Abstract
Background
To investigate the clinical characteristics of hypertriglyceridemia pancreatitis (HTGP) and evaluate the correlative risk factors for severe acute pancreatitis (SAP) in HTGP patients.
Material/Methods
A total of 1005 patients with acute pancreatitis (AP) admitted to Peking Union Medical College Hospital (PUMCH) from 1 Jan 2013 to 1 Aug 2018 were retrospectively reviewed. After screening, we enrolled 159 patients with HTGP and 172 with non-hypertriglyceridemia pancreatitis (NHTGP). We gathered and assessed demographic and blood biochemical information and analyzed the risk factors for SAP.
Results
Age, serum amylase (AMY), lipase (LIP), and serum ionized calcium (Ca2+) in the HTGP group were lower than in the NHTGP group (P<0.05), while high-sensitivity C-reactive protein (hsCRP), neutrophil–lymphocyte ratio (NLR), and body mass index (BMI) in the HTGP group were higher than in the NHTGP group (P<0.05). Among the HTGP patients, the results indicated that Ca2+ (OR=0.018, P<0.001, 95%CI: 0.002–0.129) was an independent protective factor for SAP, while higher CRP (OR=1.008, P=0.004, 95%CI: 1.003–1.013), NLR (OR=1.314, P<0.001, 95%CI: 1.161–1.488), and BMI (OR=1.597, P=0.002, 95%CI: 1.195–2.314) were independent risk factors for SAP.
Conclusions
Patients with HTGP had lower serum Ca2+ and higher hsCRP, NLR, and BMI, and these were associated with higher risk of developing SAP.
MeSH Keywords: Hypertriglyceridemia, Pancreatitis, Risk Factors
Background
Acute pancreatitis (AP) is one of the most common gastrointestinal diseases requiring hospitalization, with more than 270 000 such patients admitted annually in the United States [1]. The clinical characteristics of AP, in contrast to relatively mild and self-limited illnesses, include persistent or multisystem organ failure. With changes in lifestyle and diet, the incidence of acute hypertriglyceridemia pancreatitis (HTGP) has been gradually increased in recent decades [2,3]. The latest studies reported that HTGP accounted for about 2~5% of all cases of AP in the United States [4]. Hypertriglyceridemia (HTG) is the third leading cause of AP, after gallstones and alcohol abuse [4]. Some researchers have found that the incidence of HTGP exceeds that of alcohol-induced pancreatitis in China, which is ranked as the second leading cause of AP [2]. In addition, previous research showed that severe acute pancreatitis (SAP) accounts for 12~38% of all HTGP patients [5]. SAP easily recurs and causes a series of complications that severely threaten human health [6].
AP, which is an acute inflammatory disease caused by pancreatitis, can induce the autodigestion of pancreatic tissues. The diagnostic criteria for AP were defined in the 2012 International Association of Pancreatology (IAP) report “Atlanta Classification and Definitions (revised)” [7]. Studies have demonstrated that multiple indicators play crucial roles in detecting patients at risk for SAP and the development of complications. These indicators include serum amylase (AMY) [8], lipase (LIP) [9], low serum ionized calcium (Ca2+) [10], high-sensitivity C-reactive protein (hsCRP) [11], neutrophil-lymphocyte ratio (NLR) [12,13], and BMI [14–16]. However, to the best of our knowledge, the changes in these clinic parameters in patients with HTGP have not been previously reported.
In the present study, we performed a retrospective investigation of the medical records of HTGP patients admitted to Peking Union Medical College Hospital (PUMCH) from 1 Jan 2013 to 1 Aug 2018. We assessed the clinical characteristics, high-risk factors, and factors predicting the severity of HTGP.
Material and Methods
Study design
We retrospectively screened the medical records of 1005 patients with AP admitted to PUMCH from 1 Jan 2013 to 1 Aug 2018. The clinical data of patients were collected, including age, sex, predisposing factors, HTG medical history, local complications (LC), mortality, hospitalization time (HT), BMI and laboratory data at admission, including serum triglyceride (TG), AMY, LIP, Ca2+, hsCRP, and NLR. Furthermore, we recorded the data of patients who received mechanical ventilation, blood purification, vasoactive drugs, and renal replacement. This study was approved by the Institutional Review Board (IRB) of PUMCH, and the approval number was S-K554.
Inclusion and exclusion criteria
Inclusion criteria were: (1) age ≥18 years old; (2) clearly diagnosed as AP; and (3) admission in hospital within 1 week after AP onset. Exclusion criteria were: (1) AP combined with other severe chronic diseases such as malignant tumors and chronic organ dysfunction (OD); (2) pancreatic tumor; and (3) absence of clinical data.
Diagnostic criteria
According to the 2012 International Association of Pancreatology (IAP) report “Atlanta Classification and Definitions of AP (revised)” [7], AP can be diagnosed when a patient has 2 of the following 3 characteristics: (1) the symptoms of abdominal pain are consistent with AP, (2) the levels of AMY and/or LIP are at least 3 times above the upper limit of normal, and (3) abdominal imaging is consistent with changes in AP. For HTGP meeting the diagnosis of AP and when the level of serum TG is ≥1000 mg/dL (11.3 mmol/L) or between 500 mg/dL (5.65 mmol/L) and 1000 mg/dL (11.3 mmol/L), the diagnosis of HTGP should be considered if AP was not caused by other reasons. For SAP, there is AP with persistent OD (>48 h) [7]. For OD, there is improvement in 1 or more of the following: circulation, respiration, and kidney systems, and Marshall score ≥2 points. For diagnosis of LC, there must be acute peripancreatic fluid accumulation, acute necrosis accumulation, pancreatic pseudocysts, and parenchymal necrosis.
Statistical analysis
Statistical analysis was performed with SPSS 24.0 (SPSS, Inc., Chicago, IL). Kolmogorov-Smirnov method was used to perform the normality test of measurement data. Non-normally distributed measurement data are expressed as median (P25, P75). The Mann-Whitney U test was used for comparisons between the 2 groups. Enumeration data are expressed as numbers of cases and percentages, and were compared by χ2 test. Multivariate analysis was performed by the logistic regression model. The forward LR method was used in multivariate analysis (inclusion standard α=0.05, exclusion standard β=0.10). P<0.05 was considered as statistical significance.
Results
Case screening
Among the 1005 patients with AP, 207 (20.60%) were clearly diagnosed as having HTGP, while 755 (75.12%) were clearly diagnosed as having NHTGP. After further screening, 159 patients were included in the HTGP group and 172 patients were included in the NHTGP group (Figure 1).
Figure 1.
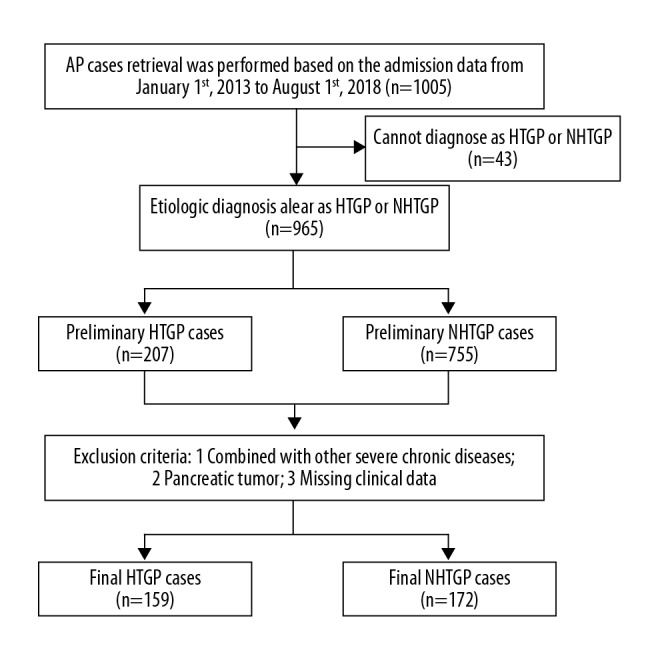
Data screening flowchart.
General condition and clinical information of patients
Basic clinical information of HTGP and NHTGP patients are shown in Table 1. Age, AMY, LIP, and Ca2+ in the HTGP group were lower than in the NHTGP group, and all differences were statistically significant (P<0.05). hsCRP, NLR, and BMI of the HTGP group were higher than these of the NHTGP group and all differences were statistically significant (P<0.05). However, there were no significant differences in sex, incidences of SAP and LC, mechanical ventilation rate (MVR), vasoactive drug usage rate (VDUR), renal replacement therapy rate (RRTR), mortality rate, and HT between the 2 groups (P>0.05).
Table 1.
General conditions and clinical data of HTGP and NHTGP patients.
| Variable | HTGP | NHTGP | χ2 | P |
|---|---|---|---|---|
| Gender/Male n (%) | 111 (69.81) | 109 (63.37) | 1.537 | 0.244 |
| Age, years; media (P25–P75) | 37.00 (31.00–45.00) | 52.00 (41.00–64.00) | 8.626 | <0.001 |
| AMY, U/L; media (P25–P75) | 223.00 (117.00–586.00) | 642.00 (259.25–1350.75) | 6.036 | <0.001 |
| LIP, U/L; media (P25–P75) | 1982.00 (883.00–5559.00) | 4334.00 (1143.50–12207.50) | 3.955 | <0.001 |
| Ca2+, mmol/L; media (P25–P75) | 2.00 (1.72–2.16) | 2.21 (1.99–2.30) | 5.966 | <0.001 |
| HsCRP, mg/L; media (P25–P75) | 173.73 (88.52–283.46) | 106.78 (31.49–211.28) | 4.827 | <0.001 |
| NLR; media (P25–P75) | 9.49 (7.51–14.12) | 8.69 (6.18–10.88) | 3.134 | 0.002 |
| SAP n (%) | 54 (33.96) | 42 (24.42) | 3.655 | 0.069 |
| LC n (%) | 41 (25.79) | 41 (23.84) | 0.168 | 0.704 |
| MVR n (%) | 28 (17.61) | 17 (9.88) | 4.199 | 0.053 |
| VDUR n (%) | 23 (14.47) | 16 (9.30) | 2.119 | 0.173 |
| RRTR n (%) | 17 (10.69) | 10 (5.81) | 2.624 | 0.113 |
| Mortality n (%) | 8 (5.03) | 4 (2.33) | 1.731 | 0.243 |
| HT, days; media (P25–P75) | 16.00 (10.00–24.00) | 13.00 (8.00–23.00) | 1.645 | 0.100 |
| BMI, kg/m2; media (P25–P75) | 26.91 (25.69–27.49) | 24.18 (22.69–25.39) | 10.153 | 0.001 |
HTGP – hypertriglyceridemia pancreatitis; NHTGP – non-hypertriglyceridemia pancreatitis; AMY – serum amylase; LIP – lipase; Ca2+ – free calcium ions; hsCRP – high-sensitivity C-reactive protein; NLR – neutrophil-lymphocyte ratio; SAP – severe acute pancreatitis; LC – local complications; MVR – mechanical ventilation rate; VDUR – vasoactive drug usage rate; RRTR – renal replacement therapy rate; HT – hospitalization time; BMI – body mass index.
Analysis for HTGP-induced factors
Among the 159 HTGP cases, 97 patients (61.01%) had an HTG medical history that accounted for of all HTGP cases. The average serum TG level of HTGP patients was 24.36 mmol/L. The factors inducing HTGP in patients were: 55 cases (34.59%) were caused by poorly controlled diabetes, 34 cases (21.38%) were caused by improper diet, 31 cases (19.50%) were caused by alcohol abuse, 18 cases (11.32%) were caused by pregnancy, 15 cases were caused by obesity (9.43%), and 6 cases (3.77%) had unknown causes (Figure 2).
Figure 2.
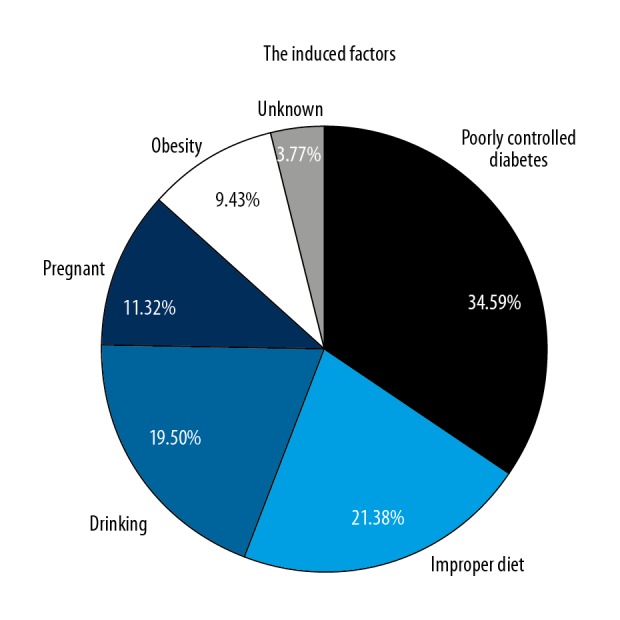
The composition of HTGP-induced factors.
Analysis of factors affecting the severity of HTGP
The HTGP patients were further divided into an SAP group and a non-SAP group. The results indicated that AMY, LIP, Ca2+, hsCRP, NLR, and BMI were significantly different between the 2 groups (P<0.001). There were no statistically significant differences in sex, age, HTG medical history, or TG level between the 2 groups (P>0.05, Table 2).
Table 2.
Univariate analysis of SAP in HTGP patients (n,%).
| Variable | SAP or non-SAP | χ2 | P | ||
|---|---|---|---|---|---|
| Non-SAP (n=105) | SAP (n=54) | ||||
| Sex n (%) | Male | 78 (74.29) | 33 (61.11) | 2.937 | 0.102 |
| Female | 27 (25.71) | 21 (38.89) | |||
| Age, years | ≤50 | 95 (90.48) | 48 (88.89) | 0.099 | 0.784 |
| >50 | 10 (9.52) | 6 (11.11) | |||
| AMY, U/L; media (P25–P75) | 195.00 (97.50–363.50) | 482.50 (152.25–936.50) | 3.548 | <0.001 | |
| LIP, U/L; media (P25–P75) | 1649.00 (643.50–3622.50) | 4086.50 (1412.25–7733.75) | 3.626 | <0.001 | |
| TG, mg/dL; media (P25–P75) | 12.30 (4.87–42.02) | 12.73 (7.60–42.05) | 0.731 | 0.465 | |
| Ca2+, mmol/L; media (P25–P75) | 2.10 (1.93–2.23) | 1.69 (1.46–1.91) | 6.309 | <0.001 | |
| hsCRP, mg/L; media (P25–P75) | 118.94 (77.30–218.47) | 286.45 (176.60–321.49) | 5.423 | <0.001 | |
| NLR; media (P25–P75) | 8.52 (7.02–9.67) | 15.39 (12.36–20.69) | 7.574 | <0.001 | |
| HTG medical history n (%) | 70 (66.67) | 27 (50.00) | 4.164 | 0.058 | |
| BMI, kg/m2; media (P25–P75) | 26.08 (25.13–27.03) | 27.57 (26.73–29.11) | 5.521 | <0.001 | |
SAP – severe acute pancreatitis; AMY – serum amylase; LIP – lipase; TG – triglyceride; Ca2+ – free calcium ions; hsCRP – high-sensitivity C-reactive protein; NLR – neutrophil-lymphocyte ratio; HTG – hypertriglyceridemia; BMI – body mass index.
The factors found to be statistically significant in univariate analysis were further analyzed by multivariate logistic regression analysis using the forward LR method. The results showed that serum Ca2+ (OR=0.018, P<0.001, 95%CI: 0.002–0.129) was an independent predictive factor for the severity of HTGP. hsCRP (OR=1.008, P=0.004, 95%CI: 1.003–1.013), NLR (OR=1.314, P<0.001, 95%CI: 1.161–1.488), and BMI (OR=1.597, P=0.002, 95%CI: 1.195–2.314) were independent risk factors (Table 3).
Table 3.
Multivariate logistic regression analysis of SAP in HTGP patients.
| Factors | OR | 95% CI | P |
|---|---|---|---|
| Ca2+ | 0.018 | 0.002–0.129 | <0.001 |
| hsCRP | 1.008 | 1.003–1.013 | 0.004 |
| NLR | 1.314 | 1.161–1.488 | <0.001 |
| BMI | 1.597 | 1.195–2.314 | 0.002 |
Ca2+ – free calcium ions; hsCRP – high-sensitivity C-reactive protein; NLR – neutrophil-lymphocyte ratio; BMI – body mass index.
Discussion
As a disease with increasing morbidity, causing severe illness and high medical cost, AP has attracted widespread attention [17]. The incidence of AP has increased by at least 20% worldwide in the past decade [18,19]. Studies show that HTG plays an important role in the morbidity of AP. Research reported that the HTG accounted for 11.8% of all etiologies of AP in the past decade in China [20]. In this study, HTG accounted for 20.60% of the total etiology of AP, which is probably related to the recent gradually increased awareness of lipid metabolism in the etiology of AP. The mechanism by which HTG leads to AP is as follows: (1) Excessive TG in the blood is hydrolyzed into free fatty acids by pancreatic LIP. When the increase of free fatty acid exceeds the binding capacity of albumin, protein kinase will be activated, which causes autodigestion of pancreatic cells. (2) Free fatty acids induce inflammatory reactions, leading to cytotoxicity and pancreatic capillary damage. (3) With the increasing blood viscosity, serum LIP particles accumulate, which can embolize pancreatic vessels and aggravate pancreatic ischemia and acidosis. (4) The acidic environment caused by ischemia further enhances the lipotoxicity of free fatty acids [21]. Previous studies suggested that, compared with AP caused by other factors, HTGP is more severe and can lead to many complications because of the intense inflammatory response induced by massive TG in the blood [22]. In this study, we found no statistically significant differences in the incidence of SAP between the 2 groups (patients with HTGP and those with NHTGP), and the incidence of SAP in the HTGP group was obviously higher than that in the NHTGP group (33.96% vs. 24.42%), which suggests that the overall condition of HTGP patients was more serious than that of NHTGP patients. However, this hypothesis needs to be tested in studies with larger samples to verify our findings. We also found that patients in the HTGP group had younger AP onset ages and lower AMY and LIP levels than those in the NHTGP group, which is consistent with the characteristics of HTGP. Early studies suggested that AMY was increased in HTGP patients. When the blood TG was indistinctly elevated, the results of AMY were false-negative due to the method of reading the calorimeter [23]. Early diagnosis is difficult owing to the clinical atypical features of HTGP. Therefore, timely imaging examination is necessary for patients with insignificant pancreatic enzyme elevation but persistent abdominal pain. Early recognition of HTGP helps improve the prognosis of these patients. As mentioned above, HTG is a key factor inducing the inflammatory response in HTGP. The average TG level at admission in this study was 24.36 mmol/L, which was far above normal levels. A cohort study demonstrated that TG levels were not only associated with the onset of HTGP, but also showed a significant dose-effect relationship [24]. Although the occurrence of AP is induced by HTG, it is controversial whether there is a direct relationship between HTG and the severity of AP. The recent study has determined that TG levels is not correlated with other indicators in estimating the severity of HTGP, such as Acute Physiology and Chronic Health Evaluation score (APACHE II), and it had no effect on prognostic indicators such as the mortality rate and the length of stay [25]. In the present study, there was no significant difference in TG between the SAP group and the non-SAP group (P>0.05), which may be related to the delayed assessment of some patients, resulting in a natural decrease in the high serum TG level. Therefore, further prospective and multicenter studies are needed to confirm whether TG is related to the severity of HTGP. Our study found that the causes of HTGP were poorly controlled diabetes (34.59%), inappropriate diet (21.38%), alcohol abuse (19.50%), pregnancy (11.32%), obesity (9.43%), and unknown causes (3.77%). In most of these patients, TG is normally at moderate levels. Under the influence of 1 or more inducing factors, the level of TG surges dramatically and quickly, which induces AP. Therefore, for patients with HTG, strict control of blood sugar, appropriate diet, alcohol abstinence, weight loss, and monitoring of TG during pregnancy could contribute to prevention of HTGP.
When HTGP patients progress into SAP, the mortality rate increases greatly. Therefore, it is of clinical significance to find early indicators to predict the severity of the disease. Our results show that low serum Ca2+ and elevated hsCRP, NLR, and BMI are independent risk factors affecting the severity of HTGP. Zahorec et al. [26] first used NLR as an index to reflect the balance between neutrophils and lymphocytes and as an inflammatory marker to evaluate systemic inflammatory response in critical patients, after which NLR was widely used in various clinical situations [27–30]. A study has shown that NLR has a significant advantage in predicting ICU hospitalization and mortality of AP patients [31]. In a cohort study, Wang et al. [32] discovered that when the NLR cut-off value was set to 10, the sensitivity and specificity for predicting severe HTGP were 90% and 82%, respectively. Multivariate analysis in the present study indicated that use of NLR in evaluation of the severity of HTGP was an independent risk factor for severe HTGP (OR=1.314, P<0.001). Elevated NRL can be used as a reliable indicator for assessing the severity of HTGP. CRP, as an acute-phase reaction protein synthesized and secreted by hepatocytes, is a non-specific inflammatory marker. The level of CRP can be distinctly increased when severe infection and tissue damage occur in the body. In the present study, hsCRP had higher sensitivity than other detection methods. In reflect of inflammatory response of the body, hsCRP shows accuracy similar to that of CRP. Previous studies have shown that CRP has a significant advantage in assessing the severity of HTGP [27]. Some scholars concluded that NLR was superior to CRP in predicting SAP and mortality [28], which is similar to our results. The most commonly used method for measuring obesity is BMI, which is calculated from a person’s weight in kilograms and height in meters (kg/m2). The literature demonstrates that BMI is a risk factor of SAP [14]. BMI higher than 30 kg/m2 can increase the risk of SAP by approximately 3-fold and can increase the risk of mortality by about 2-fold [15]. Some scholars found that elevated BMI was associated with increased incidence of chronic diseases such as cholecystitis, gallstone disease, hypertension, and hyperlipidemia, which can contribute to the occurrence and exacerbation of acute pancreatitis [16].
We found differences between HTGP and NHTGP in the severity and clinical characteristics. To prevent the occurrence of HTGP, the level of serum TG should be controlled in high-risk HTG populations, as well as avoiding the influence of the induced factors on the level of TG. Ca2+, hsCRP, NLR, and BMI have clinical value in predicting the severity of HTGP and can provide references for use in clinical practice.
Our study has certain limitations that should be considered. This was a retrospective, single-center study. We did not perform dynamic observations or analysis of the indicators affecting the severity of the disease. Moreover, the patients were all from northern China because data from southern China were not available, and we did not make an in-depth comparison of the incidence of HTGP between the northern and southern populations in China. Thus, further prospective and multicenter studies with large samples are needed to further improve diagnosis and treatment of HTGP in clinical practice.
Conclusions
We analyzed the clinical characteristics of HTGP patients in this study. Compared with NHTGP, the age of onset was younger and the levels of pancreatic enzymes were not obviously elevated in HTGP patients. Patients with HTGP tended to have low serum Ca2+ and elevated hsCRP, NLR, and BMI, and these were risk factors for developing SAP.
Footnotes
Source of support: This work was supported by the CAMS Innovation Fund for Medical Sciences (CIFMS, 2016-I2M-1-003)
Conflicts of interest
None.
References
- 1.Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 Update. Gastroenterology. 2012;143:1179–87. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kitagawa S, Sawai K. Hypertriglyceridemia-induced acute pancreatitis with normal pancreatic enzymes. Am J Med. 2018;131:E299–300. doi: 10.1016/j.amjmed.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Simons-Linares CR, Attar BM, Trick WE, et al. Is hypertriglyceridemia-induced acute pancreatitis different from other etiologies? A retrospective cohort of 460 cases. Am J Gastroenterol. 2016;111:S43. [Google Scholar]
- 4.Forsmark CE, Vege SS, Wilcox CM. Acute pancreatitis. New Engl J Med. 2016;375:1972–81. doi: 10.1056/NEJMra1505202. [DOI] [PubMed] [Google Scholar]
- 5.Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: An endocrine society clinical practice guideline. J Clin Endocr Metab. 2012;97:2969–89. doi: 10.1210/jc.2011-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adiamah A, Psaltis E, Crook M, Lobo DN. A systematic review of the epidemiology, pathophysiology and current management of hyperlipidaemic pancreatitis. Clin Nutr. 2018;37:1810–22. doi: 10.1016/j.clnu.2017.09.028. [DOI] [PubMed] [Google Scholar]
- 7.Banks PA, Bollen TL, Dervenis C, et al. Acute pancreatitis C: Classification of acute pancreatitis-2012: Revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–11. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 8.Wang CY, Zhao L, Hong YP, et al. Serum thyroid hormones levels are significantly decreased in pregnant rats with acute pancreatitis. Biochem Biophysical Res Commun. 2018;505:657–63. doi: 10.1016/j.bbrc.2018.09.180. [DOI] [PubMed] [Google Scholar]
- 9.Nie S, Cui XY, Guo JP, et al. Inhibiting role of rosiglitazone in the regulation of inflammatory response and protective effects for severe acute pancreatitis in mice. J Cell Biochem. 2019;120:799–808. doi: 10.1002/jcb.27440. [DOI] [PubMed] [Google Scholar]
- 10.Huai JP, Shao YY, Sun XC, et al. Melatonin ameliorates acute necrotizing pancreatitis by the regulation of cytosolic Ca2+ homeostasis. Pancreatology. 2012;12:257–63. doi: 10.1016/j.pan.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Cao X, Wang HM, Du H, et al. Early predictors of hyperlipidemic acute pancreatitis. Exp Ther Med. 2018;16:4232–38. doi: 10.3892/etm.2018.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abayli B, Gencdal G, Degirmencioglu S. Correlation between neutrophil/lymphocyte ratio and Ranson score in acute pancreatitis. J Clin Lab Anal. 2018;32:22437. doi: 10.1002/jcla.22437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chooklin S, Chuklin S, Shershen G, Popyk P. Neutrophil-lymphocyte ratio in acute pancreatitis: Diagnostic and prognostic role. Pancreas. 2018;47:1379. [Google Scholar]
- 14.Fei Y, Gao K, Tu J, et al. Predicting and evaluation the severity in acute pancreatitis using a new modeling built on body mass index and intra-abdominal pressure. Am J Surg. 2018;216:304–9. doi: 10.1016/j.amjsurg.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 15.Martínez J, Johnson CD, Sánchez-Payá J, et al. Obesity is a definitive risk factor of severity and mortality in acute pancreatitis: An updated meta-analysis. Pancreatology. 2006;6:206–9. doi: 10.1159/000092104. [DOI] [PubMed] [Google Scholar]
- 16.Lankisch PG, Schirren CA. Increased body weight as a prognostic parameter for complications in the course of acute pancreatitis. Pancreas. 1990;5:626–19. doi: 10.1097/00006676-199009000-00021. [DOI] [PubMed] [Google Scholar]
- 17.Peery AF, Crockett SD, Barritt AS, et al. Burden of gastrointestinal, liver, and pancreatic diseases in the United States. Gastroenterology. 2015;149:1731–41. doi: 10.1053/j.gastro.2015.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hazra N, Gulliford M. Evaluating pancreatitis in primary care: A population-based cohort study. Brit J Gen Pract. 2014;64:E295–301. doi: 10.3399/bjgp14X679732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spanier BWM, Bruno MJ, Dijkgraaf MGW. Incidence and mortality of acute and chronic pancreatitis in the Netherlands: A nationwide record-linked cohort study for the years 1995–2005. World J Gastroenterol. 2013;19:3018–26. doi: 10.3748/wjg.v19.i20.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang N, Zhang HY, Guo XH, et al. Meta-analysis of the characteristics of acute pancreatitis in China in recent ten years. Chinese J Digest Dis Imaging. 2016;6:71–75. [Google Scholar]
- 21.Kimura W, Mössner J. Role of hypertriglyceridemia in the pathogenesis of experimental acute pancreatitis in rats. Int J Gastrointest Cancer. 1996;20:177–84. doi: 10.1007/BF02803766. [DOI] [PubMed] [Google Scholar]
- 22.Nawaz H, Koutroumpakis E, Easler J, et al. Elevated serum triglycerides are independently associated with persistent organ failure in acute pancreatitis. Am J Gastroenterol. 2015;110:1497–503. doi: 10.1038/ajg.2015.261. [DOI] [PubMed] [Google Scholar]
- 23.Lesser PB, Warshaw AL. Diagnosis of pancreatitis masked by hyperlipemia. Ann Intern Med. 1975;82:795–98. doi: 10.7326/0003-4819-82-6-795. [DOI] [PubMed] [Google Scholar]
- 24.Murphy MJ, Sheng X, MacDonald TM, Wei L. Hypertriglyceridemia and acute pancreatitis. JAMA Inter Med. 2013;173(2):162–64. doi: 10.1001/2013.jamainternmed.477. [DOI] [PubMed] [Google Scholar]
- 25.Gubensek J, Buturovic-Ponikvar J, Romozi K, Ponikvar R. Factors affecting outcome in acute Hypertriglyceridemic pancreatitis treated with Plasma Exchange: An Observational Cohort Study. PLoS One. 2014;9:e102748. doi: 10.1371/journal.pone.0102748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zahorec R. Ratio of neutrophil to lymphocyte counts – rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Med J. 2001;102(1):5–14. [PubMed] [Google Scholar]
- 27.Stotz M, Gerger A, Eisner F, et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Brit J Cancer. 2013;109:416–21. doi: 10.1038/bjc.2013.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharaiha RZ, Halazun KJ, Mirza F, et al. Elevated preoperative Neutrophil: Lymphocyte ratio as a predictor of postoperative disease recurrence in esophageal cancer. Ann Surg Oncol. 2011;18:3362–69. doi: 10.1245/s10434-011-1754-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fuentes HE, Oramas DM, Paz LH, et al. Venous thromboembolism is an independent predictor of mortality among patients with gastric cancer. J Gastrointest Cancer. 2018;49(4):415–21. doi: 10.1007/s12029-017-9981-2. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Attar BM, Fuentes HE, et al. Performance of Khorana risk score for prediction of venous thromboembolism in patients with hepatocellular carcinoma. Clin Appl Thromb-Hemost. 2018;24:1–6. doi: 10.1177/1076029617699088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suppiah A, Arab T, Hamed M, et al. The prognostic value of the Neutrophil-Lymphocyte ratio (NLR) in acute pancreatitis: Iidentification of an optimal NLR. J Gastrointest Surg. 2013;17:675–81. doi: 10.1007/s11605-012-2121-1. [DOI] [PubMed] [Google Scholar]
- 32.Wang YC, Fuentes HE, Attar BM, et al. Evaluation of the prognostic value of neutrophil to lymphocyte ratio in patients with hypertriglyceridemia-induced acute pancreatitis. Pancreatology. 2017;17:893–97. doi: 10.1016/j.pan.2017.10.001. [DOI] [PubMed] [Google Scholar]


