Abstract
BACKGROUND
Surgical site infections (SSIs) following colorectal surgery (CRS) are among the most common healthcare-associated infections (HAIs). Reduction in colorectal SSI rates is an important goal for surgical quality improvement.
OBJECTIVE
To examine rates of SSI in patients with and without cancer and to identify potential predictors of SSI risk following CRS
DESIGN
American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) data files for 2011–2013 from a sample of 12 National Comprehensive Cancer Network (NCCN) member institutions were combined. Pooled SSI rates for colorectal procedures were calculated and risk was evaluated. The independent importance of potential risk factors was assessed using logistic regression.
SETTING
Multicenter study
PARTICIPANTS
Of 22 invited NCCN centers, 11 participated (50%). Colorectal procedures were selected by principal procedure current procedural technology (CPT) code. Cancer was defined by International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) codes.
MAIN OUTCOME
The primary outcome of interest was 30-day SSI rate.
RESULTS
A total of 652 SSIs (11.06%) were reported among 5,893 CRSs. Risk of SSI was similar for patients with and without cancer. Among CRS patients with underlying cancer, disseminated cancer (SSI rate, 17.5%; odds ratio [OR], 1.66; 95% confidence interval [CI], 1.23–2.26; P= .001), ASA score ≥3 (OR, 1.41; 95% CI, 1.09–1.83; P=.001), chronic obstructive pulmonary disease (COPD; OR, 1.6; 95% CI, 1.06–2.53; P= .02), and longer duration of procedure were associated with development of SSI.
CONCLUSIONS
Patients with disseminated cancer are at a higher risk for developing SSI. ASA score >3, COPD, and longer duration of surgery predict SSI risk. Disseminated cancer should be further evaluated by the Centers for Disease Control and Prevention (CDC) in generating risk-adjusted outcomes.
In 2016, an estimated 1.6 million new cases of cancer were diagnosed. Prostate, lung, and colorectal are among the most common cancers diagnosed in males. Among females, breast, lung, and colorectal cancers are the most common.1 Colorectal surgery offers the only curative option for localized cancer and resectable metastatic disease. For patients with advanced cancer, regardless of site, colorectal surgery is undertaken as a palliative modality to manage complications such as intestinal obstruction or perforation.
Surgical site infection (SSIs) is a common postoperative complication of colorectal procedures. Surgical site infections have been associated with extended postoperative hospital stay, readmission, morbidity, and death.2–5 Reduction in infectious complications is a primary goal for surgical quality programs across US hospitals.2–5 Based on the most recent estimates from the Centers for Disease Control and Prevention (CDC), 22% of all HAIs are SSIs, and 15% of these are associated with colorectal procedures.6 For these reasons, lowering SSI rates after CRS has become an observable priority.
Furthermore, SSI rates after CRS vary widely across studies, from 3% to 30%.7,8 As a leading indication for CRS, the risk of SSI exclusively for oncologic resection is less well studied. A single observational study that examined more than 600 elective CRS procedures in patients with colorectal cancer found a 30-day SSI rate of 25%.9 These findings suggest that infectious complications fall among the higher end of the spectrum for persons undergoing surgery for colorectal cancer. Multicenter standardized assessment of SSI risk among patients with cancer is not currently available.
In the early 2000s, the American College of Surgeons (ACS) extended the National Surgical Quality Improvement Program (NSQIP) method for risk-adjusted 30-day morbidity and mortality outcomes after surgery from the Veterans Affairs Healthcare system to the private sector, and SSIs are among the outcomes examined.10,11 The ACS NSQIP is broadly used by many healthcare facilities including several National Comprehensive Cancer Network (NCCN) member institutions.
The goal of this project was to examine rates of SSI following CRS in patients with and without cancer by combining ACS NSQIP datasets from NCCN member institutions. Among NCCN-NSQIP participants, pooled and facility-specific rates of SSI were examined in patients with and without cancer. For those with underlying cancer, various baseline clinical variables and intraoperative characteristics were examined as potential predictors of SSI risk following CRS for cancer.
METHODS
Data Source: American College of Surgeons National Surgical Quality Improvement Program [ACS NSQIP]
The ACS NSQIP dataset for an institution is composed of a systematic sample of major operations performed under general, spinal, or epidural anesthesia. Cases are selected through a list of current procedural terminology (CPT) codes.12 Sampling was based on consecutive selection of eligible cases in each 8-day cycle. The data collected for eligible cases included ~ 105 variables: 70 preoperative risk factors, 11 variables regarding the operation, and 24 postoperative outcome variables.13 The preoperative risk factors included demographic data and major organ-system comorbidities described below. Preoperative laboratory values closest to the time of the operation were also collected. Operative variables included CPT codes, anesthesia type, American Society of Anesthesiologists (ASA) class, wound class, and operative and anesthesia times. Postoperative outcomes included vital status at 30 days and 21 different postoperative complications including SSI, return to operating room, and length of stay.
Data Acquisition
We invited 22 National Comprehensive Cancer Network (NCCN) Member Institutions to participate in this study, and 11 centers did so: 2 standalone oncology centers and 9 large multispecialty teaching hospitals.
Patients who had undergone incision, resection, or anastomosis of the large intestine based on primary CPT codes were identified from the ACS NSQIP databases from January 1, 2011, through December 31, 2013 (Supplementary Table 1). Based on these query criteria, institutions were asked to submit deidentified ACS-NSQIP data files that were subsequently combined. A pooled oncology-specific SSI rate was derived from this dataset based on differentiation of cancer and noncancer by International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) code.
Outcomes
The primary NSQIP outcome of interest was 30-day SSI. Patients were excluded from having an SSI if the SSI was documented preoperatively. Crude rates of additional postsurgical outcomes were examined among cancer and noncancer groups. These included 30-day mortality, readmission, wound disruption at 30 days, and length of hospitalization.
Risk Adjustment Factors
Variables for risk adjustment included patient demographics, preoperative risk factors, comorbidities, procedure-related risk factors, and behavioral risk factors. Patient demographic variables included age (18–64 or ≥65 years) and gender. Preoperative risk variables included body mass index (BMI) and ASA class (1–2 or ≥3). Comorbidities included diabetes (requiring oral medication or insulin vs none), chronic obstructive pulmonary disease, ascites, cancer (postoperative diagnosis ICD code), disseminated cancer, chronic steroid use, bleeding disorders, hypertension, preoperative systemic inflammatory response syndrome/sepsis, and currently requiring or on dialysis. Procedure-related risk factors included whether it was an emergency case, wound classification (contaminated or dirty), open wound (at the time of operative procedure with or without infection), duration of procedure (continuous 1-minute units), >1 other procedure, surgical approach (defined by CPT), and surgical wound closure (no layers and only deep layers of incision closed). Behavioral risk factors included smoking status (within 1 year prior to operation). Duration of procedure was included as a continuous variable converted into 10-minute units. All other risk factors were converted into discrete categories.
Statistical Analysis
Postoperative diagnosis (ICD-9-CM code) was utilized to capture patients with underlying cancer. Overall, 3,365 procedures (53.4%) had a cancer diagnostic discharge ICD-9-CM code and were included in the cancer category. The remaining procedures formed the noncancer cohort. Univariate analysis was performed using χ2 tests for categorical variables, with significance set at P <.05. The dependent variable (SSI) was dichotomized. Multivariate logistic regression analysis was conducted for the entire cohort, for patients with underlying cancer, and for the noncancer group to determine predictors that confer risk for SSI. All statistical analyses were performed using SAS version 9.4 software (SAS Institute, Cary, NC).
RESULTS
Baseline Characteristics and Overall SSI Rates
From January 1, 2011, to December 31, 2013, a total of 5,893 colon procedures met criteria for inclusion. Among these, 652 SSIs occurred within 30 days (overall rate, 11.06%; range, 5.93%–16.67%). The age range of the 5,893 patients was 18–101 years, with a mean of 58 years (±16.35 years) and a median of 59 years, and 44% of these patients were men. Body mass index was ≥30 kg/m2 in 29% of patients. A laparoscopic approach was used in 48.6% of procedures. The mean duration of surgery was 184 minutes (±118 minutes). Mean duration for open procedures was 202 minutes (±129.4 minutes), and mean duration for laparoscopic procedures was 187.6 minutes (±105.5 minutes). Major noncancer-related comorbidities included hypertension (44%), diabetes mellitus (12. 5%), and chronic obstructive pulmonary disease (5.1%).
Oncology-Specific SSI Rates and Localized Versus Disseminated Cancer
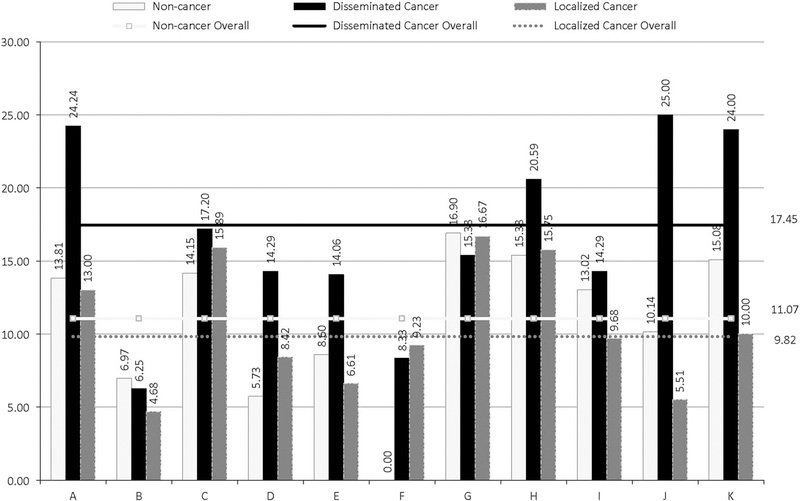
Crude facility-specific SSI rates among participating hospitals are shown in Figure 1 and Table 1. Of the 5,893 CRS patients, 3,137 (53%) had underlying cancer and 2,756 (47%) did not. Across the multispecialty centers excluding cancer-only hospitals (ie, hospitals F and H), 2,673 of 5,384 CRS (49.65%) were cancer-related procedures. The SSI rates among the cancer and noncancer cohorts were similar at 11 .06% and 11.07%, respectively (range, 4.81%–16.67% for those with cancer and 0–16.9% for those without) (Table 2). The baseline characteristics and univariate analysis are shown in Table 2.
FIGURE 1.
Facility-specific surgical site infection among 11 participating centers depicting percent of SSI in localized cancer, disseminated cancer, and noncancer CRS patients.
TABLE 1.
Facility-Specific Risk of SSI Among 11 Participating Centers Depicting Rate of Surgical Site Infection (SSI) in Localized Cancer, Disseminated Cancer, and Noncancer-Related Colorectal Surgery (CRS)
| Facility | No. of Procedures (% Cancer) (Total= 5,893) | No. of SSIs (% Cancer) (Total=652) | Noncancer, SSI Rate, % (N =2,756) | Localized Cancer, SSI Rate, % (N = 2,627) | Disseminated Cancer, SSI Rate, % (N =510) | Overall SSI Rate, % |
|---|---|---|---|---|---|---|
| A | 705 (48.65) | 102 (50.98) | 13.81 | 13.00 | 24.24 | 14.47 |
| B | 388 (48.2) | 23 (39.13) | 6.97 | 4.68 | 6.25 | 5.93 |
| C | 819 (48.23) | 124 (51.61) | 14.15 | 15.89 | 17.20 | 15.14 |
| D | 516 (45.93) | 38 (57.89) | 5.73 | 8.42 | 14.29 | 7.36 |
| E | 1510 (54.57) | 123 (52.03) | 8.60 | 6.61 | 14.06 | 8.15 |
| F | 316 (89.97) | 26 (100) | 0.00 | 9.23 | 8.33 | 8.23 |
| G | 156 (54.59) | 26 (53.85) | 16.90 | 16.67 | 15.38 | 16.67 |
| H | 193 (93.26) | 32 (93.75) | 15.38 | 15.75 | 20.59 | 16.58 |
| I | 367 (41.42) | 44 (36.36) | 13.02 | 9.68 | 14.29 | 11.99 |
| J | 323 (54.18) | 34 (55.88) | 10.14 | 5.51 | 25.00 | 10.53 |
| K | 600 (45.83) | 80 (38.75) | 15.08 | 10.00 | 24.00 | 13.33 |
| Overall | 11.06 | 9.8 | 17.5 | 11.06 |
TABLE 2.
Demographic Characteristics and Univariate Analysis
| Surgical Site Infection |
|||||
|---|---|---|---|---|---|
| Total (%) (n=5,893) | Yes, No. (%) (n=652) | No, No. (%) (n=5,241) | SSI % | P Value | |
| Demographics | |||||
| Age | |||||
| 65+ y | 2,345 (39.79) | 2,083(39.74) | 262 (40.18) | 11.17 | .8287 |
| <64 y | 3,548 (60.21) | 3,158 (60.26) | 390 (59.82) | 10.99 | |
| Gender | |||||
| Male | 2,798 (47.48) | 320 (49.08) | 2,478 (47.28) | 11.44 | .3905 |
| Female | 3,093 (52.49) | 332 (50.92) | 2,761 (52.68) | 10.73 | |
| Missing gender | 2 (0.03) | 0 (0) | 2 (0.04) | 0.00 | |
| Preoperative risk factors | |||||
| Body mass index, kg/m2 | |||||
| <18.5 | 229 (3.89) | 25 (3.83) | 204 (3.89) | 10.92 | .0006 |
| 18.5–24.9 | 1,991 (33.79) | 184 (28.22) | 1,807 (34.38) | 9.24 | |
| 25–29.9 | 1,865 (31.65) | 196 (30.06) | 1,669 (31.85) | 10.51 | |
| ≥30 | 1,715 (29.10) | 234 (35.89) | 1,481 (28.26) | 13.64 | |
| Missing BMI | 93 (1.58) | 13 (1.99) | 80 (1.53) | 13.98 | |
| ASA class | |||||
| ASA ≥3 | 2,793 (47.40) | 413 (63.34) | 2,685 (51.23) | 14.79 | <.0001 |
| ASA <3 | 3,098 (52.57) | 238 (36.5) | 2,555 (48.75) | 7.68 | |
| Missing ASA | 2 (0.03) | 1 (0.15) | 1 (0.02) | 50.00 | |
| Procedure-related risk factors | |||||
| Emergency case | |||||
| Yes | 610 (10.35) | 78 (11.96) | 532 (10.15) | 12.79 | .1519 |
| No | 5,283 (89.65) | 574 (88.04) | 4,709 (89.85) | 10.87 | |
| Wound classification | |||||
| Contaminated or dirty/infection | 1,400 (23.76) | 184 (28.22) | 1,216 (23.20) | 13.14 | .0045 |
| Clean or clean contaminated | 4493 (76.24) | 468 (71.78) | 4,025 (76.8) | 10.42 | |
| Open wound | |||||
| Yes | 175 (2.97) | 17 (2.61) | 158 (3.01) | 9.71 | .5634 |
| No | 5,718 (97.03) | 635 (97.39) | 5,083 (96.99) | 11.11 | |
| Mean duration, min | 195.07 (SD, 118.62) | 227.1 (SD, 145.9) | 191.1 (SD, 114.2) | <.0001 | |
| >1 Procedure | |||||
| >1 Other procedures | 1,384 (23.49) | 176 (26.99) | 1,208 (23.05) | 12.72 | .025 |
| ≤1 Other procedure | 4,509 (76.41) | 476 (73.01) | 4,033 (76.95) | 10.56 | |
| Surgical approach | |||||
| Open | 3,028 (51.38) | 377 (57.82) | 2,651 (50.58) | 12.45 | .0005 |
| Laparoscopic | 2,865 (48.62) | 275 (42.18) | 2,590 (49.42) | 9.60 | |
| Wound closure | |||||
| Only deep or no layers closed | 152 (2.58) | 15 (2.3) | 137 (2.61) | .7964 | |
| All layers closed | 3,887 (65.96) | 409 (62.73) | 3,478 (66.36) | ||
| Missing wound closure | 1,854 (31.46) | 228 (34.97) | 1,626 (31.02) | ||
| Comorbidities | |||||
| Diabetes | |||||
| Yes | 737 (12.51) | 123 (18.87) | 614 (11.72) | 16.69 | <.0001 |
| No | 5,156 (87.49) | 529 (81.13) | 4,627 (88.28) | 10.26 | |
| COPD | |||||
| Yes | 298 (5.06) | 55 (8.44) | 243 (4.64) | 18.46 | <.0001 |
| No | 5,595 (94.94) | 597 (91.56) | 4,498 (95.36) | 10.67 | |
| Ascites 30 days prior to surgery | |||||
| Yes | 79 (1.34) | 15 (2.3) | 64 (1.22) | 18.99 | .0238 |
| No | 5,814 (98.66) | 637 (97.7) | 5,177 (98.78) | 10.96 | |
| Cancer ICD-9 or disseminated | |||||
| Yes | 3,137 (53.23) | 347 (53.22) | 2,790 (53.23) | 11.06 | .9949 |
| No | 2,756 (46.77) | 305 (46.78) | 2,451 (46.77) | 11.07 | |
| Disseminated cancer | |||||
| Yes | 510 (8.65) | 89 (13.65) | 421 (8.03) | 17.45 | <.0001 |
| No | 5,383 (91.35) | 563 (86.85) | 4,820 (91.97) | 10.46 | |
| Chronic steroid use | |||||
| Yes | 859 (14.58) | 112 (17.18) | 747 (14.25) | 13.04 | .0459 |
| No | 5,034 (85.42) | 540 (82.82) | 4,494 (85.75) | 10.73 | |
| Bleeding disorders | |||||
| Yes | 283 (4.8) | 38 (5.83) | 245 (4.67) | 13.43 | .1939 |
| No | 5,610 (95.2) | 614 (94.17) | 4,996 (95.33) | 10.94 | |
| Hypertension | |||||
| Yes | 2,585 (43.87) | 327 (51.15) | 2,258 (43.08) | 12.65 | .0006 |
| No | 3,308 (56.13) | 325 (49.85) | 2,983 (56.92) | 9.82 | |
| Preoperative SIRS/sepsis | |||||
| Yes | 613 (10.4) | 77 (11.81) | 536 (10.23) | 12.56 | .2119 |
| No | 5,280 (89.6) | 575 (88.19) | 4,705 (89.77) | 10.89 | |
| Dialysis | |||||
| Yes | 59 (1.0) | 8 (1.23) | 51 (0.97) | 13.56 | .5391 |
| No | 5,834 (99.0) | 644 (98.77) | 5,190 (99.03) | 11.04 | |
| Behavioral risk factors | |||||
| Smoking status | |||||
| Yes | 967 (16.41) | 127 (19.48) | 840 (16.03) | 13.13 | .0248 |
| No | 4,926 (83.59) | 525 (80.52) | 4,401 (83.97) | 12.22 | |
NOTE. SSI, Surgical Site Infection; BMI, body mass index; ASA, American Society of Anesthesiologists physical status; COPD, chronic obstructive pulmonary disease; ICD-9, International Classification of Diseases, Ninth Edition; SIRS, systemic inflammatory response syndrome.
Within the cancer cohort, 510 patients (16.3%) had disseminated disease. The proportion of disseminated cancer among all oncology cases varied across centers (range, 8.5%–27.4%). Rates of SSI in those with disseminated cancer was significantly higher at 17.5% versus 9.8% in those with cancer that was not disseminated (P< .0001). Colorectal cancers constituted ~ 50% of all disseminated cancers, and the rate of SSI did not vary by site of primary cancer (colorectal versus other, 18.2% vs 16.7%; P= .60).
The most common type of SSI identified was superficial (n=325, 50%), followed by organ space (n=248, 38%) and deep incisional (n=79, 12.1%). Among cancer-related SSIs, distribution of infections by localized disease was similar to noncancer cases, with mostly superficial infections (56.6% and 52%, respectively). In patients with disseminated cancer, organspace infections accounted for most SSIs (57%) (Table 3).
TABLE 3.
Depth of Surgical Site Infection (SSI) Among 3 Groups
| Overall |
No Cancer |
Localized Cancer |
Disseminated Cancer |
|||||
|---|---|---|---|---|---|---|---|---|
| SSI Depth | Frequency | % | Frequency | % | Frequency | % | Frequency | % |
| Superficial | 325 | 49.8 | 158 | 51.8 | 146 | 56.6 | 21 | 23.6 |
| Deep | 79 | 12.1 | 35 | 11.48 | 26 | 10.1 | 18 | 20.2 |
| Organ Space | 248 | 38 | 112 | 36.72 | 86 | 33.3 | 50 | 56.1 |
| Total | 652 | 305 | 258 | 89 | ||||
Multivariate Analysis: Study Cohort
Multivariate logistic regression models were conducted separately for the entire cohort and the cancer and noncancer subgroups (Table 4). Of 5,893 colon procedures, 5,765 procedures were retained as the analytic sample due to reasons shown in Supplementary Figure 1.
TABLE 4.
Multivariate Analysis: Surgical Site Infections (SSIs) Among All Colorectal Surgery (CRS) Patients, CRS Patients With Cancer, and CRS Patients Without Cancer
| Total (n =5,765) |
Underlying Cancer (n =3,094) |
Noncancer (n =2,671) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 635 (11.01) |
341 (11.02) |
294 (11.01) |
|||||||
| No. of SSIs (%) | OR | 95% CI | P Value | Overall | No Cancer | P Value | OR | CI 95% | P Value |
| BMI, ≥30 kg/m2 vs (18.5– 24.9) | 1.349 | 1.08–1.68 | .0076 | 1.419 | 1.01–1.92 | .0236 | 1.293 | 0.93–1.79 | .1216 |
| ASA ≥3 | 1.326 | 1.10–1.61 | .0037 | 1.412 | 1.09–1.83 | .0097 | 1.206 | 0.91–1.60 | .1948 |
| History of diabetes | 1.453 | 1.15–1.85 | .0021 | 1.139 | 0.83–1.56 | .4248 | 2.028 | 1.41–2.92 | .0001 |
| COPD | 1.589 | 1.14–2.22 | .0063 | 1.639 | 1.06–2.53 | .0253 | 1.600 | 0.95–2.70 | .0803 |
| Disseminated cancer | 1.658 | 1.25–2.21 | .0005 | 1.669 | 1.23–2.26 | .001 | … | n/a | … |
| Duration of procedure | 1.002 | 1.001–1.002 | <.0001 | 1.002 | 1.001–1.002 | .0001 | 1.002 | 1.001–1.003 | <.0001 |
| Chronic steroid use | 1.317 | 1.04–1.67 | .0224 | 1.060 | 0.66–1.71 | .8075 | 1.443 | 1.09–1.92 | .0111 |
NOTE. BMI, body mass index; ASA, American Society of Anesthesiologists physical status; COPD, chronic obstructive pulmonary disease; n/a, not available.
In the entire cohort of patients, the following 7 variables were statistically significant: patients with a BMI ≥ 30 (obesity), ASA score ≥3, diabetes, COPD, steroid or immunosuppressant use, disseminated cancer, and duration of procedure (Supplementary Table 2). The risk of SSI among patients with underlying cancer was examined by comparing colorectal versus other cancer types. The site of cancer was not associated with higher risk of SSI (10.82% vs 12.07%; P = .30). However, disseminated cancer, regardless of primary site of origin, was significantly associated with higher risk of SSI (OR, 1.66; 95% CI, 1.25–2.21; P= .0005). Duration of procedure influenced the risk for SSI. Specifically, the odds of SSI were increased by 2% for every 10-minute increase in procedure duration.
Multivariate Analysis: Cancer and Noncancer Subgroups
Among CRS patients with underlying cancer, ASA score ≥3 (OR, 1.41; 95% CI, 1.09–1.83; P= .001), a history of COPD (OR, 1.6; 95% CI, 1.06–2.53; P =.02), duration of procedure (OR, 1.002; 95% CI, 1.001–1.002; P≤ .0001), and disseminated cancer (OR, 1.66; 95% CI, 1. 23–2.26; P =.001) predicted an increase in SSI risk (Table 4). Diabetes, obesity, and use of immunosuppression were not independent predictors of SSI in the subset of patients with underlying cancer.
Among colon-surgery patients without cancer, a history of diabetes (OR, 2.02; 95% CI, 1.41–2.92; P = .0001), duration of procedure (OR, 1.002; 95% CI, 1.001–1.002; P ≤ .0001), and history of steroid or immunosuppressant use (OR, 1.44; 95% CI, 1.09–1.92; P= .0111) were significant predictors of SSI.
DISCUSSION
In our multi-institutional NCCN cohort of ~ 6,000 patients, the mean 30-day SSI rate was 11.06%. Overall, 53% of patients who underwent CRS procedures had underlying cancer; 16.3% had disseminated disease. Most importantly, we found a significantly higher risk of SSI in patients with disseminated cancer. The frequency of organ-space infections was also highest among disseminated cancers when compared with the noncancer and localized cancer categories.
Intensified efforts to reduce CRS-related SSIs are being directed by several agencies including the Centers for Medicare and Medicaid Services (CMS), the Surgical Care Improvement Project (SCIP), and The Joint Commission. For benchmarking and assessing improvement in infection-related surgical outcomes, the CDC’s surveillance system, known as the National Healthcare Safety Network (NHSN), and the ACS’s NSQIP are the 2 most commonly used SSI surveillance methods.8 Each methodology has unique advantages and limitations. While no single method is ideal, the NSQIP has been widely adopted by surgical quality programs in the last decade to assess risk-adjusted performance and to identify potential predictors of SSIs.
The CDC NHSN is the nation’s most widely used healthcare-associated infection tracking system. Beginning in 2012, hospitals participating in the CMS Inpatient Prospective Payment System (IPPS) are required to report SSI data through the NHSN, which are accessible at the Hospital Compare website.14 Generally, the NHSN has used 3 patientlevel factors to determine the risk index for all SSIs: ASA score, wound classification, and procedure duration. Due to the poor performance of the NHSN risk index in predicting SSIs based on these limited variables, procedure-specific risk prediction models were subsequently created.15 Patient-specific variables, especially those related to cancer surgeries, a leading indication for colorectal resection, are not currently included.
Disseminated cancer has previously been associated with higher risk of SSI. In a report that included 740 patients undergoing CRS for neoplastic disease, Bot et al16 found the risk of all postoperative infection complications to be higher among patients with advanced tumors (stage ≥ IIB) compared to those with local disease. These differences were largely due to higher rates of anastomotic leakage in the group with advanced tumors (14.1% vs 5.6%; P=.001). The frequency of superficial SSIs was similar in both groups.16 Segal et al17 found a similar association of disseminated cancer with organ space SSIs (adjusted odds ratio [AOR], 1.21; 95% CI, 1.02–1.44).17
Data on the impact of various prevention efforts, specifically for OS SSIs are limited.18 Strictly in the context of disseminated cancer, it is plausible that the risk of SSI may be modifiable with the use of neoadjuvant strategies. However, SSI has never been formally evaluated as an outcome with such approaches. For advanced metastatic disease, this risk may not be changeable or direct meaningful modification in prevention efforts. For these reasons, a closer evaluation of disseminated cancer as a risk index in the NHSN model is essential.
This study contributes several other important findings. Apart from rates of SSI among disseminated cancer, the overall SSI rates in this study are comparable to those reported by large medical centers in the United States within a similar period.8,18 In a 2012 study by Ju et al,8 the pooled NSQIP CRS-SSI rate from 16 teaching hospitals in the United States was 13.5%. Although the study included 11 American Cancer Society (ACS)–accredited centers, cancer-specific risk was not specifically determined.8 In another large multi-institutional study from 7 multispecialty centers (~7,500 CRS procedures), the pooled SSI rate was 10.7%.18 Other variables that predicted SSI risk in our cohort were BMI >30 kg/m2 (obesity), diabetes, ASA score >3, COPD, immunosuppressant and/or steroid use, and longer duration of surgery. Many of these risk factors have previously been identified as predictors of SSI risk among patients undergoing CRS. This study further corroborates the findings from previous reports distinctively in cancerrelated CRS (Table 4). Perioperative hyperglycemia in patients with diabetes has been found to increase the risk of developing postoperative nosocomial infection.3,9–21 In this study, diabetes increased the odds of developing SSI by 2-fold in the noncancer group; however, no significant association with SSI risk was observed among patients with cancer. The small sample size, lack of an assessment of glycemic control, especially in the perioperative period, and uncertain duration of diabetes at the time of surgery are factors that may explain this nonsignificant finding.19 Larger studies are needed to further examine this relationship specifically among oncology procedures.
The strengths of this study include the fact that it leverages the NCCN infrastructure to examine cancer-related risk of SSI across several institutions. It validates disseminated cancer as a significant predictor of SSI risk among cancer patients undergoing CRS. This study has several limitations and potential biases, such as the effective “survey response rate” of 55% of invited institutions. Although hospital characteristics were not specifically collected, NSQIP hospitals are somewhat more likely to be large academic centers and, therefore, our findings are not generalizable to all US hospitals. Oncologyrelated variables including chemotherapy and radiation were not available. The breakdown of cancer versus noncancer was based on ICD-9 diagnoses and was subject to coding errors resulting in misclassification of cases. Not all risk factors by level of infection were examined. We were unable to stratify risk of SSI by stage of cancer because these data are not currently captured in NSQIP. Finally, data on best practices implemented at each center and SSI prevention initiatives were not collected, which could account for differences in observed interfacility rates and influence the SSI risk model.
In conclusion, cancer is one of the most common indications for CRS regardless of facility type (cancer hospital or other). The findings from the current study derived from the NSQIP dataset of NCCN hospitals confirm the association of disseminated cancer with risk of SSI and propose its evaluation in NHSN’s multivariate model. This is achievable with the inclusion of the disseminated cancer variable (ICD-10) with procedural entry of cancer-related surgeries.
Although standardization across 2 of the most widely used surveillance methodologies (NSQIP and NHSN) is unlikely to occur due to marked variation in data collection and analysis techniques, the information gained from one surveillance system should be validated in the other to refine existing risk-adjustment methods. Constant reevaluation with new information will ultimately provide external benchmarks that can be reliably applied to measure performance of prevention efforts.
ACKNOWLEDGMENTS
Financial support: Support for this study was provided by the NIH/NCI Cancer Center (grant no. P30 CA008748).
Footnotes
Potential conflicts of interest: All authors report no conflicts of interest related to this article.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2018.40
REFERENCES
- 1.Key statistics for colorectal cancer. American Cancer Society website. http://www.cancer.org/cancer/colonandrectumcancer/detailedguide/colorectal-cancer-key-statistics. Accessed November 20, 2016.
- 2.Klevens RM, Edwards JR, Richards CL Jr, et al. Estimating healthcare-associated infections and deaths in US hospitals, 2002. Public Health Rep 2007;122:160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson DJ, Kaye KS, Classen D, et al. Strategies to prevent surgical site infections in acute care hospitals. Infect Control Hosp Epidemiol 2008;29(Suppl 1):S51–S61. [DOI] [PubMed] [Google Scholar]
- 4.Malone DL, Genuit T, Tracy JK, Gannon C, Napolitano LM. Surgical site infections: reanalysis of risk factors. J Surg Res 2002;103:89–95. [DOI] [PubMed] [Google Scholar]
- 5.Anderson DJ, Podgorny K, Berrios-Torres SI, et al. Strategies toprevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 2014;35: 605–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magill SS, Edwards JR, Bamberg W, et al. Multistate pointprevalence survey of health care-associated infections. N Engl J Med 2014;370:1198–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang R, Chen HH, Wang YL, et al. Risk factors for surgical site infection after elective resection of the colon and rectum: a singlecenter prospective study of 2,809 consecutive patients. Ann Surg 2001;234:181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ju MH, Ko CY, Hall BL, Bosk CL, Bilimoria KY, Wick EC.A comparison of 2 surgical site infection monitoring systems. JAMA Surg 2015;150:51–57. [DOI] [PubMed] [Google Scholar]
- 9.Serra-Aracil X, Garcia-Domingo MI, Pares D, et al. Surgical site infection in elective operations for colorectal cancer after the application of preventive measures. Arch Surg 2001;146: 606–612. [DOI] [PubMed] [Google Scholar]
- 10.Khuri SF, Daley J, Henderson W, et al. The Department of Veterans Affairs NSQIP: the first national, validated, outcome-based, risk-adjusted, and peer-controlled program for the measurement and enhancement of the quality of surgical care. National VA Surgical Quality Improvement Program. Ann Surg 1998;228:491–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen ME, Ko CY, Bilimoria KY, et al. Optimizing ACS NSQIP modeling for evaluation of surgical quality and risk: patient risk adjustment, procedure mix adjustment, shrinkage adjustment, and surgical focus. J Am Coll Surg 2013;217: 336–346. [DOI] [PubMed] [Google Scholar]
- 12.Henderson WG, Daley J. Design and statistical methodology of the National Surgical Quality Improvement Program: Why is it what it is? Am J Surg 2009;198:S19–S27. [DOI] [PubMed] [Google Scholar]
- 13.Data collection, analysis, and reporting. American Cancer Society NSQIP website. https://www.facs.org/quality-programs/acsnsqip/program-specifics/data. Accessed July 25, 2016.
- 14.Healthcare-associated infections. Medicare.gov website. https://www.medicare.gov/hospitalcompare/Data/Healthcare-Associated-Infections.html. Accessed July 25, 2016.
- 15.Mu Y, Edwards JR, Horan TC, Berrios-Torres SI, Fridkin SK. Improving risk-adjusted measures of surgical site infection for the national healthcare safety network. Infect Control Hosp Epidemiol 2011;32:970–986. [DOI] [PubMed] [Google Scholar]
- 16.Bot J, Piessen G, Robb WB, Roger V, Mariette C. Advanced tumor stage is an independent risk factor of postoperative infectious complications after colorectal surgery: arguments from a case-matched series. Dis Colon Rectum 2013;56:568–576. [DOI] [PubMed] [Google Scholar]
- 17.Segal CG, Waller DK, Tilley B, Piller L, Bilimoria K. An evaluation of differences in risk factors for individual types of surgical site infections after colon surgery. Surgery 2014;156: 1253–1260. [DOI] [PubMed] [Google Scholar]
- 18.Reducing colorectal surgical site infections. Joint Commission Center for Transforming Health Care website. https://www.centerfortransforminghealthcare.org/assets/4/6/SSI_storyboard.pdf.Published 2014. Accessed February 9, 2018.
- 19.Sehgal R, Berg A, Figueroa R, et al. Risk factors for surgical site infections after colorectal resection in diabetic patients. J Am Coll Surg 2011;212:29–34. [DOI] [PubMed] [Google Scholar]
- 20.Kwon S, Thompson R, Dellinger P, Yanez D, Farrohki E, Flum D. Importance of perioperative glycemic control in general surgery: a report from the Surgical Care and Outcomes Assessment Program. Ann Surg 2013;257:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pomposelli JJ, Baxter JK 3d, Babineau TJ, et al. Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. J Parenter Enteral Nutr 1998;22:77–81. [DOI] [PubMed] [Google Scholar]