Abstract
Background
Pathologic extranodal extension (ENE) has traditionally guided the management of head and neck cancers. The prognostic value of radiographic ENE (rENE) in HPV-associated oropharyngeal squamous cell carcinoma (HPV+OPX) is uncertain.
Methods
HPV+OPX patient with adequate pre-treatment radiographic nodal evaluation, from a single institution were analyzed. rENE status was determined by neuroradiologists’ at time of diagnosis. Distant metastasis-free survival (DMFS), overall survival (OS), locoregional recurrence-free survival (LRFS) and were estimated using Kaplan-Meier methods. Cox proportional hazards models were fit to assess the impact of rENE on survival endpoints.
Results
168 patients with OPX+SCC diagnosed between April 2008 and December 2014 were included for analysis with median follow-up of 3.3 years. Eighty-eight percent of patients received concurrent chemoradiotherapy. rENE was not prognostic; its presence in HPV+OPX patients did not significantly impact OS, LRFS, or DMFS.
Conclusions
In patients with HPV+OPX, rENE was not significantly associated with OS, LRFS, or DMFS.
INTRODUCTION
There are estimated 65,000 new cases of head and neck cancer that will be diagnosed in 2018 in the US[1]. Risk stratification based on traditional clinical and pathologic variables is central to the management of locally advanced head and neck squamous cell carcinomas (SCC) [2–4]. Based on two large randomized trials and a corresponding pooled analysis, the presence of extranodal extension (ENE) and/or positive margins defined a high-risk subgroup for which concurrent chemoradiation produced improved outcomes compared to post-operative radiation therapy alone [5–7]. HPV status was unknown for patients in both trials and likely pre-dated the epidemic of HPV-associated oropharyngeal cancers. Since those pivotal studies, HPV-associated oropharyngeal squamous cell carcinoma (HPV+OPX) has become recognized as an epidemiologically and biologically distinct disease [8–11].
The prognostic value of clinically apparent extranodal extension has now been formalized into the current AJCC staging system for multiple head and neck primary sites, including p16 negative oropharynx[12]. The prognostic value of pathologic ENE (pENE), in HPV+OPX is unclear, and notably absent from its current staging system. Radiographically-defined ENE (rENE) has been used to influence treatment decisions, typically favoring definitive chemoradiation for locally advanced oropharyngeal carcinoma patients. This has been based on the principle that if neck dissection is likely to show pENE, then these patients will require concurrent chemoradiation post-operatively. Our goal was to investigate the prognostic value of rENE in patients with HPV+ oropharynx cancer.
MATERIALS AND METHODS
After institutional review board approval, consecutive patient records from a single institution were identified by pathologic diagnosis of oropharyngeal SCC (OPSCC) and reviewed for appropriateness for further analysis. Patients with radiologically positive nodal disease, and with records containing information on HPV status, were included in the analysis. Other clinical and pathologic data, including TNM classification at diagnosis(AJCC 7th edition), treatment information, smoking status, disease and mortality status at last follow-up were incorporated into a single database. HPV status was assayed by in-situ hybridization (ISH), p16 immunohistochemistry (IHC), or both. In cases of non-concordance, HPV status was determined by ISH results.
The presence and extent of rENE was reflected by each patient’s initial report as read by head and neck neuroradiologists and supplemented by multidisciplinary head and neck-specific conference notes where applicable. The interpreting neuroradiologist was recorded. Within our institution, patients underwent high-quality, preoperative computed tomography (CT) imaging of the neck with 100 cc of intravenous contrast. Images were acquired at 1.25 mm, reconstructed into axial, sagittal, and coronal series, and read at 2.5 mm. Positron emission tomography (PET) scanning was performed prior to therapy and our neuroradiologists had access to these reports and images. Preoperative images were not re-interpreted, as our purpose was to assess the prognostic value of the original diagnostic interpretations. Our neuroradiologists used irregular borders, perinodal fat stranding, and invasion of adjacent structures as indications of ENE. Based on the initial report, patients with rENE were also further described as having either ‘definite’ ENE or ‘suspicious’ for ENE, based on the strength of the interpretation. Patients with reports describing lymph node(s) ‘suspicious’ for ENE were considered positive. Patterns of interpretation between neuroradiologists who had interpreted a non-negligible number of studies were analyzed to determine whether there was significant variation between interpreters. Reports not commenting on rENE were categorized as negative. Our neuroradiologists interpreted these studies knowing the clinical significance of rENE and were thus unlikely to omit or miss finding rENE when present.
Descriptive statistics were generated for all patient characteristics. Numeric variables were summarized using mean, median, range, and standard deviation; and categorical variables were summarized using frequencies and percentages. Overall survival (OS) was defined as time from diagnosis to death or last follow-up, where those alive at last follow-up are censored at time of their last follow-up. Locoregional recurrence-free survival (LRFS) and distant metastasis-free survival (DMFS) were defined similarly. DMFS, OS, and LRFS were compared across variables using log-rank tests, and were estimated using the Kaplan-Meier method. rENE was compared across categorical patient characteristics using chi-squared test or Fisher’s exact test where appropriate, and across numeric characteristics and parameters using ANOVA. Univariate Cox proportional hazards models of DMFS, OS, and LFRS were fit for each variable. Kaplan-Meier plots were produced for rENE for DMFS, OS, and LRFS. Model assumptions were checked. The impact of rENE status was also examined with the patients with suspicious ENE by report removed (definite ENE vs negative ENE). Additional subset analyses were performed in key subgroups – by anatomic subsite: base of tongue (BOT) and tonsil; and also by method of HPV status testing: IHC and ISH. Firth’s penalized maximum likelihood method was applied in order to reduce bias in the confidence intervals and parameter estimates, and to handle empty cells in subset analyses due to smaller sample sizes [13, 14]. Statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC), and the significance level was 0.05.
RESULTS
There were 168 patients with HPV-positive oropharyngeal squamous cell carcinoma diagnosed between April 2008 and December 2014 who were included in this analysis. Median follow-up duration was 3.3 years. Median age at diagnosis was 58 years, other descriptive statistics are summarized in Table 1. Overall, patients were predominantly AJCC 7th edition group stage IVA (89.9%) or stage IVB (7.1%), with mostly N2 (89.3%) or N3 (6%) nodal classification. The majority was male (89.9%), and 58.9% were formers smokers. Palatine tonsil and base of tongue subsites constituted the majority of diagnoses, accounting for 48.8% and 49.2% of cases, respectively. Ninety-eight percent of evaluable patients received radiation therapy; 93.3% of patients were treated with chemotherapy, with 96.7% given concurrently. 132 of 133 (99.2%) patients with radiation dose information completed concurrent chemoradiation, i.e. received greater than 95% of prescribed radiation dose; 14 patients had missing information, such that cumulative radiation dose could not be confirmed.
Table 1.
Descriptive characteristics for 168 patients with HPV-associated locally advanced oropharyngeal squamous cell carcinoma
| Characteristic | (N = 168) | |
|---|---|---|
| Median Age (years) | 58 | |
| Sex | ||
| Male | 151 | 89.9% |
| Female | 17 | 10.1% |
| Race | ||
| White | 136 | 88.9% |
| Black | 14 | 9.2% |
| Other | 3 | 2% |
| T Classification | ||
| T1 | 64 | 28.1% |
| T2 | 58 | 34.5% |
| T3 | 13 | 7.7% |
| T4a | 30 | 17.9% |
| T4b | 3 | 1.8% |
| N Classification | ||
| N1 | 8 | 4.8% |
| N2a | 20 | 11.9% |
| N2b | 94 | 56% |
| N2c | 36 | 21.4% |
| N3 | 10 | 6% |
| Group Stage | ||
| III | 5 | 3% |
| IVA | 151 | 89.9% |
| IVB | 12 | 7.1% |
| Subsite | ||
| Tonsil | 82 | 48.8% |
| Base of tongue | 83 | 49.2% |
| Other | 3 | 1.8% |
| Radiation | 160/163 | 98.2% |
| Chemotherapy | 152/163 | 93.3% |
| Concurrent | 147 | 96.7% |
| Smoker at Diagnosis | ||
| Yes | 32 | 19% |
| No | 136 | 81% |
| Prior Smoker | ||
| Yes | 99 | 58.9% |
| No | 69 | 41.1% |
| Total Pack-Years | ||
| Never | 69 | 41.1% |
| >0, ≤20 | 46 | 27.4% |
| >20 | 53 | 31.5% |
| rENE | ||
| Yes | 89 | 53% |
| No | 79 | 47% |
| rENE Grade | ||
| Suspicious | 42 | 25.1% |
| Definite | 46 | 27.5% |
rENE: radiographic extranodal extension
Eighty-nine patients (53%) had rENE, and 79 patients (47%) did not. Five principle neuroradiologists interpreted 137 of 168 (81.5%) studies; 9 others interpreted the remainder of the CT scans (Table S1). There were no significant differences observed for ENE interpretation across the 5 neuroradiologists when comparing rates of definite ENE vs negative ENE (p = 0.168), and also when comparing definitive ENE vs all others (p = 0.139). When definite and suspicious for ENE were considered together as ENE positive, the pattern of interpretation differed across neuroradiologists for ENE positive vs negative (p = 0.035). There appeared to be some variation in pattern of interpretation between neuroradiologists depending on classification method. Patient characteristics were analyzed by rENE status (Table 2). Patients had similar demographic and disease characteristics regardless of rENE status, with exception being that HPV+OPX patients with rENE were associated with lower T-stage classifications (p=0.039). Age at diagnosis, race, nodal classification, treatment with chemotherapy, smoking history and current status were similar between rENE-positive and rENE-negative patients.
Table 2.
Comparison of baseline characteristics by rENE status
| Characteristic | rENE (−) (n=79) |
rENE (+) (n=89) |
P-value |
|---|---|---|---|
| Sex | 0.304 | ||
| Male | 69 (87.3%) | 82 (92.1%) | |
| Female | 10 (12.7%) | 7 (7.8%) | |
| Race | 0.491 | ||
| White | 60 (87%) | 76 (90.5%) | |
| Other | 9 (13%) | 8 (9.5%) | |
| T Classification | 0.039 | ||
| T1 | 27 (34.2%) | 37 (41.6%) | |
| T2 | 25 (31.7%) | 33 (37.1%) | |
| T3 | 11 (13.9%) | 2 (2.3%) | |
| T4 | 16 (20.3%) | 17 (19.1%) | |
| N Classification | 0.265 | ||
| N1 | 6 (7.6%) | 2 (2.3%) | |
| N2 | 69 (87.3%) | 81 (91%) | |
| N3 | 4 (5.1%) | 6 (6.7%) | |
| Chemotherapy | 0.296 | ||
| Yes | 71 (91%) | 79 (95.2%) | |
| No | 7 (9%) | 4 (4.8%) | |
| Smoker at Diagnosis | 0.680 | ||
| Yes | 14 (17.7%) | 18 (20.2%) | |
| No | 65 (82.3%) | 71 (79.8%) | |
| Prior Smoker | 0.422 | ||
| Yes | 44 (55.7%) | 55 (61.8%) | |
| No | 35 (44.3%) | 34 (38.2%) | |
| Total Pack-Years | 0.595 | ||
| Never | 35 (44.3%) | 34 (38.2%) | |
| >0, ≤20 | 22 (27.9%) | 24 (27%) | |
| >20 | 22 (27.9%) | 31 (34.8%) |
rENE: radiographic extranodal extension
Overall, there were 26 deaths, and 30 patients demonstrated disease progression. Eighteen patients died with, and 8 patients died without evidence of disease progression. rENE status did not significantly impact overall survival. The overall hazard ratio (HR) for death was 1.37 (95% CI: 0.62–3.03). The HRs were 1.42 (95% CI: 0.55–3.71) and 1.35 (95% CI 0.52–3.49) for definitive and suspicious vs no rENE respectively. 5-year OS were 83.5% vs 82.6% for rENE negative vs rENE positive cases (Figure 1). Univariate analysis of overall survival at 5 years showed that only prior smoking was significantly associated with inferior survival (Table 3). Former smoking-status predicted for inferior overall survival (HR 2.68, 95% CI: 1.07–6.72), log-rank p=0.029). rENE status was not significantly associated locoregional outcomes; HR was 1.56 (95% CI: 0.61–3.98, log-rank p=0.349). Five-year locoregional recurrence-free survival was 87.2% vs 89.8% for rENE vs no rENE (Table 4 and Figure 2). Distant metastasis-free survival were similar in patients with HPV+OPX regardless of rENE status; HR was 2.26 (95% CI: 0.78–6.59, Table 5). Five-year DMFS rates were 87.2% vs 92.6% (rENE vs no rENE, Figure 3).
Figure 1.
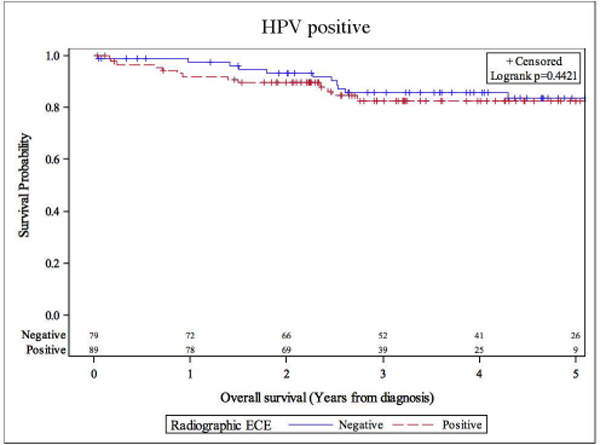
Overall survival stratified by radiographic extranodal extension status
Table 3.
Univariate analysis of overall survival
| Characteristic | HR (95% CI) | HR P-value | Log-rank P-value |
|---|---|---|---|
| rENE | 0.442 | ||
| Positive | 1.37 (0.62–3.03) | 0.444 | |
| Negative | - | - | |
| rENE Grade | 0.719 | ||
| Definite | 1.42 (0.55–3.71) | 0.468 | |
| Suspicious | 1.35 (0.52–3.49) | 0.539 | |
| Negative | - | - | |
| Sex | 0.203 | ||
| Male | 3.43 (0.46–25.67) | 0.231 | |
| Female | - | - | |
| Race | 0.075 | ||
| Other | 2.60 (0.87–7.74) | 0.086 | |
| White | - | - | |
| T Classification | 0.173 | ||
| T1 | - | - | |
| T2 | 0.89 (0.33–2.41) | 0.824 | |
| T3 | 2.79 (0.93–8.32) | 0.066 | |
| T4 | 1.02 (0.31–3.31) | 0.976 | |
| N Classification | 0.351 | ||
| N1 | - | - | |
| N2 | 1.03 (0.14–7.63) | 0.436 | |
| N3 | 2.48 (0.25–24.34) | 0.980 | |
| Smoker at Diagnosis | 0.870 | ||
| Yes | 0.91 (0.31–2.67) | 0.870 | |
| No | - | - | |
| Prior Smoker | 0.029 | ||
| Yes | 2.68 (1.07–6.72) | 0.036 | |
| No | - | - | |
| Total Pack-Years | 0.064 | ||
| Never | - | - | |
| >0, ≤20 | 2.18 (0.73–6.51) | 0.163 | |
| >20 | 3.08 (1.16–8.22) | 0.025 |
rENE: radiographic extranodal extension; HR: hazard ratio; CI: confidence interval
Table 4.
Univariate analysis of locoregional recurrence-free survival
| Characteristic | HR (95% CI) | HR P-value | Log-rank P-value |
|---|---|---|---|
| rENE | 0.349 | ||
| Positive | 1.56 (0.61–3.98) | 0.353 | |
| Negative | - | - | |
| rENE Grade | 0.600 | ||
| Definite | 1.71 (0.58–5.03) | 0.330 | |
| Suspicious | 1.44 (0.46–4.52) | 0.530 | |
| Negative | - | - | |
| Sex | 0.810 | ||
| Male | 1.20 (0.27–5.24) | 0.811 | |
| Female | - | - | |
| Race | 0.624 | ||
| Other | 1.45 (0.33–6.38) | 0.626 | |
| White | - | - | |
| T Classification | 0.175 | ||
| T1 | - | - | |
| T2 | 0.82 (0.23–2.94) | 0.766 | |
| T3 | 2.63 (0.66–10.55) | 0.173 | |
| T4 | 2.38 (0.77–7.42) | 0.133 | |
| N Classification | 0.364 | ||
| N1 | - | - | |
| N2 | 0.36 (0.08–1.57) | 0.433 | |
| N3 | 0.38 (0.03–4.28) | 0.174 | |
| Smoker at Diagnosis | 0.213 | ||
| Yes | 1.91 (0.68–5.36) | 0.221 | |
| No | - | - | |
| Prior Smoker | 0.198 | ||
| Yes | 1.87 (0.71–4.93) | 0.206 | |
| No | - | - | |
| Total Pack-Years | 0.380 | ||
| Never | - | - | |
| >0, ≤20 | 2.13 (0.71–6.37) | 0.176 | |
| >20 | 1.64 (0.53–5.09) | 0.392 |
rENE: radiographic extranodal extension; HR: hazard ratio; CI: confidence interval
Figure 2.
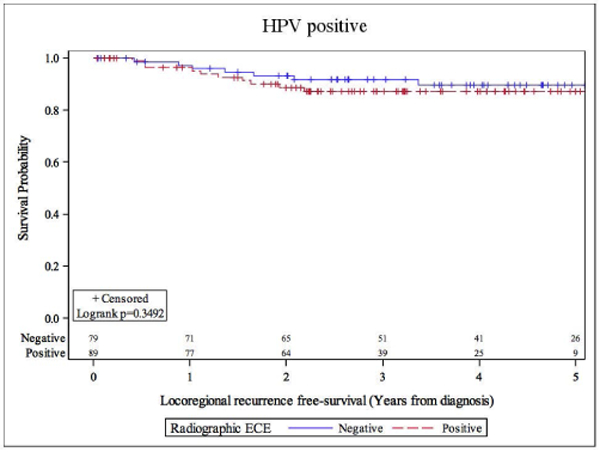
Locoregional recurrence-free survival stratified by radiographic extranodal extension status
Table 5.
Univariate analysis of distant metastasis-free survival
| Characteristic | HR (95% CI) | HR P-value | Log-rank P-value |
|---|---|---|---|
| rENE | 0.125 | ||
| Positive | 2.26 (0.78–6.59) | 0.135 | |
| Negative | - | - | |
| rENE Grade | 0.283 | ||
| Definite | 2.46 (0.74–8.17) | 0.141 | |
| Suspicious | 2.12 (0.61–7.39) | 0.239 | |
| Negative | - | - | |
| Sex | 0.419 | ||
| Male | 2.28 (0.29–17.70) | 0.431 | |
| Female | - | - | |
| Race | 0.400 | ||
| Other | 1.88 (0.42–8.42) | 0.408 | |
| White | - | - | |
| T Classification | 0.799 | ||
| T1 | - | - | |
| T2 | 0.83 (0.23–2.97) | 0.771 | |
| T3 | 1.56 (0.31–7.71) | 0.588 | |
| T4 | 1.50 (0.42–5.31) | 0.532 | |
| N Classification | 0.438 | ||
| N1 | - | - | |
| N2 | 0.57 (0.07–4.34) | 0.584 | |
| N3 | 1.41 (0.12–16.35) | 0.781 | |
| Smoker at Diagnosis | 0.389 | ||
| Yes | 1.64 (0.52–5.17) | 0.394 | |
| No | - | - | |
| Prior Smoker | 0.239 | ||
| Yes | 1.87 (0.65–5.42) | 0.246 | |
| No | - | - | |
| Total Pack-Years | 0.411 | ||
| Never | - | - | |
| >0, ≤20 | 2.22 (0.67–7.33) | 0.191 | |
| >20 | 1.59 (0.46–5.49) | 0.467 |
rENE: radiographic extranodal extension; HR: hazard ratio; CI: confidence interval
Figure 3.
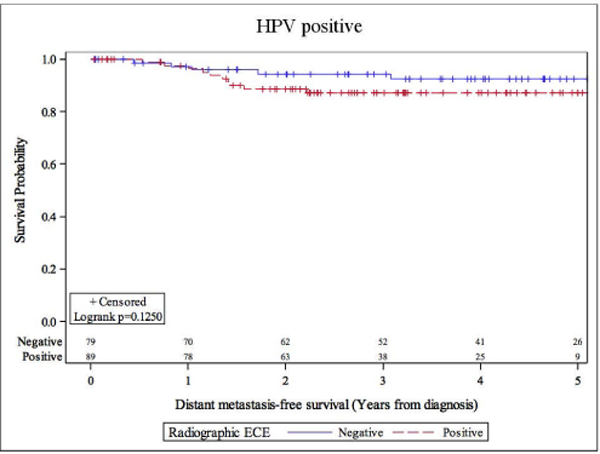
Distant metastasis-free survival stratified by radiographic extranodal extension status
The impact of rENE status on OS, LRFS and DMFS were analyzed after removing patients who were described as suspicious for rENE. The presence of definitive ENE vs no rENE was not significantly associated with any of the 3 endpoints (Figures S1–S3). Additional subset analyses stratifying on anatomic subsite and method of HPV testing were performed. rENE status did not appear to impact patients with base of tongue or tonsil as the primary subsite. Subset analyses performed examining the method of HPV detection were similar to those of the entire cohort. There were no differences by rENE status for OS, LRFS, or DMFS in patients who were tested by use of IHC, or by use of ISH. Results of testing rENE status against each endpoint for all subgroups are summarized in Table S2.
DISCUSSION
This study represents the largest single-institution series examining the role of rENE exclusively in an HPV-positive oropharynx population. HPV status was known for all cases at time of diagnosis by immunohistochemistry, in-situ hybridization, or both. rENE was seen in approximately half of the cohort, which was further described as either suspicious or definite based its reported radiographic appearance. We evaluated the prognostic relevance of rENE with respect to 3 endpoints. rENE status was not prognostic in OS, LRFS, or DMFS. 5-year differences in OS, LRFS, and DMFS based on rENE status were approximately 1%, 3% and 5%. In concordance with numerous studies reporting significantly superior prognosis for HPV+OPX, we found excellent local and distant control rates for this population. This overall difference in prognosis from HPV-negative OPX may also account, at least in part, for rENE’s apparent lack of prognostic significance here. Though DMFS was not significantly associated with rENE status based on a median follow-up length of 3.3 years, relatively late distant failures have been observed in an HPV-positive population up to 5 years [15].
The validity of pathologic ENE in HPV-associated OPX has been re-evaluated and challenged by several surgical series which found pENE neither prognostic nor predictive for treatment intensification. A series of 152 p16-positive OPSCC patients treated with primary transoral surgery and neck dissection graded pathologic extracapsular extension, including patients with high-grade extracapsular spread[16]. Neither standard ENE status, graded ENE, nor soft-tissue metastases (high-grade ENE) were significant predictors of disease-free survival (DFS), disease-specific survival or OS. Maxwell et al. reported a similar surgical series that demonstrated nodal extracapsular spread had no effect on DFS, regardless of HPV status[17].
Several publications have examined the predictive value rENE. The largest study involved 432 patients with locally advanced laryngeal and oral cavity cancers[18]. Similar to the present study, rENE status was assessed by neuroradiology at time of diagnosis without re-interpretation. Pathologic ENE was graded on a scale from 1 to 4, corresponding to the degree of involvement, from tumor reaching the nodal capsule (grade 1) to complete replacement of the lymph node (grade 4). The authors found a positive predictive value (PPV) of 82.6% and a negative predictive value (NPV) of 87.3%. Sensitivity of rENE for pathologic ENE increased with grade, from 18.8% for grade 1 to 72.2% for grade 4; specificity was 97.7%. Other single-institutional reports have found substantial variations in predictive capacity of rENE, especially in HPV-related oropharynx cancers. When examined in an exclusively HPV+OPX population, 2 series have found PPVs for rENE ranging 52.6–82% and NPVs ranging 53–100%[19, 20]. Authors who have found lower predictive values for rENE in non-oropharynx primaries with PPVs ranging 71–84% and NPVs ranging 48–70% have recommended against the use of rENE in predicting pENE[19, 21, 22]. In practice however, pathologic evaluation of the neck via pre-treatment neck dissection is not routinely performed; patients with clinically overt ENE are typically recommended to undergo definitive chemoradiotherapy. Reliance on this paradigm may complicate our ability to accurately define the predictive value of rENE. These data suggest that perhaps another reason that rENE does not appear prognostic in an HPV+OPX population is due to its uncertain predictive ability for pENE.
This study’s retrospective design is an inherent limitation. Selection and treatment biases, as well as unaccounted variables, can affect the validity of the results. Such biases may have been mitigated in this study in several ways. Patient characteristics such as age, group staging, smoking history and burden were balanced between patients with or without rENE. The vast majority of patients were treated with radiation therapy and concurrent chemotherapy. As all patients in this study were treated at a single-institution, their treatment approach, ie. risk-stratification, radiation therapy treatment planning, and choice of systemic agents were standardized. For example, the entire muscle at the appropriate levels were included in the treatment volume if there was radiographic evidence of muscle invasion. The high prevalence of chemotherapy use in this series limits our ability to isolate the effect of systemic treatment therapy in patients with rENE.
The selection of favorable cohorts for de-intensification protocols has been the subject of several recent and ongoing prospective studies. These patients are typically those with HPV+OPX with less advanced T-stage, non-bulky nodal disease with modest or no smoking history. Using a primary surgical approach, with transoral resection and neck dissection, Eastern Cooperative Oncology Group (ECOG) 3311 (NCT01898494) randomized p16-positive oropharyngeal patients with intermediate risk to standard (60Gy) or reduced dose post-operative radiation of 50Gy. Patients with low-risk factors are observed, and those with high-risk factors are treated 66Gy adjuvant radiation to 66Gy concurrent with weekly cisplatin. Of note, the study distinguishes between minimal and extensive pathologic ENE, defined as ≤1 or >1mm of spread, highlighting the importance of ENE extent for risk stratification. Those with minimal ENE, under the design of this study, are considered intermediate risk. This is a novel approach to risk stratification in HPV-associated oropharyngeal cancer which diverges from traditional risk-stratification.
CONCLUSIONS
rENE status was not a significant predictor for OS, LFRS, or DMFS in patients with locally advanced HPV-positive oropharynx squamous cell carcinoma. These findings, if properly validated, may help inform treatment-making decisions in the multidisciplinary setting.
Supplementary Material
Locoregional recurrence-free survival stratified by definite vs negative radiographic extranodal extension
Overall survival stratified by definite vs negative radiographic extranodal extension
Distant metastasis-free survival stratified by definite vs negative radiographic extranodal extension
ACKNOWLEDGEMENTS
Research reported in this publication was supported in part by the Biostatistics and Bioinformatics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Siegel RL, Miller KD, and Jemal A, Cancer statistics, 2019. CA Cancer J Clin, 2019. 69(1): p. 7–34. [DOI] [PubMed] [Google Scholar]
- 2.Ang KK, et al. , Randomized trial addressing risk features and time factors of surgery plus radiotherapy in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys, 2001. 51(3): p. 571–8. [DOI] [PubMed] [Google Scholar]
- 3.Peters LJ, et al. , Evaluation of the dose for postoperative radiation therapy of head and neck cancer: first report of a prospective randomized trial. Int J Radiat Oncol Biol Phys, 1993. 26(1): p. 3–11. [DOI] [PubMed] [Google Scholar]
- 4.Cooper JS, et al. , Precisely defining high-risk operable head and neck tumors based on RTOG #85–03 and #88–24: targets for postoperative radiochemotherapy? Head Neck, 1998. 20(7): p. 588–94. [DOI] [PubMed] [Google Scholar]
- 5.Cooper JS, et al. , Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med, 2004. 350(19): p. 1937–44. [DOI] [PubMed] [Google Scholar]
- 6.Bernier J, et al. , Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck, 2005. 27(10): p. 843–50. [DOI] [PubMed] [Google Scholar]
- 7.Bernier J, et al. , Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med, 2004. 350(19): p. 1945–52. [DOI] [PubMed] [Google Scholar]
- 8.Lassen P, et al. , The influence of HPV-associated p16-expression on accelerated fractionated radiotherapy in head and neck cancer: evaluation of the randomised DAHANCA 6&7 trial. Radiother Oncol, 2011. 100(1): p. 49–55. [DOI] [PubMed] [Google Scholar]
- 9.Posner MR, et al. , Survival and human papillomavirus in oropharynx cancer in TAX 324: a subset analysis from an international phase III trial. Ann Oncol, 2011. 22(5): p. 1071–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rischin D, et al. , Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J Clin Oncol, 2010. 28(27): p. 4142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ang KK, et al. , Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med, 2010. 363(1): p. 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lydiatt WM, et al. , Head and Neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J Clin, 2017. 67(2): p. 122–137. [DOI] [PubMed] [Google Scholar]
- 13.Firth D, Bias reduction of maximum likelihood estimates. Biometrika, 1993. 80(1): p. 27–38. [Google Scholar]
- 14.Heinze G and Schemper M, A solution to the problem of monotone likelihood in Cox regression. Biometrics, 2001. 57(1): p. 114–9. [DOI] [PubMed] [Google Scholar]
- 15.O’Sullivan B, et al. , Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J Clin Oncol, 2013. 31(5): p. 543–50. [DOI] [PubMed] [Google Scholar]
- 16.Sinha P, et al. , Extracapsular spread and adjuvant therapy in human papillomavirus-related, p16-positive oropharyngeal carcinoma. Cancer, 2012. 118(14): p. 3519–30. [DOI] [PubMed] [Google Scholar]
- 17.Maxwell JH, et al. , Extracapsular spread in head and neck carcinoma: impact of site and human papillomavirus status. Cancer, 2013. 119(18): p. 3302–8. [DOI] [PubMed] [Google Scholar]
- 18.Prabhu RS, et al. , Accuracy of computed tomography for predicting pathologic nodal extracapsular extension in patients with head-and-neck cancer undergoing initial surgical resection. Int J Radiat Oncol Biol Phys, 2014. 88(1): p. 122–9. [DOI] [PubMed] [Google Scholar]
- 19.Maxwell JH, et al. , Accuracy of computed tomography to predict extracapsular spread in p16-positive squamous cell carcinoma. Laryngoscope, 2015. 125(7): p. 1613–8. [DOI] [PubMed] [Google Scholar]
- 20.Patel MRHPA; Beitler J. J; Baugnon KL; Liu Y; Magliocca KR; Griffith C; Higgins K; Saba NF; Shin DM; Wadsworth JT; El-Deiry M; Aiken AH, Radiographic Imaging does not Reliably Predict Macroscopic Extranodal Extension (ENE) in HPV-Associated Oropharyngeal Cancer. ORL, 2018. [DOI] [PubMed] [Google Scholar]
- 21.Chai RL, et al. , Accuracy of computed tomography in the prediction of extracapsular spread of lymph node metastases in squamous cell carcinoma of the head and neck. JAMA Otolaryngol Head Neck Surg, 2013. 139(11): p. 1187–94. [DOI] [PubMed] [Google Scholar]
- 22.Carlton JA, et al. , Computed tomography detection of extracapsular spread of squamous cell carcinoma of the head and neck in metastatic cervical lymph nodes. Neuroradiol J, 2017. 30(3): p. 222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Locoregional recurrence-free survival stratified by definite vs negative radiographic extranodal extension
Overall survival stratified by definite vs negative radiographic extranodal extension
Distant metastasis-free survival stratified by definite vs negative radiographic extranodal extension


