Abstract
Screening of small-molecule libraries is a productive method for identifying both chemical probes of disease-related targets and potential starting points for drug discovery. In this article, we focus on strategies such as diversity-oriented synthesis that aim to explore novel areas of chemical space efficiently by populating small-molecule libraries with compounds containing structural features that are typically under-represented in commercially available screening collections. Drawing from over a decade’s worth of examples, we highlight how the design and synthesis of such libraries have been enabled by modern synthetic chemistry, and we illustrate the impact of the resultant chemical probes and drug leads in a wide range of diseases.
Introduction
Organic small molecules with novel mechanisms of action (nMoAs) are needed to help address today’s most challenging biomedical problems. In particular, potential disease-related molecular targets indicated by the study of human genetics are often poorly characterized and cannot be studied or modulated with existing chemical tools.1 Drugs with nMoAs are also urgently needed to address resistance to existing drugs for cancer and infectious diseases.2–4
The search for new chemical probes and drugs can begin in many places. Natural products and their derivatives, for example, have played important roles in the study and treatment of disease for thousands of years.5–9 Alternatively, when the therapeutic target is known, advances in synthetic organic chemistry, structural biology, computational modeling, and screening have enabled researchers to construct, identify, and optimize bioactive compounds via structure-based design.10,11 While these techniques and others have long histories of success in drug discovery,12–14 this article will primarily discuss high-throughput screening of small-molecule libraries, in which researchers sift through collections of compounds (103 to >106 members) to find “hits” that exhibit a desired biological activity.
The use of small-molecule screening in early-stage drug discovery expanded dramatically in the 1990s with the introduction of new screening technologies and more efficient methods to synthesize large numbers of compounds with combinatorial chemistry. Given the vastness of “chemical space,” all of the molecules synthesized to date represent only a tiny fraction of all possible compounds that have properties similar to those of existing chemical probes and drugs (Box 1). This limitation has spurred chemists to want to populate the compound “libraries” being used in screening experiments with a more optimal set of molecules. Early and insightful thoughts into valuable features of compound libraries were offered by Paul Bartlett and colleagues, who suggested that libraries can be classified in one of two ways: 1) “focused” libraries or 2) “prospecting” libraries.15 Focused libraries—also known as targeted or biased libraries—typically comprise analogs of a known bioactive to generate structure–activity relationships (SAR) that can inform optimization efforts. Conversely, prospecting libraries eschew a specific molecular architecture in favor of combinations of available starting materials that maximize the structural novelty and diversity of their products. This strategy is particularly appealing when no known bioactives exist or an nMoA compound is desired.
Box 1 |. Physicochemical properties of chemical libraries and “drug-likeness”.
Increases in screening throughput have emphasized the need for compounds that can be translated easily into drug leads and clinical candidates. Even experienced medicinal chemists, however, have difficulty identifying such molecules by manual inspection of chemical structure alone.244 In response, and pioneered originally by the late Corwin Hansch at Pomona College,245 frameworks that connect “drug-like” performance properties (e.g. solubility and oral bioavailability) to small-molecule physicochemical properties (e.g. molecular weight and lipophilicity) have emerged over the past decades. These metrics have been designed—often retrospectively—to guide chemists towards molecules with greater chances of success in both preclinical and clinical testing, but they should not been seen as strict limits or cutoffs.246
Properties correlated with success in drug discovery can also benefit the development of chemical probes.247 Compounds containing structural motifs known to cause molecules to misbehave in high-throughput assays (e.g. binding proteins non-selectively, generating spurious activity readings, or disrupting cellular membranes) are called pan-assay interference compounds (PAINS).248 Identifying and de-prioritizing these compounds can reduce rates of false positives. Validating potency alone, however, does not guarantee the success of a probe- or drug-discovery campaign. Compounds with intracellular targets must be sufficiently lipophilic to traverse at least one biological membrane en route to its appropriate cellular compartment. Simultaneously, they must be sufficiently hydrophilic to be soluble in aqueous assay buffers. Lipinski’s “rule of 5,” which was designed to predict human oral bioavailability, recommends that chemists consider factors like cLogP and molecular weight to achieve a suitable balance between solubility, membrane permeability, and other drug-like properties.171 Similarly, Veber and colleagues have proposed that a compound’s polar surface area and number of rotatable bonds constitute an orthogonal set of criteria for predicting oral bioavailability.249 Groups have also applied computational tools like machine learning algorithms to model the relationship between chemical structure and drug-likeness.250
Screening experiments with the 100,000-member diversity-oriented synthesis (DOS) collection at the Broad Institute provide an illustrative set of lab-based case studies. Because the library was not designed to address a specific disease or class of targets, it comprises hundreds of smaller sub-libraries with distinct scaffolds to maximize its biological performance diversity.251 Each sub-library was populated with compounds of “intermediate” complexity (as determined by factors like sp3 content and quantity of stereogenic elements) containing rigidifying ring systems. While the library has afforded probes for targets throughout the proteome, important lessons can be gleaned from its shortcomings. In particular, some hits have exhibited poor aqueous solubility, although medicinal chemistry efforts have been able to improve the properties of many of these compounds.79,93,117,181,205 Next-generation libraries are currently being designed that de-emphasize the large, lipophilic structures that result from many of combinatorial chemistry’s most widely-used transformations.243,252
The compounds described in this article contain chemical features that are under-represented in commercially available drug-like libraries. To begin to assess the compatibility of these types of compounds with current standards of drug-likeness, we can compute cheminformatic descriptors (via the Molinspiration molecular properties calculator) for the 11 molecules highlighted in this article:
| MW (Da) | cLogP | TPSA (Å2) | rotatable bonds | Ro5 violations | |
|---|---|---|---|---|---|
| 476.71 | 4.04 | 84.3 | 4.9 | 0.8 | |
| median | 501.63 | 4.02 | 79.9 | 5 | 1 |
These values are broadly consistent with various traditional measures of drug-likeness.171,249,253 This simple analysis suggests that under-represented chemical features (e.g. chirality and high sp3 content) are compatible with computed chemical properties that have been argued previously to be valuable in drug discovery.
Instead, initial library construction efforts were dominated by the acquisitions of increasingly larger collections from vendors because larger collections are in general thought to increase the probability of finding good starting points for drug discovery.16 Although this approach has been a productive source of drugs and probes, commercial vendors tend to increase library size by prioritizing ease-of-synthesis and availability of starting materials (e.g. by using reactions that link compounds containing planar, aromatic rings). These reaction products typically have a higher content of sp2-hydridized carbons and a lower content of sp3-hybridized carbons, leading to an unequal distribution of structural features in small-molecule libraries. Therefore, most areas of synthetically accessible chemical space remain underexplored.17
Not all compounds containing under-represented structures, however, are equally worthy of attention in bioactive discovery. Taking a cheminformatics-based approach, Shelat and Guy have proposed that compounds that are structurally distinct from the contents of commercial screening collections are more likely to act via nMoAs.18 Natural products are one such class of compounds,19,20 and they have been a productive source of chemical tools and drugs, as noted above. However, billions of years of selection pressure commonly endow natural products with exquisite specificity for their targets, which are most often essential proteins (e.g. tubulin and actin) whose inhibition will typically lead to cell death and in some cases overt toxicity.21 In one study, the molecular targets of natural products were enriched in proteins constituting the most connected nodes of protein–protein interaction networks, consistent with natural selection yielding toxins used by organisms to gain advantage over competitors.22 Furthermore, given their often high level of structural complexity, it can be challenging to synthesize many analogs of natural products to explore structure–activity relationships.
Intuitively, the high structural complexity of natural products supports the principle that the three-dimensional topography of binding sites on macromolecular targets would benefit from similarly 3D compounds. Developments in synthetic methodology over the past three decades have facilitated the construction of molecules that exhibit the intricate structural architectures and other chemical features that are common in natural products but under-represented in commercial screening collections. Some noteworthy examples include the establishment of transition metal-mediated coupling and metathesis reactions, asymmetric catalysis, and organocatalysis, all of which have been reviewed extensively.23–25 These transformations have enabled efficient syntheses of compounds that contain synthetically challenging structural features like medium-sized rings,26 non-peptidic macrocycles,27,28 and spirocyclic, fused, or bridged bi- and polycyclic ring systems,29 among many others.30–32
The concept underlying diversity-oriented synthesis (DOS), which was introduced in 2000,33 is to harness such advances to synthesize libraries of compounds that incorporate chemical features common to natural products, including sp3-hybridized basic nitrogen atoms, stereogenic elements, and novel skeletons. The resulting compounds are not analogs of specific classes of natural products, yet they have the overall appearance of natural products. In order to overcome the tendency of natural selection to select for the limited number of essential targets, this process purposefully breaks the link between natural selection and the generation of natural product-like compounds. (We use the term “natural product-like” to refer to compounds having chemical features found in ensembles of natural products, not necessarily just in specific ones.)
After a decade of developing these synthetic pathways, executing on them to make libraries of compounds that have under-represented chemical features, and testing those compounds in biological assays, in this article we focus on the remarkable activities of the resulting probes and drug leads. These studies begin to address the question of whether novel chemistry is a productive method to generate compounds that act via novel mechanisms. It is not experimentally practical, or arguably feasible, to determine whether this approach is more or less effective than other means to identify nMoA compounds, such as fragment-based drug discovery or other small-molecule synthesis and screening techniques.7,12,34 And given the vast numbers of accessible chemical structures, a diversity of approaches to find nMoA compounds could be valuable. Our aim here is simply to illustrate the value of compounds that have been identified using DOS strategies and related approaches to produce compounds that are structurally dissimilar from those in commercially available screening collections.
So, after briefly summarizing the strategic considerations behind the use of modern asymmetric synthesis to construct small-molecule screening libraries, we will focus on the performances of the resulting chemical probes and drug leads by discussing examples in a wide range of therapeutic areas.
Strategies for library synthesis
The strategy and implementation of DOS have been reviewed previously,33,35,36 but briefly, short reaction pathways are designed such that libraries of skeletally and stereochemically diverse compounds can be synthesized efficiently. Initial efforts relied upon sequences of complexity-generating reactions (e.g. multicomponent reactions, cycloadditions, and ring-opening or -closing metathesis reactions) to construct intricate scaffolds from readily available precursors rapidly.37 Pathways were designed such that diversity could stem from large arrays of building blocks (appendage diversity),38 enumeration of stereoisomers (stereochemical diversity),39 and late-stage branching points that alter scaffold topography (skeletal diversity).40,41
An early DOS pathway that furnished a selective HDAC6 inhibitor provides an illustrative case study. Inspired by the metal-binding features of natural products trichostatin A and trapoxin A, a library of 7,200 1,3-dioxanes was synthesized.42 Metal-chelating functional groups were installed to bias the library towards HDAC inhibition, and the novel 1,3-dioxane core and its appendages were conceived to impart specificity. One library member, tubacin, was found to be a domain-specific inhibitor of HDAC6.43,44 Tubacin proved to be a useful tool compound in cancer and neurodegenerative disease research,45,46 but the impracticality of its synthesis hindered its optimization (EC50 = 2.9 μM) and clinical potential. A more efficient synthetic route would have aided the elaboration of SAR and the optimization of pharmacokinetic properties for in vivo studies. Recognizing this deficiency led to a rethinking of synthetic strategies leading to novel compound collections.
Modular pathways that facilitate analog synthesis can enable chemists to meet the demands required of probe and drug molecules. These syntheses can be realized by mimicking natural product biosynthesis, in which building blocks are coupled intermolecularly and then cyclized intramolecularly—from penicillins to terpenes.47 This process introduces both topographic complexity and rigidifying structural elements into the reaction products,48 the latter of which are important for decreasing the entropic cost of binding macromolecules. In the context of DOS, this concept is known as the “build–couple–pair” strategy (discussed further in examples below).49
Target-oriented syntheses of complex molecules, especially natural products, have also benefitted tremendously from the pairing of convergent, modular synthesis with asymmetric chemistry. For example, the development of the oncology drug eribulin was made possible by the Kishi group’s efficient synthesis of the marine natural product halichondrin B and subsequent analysis of structural analogs.50,51 This strategy has been extended to the synthesis of focused libraries of natural product derivatives, which can be screened to find a molecule with improved physicochemical properties or activity against drug-resistant cells or pathogens.52 Along these lines, the Myers group has developed platforms for fully synthetic tetracycline and macrolide antibiotics.53,54 The construction of such libraries would have been impractical without advances in reaction methodology and synthetic planning.
As suggested above, however, the search for nMoA compounds is likely better served by prospecting libraries synthesized via diversity synthesis. But because nothing is known a priori about the activities of library members, the corresponding pathways should permit facile alterations throughout the molecule; ideally, each position should be amenable to functionalization. Strategic implementation of build–couple–pair can fulfill this criterion.
The remainder of this article discusses the synthesis, optimization, and biomedical impact of compounds containing under-represented chemical features. The broad range of their biological activities shows that such compounds can act as useful probes and potential drugs throughout medicine.
Probes for heritable disease targets
Advances in genotyping, sequencing, and analysis of genetic data have facilitated the large-scale study of disease-related genetic variations.55,56 Despite the substantial challenges involved in translating genomic information into therapeutic insights and new medicines,57 genetics-based “experiments of nature” can reveal a type of dose–response of gene activity that suggests the physiological consequences of modulating a target with a therapeutic agent.58 Analyses of human genomic data have revealed variants associated with type-2 diabetes,59 inflammatory bowel disease,60 and psychiatric disorders,61,62 and these variants have pointed to disease-relevant pathways not previously recognized.63
Alongside genetic approaches, chemical probes capable of potently and selectively modulating protein activity are valuable tools for exploring therapeutic hypotheses before they are tested in the clinic.64–66 If the protein target of interest is known, various in vitro binding and functional assays can enable target-based screening campaigns to identify a corresponding small-molecule modulator. Ensuing optimization efforts are then driven by secondary assays and cell-based disease models. Alternatively, phenotypic screening can identify compounds that elicit a notable effect on disease-relevant physiology, most often in human cells. This technique is particularly useful when the phenotype of interest is not strongly associated with a causal protein target, or when searching for compounds with novel MoAs. Although an in-depth discussion of the relative benefits and challenges of these two strategies is beyond the scope of this article (for more information, see REF. 67–70), the strategies can be complementary in some circumstances.67–70
The examples shown in Figure 1 illustrate how the interrogation of compound libraries synthesized using DOS and related strategies has afforded small-molecule bioactives across a wide range of heritable diseases (defined here as diseases that may be influenced by heritable genetic characteristics);71–82 notably absent are compounds for the study of genetic targets in cancer, which will be discussed in a separate section. Here, we highlight three vignettes that provide greater insight into the underlying synthetic pathways, compound libraries, and chemical biology.
Figure 1 |. A selection of compounds generated by diversity-oriented synthesis and related strategies as probes for a wide range of heritable diseases.
Studies that use δ-lactone 9, an activator of neurite growth in multiple classes of murine neurons,82 may lead to insights into neurological disorders. The pyrimidodiazepine 7, disrupts the leucyl-tRNA synthetase–RagD protein–protein interaction, which may improve our understanding of autophagy, aging, and immunosuppression via control of the mTORC1 signaling pathway.80,260 The benzannulated sultam 4 modulates lysosome acidification and thus serves as a probe for V-ATPase function,75 which has been associated with loss of bone density and decreased kidney function, among other conditions.261,262 Other compounds interact with targets implicated in Alzheimer’s disease (1),71 holoprosencephaly (2),72 schizophrenia (3),74 inflammatory diseases (5),76 lipid metabolism (6),79 and coronary artery disease (8)81. The remaining compounds shown are described in more detail in the vignettes in the main text. Properties relevant to their value as probes are discussed in Box 2.
Robotnikinin—Developmental Disorders.
The Hedgehog signaling pathway plays a role in the organization of developing vertebrates and insects.83,84 While also studied in the context of oncogenesis and tumor maintenance,85 disruptions in Hedgehog signaling in humans have been linked to developmental disorders like holoprosencephaly.86 Complementing genetic studies in model organisms, small-molecule tool compounds have greatly improved our understanding of both individual ligands and the signaling pathway as a whole.87
Prior to 2009, however, no direct modulators of Sonic Hedgehog (Shh), one of the three mammalian Hedgehog orthologs, had been discovered. To address this gap, a target-based screen was performed against ShhN (the active portion of Shh) using small-molecule microarrays.77 The microarrays consisted of ~10,000 natural products, known bioactives, and products of various diversity synthesis pathways attached to an isocyanate-functionalized glass microscope slide.88
Efforts to optimize the most promising initial hits resulted in the discovery of robotnikinin (Fig. 1), a 12-membered macrolactone that binds purified ShhN (Kd = 3.1 μM) and exhibits concentration-dependent inhibition of ShhN-mediated signaling in multiple cell lines, such as Shh-LIGHT2 and C3H10T1/2 cells (1.9–62.5 μM).77 No inhibition was observed in cell lines that lacked its transmembrane receptor, Patched. These data suggest that robotnikinin disrupts the interaction between ShhN and Patched, although the exact mechanism by which this occurs is not well understood. With regard to selectivity, later studies that revealed novel macrocyclic peptide binders also showed that robotnikinin elicits a 3- to 4-fold greater response in an ELISA-based competition assay relative to the other two Hedgehog orthologs.89
Robotnikinin was derived from a library of 12- to 14-membered chiral macrocycles.90 After an Evans asymmetric alkylation was used to set the first stereocenter, additional stereogenic elements were introduced via intermolecular couplings with chiral 1,2-aminoalcohols (Fig. 2A). The final step, a Ru-catalyzed ring-closing metathesis, effected cyclization of the linear precursor 11. The modular synthetic pathway, in addition to providing an efficient route to under-represented non-peptidic macrocycles, facilitated systematic optimization of ring size, substituents, and stereochemistry.
Figure 2 |. Key transformations along the synthetic routes to selected compounds.
The general strategy of building (or purchasing) chiral building blocks, coupling them together, and cyclizing key linear intermediates via intramolecular transformations has generated a diverse array of chemical structures and biological activities in heritable disease and other areas of medicine. A | Chiral oxizolidinone 10 was functionalized strategically to afford intermediate 11, which subsequently underwent esterification and ring-closing metathesis to afford the Shh probe robotnikinin. B | Similarly, intermediate 13 was prepared from chiral ester 12. SNAr-based cyclization afforded the benzannulated 8-membered ring (14) at the core of BRD0476, a probe for type-1 diabetes and JAK-STAT signaling. C | Intermediate 16, which was prepared from chiral acid 15 and closely resembles 13, was subjected to macrolactamization conditions to afford 12-membered macrocycle 17 en route to the autophagy probe BRD5631.
As the first direct binder of Shh, robotnikinin has been used to study the Hedgehog signaling pathway.91 On the molecular level, it is capable of distinguishing between closely related proteins and disrupting a critical protein–protein interaction required for proper signaling.
BRD0476—Diabetes.
A therapeutic agent that reverses or prevents the autoimmune destruction of the insulin-producing β-cells of the pancreas in patients with type-1 diabetes would address a significant unmet medical need. With this goal in mind, a cell-based phenotypic screen was performed to identify small molecules that inhibit cytokine-mediated β-cell apoptosis.73 The screening library comprised 6,488 chiral [6,8]-fused bicyclic lactams generated via DOS.
One of the most promising suppressors of apoptosis was optimized for potency via a medicinal chemistry campaign to afford BRD0476 (Fig. 1).73 In validation experiments, BRD0476 reduced caspase-3 activity and nitrite production in concentration-dependent manners, and it restored several aspects of normal β-cell function.73 Subsequent research involved the synthesis of analogs with improved aqueous solubility and the discovery that BRD0476 promotes β-cell survival by selectively inhibiting ubiquitin-specific peptidase 9X (USP9X)-dependent JAK-STAT signaling (significant activity at 2–5 μM in independent primary samples).92,93 Biochemical experiments revealed no significant inhibition of 96 human kinases, including JAK1/2/3 (<40% inhibition at 10 μM) or 11 other members of the DUB superfamily.92 Not only do these results provide the first example of non-kinase-mediated inhibition of JAK-STAT signaling, but they also suggest USP9X as a new potential therapeutic target for type-1 diabetes.
The synthesis of BRD0476 (Fig. 2B) begins with an auxiliary-mediated asymmetric aldol reaction and intermolecular coupling of a chiral 1,2-aminoalcohol.94 This key linear intermediate was then cyclized via SNAr cycloetherification to construct the central 8-membered ring, a structural motif that is under-represented in current screening collections.26 With the core in place, appendage elaboration afforded BRD0476. The inclusion of all possible stereoisomers in the parent library aided the triage of screening data due to the existence of built-in stereochemistry-based SAR.73
As the development of BRD0476 has shown, the value of a probe is linked to the detailed molecular understanding of its MoA. These types of studies can afford biological insights with implications for new therapeutics in the future.
BRD5631—Autophagy.
Autophagy is the catabolic mechanism by which cells shuttle macromolecules, organelles, and pathogens to the lysosome for recycling or degradation.95 Alterations in this critical maintenance process have been implicated in human genetic studies of Crohn’s disease, and additional studies have connected it to nonalcoholic fatty liver disease, Huntington’s disease, and other disorders.96,97 The development of both inhibitors and enhancers of autophagy would improve our understanding of multiple diseases and facilitate the discovery of new medicines.
One effort to search for small-molecule modulators of autophagy used a cell-based high-throughput screen of nearly 60,000 compounds from multiple DOS pathways prepared using asymmetric synthesis.78 Briefly, compound-treated HeLa cells were imaged via fluorescence microscopy to detect and quantify the presence of autophagosomes, the characteristic cellular structures of autophagy. BRD5631 (Fig. 1) was shown to increase autophagosome formation in a concentration-dependent manner (EC50 = 3.1 μM). Follow-up studies revealed that BRD5631 enhances autophagy via an mTOR-independent mechanism. While mTOR inhibitors are known to induce autophagy,98,99 their use in the clinic is limited by unwanted side-effects.100 Therefore, BRD5631 represents an additional tool for studying autophagy.
The first few steps of the BRD5631 synthesis (Fig. 2C) closely mirror those of BRD0476—an asymmetric aldol reaction followed by the intermolecular coupling of a chiral 1,2-aminoalcohol—but SNAr chemistry was incapable of closing the 12-membered ring. Instead, a head-to-tail macrolactamization successfully generated all eight stereoisomers of the core scaffold, which were then functionalized to afford a 7,936-member library that included BRD5631.101
The structural similarity of intermediates 13 and 16 highlights the power of strategically designed compounds whose functional groups can be connected in different ways to generate different molecular skeletons. This example illustrates the build–couple–pair concept that mimics the way in which biosynthetic pathways construct natural products.33,35,36,48,102 As a key tenet of DOS, the ability to access multiple scaffolds from common precursors promotes skeletal diversity.31,35,103 Skeletally diverse libraries are becoming more valuable because they are thought to be more performance diverse,104 which would aid the search for nMoA compounds.
Probes for cancer targets
Historically, cancer drug discovery was based in part on phenotypic assays that identified compounds that killed cancer cells more effectively than they killed normal cells. Thus many traditional (and still widely used) anticancer drugs affect processes important in all dividing cells, such as DNA synthesis, with their therapeutic window thought to be derived from the greater importance of such processes in rapidly dividing cancer cells.105–107 However, starting in the early 1980s, studies of the molecular basis of cancer have revealed that many cancers are driven by mutations in particular signaling proteins;108 targeting these mutated proteins—which are not present in normal cells—may enable selective cancer cell killing. Consequently, target-based screening has become an increasingly popular strategy in oncology over the past two decades.109 This era of molecularly targeted cancer therapy—enabled by technologies for investigating genetic differences between cancer cells and normal cells110,111—has substantially improved the treatment of cancer. Many indications, however, remain without a targeted therapy, and resistance to new drugs continues to arise.
Cancer drug discovery has also been advanced by the development of potent and specific small-molecule probes that help characterize the protein targets that emerge from cancer genetics research. However, these proteins are often challenging to study with either small molecules or other modalities like monoclonal antibodies. Therefore, if we are to exploit the findings of contemporary cancer research fully, the next generation of chemical probes should be capable of modulating the activities of historically challenging targets like oncogenic transcription factors and GTPases.112–114 The screening of compounds that contain under-represented structural features, generated via DOS or other methods, appears to be a promising strategy, and it has already produced several probes for cancer research (Fig. 3).115–127 The following vignettes discuss the discovery, optimization and use in cancer research of four notable tool compounds.
Figure 3 |. Chemical probes for cancer targets.
These probes include benzothiophene 18 and indoloquinolizine 20 as tools to study the cytoskeleton, a common cancer target;115,118 compounds like hydrazide 22122 and benzannulated lactam 26127 that are highly specific and can discriminate between proteins within the same sub-family; probes discovered via target-based screening that exhibit significant stereochemistry-based SAR, such as 19117 and 25125; dienone 24, which sensitizes p53-deficient cells to DNA-damaging agents;124 and probes of ostensibly “undruggable” cancer targets like transcription factors and protein–protein interactions, such as lactam 21121 and dihydropyran 23123. Other compounds shown are described in more detail in the vignettes in the main text. Properties relevant to their value as probes are discussed in Box 2.
BRD7880—Aurora Kinases B/C.
Our first example does not discuss the targeting of a novel protein or protein class, but it does illustrate an unusual degree of selectivity in the targeting of the well-known class of protein kinases with compounds resulting from DOS. Misregulation of the cell cycle, especially mitosis, is one of many hallmarks of cancer. Some of the key regulators of this process are the aurora kinases, a family of serine/threonine protein kinases that coordinate the complex cytoskeletal maneuvers that occur during cell division.128 Alterations in aurora kinase activity have been linked to oncogensis, and aurora kinases are often overexpressed in colorectal, breast, and ovarian tumors, among others.129,130 Several aurora kinase inhibitors have since entered clinical trials,131–133 but none of them has won FDA approval. Achieving selective kinase inhibition can be challenging,134 so the lack of clinical success may be a result of insufficient specificity.
An unbiased phenotypic screen of 8,000 compounds from multiple DOS pathways identified BRD7880 (Fig. 3) as a highly selective inhibitor of aurora kinases B and C.119 In this study, pools of DNA-barcoded cancer cell lines were used in a series of small-molecule screens. Cell lines were barcoded via viral transduction of DNA identifiers, pooled, treated with compounds, and assessed for viability via amplification and sequencing of the DNA barcodes.119 One of the hits from the screen that exhibited selective and concentration-dependent activity was BRD7880. After comparing its sensitivity profile—which cell lines were killed and which were not—to those of over 400 compounds with known MoAs in the Cancer Therapeutics Response Portal (CTRP), its close similarity to the sensitivity profile of pan-aurora kinase inhibitor tozasertib suggested that BRD7880 is an aurora kinase inhibitor. Follow-up biochemical assays revealed that not only was this hypothesis correct, but also that BRD7880—obtained directly from the screening collection with no further optimization—was extremely potent and considerably more selective than tozasertib (Fig. 4), which had previously been tested in clinical trials.133 The only two kinases (out of 308) to suffer >50% inhibition when treated with 30 nM BRD7880 were aurora kinases B (IC50 = 7 nM) and C (IC50 = 12 nM).119
Figure 4 |. Kinase affinity profiling reveals that BRD7880 is significantly more selective than tozasertib.
The KinomeScan assay reveals kinases for which a given small molecule shows significant affinity (compound decreases binding of control by >75%). Aurora kinases A, B, and C are shown in blue; other kinase binding partners are shown in red. Reproduced with permission from REF. 119.
The synthesis of BRD7880 (Fig. 5A) closely resembles that of BRD0476, a structurally similar benzannulated lactam (Fig. 2B). However, an important difference is the relative stereochemistry of the methyl group and the silyl ether in linear intermediate 28. An Evans asymmetric aldol reaction was performed to install the desired 1,2-syn stereodiad.94 Cyclization via SNAr followed by functionalization of orthogonally protected lactam 29 furnished BRD7880.
Figure 5 |. Key transformations along the synthetic routes to selected compounds.
A | In a synthesis that resembles that of BRD0476, chiral oxazolidinone 27 was functionalized strategically to afford intermediate 28, which underwent SNAr-based cyclization and further elaboration to provide BRD7880, a remarkably selective aurora kinase inhibitor. B | Fully synthetic analogs of rocaglate natural products originated from hydroxyflavone 30. Rohinitib, a probe for HSF1 activity, and other rocaglate derivatives were synthesized via a biomimetic approach, proceeding through key intermediate 31. C | Starting from imine 32, linear intermediate 33 was generated via a diastereo- and enantioselective nitro-Mannich reaction. After cyclization to afford lactam 34, appendage decoration furnished the BRD7/9 inhibitor LP99 as a single stereoisomer. D | The asymmetric synthesis of ABL127, a potent and selective probe of PME-1, was achieved via generation of ketene 36 from carboxylic acid 35, followed by a [2+2] cycloaddition that was rendered enantioselective via a chiral nucleophilic catalyst.
BRD7880 is a particularly striking example of the impact of screening compounds that are only accessible via modern stereoselective synthesis. Because the aurora kinases had already been the subject of multiple drug-discovery campaigns, it is remarkable that the most specific inhibitor to date was identified directly from a screen of 8,000 compounds. This result highlights the potential efficiency of exploring new regions of chemical space made accessible by DOS strategies and advances in synthetic methodology. The rapid determination of the MoA of BRD7880 via high-dimensional assay data also provides a valuable precedent for future phenotypic screening campaigns. But the most direct impact of BRD7880 may stem from its use in studying the therapeutic hypothesis of aurora kinase inhibition more rigorously than had been possible before with chemical tools.
Rohinitib—HSF1.
Heat shock factor 1 (HSF1), a transcription factor that coordinates the heat shock response in human cells,135 is a regulator of ribosome biosynthesis that has also been associated with oncogenesis and poor outcomes in cancer patients.136–138 Exploring the connections between HSF1 activity, translational flux, and maintenance of the tumorigenic cell state could reveal new therapeutic targets and strategies for cancer.
While studying the effects of varying ribosomal activity on transcription, Santagata and colleagues discovered a potent and selective inhibitor of HSF1.116 They first observed that inhibition of translation elongation significantly decreased HSF1 activity, suggesting that HSF1 is involved in ribosome-mediated regulation of transcription. A screen of more than 300,000 compounds from the NIH Molecular Libraries Probe Production Centers Network (MLPCN) collection was then performed to identify small-molecule inhibitors of HSF1 activity. The resulting ~2,500 hits were then subjected to a dual reporter-based secondary screen to eliminate nonspecific compounds; remarkably, several ostensibly selective HSF1 inhibitors suppressed both reporters,116,139 which underscores the challenges associated with modulating transcription factor activity.140 The most potent and selective (>20-fold) hit, a natural product named rocaglamide A, was optimized to afford rohinitib (IC50 = ~20 nM; Fig. 3). This unique chemical probe was then used to characterize extensively the link between protein translation and HSF1 activity in cancer cells. In addition to illuminating a number of fundamental insights into cancer biology, rohinitib suppressed tumor growth both in vitro and in vivo.116
The optimization of rocaglamide A involved both fully synthetic analogs and additional rocaglate natural products. All synthetic analogs, including rohinitib, were derived from the same 3-hydroxyflavone derivative, 30. The fused tricyclic rocaglate core was constructed via a biomimetic photocycloaddition/alpha-ketol rearrangement/reduction sequence (Fig. 5B).141,142 The use of a chiral Brønsted acid can render the [3+2] cycloaddition enantioselective;143 alternatively, the desired enantiomer can be accessed via chiral resolution.144 This synthetic pathway demonstrates the utility of oft-underutilized photochemical transformations in generating natural product-like scaffolds from flat, sp2-rich precursors.145
LP99—BRD7/9.
SWI/SNF chromatin remodeling complexes are multi-protein complexes that regulate transcriptional processes involved in proliferation, DNA repair, and other critical cellular functions. Loss of overall SWI/SNF function via inactivation of specific subunits has been linked to tumorigenesis, and SWI/SNF subunit mutations are found in roughly 20% of human cancers.146,147 One such subunit is bromodomain-containing protein 7 (BRD7), which is an essential cofactor for p53-mediated tumor suppression.148 Conversely, a subunit known as BRD9 is upregulated in some cancers and promotes the growth of acute myeloid leukemia cell lines.149 Because the human proteome contains dozens of bromodomain-containing proteins, selective inhibitors are needed to assess the viability of BRD7 and BRD9 as therapeutic targets.
Looking to develop the first selective BRD7/9 inhibitor, Clark and colleagues found that 1-methylquinolone could be elaborated into a potent and selective probe via asymmetric synthesis.126 1-methylquinolone had previously been identified via a crystallographic fragment screen against a BRD9-related protein called ATAD2.150 From this promising starting point, additional structure-based and biophysical data informed the design of analogs that bind BRD9 with sub-micromolar affinity. The most potent compound, LP99 (KD = 99 nM; Fig. 3), stabilized only two of the 48 expressible human bromodomain-containing proteins at 10 μM as measured via differential scanning fluorimetry (BRD7/9, ΔTm > 4 °C; all others, ΔTm < 1 °C).126 LP99 was shown to disrupt the BRD7/9–chromatin interaction in cells without causing indiscriminate cell death. Preliminary experiments using LP99 as a tool compound found that BRD7 and BRD9 regulate inflammatory cytokine production, suggesting a novel anti-inflammatory therapeutic strategy.
While early analogs were synthesized as racemates and resolved via preparative-scale chiral chromatography, the active enantiomers of the most potent compounds, including LP99, were prepared using asymmetric synthesis. The key transformation in the pathway (Fig. 5C) is achieved via an organocatalyzed nitro-Mannich reaction that furnishes the desired intermediate 33 with high diastero- and enantioselectivity (d.r. = 7:1, e.r. = 19:1).126 Furthermore, this reaction can be performed on gram scale, which facilitates the generation of additional analogs and the stockpiling of material for biological studies. Installation and decoration of the lactam core allowed the full synthesis of LP99 to be realized in just eight steps from commercially available reagents.
LP99 is a potent and selective chemical probe that has already uncovered previously unknown—and therapeutically promising—biology. Its development leveraged the interplay of many techniques, such as asymmetric catalysis and X-ray crystallography, and demonstrates that fragment-based drug design12 is also capable of generating compounds with under-represented chemical features.
ABL127—PME-1.
The widespread development of kinase inhibitors to study and treat cancer demonstrates the importance of the phosphoproteome in oncology.134 Various protein phosphatases regulate cellular dynamics by catalyzing the dephosphorylation of serine, threonine, and tyrosine residues. But despite the well-characterized link between aberrant phosphatase activity and cancer,151,152 many fewer chemical probes exist for protein phosphatases than for their kinase counterparts, contributing to the belief that phosphatases are largely “undruggable.”153,154 Therefore, compounds that selectively modulate protein phosphatase activity, either directly or indirectly, would be useful small-molecule probes.
Protein phosphatase 2A (PP2A), a potent tumor suppressor that is responsible for the majority of serine/threonine dephosphorylation in eukaryotic cells, is inactivated in a variety of cancers.155,156 Its negative regulation is mediated in part by protein phosphatase methylesterase-1 (PME-1),157 a serine hydrolase whose overexpression has been linked to increased cellular proliferation and other disease phenotypes.158 PME-1 inhibition, therefore, is a potential anti-cancer therapeutic strategy.
A collaboration between the Cravatt and Fu laboratories afforded the first small-molecule inhibitor of PME-1.120 After a high-throughput fluorescence polarization-activity-based protein profiling assay of PME-1 activity was developed, a screen of more than 315,000 members of the NIH MLPCN collection, a small fraction of which included compounds submitted by academic labs practicing asymmetric synthesis (e.g. BRD7880 above), was conducted. This experiment revealed a set of four aza-β-lactams, including ABL127 (IC50 = 4.2 nM; Fig. 3), that inhibited PME-1. 22 other aza-β-lactams (including the enantiomers of three of the four hits) were significantly less active, which suggested that the observed PME-1 inhibition resulted from a specific interaction. Follow-up studies showed that ABL127 inhibits PME-1 in both cells (IC50 = 6.4–11.1 nM) and mice (50 mg/kg), and that this inhibition decreases demethylated PP2A levels. Additional activity-based protein profiling and “click” chemistry-based experiments revealed that ABL127 exhibits selectivity across the entire proteome,120 further increasing its value as an nMoA chemical tool to study both PME-1 and PP2A.
Enantioselective nucleophilic catalysis enables a three-step asymmetric synthesis of ABL127 (Fig. 5D). After generation of ketene 36, a [2+2] cycloaddition with dimethyl azodicarboxylate affords ABL127. The cyclization is rendered enantioselective via the inclusion of a planar-chiral 4-pyrrolidinopyridine catalyst, and the transformation is compatible with a broad range of ketene and azo coupling partners.159 An extension of this methodology was used to synthesize oxo-β-lactams and develop a general platform for crafting specific serine hydrolase inhibitors.160
Probes for infectious disease targets
Because agents of infectious disease are distinct from the host organism, the search for antimicrobials presents unique sets of opportunities and challenges. For the most common strategy of targeting the pathogen directly—rather than host-based mechanisms—on-target toxicity can be less of a concern because the drug target in the pathogen may not be found or may be significantly altered in the human patient. On the other hand, many pathogens can rapidly acquire drug resistance due to high rates of reproduction and DNA mutation.161,162 Accordingly, high-throughput phenotypic screening for compounds that kill pathogens but not human cells is commonly used to find potential drug leads for infectious diseases.163 Once a lead has emerged from screening, initial clues regarding the MoA of the compound can be gained via genetic methods. One approach involves incubating pathogens with sub-lethal concentrations of the compound to select for resistant strains that have altered sequences or copy numbers of the compound’s target (as revealed by genome sequencing).164,165 Alternatively, systematically treating panels of pathogens in which a single gene is either deleted or overexpressed in each clone can point to relevant biology.166
Reflecting their often evolutionary origin in competition between microbes, natural products have been a prolific source of leads and drugs against infectious diseases, but the emergence of resistance and the exhaustion of “low-hanging fruit” have led this strategy to become less productive in recent decades.7,8,167,168 For some diseases, such as HIV/AIDS and hepatitis C, target-based drug discovery (often aided by knowledge of pathogen genetics) has made major contributions to the development of new medicines.169,170 But in others, particularly in the search for novel antibiotics, such strategies have been much less fruitful. In these project areas, the compounds typically available in both commercial libraries and industry collections could be a major limiting factor in anti-infective drug discovery. For example, “Rule-of-Five” compliance continues to shape the contents of many screening collections despite it having little bearing on the success of antimicrobials.168,171
One strategy to address these issues is to synthesize derivatives of known natural product-based antimicrobials.53,54,172,173 Alternatively, advances in synthetic methodology may be leveraged to access scaffolds that harbor chemical features frequently found in natural products without directly mimicking any specific class. Many promising next-generation antimicrobial agents contain such structural features (Fig. 6).174–185 The following four vignettes provide more detailed examples of the value of this approach in generating compounds that can be used to study and possibly treat infectious diseases.
Figure 6 |. Chemical probes and clinical candidates for infectious disease targets.
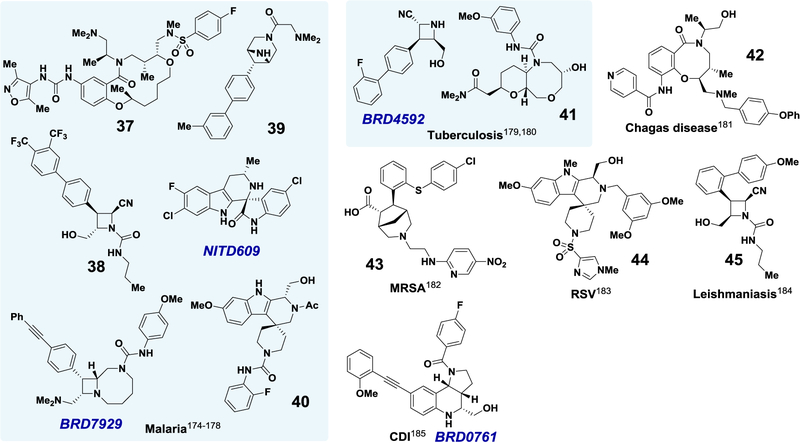
A library of chiral azetidines originally designed to mimic CNS-active compounds206 has afforded promising compounds against parasites like Leishmania donovani (the causative agent of visceral leishmaniasis; 45) and Plasmodium falciparum (the causative agent of malaria; 38–39, BRD7929).175,176,178,184 Macrocycle 37 (malaria) and benzannulated lactam 42 (Chagas disease) also exhibit antiparasitic activity.174,181 Target-based and phenotypic screening strategies were used to identify oxazocane 41 and bridged bicyclic 43, which have bactericidal properties.180,182 The Pictet-Spengler-derived spirocycles 40 and 44 show antimalarial and antiviral activity, respectively.178,183 Other compounds shown are described in more detail in the vignettes in the main text. Properties relevant to their value as probes are discussed in Box 2.
NITD609—Malaria.
Malaria is caused by several species of mosquito-borne Plasmodium parasites, with P. falciparum being responsible for the majority of fatalities.186 But most antimalarials only treat the asexual blood stage of Plasmodium,187 sparing the liver- and sexual blood-stage parasites that can sit dormant in the body and cause relapse at a later date.188 Furthermore, the risk of drug resistance189–191 necessitates easy-to-follow dosing regimens that maximize patient compliance—ideally a single-dose cure with prophylaxis and transmission-blocking activities.187 nMoA compounds that inhibit Plasmodium growth at multiple life stages are needed to address these challenges.
Prompted by the emergence of malaria-causing parasites resistant to artemisinin, an endoperoxide natural product,192,193 scientists at the Novartis Institute for Tropical Diseases and their collaborators performed phenotypic screens with Plasmodium to find nMoA antimalarial leads.177 After a screen of ~12,000 natural products and natural product-like synthetic compounds, subsequent optimization yielded NITD609 (Fig. 6), a chiral spiroindolone that exhibits potent in vitro activity (IC50 = ~0.5–10 nM) against several strains of P. falciparum and P. vivax via a novel MoA. Its molecular target, as first suggested by genomic sequencing of resistant parasites, was confirmed to be PfATP4, a cation-transporting P-type adenosine triphosphatase that maintains Plasmodium sodium homeostasis.177,187,194,195 Studies measuring toxicity (CC50/IC50 > 104; hERG IC50 > 30 μM), pharmacokinetics (100% bioavailability), and in vivo efficacy (single-dose cure in a P. berghei mouse model at 100 mg/kg) suggested that NITD609 would be compatible with once-daily oral dosing,177 which was critical for its advancement into human trials as the first nMoA antimalarial clinical candidate in two decades.187 Since entering the clinic, not only has NITD609 exhibited impressive efficacy,196 but it has also been shown to prevent P. falciparum transmission from humans back to mosquitos.197 Its success has prompted the development of additional antimalarials that target PfATP4.198–200
Advances in asymmetric catalysis have made the synthesis of NITD609 efficient. Starting from 6-chloro-5-fluoroindole, the original synthetic route afforded racemic NITD609 in eight steps; chiral chromatography was needed to isolate the active enantiomer.177 Due to the high cost of preparative scale chiral chromatography, asymmetric routes would likely be more practical. The last step of the original synthesis, a Pictet-Spengler reaction, is highly diastereoselective but necessarily produces a racemate because the reaction lacks a chiral influence. Use of enantioenriched tryptamine derivatives or inclusion of a chiral phosphoric acid catalyst have been shown to furnish the appropriate enantiomer.201,202 Alternative routes that do not rely upon the Pictet-Spengler reaction have also been devised. One such pathway incorporates a Ni-catalyzed enantioselective aza-Diels–Alder reaction to construct NITD609’s key spirocyclic ring system (Fig. 7A), affording the final product in just three steps from indole 46 and ketimine 47.203
Figure 7 |. Key transformations along the synthetic routes to selected compounds.
A | One synthetic route to NITD609, a potent nMoA antimalarial, involved an enantioselective aza-Diels–Alder reaction between indole 46 and ketimine 47 to afford spirocycle 48, which is one step from NITD609. B | The synthesis of antimalarial BRD7929 contained two key cyclizations. First, treatment of chloride 50 with strong base supplied azetidine 51. Subsequent functionalization furnished RCM substrate 52, which was cyclized to diazocine 53 en route to BRD7929. C | From aniline 54, imine 55 was an ideal substrate for a diastereo- and enantioselective Povarov reaction. The resulting tetrahydroquinoline 56 was readily converted to BRD0761, which shows nMoA activity against C. difficile. D | Similar to BRD7929, the tuberculosis probe BRD4592 was accessed via a chiral azetidine (59), which in turn was generated from chloride 58 and amino alcohol 57.
BRD7929—Malaria.
Buoyed by the success of earlier pilot experiments,174,204,205 another effort to develop multistage antimalarial compounds began with a high-throughput screen of ~100,000 DOS-derived small molecules against a multidrug-resistant strain of blood-stage P. falciparum.178 These compounds represent hundreds of scaffolds—several of which were described above—not currently found outside of the Broad Institute. Hits from the primary screen were subjected to a panel of counter-screens to identify nMoA compounds that exhibited multistage activity. This analysis revealed clusters of structurally related bioactives, most notably a series of bicyclic azetidines that showed nanomolar activity against all three Plasmodium life stages. For this series, genome sequencing of intentionally-evolved resistant parasites showed mutations in the genetic locus predicted to encode phenylalanyl-tRNA synthetase (Pf PheRS)—a novel antimalarial target. Follow-up experiments confirmed that an optimized analog of the original hit, BRD7929 (Fig. 6), was not only a potent inhibitor of Pf PheRS activity in vitro (IC50 = 23 nM), but also a single-dose cure, prophylactic, and inhibitor of disease transmission in mouse models of malaria when dosed at 25 mg/kg.178
The unusual bicyclic core of BRD7929 was constructed via two non-successive cyclizations (Fig. 7B). First, the azetidine was installed via treatment of chloride 50 with strong base to effect intramolecular ring closure, affording a 6:5 mixture of epimers that were separated by chromatography.206 Then, after functionalization of azetidine 51, ring-closing metathesis constructed the 8-membered diazocine. Subsequent Sonogashira coupling, isocyanate condensation, and Mitsunobu-mediated amination afforded BRD7929.178 While not currently applicable to BRD7929, recent advances in stereospecific C-H arylation methodology have improved the efficiency of synthetic pathways to bicyclic azetidines that lack functionalization at the 4-position.207
The development of NITD609 and BRD7929 highlight the power of combining asymmetric synthesis with phenotypic screening to discover new antimalarials. Multiple chemotypes showed potent activity against multidrug-resistant Plasmodium directly out of the screening deck; data surrounding many of these are freely available at the Malaria Therapeutics Response Portal (MTRP). And once optimized, both NITD609 and BRD7929 showed remarkable in vivo results that build upon an expanding pipeline of antimalarial compounds making their way through pre-clinical and clinical trials.208,209
BRD0761—C. difficile Infection.
Narrow-spectrum nMoA antibiotics could have an important role in addressing the growing threat posed by the emergence of drug resistance in the Gram-positive bacterium Clostridium difficile, an opportunistic pathogen that can cause severe diarrhea, sepsis, and death.210,211 A high-throughput screen of the same ~100,000-member DOS library described above against eight bacterial organisms revealed potent and selective inhibitors of C. difficile growth.185 Two structurally distinct series of compounds, one of which is represented by BRD0761 (Fig. 6), were explored further. Compounds from both series were more selective than fidaxomicin, an FDA-approved antibiotic that was expressly approved for its selective activity against C. difficile.212 Furthermore, BRD0761 exhibited greater potency against several C. difficile isolates (MIC = 0.06–1 μg/mL) than did vancomycin, which is used to treat severe cases of C. difficile infection.210,213 Genomic analysis of resistant mutants and in silico modeling suggested that the target of BRD0761 is glutamate racemase, an essential protein involved in cell wall biosynthesis. While this enzyme is a known target in Helicobacter pylori, Mycobacterium tuberculosis (Mtb), and other Gram-positive bacteria,214 it had not yet been validated in C. difficile.185
The preparation of BRD0761 (Fig. 7C) demonstrates the value of combining complexity-generating transformations with asymmetric catalysis. An organocatalyzed Povarov reaction developed in the Jacobsen laboratory was identified as an efficient method to generate tetrahydroquinoline-containing compounds with three contiguous stereocenters. This reaction was used to synthesize tetrahydroquinoline 56 with high diastereo- and enantioselectivity.215 Prepared in only two steps from commercially available starting materials, the resulting scaffold was readily elaborated into a 2,328-membered library that contained BRD0761.185
Despite their structural and biological differences, as described above BRD7929 and BRD0761 were derived from the same screening collection. These results highlight the ability of prospecting libraries to generate nMoA leads with diverse chemotypes across different diseases.
BRD4592—Tuberculosis (TB).
Recent years have seen the alarming rise of multidrug-resistant (MDR), extensively drug-resistant (XDR), and totally drug-resistant (TDR) strains of Mtb,216–218 so there is a substantial need for nMoA anti-TB compounds. Hoping to discover such compounds, a collaboration between researchers at the Broad Institute, the University of Chicago, and Argonne National Laboratory screened roughly 83,000 compounds of the Broad DOS collection for activity against GFP-expressing Mtb.179 This screen identified BRD4592 (Fig. 6), a chiral azetidine with three contiguous stereocenters, as an inhibitor of Mtb growth (MIC90 = 3 μM). Notably, its other seven stereoisomers were not scored as active, which suggests that its bactericidal activity is due to specific target engagement. Genetic analysis of resistant mutants followed by extensive kinetic, thermodynamic, and biochemical characterization revealed the molecular target to be both subunits of tryptophan synthase (IC50α = 71 nM; IC50β = 23 nM), a previously untargeted essential metabolic enzyme.179,219 BRD4592 stabilizes multiple enzyme conformations via allosteric binding (Fig. 8), which likely contributes to its high specificity; in contrast, substrate mimetics that target enzyme active sites are likely to exhibit substantial off-target activity.220 BRD4592 was then shown to inhibit Mycobacterium marinum growth at 15 μM in zebrafish embryos, a common in vivo model of TB.221
Figure 8 |. Stereo view of BRD4592 (cyan) bound at the interface of the α and β subunits of tryptophan synthase.
X-ray crystallography was used to solve the co-crystal structure of BRD4592 and tryptophan synthase. Hydrogen bonds and water molecules shown as dashed lines and red spheres, respectively. Reproduced with permission from REF. 179.
The versatile azetidine-based synthetic pathway that afforded BRD7929 can be redirected to generate BRD4592 (Fig. 7D). As before, cyclization of chloride 58 produced a near-equal mixture of epimers at the 2-position that were easily separated via chromatography.206 Suzuki cross-coupling and functional group deprotections then furnished BRD4592. BRD7929 and BRD4592 highlight just two of the many distinct skeletons that can be synthesized from azetidines 51, 59, and their stereoisomers; the full complement of bridged, fused, and spirocyclic ring systems that can be accessed via this chemistry is discussed elsewhere.206
The discovery of BRD4592 offers important lessons for antimicrobial drug discovery. The use of phenotypic screening both yielded membrane-permeable hits (a significant issue in finding inhibitors of Mtb164,222,223) and allowed for the discovery of a new therapeutic target outside of previously drugged pathways.179 Furthermore, while the similarities between the syntheses and chemical structures of BRD4592 and BRD7929 are striking, we hesitate to call the azetidine a “privileged scaffold”; for example, the corresponding pyrrolidines were not included in the library and are therefore not available for comparison. Rather, we propose that these two vignettes are simply evidence that compounds that are structurally dissimilar from those in screening collections are likely to lead to novel biological MoAs and therapeutic insights—different chemistry yields different outcomes.
Conclusions and outlook
The ability to modulate biological systems reversibly, selectively, and with temporal control is one of the most powerful weapons in the chemical biologist’s arsenal. By advancing our understanding of disease biology, high-quality small-molecule probes have catalyzed new research efforts to test therapeutic hypotheses and develop new medicines.66 And yet, we must be wary of compounds whose activities have not been sufficiently or accurately defined (Box 2).65,224 Furthermore, much of our currently available chemical matter is both structure- and performance-redundant,225 which hampers our ability to develop nMoA compounds that can exploit insights from human genetics or combat drug-resistant pathogens.
Box 2 |. Chemical probes: promises and pitfalls.
Chemical probes can contribute to the development of drug leads by gauging the chemical tractability and physiological relevance of a potential therapeutic target.254 But these two classes of molecules serve fundamentally different purposes. Unlike drugs, probes need not be optimized for safety and therapeutic efficacy in human patients—they are designed to answer a specific biological question in models of disease. Therefore, structural properties related to traditional measures of “drug-likeness” like oral bioavailability and metabolic stability are not essential. For their corresponding biological results to be compelling, however, probes must be potent, selective, and act via known MoAs, among many other factors.224 Failure to understand fully the biological consequences of probe treatment could result in the mis-assignment of molecular cause to phenotypic effect. Such mistakes have likely contributed to the reproducibility crisis in biomedical research.255
The growing popularity and importance of small-molecule probes has prompted systematic reviews of probe quality. Established mechanisms to correct the scientific literature are often not sufficiently nimble or comprehensive to curb errors that can propagate at an exponential rate. In response, groups like the Structural Genomics Consortium (SGC) have established guidelines for describing valuable, high-quality tool compounds. Originally founded in 2008, the SGC is a public–private partnership that curates collections of probes related primarily to epigenetic signaling. To be included in the SGC database, probes must demonstrate potency both in vitro (IC50 or Kd < 100 nM) and in cellulo (on-target activity at 1 μM) and greater than 30-fold selectivity over other protein family members. As an alternative to objective but strict guidelines, Paul Workman and colleagues have proposed a “fit-for-purpose” model that emphasizes a series of principles that are more practical to implement.256,257 These factors fall into a handful of broad categories relating to chemistry (e.g. solubility, stability, and membrane permeability), potency (e.g. in biochemical, cellular, or in vivo assays), and other properties. Probes that do not fulfill all of these criteria can illuminate remarkable biology,256 but the conclusions they suggest must always be placed in the appropriate context.
To complement the guidelines established by the SGC, Workman, and others, various web-based resources have recently been established. These databases allow researchers to sift through experimental data and recommendations from the scientific community to identify the best compound for their particular research project. One such example is the Chemical Probes Portal,65 which features reviews of several hundred probes from members of its scientific advisory board. The Probes & Drugs Portal, on the other hand, provides experimental data relating to ~30,000 bioactive compounds (updated on a monthly basis).258 Finally, Probe Miner is an ambitious effort to centralize data from the vast medicinal chemistry literature.259 Analysis of >3.9 million experiments of >1.8 million compounds led to in-depth scoring of hundreds of thousands of probes against 2,220 human protein targets. These and similar resources should be used early and often in biomedical research projects that hope to draw biological conclusions from small molecule-based experiments.
As this article has attempted to illustrate, advances in synthesis have enabled the construction of libraries of natural product-like compounds. These novel chemistries have afforded nMoA compounds with potential therapeutic value across a broad range of diseases. While “novel” does not necessarily mean “better,” probe- and drug-discovery campaigns are profoundly influenced by the quality of their starting points (“front loading”).226 Earlier analyses, such as those conducted by Michael Hann and colleagues,227,228 have suggested that libraries enriched in these complex compounds are more likely to be inefficient in high-throughput screening experiments. However, the probes and drug leads discussed in this article were all derived from screens of 8,000 to ~315,000 compounds, suggesting that such libraries can generate useful hits and leads at least as efficiently as the large libraries used in the pharmaceutical industry.229
Biomedical research would also benefit from general, high-throughput methods for discovering small-molecule binders to the novel therapeutic targets uncovered by human and microbial genetics, molecular and cellular biology, and chemical biology. Even if they do not alter protein activity, binders can be used to study how biological systems respond to various perturbations.230,231 For example, they can be applied to discover compounds that promote new protein–protein interactions, or stabilize or destabilize their cellular targets. In addition, the binders can be equipped with additional chemical motifs that promote the cellular mis-localization or degradation of targeted proteins.232,233 Unfortunately, methods for discovering binders are often time-consuming and resource-intensive.234,235 Affinity enrichment-based screening of libraries of DNA-barcoded compounds, however, is a promising technique that is gaining in popularity due to advances in DNA sequencing, among other factors.236 Current DNA-encoded libraries are largely populated by peptidomimetics and sp2-rich compounds,237 similar to commercial libraries of “conventional” small molecules. Perhaps, then, DNA-encoded libraries could also benefit from the inclusion of compounds that contain under-represented structural features. This concept poses a future challenge for synthetic organic synthesis because modern chemistries are needed that are compatible with the conditions required for concomitant DNA barcoding.
Looking ahead, the building of performance-diverse small-molecule libraries would be more efficient if a rapid feedback mechanism were to exist between synthetic organic chemistry and biology. The current multi-year retrospective and time-consuming method for assessing small-molecule bioactivity precludes biological performance from influencing library design,225 and structural diversity alone has been shown to be a poor predictor of biological performance diversity.238 On the other hand, high-dimensional (multiplexed) biological assays that track changes in gene expression,239 cell morphology,240 and other cellular features241 can provide chemists with near-immediate feedback on their synthetic decisions. By associating each compound with thousands of biological changes, these assays constitute the backbone of a means for “real-time” biological annotation of reaction products.242,243 Such a feedback loop would indicate during library construction—rather than years later—the reactions, scaffolds, and synthetic pathways worth pursuing for maximal performance diversity. They also enable the hand picking of small clusters of compounds with each cluster having either known or novel MoAs, and thus collectively provide an empirical means for the construction of performance-diverse compound collections for probe and drug development. Chemists have neither the time nor the resources to synthesize every possible compound, so a prudent approach to bioactive discovery would incorporate the principles of the scientific method—hypotheses, experiments, observations, and conclusions—whenever possible.
Acknowledgments
The authors thank B. Melillo, T. Sharifnia, and B. Wagner for providing critical feedback on the manuscript. C.J.G. is supported by a National Science Foundation Graduate Research Fellowship (DGE1144152). S.L.S. is an Investigator at the Howard Hughes Medical Institute. Research in his lab in the area covered in this Review has been generously supported for three decades by the National Institute of General Medical Sciences (GM038627).
References
- 1.Plenge RM Disciplined approach to drug discovery and early development. Sci. Transl. Med 8, 349ps15 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Holohan C, Van Schaeybroeck S, Longley DB & Johnston PG Cancer drug resistance: an evolving paradigm. Nat. Rev. Cancer 13, 714–726 (2013). [DOI] [PubMed] [Google Scholar]
- 3.Spellberg B, Bartlett JG & Gilbert DN The Future of Antibiotics and Resistance. N. Engl. J. Med 368, 299–302 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blair JMA, Webber MA, Baylay AJ, Ogbolu DO & Piddock LJV Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol 13, 42–51 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Koehn FE & Carter GT The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov 4, 206–220 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Gerwick WH & Moore BS Lessons from the past and charting the future of marine natural products drug discovery and chemical biology. Chem. Biol 19, 85–98 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cragg GM & Newman DJ Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta 1830, 3670–3695 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ganesan A The impact of natural products upon modern drug discovery. Curr. Opin. Chem. Biol 12, 306–317 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Newman DJ & Cragg GM Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod 79, 629–661 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Ferreira LG, Dos Santos RN, Oliva G & Andricopulo AD Molecular docking and structure-based drug design strategies. Molecules 20, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moitessier N et al. Medicinal Chemistry Projects Requiring Imaginative Structure-Based Drug Design Methods. Acc. Chem. Res 49, 1646–1657 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Erlanson DA, Fesik SW, Hubbard RE, Jahnke W & Jhoti H Twenty years on: The impact of fragments on drug discovery. Nat. Rev. Drug Discov 15, 605–619 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Lionta E, Spyrou G, Vassilatis DK & Cournia Z Structure-Based Virtual Screening for Drug Discovery: Principles, Applications and Recent Advances. Curr. Top. Med. Chem 14, 1923–1938 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sliwoski G, Kothiwale S, Meiler J & Lowe EW Computational Methods in Drug Discovery. Pharmacol. Rev 66, 334–395 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spaller MR, Burger MT, Fardis M & Bartlett PA Synthetic strategies in combinatorial chemistry. Curr. Opin. Chem. Biol 1, 47–53 (1997). [DOI] [PubMed] [Google Scholar]
- 16.Dandapani S & Marcaurelle LA Accessing new chemical space for ‘undruggable’ targets. Nat. Chem. Biol 6, 861–863 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Reymond JL The Chemical Space Project. Acc. Chem. Res 48, 722–730 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Shelat AA & Guy RK Scaffold composition and biological relevance of screening libraries. Nat. Chem. Biol 3, 442–446 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Clemons PA et al. Small molecules of different origins have distinct distributions of structural complexity that correlate with protein-binding profiles. Proc. Natl. Acad. Sci 107, 18787–18792 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clemons PA et al. Quantifying structure and performance diversity for sets of small molecules comprising small-molecule screening collections. Proc. Natl. Acad. Sci 108, 6817–6822 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salvador-Reyes LA & Luesch H Biological targets and mechanisms of action of natural products from marine cyanobacteria. Nat. Prod. Rep 32, 478–503 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dančík V, Seiler KP, Young DW, Schreiber SL & Clemons PA Distinct Biological Network Properties between the Targets of Natural Products and Disease Genes. J. Am. Chem. Soc 132, 9259–9261 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalko PI & Moisan L Enantioselective Organocatalysis. Angew. Chemie - Int. Ed 40, 3726–3748 (2001). [DOI] [PubMed] [Google Scholar]
- 24.Taylor MS & Jacobsen EN Asymmetric catalysis by chiral hydrogen-bond donors. Angew. Chemie - Int. Ed 45, 1520–1543 (2006). [DOI] [PubMed] [Google Scholar]
- 25.Fürstner A Olefin Metathesis and Beyond. Angew. Chemie - Int. Ed 39, 3012–3043 (2000). [PubMed] [Google Scholar]
- 26.Bauer RA, Wenderski TA & Tan DS Biomimetic diversity-oriented synthesis of benzannulated medium rings via ring expansion. Nat. Chem. Biol 9, 21–29 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kopp F, Stratton CF, Akella LB & Tan DS A diversity-oriented synthesis approach to macrocycles via oxidative ring expansion. Nat. Chem. Biol 8, 358–365 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nie F et al. A Multidimensional Diversity-Oriented Synthesis Strategy for Structurally Diverse and Complex Macrocycles. Angew. Chemie - Int. Ed 55, 11139–11143 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antonchick AP et al. Highly enantioselective synthesis and cellular evaluation of spirooxindoles inspired by natural products. Nat. Chem 2, 735–740 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Moura-Letts G, Diblasi CM, Bauer RA & Tan DS Solid-phase synthesis and chemical space analysis of a 190-membered alkaloid/terpenoid-like library. Proc. Natl. Acad. Sci 108, 6745–6750 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morton D, Leach S, Cordier C, Warriner S & Nelson A Synthesis of natural-product-like molecules with over eighty distinct scaffolds. Angew. Chemie - Int. Ed 48, 104–109 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siau WY & Bode JW One-step synthesis of saturated spirocyclic N-heterocycles with stannyl amine protocol (SnAP) reagents and ketones. J Am Chem Soc 136, 17726–17729 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Schreiber SL Target-oriented and diversity-oriented organic synthesis in drug discovery. Science 287, 1964–1969 (2000). [DOI] [PubMed] [Google Scholar]
- 34.Wetzel S, Bon RS, Kumar K & Waldmann H Biology-oriented synthesis. Angew. Chemie - Int. Ed 50, 10800–10826 (2011). [DOI] [PubMed] [Google Scholar]
- 35.Nielsen TE & Schreiber SL Towards the optimal screening collection: A synthesis strategy. Angew. Chemie - Int. Ed 47, 48–56 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burke MD & Schreiber SL A Planning Strategy for Diversity-Oriented Synthesis. Angew. Chemie - Int. Ed 43, 46–58 (2004). [DOI] [PubMed] [Google Scholar]
- 37.Lee D, Sello JK & Schreiber SL Pairwise Use of Complexity-Generating Reactions in Diversity-Oriented Organic Synthesis. Org. Lett 2, 709–712 (2000). [DOI] [PubMed] [Google Scholar]
- 38.Tan DS, Foley MA, Stockwell BR, Shair MD & Schreiber SL Synthesis and Preliminary Evaluation of a Library of Polycyclic Small Molecules for Use in Chemical Genetic Assays. J. Am. Chem. Soc 121, 9073–9087 (1999). [Google Scholar]
- 39.Lee D, Sello JK & Schreiber SL A Strategy for Macrocyclic Ring Closure and Functionalization Aimed toward Split-Pool Syntheses. J. Am. Chem. Soc 121, 10648–10649 (1999). [Google Scholar]
- 40.Oguri H & Schreiber SL Skeletal Diversity via a Folding Pathway: Synthesis of Indole Alkaloid-Like Skeletons. Org. Lett 7, 47–50 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Kumagai N, Muncipinto G & Schreiber SL Short synthesis of skeletally and stereochemically diverse small molecules by coupling petasis condensation reactions to cyclization reactions. Angew Chem Int Ed Engl 45, 3635–3638 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Sternson SM, Wong JC, Grozinger CM & Schreiber SL Synthesis of 7200 Small Molecules Based on a Substructural Analysis of the Histone Deacetylase Inhibitors Trichostatin and Trapoxin. Org. Lett 3, 4239–4242 (2001). [DOI] [PubMed] [Google Scholar]
- 43.Haggarty SJ, Koeller KM, Wong JC, Butcher RA & Schreiber SL Multidimensional Chemical Genetic Analysis of Diversity-Oriented Synthesis-Derived Deacetylase Inhibitors Using Cell-Based Assays. Chem. Biol 10, 383–396 (2003). [DOI] [PubMed] [Google Scholar]
- 44.Haggarty SJ, Koeller KM, Wong JC, Grozinger CM & Schreiber SL Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc. Natl. Acad. Sci 100, 4389–4394 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hideshima T et al. Small-molecule inhibition of proteasome and aggresome function induces synergistic antitumor activity in multiple myeloma. Proc. Natl. Acad. Sci 102, 8567–8572 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tan DS Diversity-oriented synthesis: exploring the intersections between chemistry and biology. Nat. Chem. Biol 1, 74–84 (2005). [DOI] [PubMed] [Google Scholar]
- 47.Schreiber SL Organic chemistry: Molecular diversity by design. Nature 457, 153–154 (2009). [DOI] [PubMed] [Google Scholar]
- 48.Schreiber SL Organic synthesis toward small-molecule probes and drugs. Proc. Natl. Acad. Sci 108, 6699–6702 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dandapani S & Marcaurelle LA Current strategies for diversity-oriented synthesis. Curr. Opin. Chem. Biol 14, 362–370 (2010). [DOI] [PubMed] [Google Scholar]
- 50.Aicher TD et al. Total synthesis of halichondrin B and norhalichondrin B. J. Am. Chem. Soc 114, 3162–3164 (1992). [Google Scholar]
- 51.Towle MJ et al. In vitro and in vivo anticancer activities of synthetic macrocyclic ketone analogues of halichondrin B. Cancer Res. 61, 1013–1021 (2001). [PubMed] [Google Scholar]
- 52.Paterson I & Anderson EA The Renaissance of Natural Products as Drug Candidates. Science (80-.). 310, 451–453 (2005). [DOI] [PubMed] [Google Scholar]
- 53.Charest MG, Lerner CD, Brubaker JD, Siegel DR & Myers AG A Convergent Enantioselective Route to Structurally Diverse 6-Deoxytetracycline Antibiotics. Science (80-.). 308, 395–398 (2005). [DOI] [PubMed] [Google Scholar]
- 54.Seiple IB et al. A platform for the discovery of new macrolide antibiotics. Nature 533, 338–345 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lek M et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 536, 285–291 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Altshuler D, Daly MJ & Lander ES Genetic Mapping in Human Disease. Science (80-.). 322, 881–888 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dugger SA, Platt A & Goldstein DB Drug development in the era of precision medicine. Nat. Rev. Drug Discov (2017). doi: 10.1038/nrd.2017.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Plenge RM, Scolnick EM & Altshuler D Validating therapeutic targets through human genetics. Nat. Rev. Drug Discov 12, 581–594 (2013). [DOI] [PubMed] [Google Scholar]
- 59.The SIGMA Type 2 Diabetes Consortium. Sequence variants in SLC16A11 are a common risk factor for type 2 diabetes in Mexico. Nature 506, 97–101 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Momozawa Y et al. Resequencing of positional candidates identifies low frequency IL23R coding variants protecting against inflammatory bowel disease. Nat. Genet 43, 43–47 (2011). [DOI] [PubMed] [Google Scholar]
- 61.Cross-Disorder Group of the Psychiatric Genomics Consortium. Identification of risk loci with shared effects on five major psychiatric disorders: A genome-wide analysis. Lancet 381, 1371–1379 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Psychiatric GWAS Consortium Bipolar Disorder Working Group. Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat. Genet 43, 977–83 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sekar A et al. Schizophrenia risk from complex variation of complement component 4. Nature 530, 177–183 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bunnage ME, Piatnitski Chekler EL & Jones LH Target validation using chemical probes. Nat. Chem. Biol 9, 195–199 (2013). [DOI] [PubMed] [Google Scholar]
- 65.Arrowsmith CH et al. The promise and peril of chemical probes. Nat. Chem. Biol 11, 536–541 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schreiber SL et al. Advancing biological understanding and therapeutics discovery with small-molecule probes. Cell 161, 1252–1265 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Swinney DC Phenotypic vs. Target-Based Drug Discovery for First-in-Class Medicines. Clin. Pharmacol. Ther 93, 299–301 (2013). [DOI] [PubMed] [Google Scholar]
- 68.Moffat JG, Vincent F, Lee JA, Eder J & Prunotto M Opportunities and challenges in phenotypic drug discovery: an industry perspective. Nat. Rev. Drug Discov 16, 531–543 (2017). [DOI] [PubMed] [Google Scholar]
- 69.Gilbert IH Drug Discovery for Neglected Diseases: Molecular Target-Based and Phenotypic Approaches. J. Med. Chem 56, 7719–7726 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zheng W, Thorne N & McKew JC Phenotypic screens as a renewed approach for drug discovery. Drug Discov. Today 18, 1067–1073 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Y et al. Diversity-Oriented Synthesis as a Strategy for Fragment Evolution against GSK3β. ACS Med. Chem. Lett 7, 852–856 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schaefer GI et al. Discovery of Small-Molecule Modulators of the Sonic Hedgehog Pathway. J. Am. Chem. Soc 135, 9675–9680 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chou DH-C et al. Synthesis of a Novel Suppressor of β-Cell Apoptosis via Diversity-Oriented Synthesis. ACS Med. Chem. Lett 2, 698–702 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gray BL, Wang X, Brown WC, Kuai L & Schreiber SL Diversity synthesis of complex pyridines yields a probe of a neurotrophic signaling pathway. Org. Lett 10, 2621–2624 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aldrich LN et al. Discovery of a small-molecule probe for V-ATPase function. J. Am. Chem. Soc 137, 5563–5568 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cruz D, Wang Z, Kibbie J, Modlin R & Kwon O Diversity through phosphine catalysis identifies octahydro-1,6-naphthyridin-4-ones as activators of endothelium-driven immunity. Proc. Natl. Acad. Sci 108, 6769–6774 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stanton BZ et al. A small molecule that binds Hedgehog and blocks its signaling in human cells. Nat. Chem. Biol 5, 154–156 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kuo S-Y et al. Small-molecule enhancers of autophagy modulate cellular disease phenotypes suggested by human genetics. Proc. Natl. Acad. Sci 112, E4281–E4287 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dockendorff C et al. Benzo-fused lactams from a diversity-oriented synthesis (DOS) library as inhibitors of scavenger receptor BI (SR-BI)-mediated lipid uptake. Bioorganic Med. Chem. Lett 25, 2100–2105 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim J et al. Diversity-oriented synthetic strategy for developing a chemical modulator of protein–protein interaction. Nat. Commun 7, 13196 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nagiec MM et al. Modulators of hepatic lipoprotein metabolism identified in a search for small-molecule inducers of tribbles pseudokinase 1 expression. PLoS One 10, 1–26 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dakas PY et al. Discovery of neuritogenic compound classes inspired by natural products. Angew. Chemie - Int. Ed 52, 9576–9581 (2013). [DOI] [PubMed] [Google Scholar]
- 83.Chiang C et al. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature 383, 407–413 (1996). [DOI] [PubMed] [Google Scholar]
- 84.Ingham PW & McMahon AP Hedgehog signaling in animal development : paradigms and principles Hedgehog signaling in animal development : paradigms and principles. Genes Dev. 15, 3059–3087 (2001). [DOI] [PubMed] [Google Scholar]
- 85.Thayer SP et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature 425, 851–6 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Roessler E et al. Mutations in the human Sonic Hedgehog gene cause holoprosencephaly. Nat. Genet 14, 357–360 (1996). [DOI] [PubMed] [Google Scholar]
- 87.Stanton BZ & Peng LF Small-molecule modulators of the Sonic Hedgehog signaling pathway. Mol. BioSyst 6, 44–54 (2010). [DOI] [PubMed] [Google Scholar]
- 88.Bradner JE et al. A Robust Small-Molecule Microarray Platform for Screening Cell Lysates. Chem. Biol 13, 493–504 (2006). [DOI] [PubMed] [Google Scholar]
- 89.Owens AE et al. Design and Evolution of a Macrocyclic Peptide Inhibitor of the Sonic Hedgehog/Patched Interaction. J. Am. Chem. Soc 139, 12559–12568 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Peng LF, Stanton BZ, Maloof N, Wang X & Schreiber SL Syntheses of aminoalcohol-derived macrocycles leading to a small-molecule binder to and inhibitor of Sonic Hedgehog. Bioorganic Med. Chem. Lett 19, 6319–6325 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Justilien V & Fields AP Molecular pathways: Novel approaches for improved therapeutic targeting of hedgehog signaling in cancer stem cells. Clin. Cancer Res 21, 505–513 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chou DHC et al. Kinase-Independent Small-Molecule Inhibition of JAK-STAT Signaling. J. Am. Chem. Soc 137, 7929–7934 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Scully SS et al. Small-molecule inhibitors of cytokine-mediated STAT1 signal transduction in beta-cells with improved aqueous solubility. J Med Chem 56, 4125–4129 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Marcaurelle LA et al. An aldol-based build/couple/pair strategy for the synthesis of medium- and large-sized rings: Discovery of macrocyclic histone deacetylase inhibitors. J. Am. Chem. Soc 132, 16962–16976 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Feng Y, He D, Yao Z & Klionsky DJ The machinery of macroautophagy. Cell Res. 24, 24–41 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hidvegi T et al. An Autophagy-Enhancing Drug Promotes Degradation of Mutant a1-Antitrypsin Z and Reduces Hepatic Fibrosis. Science (80-.). 329, 229–232 (2010). [DOI] [PubMed] [Google Scholar]
- 97.Rubinsztein DC, Codogno P & Levine B Autophagy modulation as a potential therapeutic target for diverse diseases. Nat. Rev. Drug Discov 11, 709–730 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim J, Kundu M, Viollet B & Guan K-L AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol 13, 132–41 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ravikumar B et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat. Genet 36, 585–95 (2004). [DOI] [PubMed] [Google Scholar]
- 100.Martins F et al. A review of oral toxicity associated with mTOR inhibitor therapy in cancer patients. Oral Oncol. 49, 293–298 (2013). [DOI] [PubMed] [Google Scholar]
- 101.Fitzgerald ME et al. Build/couple/pair strategy for the synthesis of stereochemically diverse macrolactams via head-to-tail cyclization. ACS Comb. Sci 14, 89–96 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Galloway WRJD, Isidro-Llobet A & Spring DR Diversity-oriented synthesis as a tool for the discovery of novel biologically active small molecules. Nat. Commun 1, 1–13 (2010). [DOI] [PubMed] [Google Scholar]
- 103.Burke MD, Berger EM & Schreiber SL A synthesis strategy yielding skeletally diverse small molecules combinatorially. J. Am. Chem. Soc 126, 14095–14104 (2004). [DOI] [PubMed] [Google Scholar]
- 104.Sauer WHB & Schwarz MK Size doesn’t matter: Scaffold diversity, shape diversity and biological activity of combinatorial libraries. Chimia (Aarau). 57, 276–283 (2003). [Google Scholar]
- 105.Moffat JG, Rudolph J & Bailey D Phenotypic screening in cancer drug discovery - past, present and future. Nat. Rev. Drug Discov 13, 588–602 (2014). [DOI] [PubMed] [Google Scholar]
- 106.Fesik SW Promoting apoptosis as a strategy for cancer drug discovery. Nat. Rev. Cancer 5, 876–885 (2005). [DOI] [PubMed] [Google Scholar]
- 107.Hanahan D & Weinberg RA Hallmarks of cancer: The next generation. Cell 144, 646–674 (2011). [DOI] [PubMed] [Google Scholar]
- 108.Fruman DA & Rommel C PI3K and cancer: Lessons, challenges and opportunities. Nat. Rev. Drug Discov 13, 140–156 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sawyers C Targeted cancer therapy. Nature 432, 294–297 (2004). [DOI] [PubMed] [Google Scholar]
- 110.Bredel M & Jacoby E Chemogenomics: an emerging strategy for rapid target and drug discovery. Nat. Rev. Genet 5, 262–275 (2004). [DOI] [PubMed] [Google Scholar]
- 111.Jones S et al. Personalized genomic analyses for cancer mutation discovery and interpretation. Sci. Transl. Med 7, 283ra53 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cox AD, Fesik SW, Kimmelman AC, Luo J & Der CJ Drugging the undruggable RAS: Mission Possible? Nat. Rev. Drug Discov 13, 828–51 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Verdine GL & Walensky LD The challenge of drugging undruggable targets in cancer: Lessons learned from targeting BCL-2 family members. Clin. Cancer Res 13, 7264–7270 (2007). [DOI] [PubMed] [Google Scholar]
- 114.Yan C & Higgins PJ Drugging the undruggable: Transcription therapy for cancer. Biochim. Biophys. Acta - Rev. Cancer 1835, 76–85 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ibbeson BM et al. Diversity-oriented synthesis as a tool for identifying new modulators of mitosis. Nat. Commun 5, 1–8 (2014). [DOI] [PubMed] [Google Scholar]
- 116.Santagata S et al. Tight Coordination of Protein Translation and HSF1 Activation Supports the Anabolic Malignant State. Science (80-.). 341, 1238303 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Law JM et al. Discovery of 8-Membered Ring Sulfonamides as Inhibitors of Oncogenic Mutant Isocitrate Dehydrogenase 1. ACS Med. Chem. Lett 7, 944–949 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dückert H et al. Natural product–inspired cascade synthesis yields modulators of centrosome integrity. Nat. Chem. Biol 8, 179–184 (2011). [DOI] [PubMed] [Google Scholar]
- 119.Yu C et al. High-throughput identification of genotype-specific cancer vulnerabilities in mixtures of barcoded tumor cell lines. Nat. Biotechnol 34, 419–423 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bachovchin DA et al. Academic cross-fertilization by public screening yields a remarkable class of protein phosphatase methylesterase-1 inhibitors. Proc. Natl. Acad. Sci 108, 6811–6816 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ng PY, Tang Y, Knosp WM, Stadler HS & Shaw JT Synthesis of diverse lactam carboxamides leading to the discovery of a new transcription-factor inhibitor. Angew. Chemie - Int. Ed 46, 5352–5355 (2007). [DOI] [PubMed] [Google Scholar]
- 122.Tang W, Luo T, Greenberg EF, Bradner JE & Schreiber SL Discovery of histone deacetylase 8 selective inhibitors. Bioorganic Med. Chem. Lett 21, 2601–2605 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Koehler AN, Shamji AF & Schreiber SL Discovery of an inhibitor of a transcription factor using small molecule microarrays and diversity-oriented synthesis. J. Am. Chem. Soc 125, 8420–8421 (2003). [DOI] [PubMed] [Google Scholar]
- 124.Huryn DM et al. Chemical methodology as a source of small-molecule checkpoint inhibitors and heat shock protein 70 (Hsp70) modulators. Proc. Natl. Acad. Sci 108, 6757–6762 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fang C et al. Single diastereomer of a macrolactam core binds specifically to myeloid cell leukemia 1 (MCL1). ACS Med. Chem. Lett 5, 1308–1312 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Clark PGK et al. LP99: Discovery and synthesis of the first selective BRD7/9 bromodomain inhibitor. Angew. Chemie - Int. Ed 54, 6217–6221 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Frumm SM et al. Selective HDAC1/HDAC2 inhibitors induce neuroblastoma differentiation. Chem. Biol 20, 713–725 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Carmena M & Earnshaw WC The cellular geography of Aurora kinases. Nat Rev Mol Cell Biol 4, 842–854 (2003). [DOI] [PubMed] [Google Scholar]
- 129.Bischoff JR et al. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J. 17, 3052–3065 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Harrington EA et al. VX-680, a potent and selective small-molecule inhibitor of the Aurora kinases, suppresses tumor growth in vivo. Nat. Med 10, 262–267 (2004). [DOI] [PubMed] [Google Scholar]
- 131.Bavetsias V & Linardopoulos S Aurora Kinase Inhibitors: Current Status and Outlook. Frontiers in Oncology 5, 278 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Melichar B et al. Safety and activity of alisertib, an investigational aurora kinase A inhibitor, in patients with breast cancer, small-cell lung cancer, non-small-cell lung cancer, head and neck squamous-cell carcinoma, and gastro-oesophageal adenocarcinoma: a five-arm ph. Lancet Oncol. 16, 395–405 (2015). [DOI] [PubMed] [Google Scholar]
- 133.Dar AA, Goff LW, Majid S, Berlin J & El-Rifai W Aurora Kinase Inhibitors - Rising Stars in Cancer Therapeutics? Mol. Cancer Ther 9, 268–278 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang J, Yang PL & Gray NS Targeting cancer with small molecule kinase inhibitors. Nat Rev Cancer 9, 28–39 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sonna LA, Fujita J, Gaffin SL & Lilly CM Invited Review: Effects of heat and cold stress on mammalian gene expression. J. Appl. Physiol 92, 1725–1742 (2002). [DOI] [PubMed] [Google Scholar]
- 136.Santagata S et al. High levels of nuclear heat-shock factor 1 (HSF1) are associated with poor prognosis in breast cancer. Proc. Natl. Acad. Sci 108, 18378–18383 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dai C, Whitesell L, Rogers AB & Lindquist S Heat Shock Factor 1 Is a Powerful Multifaceted Modifier of Carcinogenesis. Cell 130, 1005–1018 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Meng L, Gabai VL & Sherman MY Heat-shock transcription factor HSF1 has a critical role in human epidermal growth factor receptor-2-induced cellular transformation and tumorigenesis. Oncogene 29, 5204–5213 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Whitesell L & Lindquist S Inhibiting the transcription factor HSF1 as an anticancer strategy. Expert Opin. Ther. Targets 13, 469–478 (2009). [DOI] [PubMed] [Google Scholar]
- 140.Koehler AN A complex task? Direct modulation of transcription factors with small molecules. Curr. Opin. Chem. Biol 14, 331–340 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gerard B, Jones G & Porco JA A Biomimetic Approach to the Rocaglamides Employing Photogeneration of Oxidopyryliums Derived from 3-Hydroxyflavones. J. Am. Chem. Soc 126, 13620–13621 (2004). [DOI] [PubMed] [Google Scholar]
- 142.Roche SP, Cencic R, Pelletier J & Porco JA Biomimetic photocycloaddition of 3-hydroxyflavones: Synthesis and evaluation of rocaglate derivatives as inhibitors of eukaryotic translation. Angew. Chemie - Int. Ed 49, 6533–6538 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gerard B, Sangji S, O’Leary DJ & Porco JA Enantioselective Photocycloaddition Mediated by Chiral Brønsted Acids: Asymmetric Synthesis of the Rocaglamides. J. Am. Chem. Soc 128, 7754–7755 (2006). [DOI] [PubMed] [Google Scholar]
- 144.Rodrigo CM, Cencic R, Roche SP, Pelletier J & Porco JA Synthesis of rocaglamide hydroxamates and related compounds as eukaryotic translation inhibitors: Synthetic and biological studies. J. Med. Chem 55, 558–562 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kärkäs MD et al. Photochemical Approaches to Complex Chemotypes: Applications in Natural Product Synthesis. Chem. Rev 116, 9683–9747 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wilson BG & Roberts CWM SWI/SNF nucleosome remodellers and cancer. Nat. Rev. Cancer 11, 481–492 (2011). [DOI] [PubMed] [Google Scholar]
- 147.Reisman D, Glaros S & Thompson EA The SWI/SNF complex and cancer. Oncogene 28, 1653–1668 (2009). [DOI] [PubMed] [Google Scholar]
- 148.Drost J et al. BRD7 is a candidate tumour suppressor gene required for p53 function. Nat. Cell Biol 12, 380–389 (2010). [DOI] [PubMed] [Google Scholar]
- 149.Hohmann AF et al. Sensitivity and engineered resistance of myeloid leukemia cells to BRD9 inhibition. Nat. Chem. Biol 12, 672–679 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chaikuad A, Petros AM, Fedorov O, Xu J & Knapp S Structure-based approaches towards identification of fragments for the low-druggability ATAD2 bromodomain. Medchemcomm 5, 1843–1848 (2014). [Google Scholar]
- 151.Saha S et al. A Phosphatase Associated with Metastasis of Colorectal Cancer. Science (80-.). 294, 1343–1346 (2001). [DOI] [PubMed] [Google Scholar]
- 152.Li J et al. PTEN, a Putative Protein Tyrosine Phosphatase Gene Mutated in Human Brain, Breast, and Prostate Cancer. Science (80-.). 275, 1943–1947 (1997). [DOI] [PubMed] [Google Scholar]
- 153.Tonks NK Protein tyrosine phosphatases – from housekeeping enzymes to master regulators of signal transduction. FEBS J. 280, 346–378 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lazo JS & Sharlow ER Drugging Undruggable Molecular Cancer Targets. Annu. Rev. Pharmacol. Toxicol 56, 23–40 (2016). [DOI] [PubMed] [Google Scholar]
- 155.Khanna A, Pimanda JE & Westermarck J Cancerous Inhibitor of Protein Phosphatase 2A, an Emerging Human Oncoprotein and a Potential Cancer Therapy Target. Cancer Res. 73, 6548–6553 (2013). [DOI] [PubMed] [Google Scholar]
- 156.Ciccone M, Calin GA & Perrotti D From the Biology of PP2A to the PADs for Therapy of Hematologic Malignancies. Frontiers in Oncology 5, 21 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Xing Y et al. Structural Mechanism of Demethylation and Inactivation of Protein Phosphatase 2A. Cell 133, 154–163 (2008). [DOI] [PubMed] [Google Scholar]
- 158.Wandzioch E et al. PME-1 Modulates Protein Phosphatase 2A Activity to Promote the Malignant Phenotype of Endometrial Cancer Cells. Cancer Res. 74, 4295 LP–4305 (2014). [DOI] [PubMed] [Google Scholar]
- 159.Berlin JM & Fu GC Enantioselective nucleophilic catalysis: The synthesis of aza-β-lactams through [2+2] cycloadditions of ketenes with azo compounds. Angew. Chemie - Int. Ed 47, 7048–7050 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zuhl AM et al. Competitive Activity-Based Protein Profiling Identifies Aza-β-Lactams as a Versatile Chemotype for Serine Hydrolase Inhibition. J. Am. Chem. Soc 134, 5068–5071 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Little SJ et al. ANTIRETROVIRAL-DRUG RESISTANCE AMONG PATIENTS RECENTLY INFECTED WITH HIV. N. Engl. J. Med 347, 385–394 (2002). [DOI] [PubMed] [Google Scholar]
- 162.Cohen ML Epidemiology of drug resistance: inplications for a post-antimicrobial era. Science (80-.). 257, 1050–1055 (1992). [DOI] [PubMed] [Google Scholar]
- 163.Katsuno K et al. Hit and lead criteria in drug discovery for infectious diseases of the developing world. Nat. Rev. Drug Discov 14, 751–758 (2015). [DOI] [PubMed] [Google Scholar]
- 164.Lewis K Platforms for antibiotic discovery. Nat. Rev. Drug Discov 12, 371–87 (2013). [DOI] [PubMed] [Google Scholar]
- 165.Davies J & Davies D Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev 74, 417–433 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Farha MA & Brown ED Strategies for target identification of antimicrobial natural products. Nat. Prod. Rep 33, 668–680 (2016). [DOI] [PubMed] [Google Scholar]
- 167.Chu J et al. Discovery of MRSA active antibiotics using primary sequence from the human microbiome. Nat Chem Biol 12, 1004–1006 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Payne DJ, Gwynn MN, Holmes DJ & Pompliano DL Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat. Rev. Drug Discov 6, 29–40 (2007). [DOI] [PubMed] [Google Scholar]
- 169.Wensing AMJ, van Maarseveen NM & Nijhuis M Fifteen years of HIV Protease Inhibitors: raising the barrier to resistance. Antiviral Res. 85, 59–74 (2010). [DOI] [PubMed] [Google Scholar]
- 170.Scheel TKH & Rice CM Understanding the hepatitis C virus life cycle paves the way for highly effective therapies. Nat. Med 19, 837–849 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lipinski CA, Lombardo F, Dominy BW & Feeney PJ Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev 23, 3–25 (1997). [DOI] [PubMed] [Google Scholar]
- 172.Fischbach MA & Walsh CT Antibiotics for Emerging Pathogens. Science (80-.). 325, 1089–1093 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wright PM, Seiple IB & Myers AG The evolving role of chemical synthesis in antibacterial drug discovery. Angew. Chemie - Int. Ed 53, 8840–8869 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lukens AK et al. Diversity-oriented synthesis probe targets plasmodium falciparum cytochrome b ubiquinone reduction site and synergizes with oxidation site inhibitors. J. Infect. Dis 211, 1097–1103 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Maetani M et al. Discovery of Antimalarial Azetidine-2-carbonitriles That Inhibit P. falciparum Dihydroorotate Dehydrogenase. ACS Med. Chem. Lett 8, 438–442 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Plouffe DM et al. High-Throughput Assay and Discovery of Small Molecules that Interrupt Malaria Transmission. Cell Host Microbe 19, 114–126 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rottmann M et al. Spiroindolones, a Potent Compound Class for the Treatment of Malaria. Science (80-.). 329, 1175–1180 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kato N et al. Diversity-oriented synthesis yields novel multistage antimalarial inhibitors. Nature 538, 344–349 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wellington S et al. A small-molecule allosteric inhibitor of Mycobacterium tuberculosis tryptophan synthase. Nat. Chem. Biol 13, 943–950 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Park SW et al. Target-based identification of whole-cell active inhibitors of biotin biosynthesis in mycobacterium tuberculosis. Chem. Biol 22, 76–86 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dandapani S et al. Diversity-Oriented Synthesis Yields a New Drug Lead for Treatment of Chagas Disease. ACS Med. Chem. Lett 5, 149–153 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Thomas GL et al. Anti-MRSA agent discovery using diversity-oriented synthesis. Angew. Chemie - Int. Ed 47, 2808–2812 (2008). [DOI] [PubMed] [Google Scholar]
- 183.Duvall JR et al. Novel diversity-oriented synthesis-derived respiratory syncytial virus inhibitors identified via a high throughput replicon-based screen. Antiviral Res. 131, 19–25 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Nuhs A et al. Development and Validation of a Novel Leishmania donovani Screening Cascade for High-Throughput Screening Using a Novel Axenic Assay with High Predictivity of Leishmanicidal Intracellular Activity. PLoS Negl. Trop. Dis 9, 1–17 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Duvall JR et al. Identification of Highly Specific Diversity-Oriented Synthesis-Derived Inhibitors of Clostridium difficile. ACS Infect. Dis 3, 349–359 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Baniecki ML, Wirth DF & Clardy J High-throughput Plasmodium falciparum growth assay for malaria drug discovery. Antimicrob. Agents Chemother 51, 716–723 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Flannery EL, Chatterjee AK & Winzeler EA Antimalarial drug discovery - approaches and progress towards new medicines. Nat. Rev. Microbiol 11, 849–62 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Campo B, Vandal O, Wesche DL & Burrows JN Killing the hypnozoite - drug discovery approaches to prevent relapse in Plasmodium vivax. Pathog. Glob. Health 109, 107–22 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.International Artemisinin Study Group. Artesunate combinations for malaria. Lancet 363, 9–17 (2004). [DOI] [PubMed] [Google Scholar]
- 190.White NJ Antimalarial drug resistance. J. Clin. Invest 113, 1084–1092 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Winzeler EA & Manary MJ Drug resistance genomics of the antimalarial drug artemisinin. Genome Biol. 15, 544 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Klayman DL Qinghaosu (artemisinin): an antimalarial drug from China. Science (80-.). 228, 1049–1055 (1985). [DOI] [PubMed] [Google Scholar]
- 193.Dondorp AM et al. Artemisinin Resistance in Plasmodium falciparum Malaria. N. Engl. J. Med 361, 455–467 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Dyer M, Jackson M, McWhinney C, Zhao G & Mikkelsen R Analysis of a cation-transporting ATPase of Plasmodium falciparum. Mol. Biochem. Parasitol 78, 1–12 (1996). [DOI] [PubMed] [Google Scholar]
- 195.Spillman NJ et al. Na+ Regulation in the Malaria Parasite Plasmodium falciparum Involves the Cation ATPase PfATP4 and Is a Target of the Spiroindolone Antimalarials. Cell Host Microbe 13, 227–237 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.White NJ et al. Spiroindolone KAE609 for Falciparum and Vivax Malaria. N. Engl. J. Med 371, 403–410 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Van Pelt-Koops JC et al. The spiroindolone drug candidate NITD609 potently inhibits gametocytogenesis and blocks Plasmodium falciparum transmission to Anopheles mosquito vector. Antimicrob. Agents Chemother 56, 3544–3548 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Flannery EL et al. Mutations in the P-Type Cation-Transporter ATPase 4, PfATP4, Mediate Resistance to Both Aminopyrazole and Spiroindolone Antimalarials. ACS Chem. Biol 10, 413–420 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Vaidya AB et al. Pyrazoleamide compounds are potent antimalarials that target Na(+) homeostasis in intraerythrocytic Plasmodium falciparum. Nat. Commun 5, 5521 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Jiménez-Díaz MB et al. (+)-SJ733, a clinical candidate for malaria that acts through ATP4 to induce rapid host-mediated clearance of Plasmodium. Proc. Natl. Acad. Sci 111, E5455–E5462 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ang SH et al. SPIRO-INDOLE DERIVATIVES FOR THE TREATMENT OF PARASITIC DISEASES. (2009).
- 202.Badillo JJ, Silva-García A, Shupe BH, Fettinger JC & Franz AK Enantioselective Pictet-Spengler reactions of isatins for the synthesis of spiroindolones. Tetrahedron Lett. 52, 5550–5553 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zheng H et al. Regio- and Enantioselective Aza-Diels-Alder Reactions of 3-Vinylindoles: A Concise Synthesis of the Antimalarial Spiroindolone NITD609. Angew. Chemie - Int. Ed 54, 10958–10962 (2015). [DOI] [PubMed] [Google Scholar]
- 204.Heidebrecht RW Jr et al. Diversity-Oriented Synthesis Yields a Novel Lead for the Treatment of Malaria. ACS Med. Chem. Lett 3, 112–117 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Comer E et al. Diversity-oriented synthesis-facilitated medicinal chemistry: Toward the development of novel antimalarial agents. J. Med. Chem 57, 8496–8502 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lowe JT et al. Synthesis and profiling of a diverse collection of azetidine-based scaffolds for the development of CNS-focused lead-like libraries. J. Org. Chem 77, 7187–7211 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Maetani M et al. Synthesis of a bicyclic azetidine with in vivo antimalarial activity enabled by stereospecific, directed C(sp 3)-H arylation. J. Am. Chem. Soc 139, 11300–11306 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Younis Y et al. 3,5-Diaryl-2-Aminopyridines As a Novel Class of Orally Active Antimalarials Demonstrating Single Dose Cure in Mice and Clinical Candidate Potential. J. Med. Chem 55, 3479–3487 (2012). [DOI] [PubMed] [Google Scholar]
- 209.Baragaña B et al. A novel multiple-stage antimalarial agent that inhibits protein synthesis. Nature 522, 315–320 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Cohen SH et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults: 2010 Update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect. Control Hosp. Epidemiol 31, 431–455 (2010). [DOI] [PubMed] [Google Scholar]
- 211.Kelly CP, Pothoulakis C & LaMont JT Clostridium difficile Colitis. N. Engl. J. Med 330, 257–262 (1994). [DOI] [PubMed] [Google Scholar]
- 212.Louie TJ, Emery J, Krulicki W, Byrne B & Mah M OPT-80 eliminates Clostridium difficile and is sparing of Bacteroides species during treatment of C. difficile infection. Antimicrob. Agents Chemother 53, 261–263 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Smits WK, Lyras D, Lacy DB, Wilcox MH & Kuijper EJ Clostridium difficile infection. Nat. Rev. Dis. Prim 2, 16020 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Fisher SL Glutamate racemase as a target for drug discovery. Microb. Biotechnol 1, 345–360 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Gerard B et al. Application of a catalytic asymmetric povarov reaction using chiral ureas to the synthesis of a tetrahydroquinoline library. ACS Comb. Sci 14, 621–630 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.World Health Organization. Global Tuberculosis Report 2016. (2016). doi:ISBN 978 92 4 156539 4 [Google Scholar]
- 217.Dheda K, Barry CE & Maartens G Tuberculosis. Lancet 387, 1211–1226 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Munro SA et al. Patient Adherence to Tuberculosis Treatment: A Systematic Review of Qualitative Research. PLOS Med. 4, e238 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Dunn MF Allosteric regulation of substrate channeling and catalysis in the tryptophan synthase bienzyme complex. Arch. Biochem. Biophys 519, 154–166 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Nussinov R & Tsai C-J Allostery in Disease and in Drug Discovery. Cell 153, 293–305 (2013). [DOI] [PubMed] [Google Scholar]
- 221.Meijer AH Protection and pathology in TB: learning from the zebrafish model. Semin. Immunopathol 38, 261–273 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Stanley SA et al. Identification of Novel Inhibitors of M. tuberculosis Growth Using Whole Cell Based High-Throughput Screening. ACS Chem. Biol 7, 1377–1384 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.J.Silhavy T, Kahne D & Walker S The bacterial cell envelope. Cold Spring Harb. Perspect. Biol 2, 1–17 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Frye SV The art of the chemical probe. Nat. Chem. Biol 6, 159–161 (2010). [DOI] [PubMed] [Google Scholar]
- 225.Wawer MJ et al. Toward performance-diverse small-molecule libraries for cell-based phenotypic screening using multiplexed high-dimensional profiling. Proc. Natl. Acad. Sci 111, 10911–10916 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Drews J Drug Discovery: A Historical Perspective. Science (80-.). 287, 1960–1964 (2000). [DOI] [PubMed] [Google Scholar]
- 227.Hann MM, Leach AR & Harper G Molecular Complexity and Its Impact on the Probability of Finding Leads for Drug Discovery. J. Chem. Inf. Comput. Sci 41, 856–864 (2001). [DOI] [PubMed] [Google Scholar]
- 228.Hann MM & Oprea TI Pursuing the leadlikeness concept in pharmaceutical research. Curr. Opin. Chem. Biol 8, 255–263 (2004). [DOI] [PubMed] [Google Scholar]
- 229.Kogej T et al. Big pharma screening collections: more of the same or unique libraries? The AstraZeneca–Bayer Pharma AG case. Drug Discov. Today 18, 1014–1024 (2013). [DOI] [PubMed] [Google Scholar]
- 230.Terai T & Nagano T Small-molecule fluorophores and fluorescent probes for bioimaging. Pflügers Arch. - Eur. J. Physiol 465, 347–359 (2013). [DOI] [PubMed] [Google Scholar]
- 231.Sakamoto KM et al. Protacs: Chimeric molecules that target proteins to the Skp1–Cullin–F box complex for ubiquitination and degradation. Proc. Natl. Acad. Sci 98, 8554–8559 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Winter GE et al. Phthalimide conjugation as a strategy for in vivo target protein degradation. Science (80-.). (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Bondeson DP et al. Catalytic in vivo protein knockdown by small-molecule PROTACs. Nat. Chem. Biol 11, 611–617 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Meyer B & Peters T NMR Spectroscopy Techniques for Screening and Identifying Ligand Binding to Protein Receptors NMR Spectroscopy Techniques for Screening and Identifying Ligand Binding to Protein Receptors. Angew. Chem. Int. Ed 42, 864–890 (2003). [DOI] [PubMed] [Google Scholar]
- 235.Arkin MR & Wells JA Small-molecule inhibitors of protein-protein interactions: Progressing towards the dream. Nat. Rev. Drug Discov 3, 301–317 (2004). [DOI] [PubMed] [Google Scholar]
- 236.Salamon H, Klika Skopic M, Jung K, Bugain O & Brunschweiger A Chemical Biology Probes from Advanced DNA-encoded Libraries. ACS Chem. Biol 11, 296–307 (2016). [DOI] [PubMed] [Google Scholar]
- 237.Franzini RM & Randolph C Chemical Space of DNA-Encoded Libraries. J. Med. Chem 59, 6629–6644 (2016). [DOI] [PubMed] [Google Scholar]
- 238.Petrone PM et al. Biodiversity of small molecules – a new perspective in screening set selection. Drug Discov. Today 18, 674–680 (2013). [DOI] [PubMed] [Google Scholar]
- 239.Subramanian A et al. A Next Generation Connectivity Map: L1000 Platform And The First 1,000,000 Profiles. bioRxiv (2017). doi: 10.1101/136168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Gustafsdottir SM et al. Multiplex Cytological Profiling Assay to Measure Diverse Cellular States. PLoS One 8, e80999 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kang J et al. Improving drug discovery with high-content phenotypic screens by systematic selection of reporter cell lines. Nat. Biotechnol 34, 70–77 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Gerry CJ et al. Real-Time Biological Annotation of Synthetic Compounds. J. Am. Chem. Soc 138, 8920–8927 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Nelson SD Jr., Wawer MJ & Schreiber SL Divergent Synthesis and Real-Time Biological Annotation of Optically Active Tetrahydrocyclopenta[c]pyranone Derivatives. Org. Lett 18, 6280–6283 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Lajiness MS, Maggiora GM & Shanmugasundaram V Assessment of the Consistency of Medicinal Chemists in Reviewing Sets of Compounds. J. Med. Chem 47, 4891–4896 (2004). [DOI] [PubMed] [Google Scholar]
- 245.Hansch C The advent and evolution of QSAR at Pomona College. J. Comput. Aided. Mol. Des 25, 495–507 (2011). [DOI] [PubMed] [Google Scholar]
- 246.Kirkpatrick P Chris Lipinski. Nat. Rev. Drug Discov 11, 900–901 (2012). [DOI] [PubMed] [Google Scholar]
- 247.Lipinski C & Hopkins A Navigating chemical space for biology and medicine. Nature 432, 855–861 (2004). [DOI] [PubMed] [Google Scholar]
- 248.Baell J & Walters MA Chemistry: Chemical con artists foil drug discovery. Nature 513, 481–483 (2014). [DOI] [PubMed] [Google Scholar]
- 249.Veber DF et al. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem 45, 2615–2623 (2002). [DOI] [PubMed] [Google Scholar]
- 250.Tian S et al. The application of in silico drug-likeness predictions in pharmaceutical research. Adv. Drug Deliv. Rev 86, 2–10 (2015). [DOI] [PubMed] [Google Scholar]
- 251.Blake JF Integrating cheminformatic analysis in combinatorial chemistry. Curr. Opin. Chem. Biol 8, 407–411 (2004). [DOI] [PubMed] [Google Scholar]
- 252.Haftchenary S et al. Efficient Routes to a Diverse Array of Amino Alcohol-Derived Chiral Fragments. ACS Comb. Sci 18, 569–574 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Ghose AK, Viswanadhan VN & Wendoloski JJ A Knowledge-Based Approach in Designing Combinatorial or Medicinal Chemistry Libraries for Drug Discovery. 1. A Qualitative and Quantitative Characterization of Known Drug Databases. J. Comb. Chem 1, 55–68 (1999). [DOI] [PubMed] [Google Scholar]
- 254.Garbaccio RM & Parmee ER The Impact of Chemical Probes in Drug Discovery: A Pharmaceutical Industry Perspective. Cell Chem. Biol 23, 10–17 (2016). [DOI] [PubMed] [Google Scholar]
- 255.Frye SV et al. Tackling reproducibility in academic preclinical drug discovery. Nat. Rev. Drug Discov 14, 733–734 (2015). [DOI] [PubMed] [Google Scholar]
- 256.Workman P & Collins I Probing the Probes: Fitness Factors For Small Molecule Tools. Chem. Biol 17, 561–577 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Blagg J & Workman P Choose and Use Your Chemical Probe Wisely to Explore Cancer Biology. Cancer Cell 32, 9–25 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Skuta C et al. Probes & Drugs portal: an interactive, open data resource for chemical biology. Nat. Methods 14, 759–760 (2017). [DOI] [PubMed] [Google Scholar]
- 259.Antolin AA et al. Objective, Quantitative, Data-Driven Assessment of Chemical Probes. Cell Chem. Biol 25, 194–205 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Cornu M, Albert V & Hall MN mTOR in aging, metabolism, and cancer. Curr. Opin. Genet. Dev 23, 53–62 (2013). [DOI] [PubMed] [Google Scholar]
- 261.Batlle D & Haque SK Genetic causes and mechanisms of distal renal tubular acidosis. Nephrol. Dial. Transplant 27, 3691–3704 (2012). [DOI] [PubMed] [Google Scholar]
- 262.Bhargava A et al. Osteopetrosis mutation R444L causes endoplasmic reticulum retention and misprocessing of vacuolar H+-ATPase a3 subunit. J. Biol. Chem 287, 26829–26839 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]