjunct zonisamide to levodopa for DLB parkinsonism: A randomized double-blind phase 2 study. Neurology 2018;90(8):e664–e672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Espay AJ, Brundin P, Lang AE. Precision medicine for disease modification in Parkinson disease. Nat Rev Neurol 2017;13(2):119–126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. High-order thalamic modulation of cortical activity. The scheme depicts the modulation exerted by the high-order thalamic nuclei on the DMN upon physiological and pathological conditions. The red boxes indicate overactivation, the blue boxes inhibition. Abbreviations: Default Mode Network, DMN; pedunculopontine nucleus, PPN; thalamic reticular nucleus, TNR.