Abstract
Objectives
To examine the effectiveness and safety of accelerated corneal collagen cross-linking (CXL) for keratoconus over a 24-month period and to explore potential prognostic factors for post-treatment visual outcome and progression.
Methods
A retrospective, non-comparative, interventional case series. All patients who underwent accelerated epithelium-off CXL, using 9 mW/cm2 ultraviolet-A irradiation for 10 min, for progressive keratoconus in Sunderland Eye Infirmary, UK, between May 2014 and July 2016 were included. All patients completed 24 months’ post-CXL follow-up. Significant post-CXL progression of keratoconus was defined as >1 D increase in Kmax from preoperative to 24-month visit.
Results
Fifty-two eyes of 48 patients were included. At 24-month post-CXL, there was a significant improvement in corrected-distance visual acuity (CDVA; −0.05 LogMAR; p = 0.026), Kmax (−1.68 D; p < 0.001), K1 (−0.64 D; p = 0.002) and Kmean (−0.50 D; p = 0.009). The proportion of eyes with CDVA ≥ 0.3 LogMAR significantly improved from 43 (82.7%) eyes preoperatively to 50 (96.2%) eyes at 24 months (p = 0.026). Corneal haze (12, 23.1%) was the only postoperative complication and no adverse event was noted. Final CDVA was associated with lower CDVA (p = 0.002) and greater Kmax (p = 0.018) at baseline. Post-CXL progression of keratoconus was associated with greater preoperative Kmax (p = 0.12) and Kmean (p = 0.11), though statistical significances were not achieved.
Conclusions
Accelerated CXL (9 mW/cm2) serves as an effective and safe treatment for halting the progression of keratoconus and stabilising the vision over a 24-month period. Our observation suggests that accelerated CXL might be more effective in stabilising keratoconus of milder severity; however further larger studies are required to elucidate this finding.
Subject terms: Corneal diseases, Risk factors
Introduction
Keratoconus is a bilateral, progressive, degenerative corneal condition. It is the most common corneal ectatic disorder with an estimated prevalence of 2–3 per 1000 people in the developed world [1]. It is characterised by progressive myopia and irregular astigmatism associated with central or paracentral stromal thinning and apical protusion [2]. Traditionally keratoconus is managed by glasses, soft and rigid gas-permeable contact lens depending on the severity of the disease, and ultimately with corneal transplantation if all conservative measures fail. Although only a small number of patients progress to needing corneal transplant, keratoconus remains the leading indication for corneal transplant in many countries [3–5].
In 2003 Wollensak et al. [6]. introduced the use of corneal collagen cross-linking (CXL) in managing progressive keratoconus. This novel, minimally invasive surgical technique utilises the combination of ultraviolet-A (UVA) light of 370 nm and photosensitising agent riboflavin to strengthen the corneal biomechanical stability and rigidity. In 2009 the National Institute for Health and Clinical Excellence (NICE) has recommended the use of CXL for progressive keratoconus in the UK. So far this is the only treatment that stabilises the progression of ectasia. The original epithelium-off Dresden protocol for CXL requires a 30-min instillation of topical riboflavin 0.1% solution containing 20% dextran followed by UVA irradiation at 3 mW/cm2 for 30 min (fluence of 5.4 J/cm2) [6]. Over the past decade, various modifications have been proposed to shorten the original treatment regime. These included the use of accelerated CXL protocol with higher irradiation energy in shorter time span and shorter instillation time for riboflavin drops [7].
The effectiveness and safety of conventional CXL protocol using 30 min of 3 mW/cm2 UVA irradiation for progressive keratoconus has been well established by many long-term studies [8–12]. There is currently an increasing number of studies demonstrating comparable effectiveness and safety between conventional and various accelerated protocols; however, only a few studies reported outcomes beyond 1 year follow-up [13–17]. In addition some studies demonstrated that the corneal flattening effect following CXL was more significant in advanced keratoconic eyes than in mild-moderate cases [18–20], albeit not consistently observed across all studies [17]. In this study, we aim to examine the effectiveness and safety of accelerated CXL for progressive keratoconus over a 24-month period and to determine the prognostic factors for visual outcome and progression following CXL.
Materials and methods
This was a retrospective, non-comparative, interventional case series. All eyes that underwent accelerated epithelium-off CXL for progressive keratoconus in Sunderland Eye Infirmary, UK, between May 2014 and July 2016 were included. Ethical approval was not required as the treatment provided was part of the standard clinical care. This study was approved as a service evaluation by the Local Clinical Governance Department (Sunderland, UK) and the conduct of study adhered to the tenets of Declaration of Helsinki.
Diagnosis and definition of disease progression
The diagnosis of keratoconus was made on both clinical and corneal tomographic grounds. Clinical signs included Fleischer ring, Vogt’s striae, central/paracentral stromal thinning and scissor reflex on retinoscopy. Corneal tomographic measurements, including Kmax (steepest keratometric point), K1 (flat keratometric meridian), K2 (steep keratometric meridian), astigmatism and thinnest corneal pachymetry (TCP), were obtained from a Scheimpflug camera (OCULUS Pentacam, Wetzlar, Germany) and were analysed. Belin-Ambrosio Enhanced Ectasia Display was used to aid the diagnosis in borderline cases. Amsler–Krumeich classification was used to grade the severity of keratoconus; stage 1–Kmean of <48 dioptres (D) and TCP > 400 μm, stage 2—Kmean of <53 D and/or TCP > 400 μm, and stage 3—Kmean of >53 D and/or TCP of 300–400 μm [21]. All patients were requested to leave out their soft contact lens for a week and hard contact lens for 2 weeks prior to corneal tomography. In patients who were contact lens wearer, if there was no best-spectacle-corrected visual acuity available on that particular clinic visit or there was an unexpected dropped of visual acuity from the previous visit which was not explained by clinical or tomographic changes, the contact lens-corrected visual acuity within the 3 months of that particular visit was used instead to overcome the limitation of this retrospective study.
Progression of keratoconus before CXL was defined by the presence of two or more of the following criteria: a decrease in subjective or objective visual acuity [either uncorrected-distance visual acuity (UDVA) or corrected-distance visual acuity (CDVA)] attributed to keratoconus, an increase of ≥1 D in K2, or an increase of ≥1.0 D in Kmax over the preceding 12 months [13]. The patients were initially reviewed at every 3–4 months after their first clinic visit during the first year and at every 6–9 monthly thereafter at the clinicians’ discretion. Poor visual outcome was defined as CDVA ≤ 0.3 LogMAR (or ≤6/12 Snellen vision) at 24 months following CXL. Significant post-CXL progression of keratoconus was defined as >1 D increase in Kmax from preoperative to 24 months following CXL.
Surgical technique
On the day of surgery, corneal tomography was repeated if the last measurement was obtained more than 2 months prior to the CXL. The majority of the cases were done under topical local anaesthesia unless general anaesthesia was specifically required (e.g. patient’s request, patient with learning disability, and paediatric cases). Briefly, topical tetracaine 1% and povidone-iodine 5% were instilled prior to the procedure. After surgical draping and insertion of a lid speculum, 18% alcohol was used for 30 s to loosen the corneal epithelium which was then removed by dry corneal spears to achieve a 7–8 mm diameter treatment area (incorporating the Fleischer ring, if present). Lid speculum was subsequently removed (to reduce corneal desiccation) [22] and topical 0.1% riboflavin drops with 1.1% hydroxypropyl methylcellulose without dextran (MedioCROSS M isotonic; Medio-Haus Medizinprodukte GmbH, Germany) were instilled onto the cornea every 3 min for 30 min. In eyes with preoperative TCP of <400 μm, topical hypotonic riboflavin drops (MedioCROSS H; Medio-Haus Medizinprodukte GmbH, Germany) was used as per the manufacturer’s guidance. Pachymetry was performed to ensure that the TCP was more than 400 μm before the UVA application. UVA (365 nm) irradiation with an energy release of 9 mW/cm2 for 10 min (fluence of 5.4 J/cm2) was then applied using the Intacs XL system (NanoSigma Biotech. Co., Ltd, Taiwan). At the end of the procedure, a bandage contact lens (BCL) was inserted (and removed within a week after CXL). The postoperative treatment regime included topical levofloxacin 0.5% (minims) QID for 2 weeks, topical diclofenac 0.1% (minims) TID as required for 2 days, topical dexamethasone 0.1% (minims) QID for 1 week followed by topical prednisolone 0.5% QID for 4–12 weeks (depending on the presence and severity of the corneal haze), and oral co-codamol 10/500 1–2 tablets QID as required for 2 days.
Statistical analysis
Corrected-distance visual acuity (CDVA) was analysed in LogMAR, with 0.0 and 1.0 being equivalent to Snellen vision of 6/6 and 6/60, respectively. Student’s t-test (paired and unpaired) and Fisher exact test/Chi square test were used for analysis of the mean difference and categorical variables between two groups, respectively. P-value of <0.05 was considered statistically significant.
Results
A total of 52 eyes of 48 patients were included in this study; mean age was 24.6 ± 6.0 years and 31 (59.6%) were male. All eyes completed 24 months’ post-CXL follow-up. Based on the Amsler–Krumeich classification, 27 (51.9%), 15 (28.8%), and 10 (19.2%) were of stage 1, stage 2, and stage 3 keratoconus, respectively.
Visual outcome
At the end of the study period, 41 (78.8%) eyes were corrected with spectacles and 11 (21.2%) eyes were corrected with rigid contact lens. Contact lens-corrected visual acuity was used in seven (13.5%) eyes at the 24-month visit based on the reason mentioned in the “Methods section”. There was a significant improvement of CDVA from preoperative (0.22 ± 0.18) to 24-month postoperative (0.17 ± 0.12; p = 0.026; Table 1). The majority of eyes maintained or improved the CDVA following CXL: 7 (13.5%), 11 (21.2%), 26 (50.0%), 7 (13.5%) and 1 (1.9%) eyes had >2-line gain, 1-to-2-line gain, no change (within 1-line gain/loss), 1-to-2-line loss and >2-line loss at final follow-up, respectively. The number of eyes achieving CDVA of 0.3 LogMAR or better significantly increased from 43 (82.7%) preoperatively to 50 (96.2%) at 24-month postoperative (p = 0.026; Fig. 1). Final CDVA was associated with various preoperative parameters, including lower CDVA (p = 0.002) and greater Kmax (p = 0.018; Table 2).
Table 1.
Summary of visual and corneal tomographic outcomes at baseline, at 12 months and at 24 months following accelerated (9 mW/cm2) corneal collagen cross-linking for progressive keratoconus
| Parameters | Baseline | 12 months | Changesa | P-value | 24 months | Changesa | P-value |
|---|---|---|---|---|---|---|---|
| LogMAR | 0.22 ± 0.18 | 0.19 ± 0.12 | −0.03 ± 0.14 | 0.10 | 0.17 ± 0.12 | −0.05 ± 0.16 | 0.026 |
| Kmax, D | 60.14 ± 6.19 | 59.30 ± 6.35 | −0.84 ± 2.23 | 0.009 | 58.46 ± 5.94 | −1.68 ± 2.95 | < 0.001 |
| K1, D | 47.53 ± 4.19 | 47.13 ± 4.16 | −0.41 ± 1.23 | 0.020 | 46.89 ± 4.06 | −0.64 ± 1.41 | 0.002 |
| K2, D | 51.03 ± 4.46 | 50.78 ± 4.12 | −0.25 ± 1.30 | 0.17 | 50.68 ± 4.10 | −0.35 ± 1.46 | 0.09 |
| Kmean, D | 49.28 ± 4.26 | 48.95 ± 4.06 | −0.33 ± 1.14 | 0.041 | 48.78 ± 4.00 | −0.50 ± 1.33 | 0.009 |
| Astigmatism, D | 3.49 ± 1.56 | 3.47 ± 1.98 | −0.03 ± 1.55 | 0.90 | 3.55 ± 1.62 | 0.06 ± 1.52 | 0.79 |
| TCP, μm | 447.31 ± 35.40 | 436.69 ± 38.53 | −10.62 ± 18.52 | <0.001 | 437.02 ± 38.74 | −10.29 ± 20.02 | <0.001 |
Mean values are presented in mean ± standard deviation (SD). Significant p-values (<0.05) are underlined. Paired t-test was used for statistical analysis
CDVA corrected-distance visual acuity, D dioptres, Kmax , maximum keratometric reading, K1 flattest keratometric meridian, K2 steepest keratometric meridian, TCP thinnest corneal pachymetry
aChanges refers to the difference between baseline and respective postoperative time points
Fig. 1.
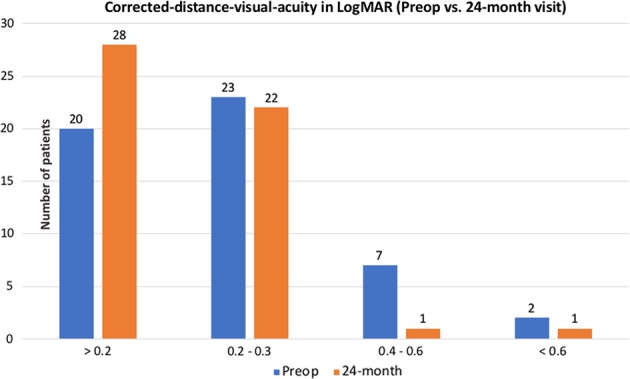
Summary of preoperative and final postoperative corrected-distance visual acuity of progressive keratoconic eyes that underwent accelerated corneal collagen cross-linking
Table 2.
Prognostic factors for poor visual outcome at final postoperative visit following accelerated corneal collagen cross-linking for progressive keratoconus
| Parameters | CDVA > 0.3 LogMAR | CDVA ≤ 0.3 LogMAR | P-value |
|---|---|---|---|
| (N = 42) | (N = 10) | ||
| Age, years | 24.23 ± 5.38 | 26.30 ± 8.09 | 0.33 |
| Gender | 0.28 | ||
| Female | 15 | 6 | |
| Male | 27 | 4 | |
| Laterality | 0.48 | ||
| Right eye | 20 | 6 | |
| Left eye | 22 | 4 | |
| Preoperative CDVA, LogMAR | 0.18 ± 0.16 | 0.37 ± 0.20 | 0.002 |
| Preop Kmax, D | 59.16 ± 5.57 | 64.26 ± 7.23 | 0.018 |
| Preop K1, D | 47.24 ± 4.04 | 48.79 ± 4.81 | 0.30 |
| Preop K2, D | 50.80 ± 4.42 | 52.02 ± 4.75 | 0.44 |
| Preop Kmean, D | 49.01 ± 4.16 | 50.41 ± 4.71 | 0.36 |
| Preop astigmatism, D | 3.56 ± 1.57 | 3.23 ± 1.58 | 0.56 |
| Preop TCP, μm | 449.43 ± 34.59 | 438.40 ± 39.28 | 0.38 |
Mean values are presented in mean ± standard deviation (SD). Significant p-values (<0.05) are underlined. Unpaired t-test and Fisher exact test were used for statistical analysis
CDVA corrected-distancevisual acuity, Kmax maximum keratometric reading, D dioptres, K1 flattest keratometric meridian, K2 steepest keratometric meridian, Kmean mean keratometry, TCP thinnest corneal pachymetry
Corneal tomographic outcomes
From preoperative to 24-month postoperative, there was a significant improvement in Kmax (−1.68 ± 2.95 D; p < 0.001), K1 (−0.64 ± 1.41 D; p = 0.002) and Kmean (−0.50 ± 1.33 D; p = 0.009) following accelerated CXL (Table 1). There was a mild, but statistically significant, decrease in TCP from preoperative to final postoperative visit (−10.29 ± 20.02 μm; −2.3%; p < 0.001) whereas K2 and astigmatism remained stable at 24 months postoperative.
Complications
There were 12 (23.1%) cases of mild post-CXL corneal haze, all of which settled by 6 months postoperative. There were no incidents of serious complications such as corneal scarring, infection, persistent epithelial defect and corneal decompensation.
Prognostic factors for post-treatment progression
Various potential prognostic factors for significant post-treatment progression of keratoconus were examined (Table 3). Although eyes that developed significant post-treatment progression were associated with higher Kmax (63.87 ± 7.95 vs. 59.66 ± 5.85 D; p = 0.12) and higher Kmean (51.92 ± 4.98 D vs. 48.94 ± 4.10 D; p = 0.11) compared to eyes that did not progress, none of these factors were statistically significant. We also examined for potential prognostic factors for post-treatment progression using Kmean and K2 as dependent variables but none was found to be statistically significant (not presented herein).
Table 3.
Prognostic factors for significant post-treatment progression of keratoconus, defined by >1 D increase in Kmax from preoperative to 24 months following corneal collagen cross-linking
| Parameters | ≤1 D change | >1 D change | P-value |
|---|---|---|---|
| (N = 46) | (N = 6) | ||
| Age, years | 24.37 ± 6.08 | 26.64 ± 4.84 | 0.38 |
| Gender | 0.68 | ||
| Female | 28 | 3 | |
| Male | 18 | 3 | |
| Laterality | 0.39 | ||
| Right eye | 22 | 4 | |
| Left eye | 24 | 2 | |
| Preop Kmax, D | 59.66 ± 5.85 | 63.87 ± 7.95 | 0.12 |
| Preop K1, D | 47.18 ± 4.01 | 50.25 ± 4.98 | 0.09 |
| Preop K2, D | 50.70 ± 4.32 | 53.58 ± 5.12 | 0.14 |
| Preop Kmean, D | 48.94 ± 4.10 | 51.92 ± 4.98 | 0.11 |
| Preop astigmatism, D | 3.52 ± 1.56 | 3.33 ± 1.70 | 0.79 |
| Preop TCP, μm | 448.15 ± 35.08 | 440.83 ± 40.69 | 0.64 |
Mean values are presented in mean ± standard deviation (SD). Unpaired t-test and Fisher exact test were used for statistical analysis
Kmax maximum keratometric reading, K1 flattest keratometric meridian, K2 steepest keratometric meridian, D dioptres, Kmean mean keratometry, TCP thinnest corneal pachymetry
Discussion
In this study we highlight the effectiveness and safety of accelerated CXL (9 mW/cm2 for 10 min) for progressive keratoconus over a 24-month period. We have also explored various potential prognostic factors for post-treatment visual outcome and progression of keratoconus. The concept of accelerated CXL was first proposed and validated in an animal model in which Schumacher et al. [23] demonstrated comparable corneal stiffening effect between conventional CXL (3 mW/cm2 for 30 min) and accelerated CXL (10 mW/cm2 for 9 min). The accelerated protocol allows the shortening of the irradiation treatment time while maintaining the same fluence of 5.4 J/cm2. Since then various accelerated protocols, including 9 mW/cm2 irradiation for 10 min, 18 mW/cm2 irradiation for 5 min and 30 mW/cm2 irradiation for 3 min [13–17, 24–27], have been proposed and studied.
Visual outcomes
To date there were only few studies in the literature reporting the outcome of accelerated CXL using 9 mW/cm2 irradiation for 10 min beyond 1-year follow-up [14–17], with two small studies limited to 12 eyes (mean follow-up of 13.9 months) [14] and 21 eyes (mean follow-up of 20.5 months) [15], one medium study of 49 eyes with 24 months follow-up [16], and one large study consisting of 282 eyes with 36 months follow-up [17]. All these studies consistently demonstrated non-significant changes in the CDVA, ranging from −0.06 to +0.02 LogMAR vision [14–17]. In our study, we observed a significant improvement of CDVA in 52 eyes at 24 months follow-up and the magnitude of the visual changes was similar to these studies (mean change of −0.05 LogMAR). Although one of the studies (n = 12 eyes) demonstrated significant improvement in CDVA following conventional CXL as compared to accelerated CXL [14], three other larger studies did not demonstrate any significant difference in CDVA postoperatively between the two protocols [15–17].
On the other hand, Hashemi et al. [10] and Wittig-Silva et al. [11]. reported a significant improvement of CDVA of 0.12 LogMAR (from 0.31 preoperative to 0.19 LogMAR at 5-year postoperative) and 0.09 LogMAR (from 0.33 preoperative to 0.24 LogMAR at 3-year postoperative), respectively, following conventional CXL. The greater extent of improvement of CDVA following CXL demonstrated in their studies might be attributed to the lower preoperative CDVA as compared to our study (mean preoperative CDVA of 0.22 LogMAR). Previous studies have reported that eyes with CDVA of 0.3 LogMAR (or 6/12 Snellen vision) or worse were more likely to achieve improvement of vision following CXL [28, 29]. The better preoperative CDVA observed in our study might also reflect an earlier detection and treatment of progressive keratoconus, potentially limiting the magnitude of improvement in CDVA following CXL. We also noted that postoperative CDVA was associated with lower preoperative CDVA and greater preoperative Kmax, which were all indicators of more advanced keratoconus. This highlights the potential benefit of early administration of CXL for progressive keratoconus in order to achieve good long-term visual outcome. We did not examine the prognostic factors for visual gain/loss following CXL because 84.6% patients maintained their vision (less than 2-line change) and only 1 patient lost more than 2 lines of vision. Recently Gore et al. [30] reported promising results of combined wavefront-guided transepithelial photorefractive keratectomy and CXL for visual rehabilitation in early-stage keratoconus whereby the mean CDVA improved from 0.28 LogMAR preoperatively to 0.15 LogMAR at 24 months postoperative.
Corneal tomographic/topographic outcomes
In addition to the improvement of CDVA, we observed a significant reduction of Kmax (−1.68 D), K1 (−0.64 D) and Kmean (−0.50 D) at 24-month follow-up. Significant improvement of Kmax, ranging from −0.3 D to −1.4 D, has been demonstrated in several long-term studies, either following conventional CXL [11, 17] or accelerated CXL using 9 mW/cm2 for 10 min [16, 17]. In addition Vounotrypidis et al. [17] reported a non-significant improvement of Kmean following accelerated CXL (10 mW/cm2 for 9 min) with significant difference between accelerated (−0.4 D) and conventional CXL (+0.1 D) at 3 years postoperative. Wang et al. [13] similarly reported a non-significant improvement of Kmean following accelerated CXL (18 mW/cm2 for 5 min) at a medium duration of 29 months (ranged 6–66 months) postoperative. On the other hand, we observed a mild but significant decline in the TCP (−10 μm or around 2.3% reduction) postoperatively. This finding has been previously reported in progressive keratoconic eyes following conventional CXL [11, 16, 17] and accelerated CXL [16, 26]. However this corneal thinning effect was not consistently demonstrated in other studies [10, 13, 15]. The difference in postoperative changes in TCP might be attributed to the heterogeneous patient cohort, types of CXL machine used for UVA irradiation and surgical technique employed among various studies. It is noteworthy to mention that though there was a significant decrease in TCP observed in our study, the extent of change was small and all other tomographic measurements have gained either significant or non-significant improvement postoperatively.
Prognostic factors for progression of keratoconus following CXL
Although we noted that keratoconic eyes that developed post-treatment progression were associated with higher preoperative Kmax and Kmean, none of these factors reached statistical significance. This might be related to the relatively small sample size that could have resulted in a type 2 error. Asri et al. [31] observed that preoperative Kmax > 58 D, age more than 35 years, and female gender significantly increased the rate of CXL treatment failure, which was defined as an increase of Kmax of >2 D at 1-year post-treatment. Nonetheless Vounotrypidis et al. [17] and Toprak et al. [29] did not observe any influence of preoperative Kmax on the outcome of postoperative tomographic parameters whereas other studies found that eyes with greater preoperative Kmax achieved more tomographic/topographic flattening following CXL [18–20].
Safety
The long-term safety of conventional and accelerated CXL has been well established in many studies. In our study, we observed 23.1% cases of mild post-CXL corneal haze, which were all transient in nature and resolved by 6 months postoperative after an initial course of topical steroids. Although our rate of corneal haze was higher than the incidence of postoperative corneal haze (median of 9.8%) reported in a systematic review [32], none of our patients experienced any significant visual loss (>2 Snellen lines) related to the corneal haze. Significant adverse events such as corneal infection, scarring and perforation have been previously reported in the literature following CXL [32, 33]; fortunately, these complications are uncommon and were not noted in our case series.
One of the limitations of our study was the lack of a control group for direct comparison of the efficacy between accelerated and conventional CXL. In addition, it is noteworthy to mention that subjective refraction in keratoconus, especially the advanced cases, could sometimes be difficult and prone to a degree of bias. However, the stabilisation of CDVA and significant improvement of Kmax observed in our study were consistent with other comparative studies, suggesting that this CXL protocol is effective and safe for stabilising progressive keratoconus. The other study limitation was that we did not examine the corneal biomechanical properties or the endothelial cell count pre- and post-CXL but there was no clinically detectable complication such as corneal decompensation or oedema noted in our study. In addition, we explored various potential prognostic factors for post-treatment progression of keratoconus but none of the parameters, including Kmax and Kmean, were statistically significant.
In summary our study demonstrates the effectiveness and safety of accelerated CXL on halting the progression of keratoconus, and stabilisation of vision over a 24-month period. We recommend the use of this CXL protocol in the clinical practice and our observation suggests that accelerated CXL might be more effective in stabilising keratoconus of milder severity, highlighting the potential benefit of early treatment; however further larger studies are required to elucidate this finding. Further randomised controlled trials with larger sample size and longer-term follow-up will help compare and validate the long-term effectiveness between accelerated CXL and conventional CXL.
Summary
What was known before
Accelerated corneal collagen cross-linking treatment (CXL), using the 9 mW/cm2 for 10 min protocol, was shown to be effective in stabilising progressive keratoconus; however, there are limited studies reporting outcomes beyond the 1 year time point. Various factors, including preoperative Kmax and age, were found to predict the risk of progression of keratoconus following CXL but these effects were inconsistently observed across different studies.
What this study adds
Our study supports the effectiveness and safety of accelerated CXL, using the 9 mW/cm2 for 10 min protocol, for halting the progression of keratoconus and stabilising the vision over a 24-month period. Post-CXL progression of keratoconus was associated with higher preoperative Kmax and Kmean, albeit statistical significances were not achieved in our study.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Godefrooij DA, de Wit GA, Uiterwaal CS, Imhof SM, Wisse RP. Age-specific incidence and prevalence of keratoconus: a nationwide registration study. Am J Ophthalmol. 2017;175:169–72. doi: 10.1016/j.ajo.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 2.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42:297–319. doi: 10.1016/S0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 3.Al-Towerki AE, Gonnah elS, Al-Rajhi A, Wagoner MD. Changing indications for corneal transplantation at the King Khaled Eye Specialist Hospital (1983-2002) Cornea. 2004;23:584e8. doi: 10.1097/01.ico.0000121708.58571.5b. [DOI] [PubMed] [Google Scholar]
- 4.Fasolo A, Frigo AC, Böhm E, Genisi C, Rama P, Spadea L, et al. The CORTES study: corneal transplant indications and graft survival in an Italian cohort of patients. Cornea. 2006;25:507e15. doi: 10.1097/01.ico.0000214211.60317.1f. [DOI] [PubMed] [Google Scholar]
- 5.Ting DS, Sau CY, Srinivasan S, Ramaesh K, Mantry S, Roberts F. Changing trends in keratoplasty in the West of Scotland: a 10-year review. Br J Ophthalmol. 2012;96:405–8. doi: 10.1136/bjophthalmol-2011-300244. [DOI] [PubMed] [Google Scholar]
- 6.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-A-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135:620–7. doi: 10.1016/S0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 7.Toker E, Çerman E, Özcan DOuml, Seferoğlu OumlB. Efficacy of different accelerated corneal crosslinking protocols for progressive keratoconus. J Cataract Refract Surg. 2017;43:1089–99. doi: 10.1016/j.jcrs.2017.05.036. [DOI] [PubMed] [Google Scholar]
- 8.Caporossi A, Mazzotta C, Baiocchi S, Caporossi T. Long-term results of riboflavin ultraviolet a corneal collagen cross-linking for keratoconus in Italy: the Siena eye cross study. Am J Ophthalmol. 2010;149:585–93. doi: 10.1016/j.ajo.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 9.Mazzotta C, Traversi C, Baiocchi S, Bagaglia S, Caporossi O, Villano A, et al. Corneal collagen cross-linking with riboflavin and ultraviolet A light for paediatric keratoconus: ten-year results. Cornea. 2018;37:560–6. doi: 10.1097/ICO.0000000000001505. [DOI] [PubMed] [Google Scholar]
- 10.Hashemi H, Seyedian MA, Miraftab M, Fotouhi A, Asgari S. Corneal collagen cross-linking with riboflavin and ultraviolet A irradiation for keratoconus: long-term results. Ophthalmology. 2013;120:1515–20. doi: 10.1016/j.ophtha.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Wittig-Silva C, Chan E, Islam FM, Wu T, Whiting M, Snibson GR. A randomized, controlled trial of corneal collagen cross-linking in progressive keratoconus: three-year results. Ophthalmology. 2014;121:812–21. doi: 10.1016/j.ophtha.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 12.Raiskup F, Theuring A, Pillunat LE, Spoerl E. Corneal collagen crosslinking with riboflavin and ultraviolet-A light in progressive keratoconus: ten-year results. J Cataract Refract Surg. 2015;41:41–6. doi: 10.1016/j.jcrs.2014.09.033. [DOI] [PubMed] [Google Scholar]
- 13.Wang YM, Chan TC, Yu MCY, Jhanji V. Comparative evaluation of progression rate in keratoconus before and after collagen crosslinking. Br J Ophthalmol. 2018;102:1109–13. doi: 10.1136/bjophthalmol-2017-311017. [DOI] [PubMed] [Google Scholar]
- 14.Ng AL, Chan TC, Cheng AC. Conventional versus accelerated corneal collagen cross-linking in the treatment of keratoconus. Clin Exp Ophthalmol. 2016;44:8–14. doi: 10.1111/ceo.12571. [DOI] [PubMed] [Google Scholar]
- 15.Males JJ, Viswanathan D. Comparative study of long-term outcomes of accelerated and conventional collagen crosslinking for progressive keratoconus. Eye (Lond) 2018;32:32–8. doi: 10.1038/eye.2017.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarac Ozge, Caglayan Mehtap, Uysal Betul Seher, Uzel Ayse Guzin Taslipinar, Tanriverdi Burak, Cagil Nurullah. Accelerated versus standard corneal collagen cross-linking in pediatric keratoconus patients: 24 months follow-up results. Contact Lens and Anterior Eye. 2018;41(5):442–447. doi: 10.1016/j.clae.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Vounotrypidis E, Athanasiou A, Kortüm K, Kook D, Shajari M, Priglinger S, et al. Long-term database analysis of conventional and accelerated crosslinked keratoconic mid-European eyes. Graefes Arch Clin Exp Ophthalmol. 2018;256:1165–72. doi: 10.1007/s00417-018-3955-3. [DOI] [PubMed] [Google Scholar]
- 18.Koc M, Uzel MM, Tekin K, Kosekahya P, Ozulken K, Yilmazbas P. Effect of preoperative factors on visual acuity, corneal flattening, and corneal haze after accelerated corneal crosslinking. J Cataract Refract Surg. 2016;42:1483–9. doi: 10.1016/j.jcrs.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 19.Chan TC, Chow VW, Jhanji V, Wong VW. Different topographic response between mild to moderate and advanced keratoconus after accelerated collagen cross-linking. Cornea. 2015;34:922–7. doi: 10.1097/ICO.0000000000000483. [DOI] [PubMed] [Google Scholar]
- 20.Chang CY, Hersh PS. Corneal collagen cross-linking: a review of 1-year outcomes. Eye Contact Lens. 2014;40:345–52. doi: 10.1097/ICL.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 21.Krumeich JH, Daniel J, Knulle A. Live-epikeratophakia for keratoconus. J Cataract Refract Surg. 1998;24:456–63. doi: 10.1016/S0886-3350(98)80284-8. [DOI] [PubMed] [Google Scholar]
- 22.Schmidinger G, Pachala M, Prager F. Pachymetry changes during corneal crosslinking: effect of closed eyelids and hypotonic riboflavin solution. J Cataract Refract Surg. 2013;39:1179–83. doi: 10.1016/j.jcrs.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 23.Schumacher S, Oeftiger L, Mrochen M. Equivalence of biomechanical changes induced by rapid and standard corneal cross-linking, using riboflavin and ultraviolet radiation. Invest Ophthalmol Vis Sci. 2011;52:9048–52. doi: 10.1167/iovs.11-7818. [DOI] [PubMed] [Google Scholar]
- 24.Shetty R, Pahuja NK, Nuijts RM, Ajani A, Jayadev C, Sharma C, et al. Current protocols of corneal collagen cross-linking: visual, refractive, and tomographic outcomes. Am J Ophthalmol. 2015;160:243–9. doi: 10.1016/j.ajo.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 25.Tomita M, Mita M, Huseynova T. Accelerated versus conventional corneal collagen crosslinking. J Cataract Refract Surg. 2014;40:1013–20. doi: 10.1016/j.jcrs.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 26.Hashemi H, Miraftab M, Seyedian MA, Hafezi F, Bahrmandy H, Heidarian S, et al. Long-term results of an accelerated corneal cross-linking protocol (18 mW/cm2) for the treatment of progressive keratoconus. Am J Ophthalmol. 2015;160:1164–70. doi: 10.1016/j.ajo.2015.08.027. [DOI] [PubMed] [Google Scholar]
- 27.Kymionis GD, Kontadakis GA, Hashemi KK. Accelerated versus conventional corneal crosslinking for refractive instability: an update. Curr Opin Ophthalmol. 2017;28:343–7. doi: 10.1097/ICU.0000000000000375. [DOI] [PubMed] [Google Scholar]
- 28.Greenstein SA, Hersh PS. Characteristics influencing outcomes of corneal collagen crosslinking for keratoconus and ectasia: implications for patient selection. J Cataract Refract Surg. 2013;39:1133–40. doi: 10.1016/j.jcrs.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Toprak I, Yaylalı V, Yildirim C. Factors affecting outcomes of corneal collagen crosslinking treatment. Eye (Lond) 2014;28:41–6. doi: 10.1038/eye.2013.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gore DM, Leucci MT, Anand V, Fernandez-Vega Cueto L, Arba Mosquera S, et al. Combined wavefront-guided transepithelial photorefractive keratectomy and corneal crosslinking for visual rehabilitation in moderate keratoconus. J Cataract Refract Surg. 2018;44:571–80. doi: 10.1016/j.jcrs.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 31.Asri D, Touboul D, Fournie P, Malet F, Garra C, Gallois A, et al. Corneal collagen crosslinking in progressive keratoconus: multicenter results from the French National Reference Center for Keratoconus. J Cataract Refract Surg. 2011;37:2137–43. doi: 10.1016/j.jcrs.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 32.Shalchi Z, Wang X, Nanavaty MA. Safety and efficacy of epithelium removal and transepithelial corneal collagen crosslinking for keratoconus. Eye (Lond) 2015;29:15–29. doi: 10.1038/eye.2014.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohammadpour M, Masoumi A, Mirghorbani M, Shahraki K, Hashemi H. Updates on corneal collagen cross-linking: indications, techniques and clinical outcomes. J Curr Ophthalmol. 2017;29:235–47. doi: 10.1016/j.joco.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


