Abstract
Background
Increasing evidence suggests that glutathione peroxidase 2 (GPX2) plays important roles in the tumorigenesis and progression of various human cancers, such as colorectal carcinomas and lung adenocarcinomas. However, the role of GPX2 in cervical cancer is unclear. In this study, we identified the role of GPX2 in cervical cancer tissues and cell lines.
Materials and methods
The basal mRNA and protein expression of GPX2 in cervical cancer cells and a series of key molecules in the epithelial to mesenchymal transition (EMT) and WNT/β-catenin pathways were examined via real time fluorescence quantitative PCR (qRT-PCR) and Western blot assays. The biological phenotype of the cervical cancer cell lines was detected by the cloning formation and transwell assays, and intracellular reactive oxygen species (ROS) levels were detected by flow cytometry. Finally, the GPX2 expression level in 100 clinical cervical tissues was examined by immunohistochemistry.
Results
We found that GPX2 was highly expressed in cervical cancer tissues compared to normal individuals and promoted the proliferation and metastasis of cervical cancer cells, and this promotion correlated with the activation of EMT and WNT/β-catenin signaling in vitro. GPX2 was determined to reduce apoptotic damage by reducing hydroperoxides. According to the characteristics and verification of GPX2, this series of phenotypes is clearly related to oxidative stress in cells. Furthermore, we verified that GPX2 was highly expressed in cervical cancer tissues and promoted the metastasis of cervical cancer.
Conclusion
In summary, we found that GPX2 was highly expressed in cervical cancer cells and promoted the proliferation and metastasis of cervical cancer by affecting oxidative stress. Our study provides a new target for the clinical treatment of cervical cancer.
Keywords: GPX2, reactive oxygen species, epithelial to mesenchymal transition, metastasis
Introduction
Cervical cancer is the second most common cancer in women, and the majority of female cancer deaths occur in developing countries.1 Various treatments based on the tumor grade and stage, including surgery and radiation therapy, are available for cervical cancer. Therefore, the identification and establishment of molecular markers for cervical cancer are highly necessary for improved diagnoses and the development of therapeutic targets.2,3 Glutathione peroxidase 2 (GPX2), also known as gastrointestinal GPX, belongs to the antioxidant enzyme GPX family. In mammals, the antioxidant enzyme family consists of eight different isoforms of glutathione peroxidase (GPX1–8).4,5 Five of the eight human glutathione peroxidases (GPXes) are selenoproteins, and their expression depends on the selenium (Se) supply. Most Se-dependent GPXes are downregulated in tumor cells; only GPX2 is considerably upregulated.6 A substantial amount of research on the role of GPXes in cancer progression has been performed. The major biochemical role of a hydroperoxide is to regulate the characteristics of cancer cells, including their proliferation, migration, invasion, angiogenesis and apoptosis. Oxidative stress due to the accumulation of reactive oxygen species (ROS), such as superoxide or hydrogen peroxide, is commonly observed in many types of cancer cells. Specifically, H2O2 can act as a second messenger in protumorigenic signaling pathways.7,8 GPX2 is believed to regulate cancer progression by regulating the level of hydroperoxides inside cells.9 The epithelial to mesenchymal transition (EMT) is an important molecular mechanism that promotes cancer metastasis,10–12 and it can be initiated by multiple signaling pathways, including the WNT/β-catenin signaling pathway, which plays key roles in the induction of cell growth, differentiation and metastasis. Moreover, previous studies have suggested that β-catenin can activate the promoter of GPX2,13 and the upregulated expression of β-catenin in the nucleus is associated with a poor prognosis.14–16
In this study, we explored the role of GPX2 in the progression of cervical cancer and confirmed the relationship between GPX2 expression and clinical pathological stages. Furthermore, we demonstrate that GPX2 activates EMT via the WNT/β-catenin signaling pathway leading to poor tumor prognosis.
Materials and methods
Human tumor tissues
All cervical cancer primary tissues were collected from the Pathology Department of the First Affiliated Hospital of Dalian Medical University between 2016 and 2018. All diagnoses of cervical cancer were confirmed by a professional pathologist. None of the patients received hormonal therapy, chemotherapy or radiotherapy before tumor resection. The tumor tissue was stored in formalin until use. All patients provided written informed consent, in accordance with the Declaration of Helsinki, and was approved by the Ethics Committee of the First Affiliated Hospital of Dalian Medical University.
Cell lines and cell culture
Human cervical cancer cell lines ME180 and HeLa were purchased from the American Type Culture Collection. The HeLa cell line was cultured in DMEM supplemented with 10% fetal bovine serum (FBS); and the ME180 cell line was cultured in McCoy’s 5A medium supplemented with 10% FBS. All cells were incubated at 37 °C with a 5% CO2 atmosphere.
Quantitative RT-PCR
Total RNA was extracted from cervical cancer cell lines with RNAiso Plus (TaKaRa Biotechnology, Dalian, China). RNA reverse transcription was performed using a PrimeScript™ RT reagent kit with gDNA Eraser (TaKaRa Biotechnology, Dalian, China). qRT-PCR analyses of GPX2, TCF-1 and cyclin D1 were performed using a SYBR Green qRT-PCR kit (TaKaRa Biotechnology, Dalian, China) according to the manufacturer’s protocol. Specificity was determined by melting curve analysis, and β-actin was used as an internal control for the normalization of mRNA levels. The primers used for GPX2 were 5-GACACGAGGAAACCGAAGCA-3 and 5-GGCCCTTCACAACGTCT-3; those used for TCF-1 were 5-GGTCCTACGTTCACCAACACA-3 and 5-CTCTGGGTCACATGGCTCT; and those used for cyclin D1 were 5-TGGAGCCCGTGAAAAAGAGC-3 and 5-TCTCCTTCATCTTAGAGGCCAC-3. The primers used for β-actin were 5-CATGTACGTTGCTATCCAGGC-3 and 5-CTCCTTAATGTCACGGACGAT-3 (GenePharma, Suzhou, China). All experiments were performed three times. Data analysis was performed using the 2−ΔΔCt method.
Transient transfection
The GPX2 overexpression sequence was inserted into the Pex-6(pGCMV/MCS/REP/Neo) vector. HeLa cells transfected with the GPX2 overexpression vector are described as the GPX2-Pex-6 group. Furthermore, HeLa cells transfected with the Pex-6 empty vector are described as the Pex-6 group. The cell line ME180 with transient silencing of GPX2 was classified into 3 groups: control (cells transfected with the negative control, sense: 5-UUCUCCGAACGUGUCACGUTT-3 and antisense: 5-AGCUGACACGUUCGGAGAATT-3); shGPX2#5 (cells transfected with GPX2-homo-593, sense: 5-GCUCAUCAUUUGGAGCCCUTT and antisense: 5-AGGGCUCCAAAUGAUGAGCTT-3); and shGPX2#8 (cells transfected with GPX2-homo-841, sense: 5-CCUCAGCAUUCCCUUGAUATT and antisense: UAUCAAGGGAAUGCUGAGGTT-3) (GenePharma, Suzhou, China). Transfection was performed using a Lipofectamine 2000 Transfection kit (Invitrogen, USA). The ratio of siRNA/DNA with Lipofectamine 2000 was determined and the transfection process was performed according to the manufacturer’s protocol. Subsequent experiments were performed 48 h after cell transfection.
Immunoblot analysis
Protein was extracted from cervical cancer cell lines using RIPA buffer (Solarbio, Beijing, China) supplemented with 1% protease/phosphatase inhibitor PMSF (Solarbio, Beijing, China). Protein separation was conducted by SDS/PAGE at 80 V for the spacer gel and 100 V for the separation gel, and proteins were transferred at 300 mA for 90 mins. The following primary antibodies were used: GPX2 (1:2000 dilution, ab140130, Abcam), β-actin (1:10,000 dilution, 14395-1-AP, Proteintech), GAPDH (1:4000 dilution, 60004-1-lg, Proteintech). E-cadherin (1:10,000 dilution, ab40772, Abcam), Vimentin (1:2000 dilution, 10366-1-AP, Proteintech), and Caspase-3 (1:1000 dilution, 19677-1-AP, Proteintech).
Measurement of ROS
To assay intracellular ROS, cells were incubated with 10 mmol/L DCF-DA (Nanjing Jiancheng, China) for 25 mins at 37 °C. The increase in fluorescence resulting from the oxidation of DCFH to DCF was measured by flow cytometry.
Colony formation assay
After transfection, cells (5,000 cells/well) were inoculated in a 12-well plate and cultured in medium in a 5% CO2 incubator at 37 °C for 1 week. The medium was changed every three days and supplemented with or without 0.2 mmol/L NAC or 400 µmol/L H2O2. Clones that were visible to the naked eye were washed three times with PBS. Then, the clones were stained with 0.1% crystal violet (Sigma-Aldrich) solution for 10 mins. The sum of the clone area was calculated by Image-Pro Plus 6.0.
Migration and invasion assays
The experiment was performed using transwell chambers with 8 μm pore membranes (Millipore). For the migration assay, cells were seeded into the top chamber in FBS-free medium at a density of 8×104 cells per chamber, and 600 μL of 10% FBS-containing medium was placed in the bottom chamber as a chemoattractant. For the invasion assay, 8×104 cells per chamber were plated on the top chamber with a Matrigel-coated membrane. After 48 h, the cells were stained with 0.1% crystal violet solution and counted under an inverted microscope (Leica).
Immunohistochemistry
Immunohistochemistry (IHC) analyses were carried out on paraffin sections of cervical tissues to investigate alterations in GPX2 protein expression using primary antibodies against GPX2 (1:100, ab140130, Abcam). Image-Pro Plus 6.0 was used to evaluate the mean integrated optical density (IOD) of the immunohistochemical results. Briefly, the results of the IHC were classified as follows: negative (0≤mean of the IOD<50); weak (50≤mean of the IOD<100); moderate (100≤mean of the IOD<200) and strong (200≤mean of the IOD). Five visual fields from different areas of each tumor were used for IOD evaluation, with mean IOD≤100 defined as low GPX2 expression and mean IOD≥100 defined as high GPX2 expression.
Statistical analysis
Values were obtained from at least three independent experiments, and differences between each group were expressed as the mean ± S.D. and analyzed by one-way ANOVA. Statistical analysis was performed using SPSS 15.0 software. A p-value<0.05 was considered significant.
Results
GPX2 expression in cervical squamous cell carcinoma (CESE) clinical specimens obtained from the TCGA database
GPX2 is upregulated in CESE. The mRNA expression of GPX2 was comparatively investigated in 3 normal cervix and 305 cervical cancer tissues. We found that GPX2 mRNA expression was significantly increased in CESE tissues compared to normal cervical tissues in the TCGA database (p<0.001, independent sample t-test, Figure 1A). However, in this study, we focused not only on CESE but also on adenocarcinoma. Therefore, in the subsequent experiments, we selected different types of cervical cancer cell lines.
Figure 1.
Expression of GPX2 in cervical clinical specimens and cell lines.
Notes: (A) GPX2 expression in cervical squamous cell carcinoma (CESE) clinical specimens obtained from the TCGA database. (B) GPX2 expression in cervical carcinoma cell lines ME180 and HeLa. (C) Western blot assay and qRT-PCR assay verify the transfection effect. Means ± S.D. for three independent experiments are shown. **P<0.01; ***P<0.001; ns, not significant.
Expression of GPX2 in cervical cancer cell lines
Studies based on the TCGA database led us to investigate the potential role of GPX2 in cervical cancer cell lines. First, we used qRT-PCR and Western blot analysis to examine GPX2 expression in ME180 and HeLa cells. Significantly higher mRNA and protein levels of GPX2 were found in epidermoid carcinoma-like ME180 cells than in HeLa cells, which exhibit an adenocarcinoma-like phenotype (p<0.001, t-test, Figure 1B). We transiently transfected the GPX2 overexpression vector into the HeLa cell line and silenced GPX2 expression in the ME180 cell line with two different sequences. After transfection, the levels of GPX2 in these two cell lines were verified by qRT-PCR and Western blot (Figure 1C). The results showed that the transfection was successful, and significant differences were observed.
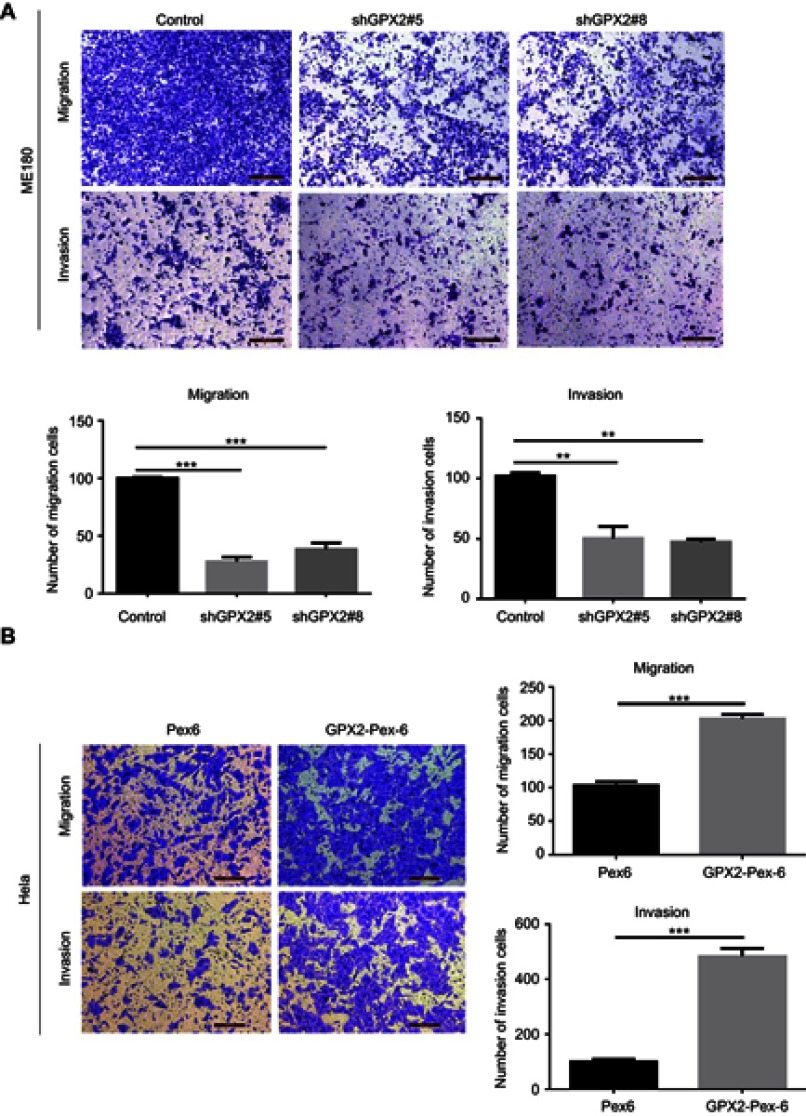
GPX2 promotes the migration and invasion of ME180 and HeLa cells
In the process of cervical cancer metastasis, cancer cell migration and invasion are critical initiation steps and can promote a micrometastasis to form a larger secondary tumor. To define the role of GPX2 in cancer cell metastasis, we examined cell migration and invasion in two cervical cancer cell lines, ME180 and HeLa. ME180 and HeLa cells were transfected for 24 h, and Boyden’s chamber assays were performed. As shown, silencing GPX2 in ME180 cells attenuated migratory and invasive abilities through the transwell assay with or without Matrigel (p<0.001, p<0.01, t-test, Figure 2A). In contrast, overexpressing GPX2 dramatically enhanced the migratory and invasive capacities of HeLa cells (p<0.001, p<0.001, t-test, Figure 2B). Taken together, these data indicate that GPX2 is a major determinant of migration and invasion. GPX2 promotes migratory and invasive behaviors in cervical cancer cells.
Figure 2.
GPX2 promotes the migration and invasion of ME180 and HeLa cells.
Notes: (A, B) The cellular migration and invasion potential of ME180 and HeLa were assessed using transwell assay, cell numbers on the underside of the filter was calculated as the total count from 10 random fields per filter. Means ± S.D. for three independent experiments are shown. **P<0.01; ***P<0.001.
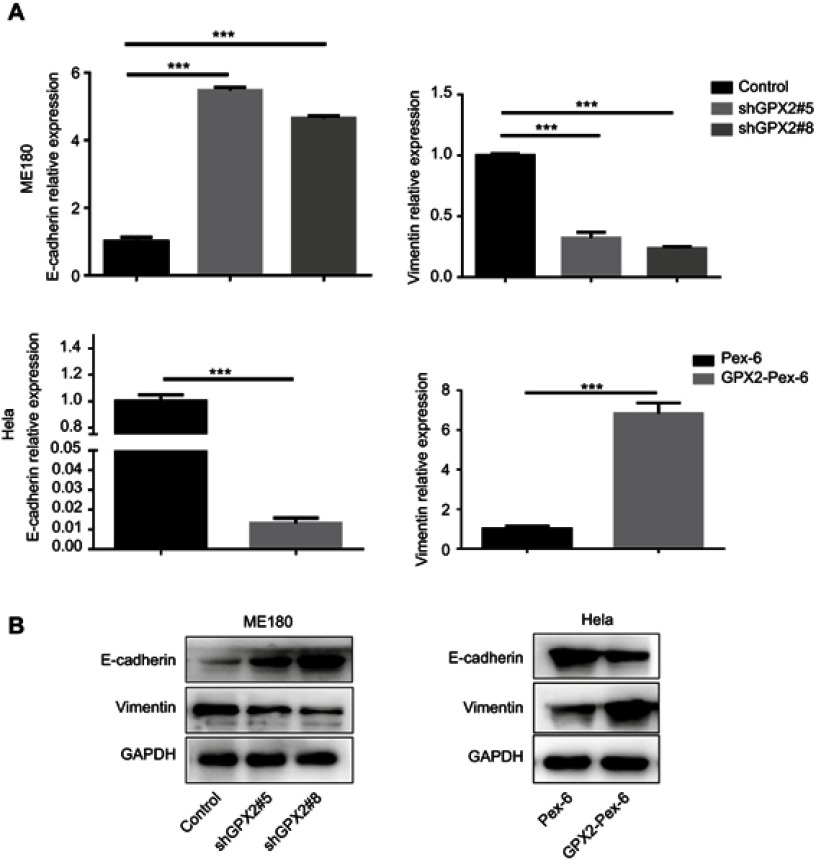
GPX2 promotes migration and invasion by activating the EMT
The primary cause of cancer-associated mortality is the ability of tumor cells to disseminate to distant sites. Studies have indicated that the EMT is a necessary prerequisite for tumor metastasis. To investigate how GPX2 positively regulates migration and invasion in cervical cancer cells, we measured the two most important EMT correlative markers, E-cadherin and Vimentin. The qRT-PCR results showed that GPX2 knockdown increased E-cadherin (epithelial marker) and decreased Vimentin (mesenchymal marker) at the mRNA and protein levels in the ME180 cell line (p<0.001, t-test, Figure 3A and B). In contrast, overexpression of GPX2 decreased E-cadherin and increased Vimentin at the mRNA and protein levels in the HeLa cell line (p<0.001, t-test, Figure 3A and B). These results suggest that GPX2 promotes migration and invasion by activating the EMT pathway.
Figure 3.
GPX2 affects the expression of EMT markers in cervical cancer cell lines.
Notes: (A, B) qRT-PCR and Western blot for E-cadherin and Vimentin in ME180 and HeLa cells after transfection. Means ± S.D. for three independent experiments are shown. ***P<0.001.
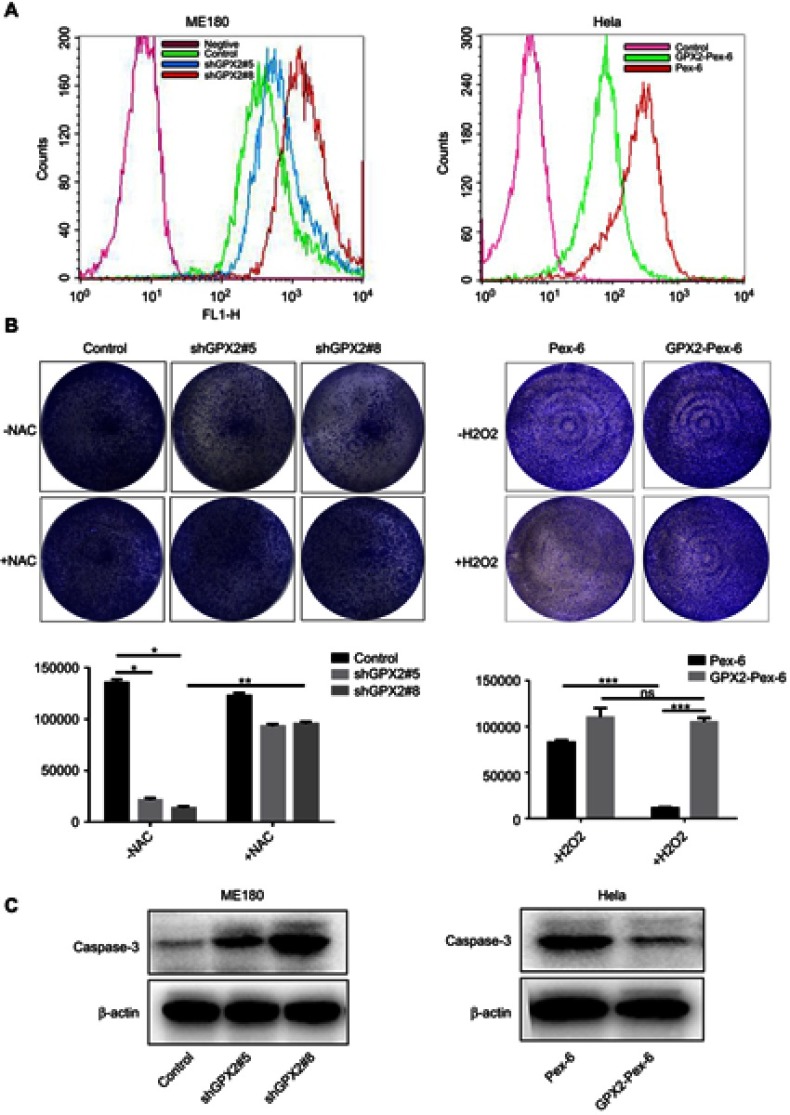
Identification of GPX2 as a key regulator of intracellular H2O2 levels and an inhibitor of apoptosis
According to previous research, GPX2 is expected to regulate cancer progression by regulating the levels of hydroperoxides inside cells. First, we assessed how the depletion of GPX2 would affect intracellular redox status by using the 2ʹ-7ʹdichlorofluorescein (DCF-DA) probe in cervical cancer cells. The results were consistent with previous studies.17 GPX2-knockdown ME180 cells contained significantly higher basal levels of ROS than control ME180 cells. Inversely, GPX2-overexpressing HeLa cells contained significantly lower basal levels of ROS than control HeLa cells (Figure 4A). Next, we tested how GPX2 knockdown affects the clone-forming potential in vitro. GPX2 silencing strongly reduced the proliferation capacity of the ME180 cell line, whereas the opposite result was found in HeLa cells overexpressing GPX2. Next, we examined whether elevated ROS levels were the cause of reduced proliferation and clone-forming potential in GPX2-knockdown tumor cells by using the ROS scavenger N-acetylcysteine (NAC). NAC treatment largely restored the clone-forming capacity of GPX2-knockdown cells. However, high expression of GPX2 in HeLa cells promoted the ability to resist the oxidative stress effect of H2O2 compared with the control group (Figure 4B). At the same time, we detected how GPX2 affects cell apoptosis. We found that the level of caspase-3, an indicator of apoptosis, was increased in ME180 cells with GPX2 knockdown. According to the Western blot analysis, the level of activated caspase-3 was decreased in HeLa cells with GPX2 overexpression (Figure 4C). In conclusion, we observed an effect of GPX2 suppression on tumor cell apoptosis.
Figure 4.
Identification of GPX2 as a key regulator of intracellular H2O2 levels and an inhibitor of apoptosis.
Notes: (A) FACS analysis of ROS levels using DCF-DA probe in ME180 and HeLa cell lines after transfection. (B) Colony formation assay of ME180 and HeLa cells after transfection in the presence or absence of 0.2 mmol/L NAC and 400 µmol/L H2O2; the sum of the clone area was calculated by the Image-Pro Plus 6.0. (C) Western blot analysis of the activated caspase-3 protein levels in ME180 and HeLa cells after transfection. Means ± S.D. for three independent experiments are shown. *P<0.05; **P<0.01; ***P<0.001; ns, not significant.
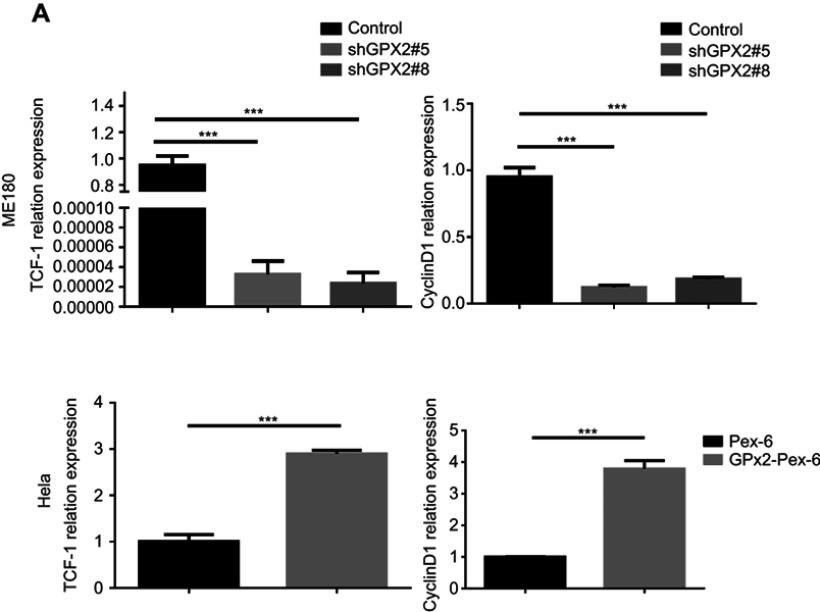
GPX2 activates the WNT/β-catenin pathway in cervical cancer cells
According to previous research, the GPX2 promoter is activated by β-catenin in gastrointestinal adenocarcinomas, which might explain the specific expression pattern of GPX2. To reveal the potential mechanisms by which GPX2 affects cervical cancer cells, we examined key molecules involved in the WNT/β-catenin pathway, ie, TCF-1 and cyclin D1. We found that the expression of TCF-1 and cyclin D1 decreased in ME180 cells under low GPX2 expression. However, the qRT-PCR assay showed that the expression of TCF-1 and cyclin D1 increased in HeLa cells under high GPX2 expression (p<0.001, t-test, Figure 5A). Thus, our results are consistent with previous studies13 and showed that GPX2 can activate the WNT/β-catenin pathway, thus leading to changes in other molecules involved in the pathway.
Figure 5.
GPX2 activates WNT/β-catenin pathway in cervical cancer cells.
Notes: (A) The relative expression of TCF-1 and Cyclin D1 was measured by qRT-PCR. Means ± S.D. for three independent experiments are shown. ***P<0.001.
Increased GPX2 is associated with accelerated metastasis in cervical cancer
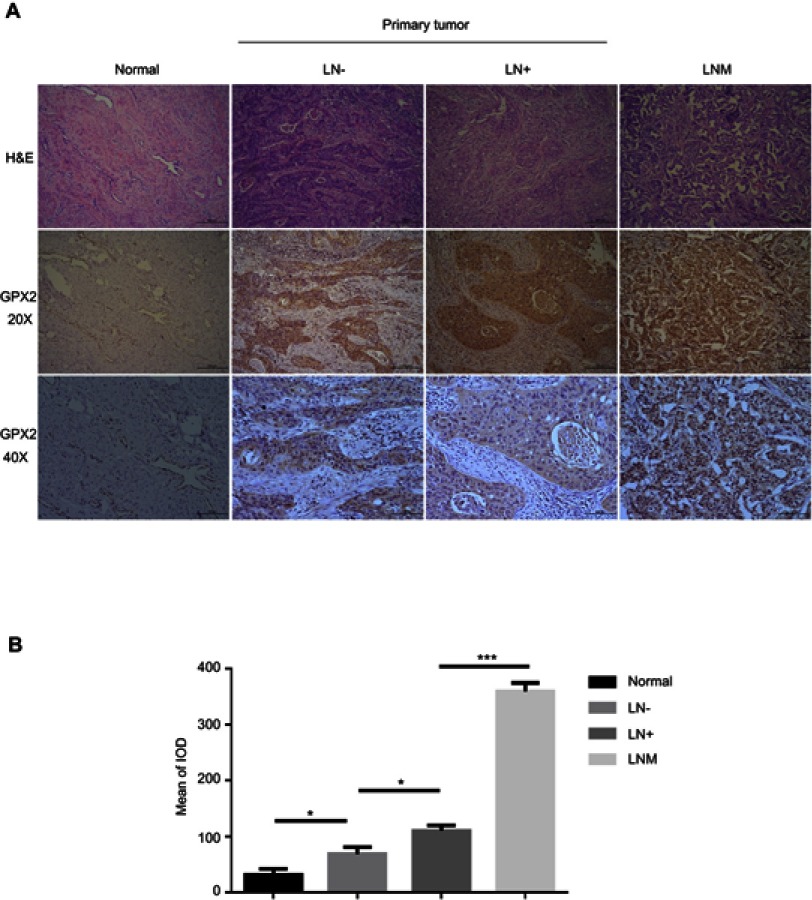
To demonstrate the biological significance of GPX2 in cervical cancer, we examined the immunohistochemical expression of GPX2 in 100 cervical specimens, including normal cervical tissues (normal), nonlymph metastatic primary cervical cancer tissues (LN-), lymph metastatic cervical cancer tissues (LN+) and distal metastatic lymph nodes (LNM). All specimens were collected from the Pathology Department of the First Affiliated Hospital of Dalian Medical University between 2016 and 2018. IHC images showed that the staining intensity of distant metastatic tissue was higher than that of regional lymph node metastatic tissue (Figure 6A). A quantitative IHC analysis revealed that GPX2 expression in normal cervical tissues (normal) was lower than that in nonlymph metastatic primary cervical cancer tissues (LN-) (p<0.05, t-test, Figure 6B) and lower in LN- than that in lymph metastatic cervical cancer tissues (LN+) (p<0.05, t-test, Figure 6B). All cervical tissues exhibited lower GPX2 expression than distal metastatic lymph nodes (LNM) (p<0.001, t-test, Figure 6B). We also analyzed the association between the pathological stage and GPX2 protein expression in 100 cervical cancer patients according to the standard of International Federation of Gynaecology and Obstetrics (FIGO) stage. The expression of GPX2 protein was associated with greater lymph node metastasis (p<0.001, x2-test, Table 1), although it was not associated with age, tumor size or histological type in cervical cancer patients. Taken together, these results suggest that GPX2 is closely related to tumor metastasis and the pathological stage in cervical cancer.
Figure 6.
Increase in GPX2 is associated with accelerated metastasis in cervical cancer.
Notes: (A) Pictures of H&E and GPX2 IHC in human cervical cancer tissues and metastatic lymph nodes (20X and 40X microscopes). Normal cervical tissues (Normal), nonlymph metastatic primary cervical cancer tissues (LN-), lymph metastatic cervical cancer tissues (LN+) and distal metastatic lymph nodes (LNM). (B) Image-Pro Plus 6.0 was used to evaluate the mean of the integrated optical density (IOD) of the immunohistochemical results. Means ± S.D. for three independent experiments are shown. *P<0.05; ***P<0.001.
Table 1.
Relationship between GPX2 expression and clinicopathological characteristics of cervical patient
| Characteristics | Total | IOD (mean) ≤100 | IOD (mean) ≥100 | P-value |
|---|---|---|---|---|
| Age (years) | 0.904 | |||
| <53 | 55 | 30 | 25 | |
| ≥53 | 45 | 24 | 21 | |
| Size (cm) | 0.450 | |||
| ≤2 | 35 | 20 | 15 | |
| >2 | 65 | 32 | 33 | |
| FIGO stage | <0.001*** | |||
| 35 | 30 | 5 | ||
| 32 | 10 | 22 | ||
| 20 | 4 | 16 | ||
| 13 | 1 | 12 | ||
| Histological type | 0.346 | |||
| Squamous cell carcinoma | 82 | 36 | 46 | |
| Adenocarcinoma | 17 | 10 | 7 | |
| Adeno-acanthoma | 1 | 0 | 1 | |
| Lymph node metastasis | <0.001*** | |||
| Absent | 67 | 40 | 27 | |
| Present | 33 | 5 | 28 |
Notes: Cervical patient were divided into GPX2 low and GPX2 high group according to the cut off value. (n = 100). p< 0.05 was considered statistic significant. Differences among variables were evaluated SPSS application. ***p<0.001.
Abbreviations: GPX2, Glutathione peroxidase; IOD (mean), the mean of integrated optical density; IOD ≤100, low GPX2 expression.
Discussion
GPX2 is a member of the GPX antioxidant enzyme family, and reports have shown the involvement of GPX2 in several types of cancer.18 However, the role of GPX2 in the development of cervical cancer is still unclear, although the role of GPX3 in cervical cancer has been reported.2 In this study, we focused on the relationship between GPX2 and the development of cervical cancer. Compared to other GPXes, GPX2 is mostly upregulated in tumor tissue, indicating that GPX2 expression is associated with high proliferation.6 In data extracted from the TCGA database, we found that GPX2 was highly expressed in CESE compared with normal tissues (Figure 1A). We also examined the relationship between GPX2 expression and cell proliferation in different types of cervical cancer cells. Similarly, we found that cells with high GPX2 expression were more likely to proliferate than those with low GPX2 expression (Figure 4B). According to previous research, GPX2 is specifically localized to the epithelial cells of different tissues rather than ubiquitously expressed.18,19 We observed this phenomenon in our IHC experiments (data not shown). GPX2 is most often detected within the gastrointestinal tract in humans, and it is localized at the crypt base, where stem cells and transit amplifying cells reside. Thus, the location of GPX2 indicated its connection to stem cell-like cells. Indeed, human-induced pluripotent stem cells appear to ensure their genomic integrity by upregulating GPX2 expression.20 GPXes are a family of antioxidant enzymes that promote the reduction of hydroperoxides by means of glutathione (2 glutathiones + H2O2←→glutathione disulfide +2H2O).2 GPX2 is the primary enzyme responsible for scavenging lipid hydrogen peroxide and ROS produced by intestinal inflammation primarily caused by pathogenic and nonpathogenic bacteria.21 GPX2 has an anti-inflammatory and antitumor effect in the course of tumorigenesis.22 According to our research, ME180 cells with GPX2 knockdown have higher dichlorodihydrofluorescein (DCF) fluorescence, indicating higher H2O2 levels than GPX2 competent control cells. HeLa cells with GPX2 overexpression showed the opposite result (Figure 4A). NAC treatment largely restored the clone-forming capacity of GPX2-knockdown cells. However, in HeLa cells, high expression of GPX2 was more beneficial to resisting the oxidative stress effect of H2O2 on cells compared with the control group (Figure 4B). In general, ROS generation supports tumor growth but also leads to vulnerabilities that may be exploited therapeutically.23 Thus, these characteristics would classify GPX2 as a potent anticarcinogenic agent. However, the same properties would also classify GPX2 as a procarcinogenic agent because the removal of hydroperoxides helps cells evade apoptosis, especially damaged cells that have progressed into a precancerous state.24 Many reports have shown that the ROS-mediated regulation of cell death is in the form of apoptosis, and the central mediator of the intrinsic apoptosis pathway is the mitochondria.25–27 According to the Western blot assay, we found that the level of activated caspase-3, an indicator of apoptosis, was increased in ME180 cells with GPX2 knockdown and decreased in HeLa cells with GPX2 overexpression (Figure 4C). The EMT is a crucial step in cancer metastasis because it is a phenotype that causes cancer cell migration and invasion by breaking cell–cell junctions.28,29 ROS act as inducers of matrix metalloproteinase (MMP) production and regulators in the EMT process, which are both related to cancer metastatic progression.30,31 Therefore, we speculated that GPX2 could regulate metastasis, including the migration and invasion of cervical cancer cells, by regulating ROS. To elucidate the relationship between GPX2 and the EMT process in cervical cancer, we conducted phenotypic experiments on cervical cancer cell lines, and the results showed that GPX2 promoted cell metastasis (Figure 2A and B). Then, we examined the EMT-related molecules E-cadherin and Vimentin in transgenic cervical cancer cell lines, and the results suggested that GPX2 contributed to cellular mesenchymal transformation. A possible link between GPX2 and the WNT pathway was first observed by van de Wetering et al32. Based on this finding, a functional TCF-binding element was identified in the GPX2 promoter, which responded to WNT signals. Thus, GPX2 might be a novel target of the WNT pathway. We examined WNT pathway-related molecules, and the results are presented in Figure 5A. However, the mechanism by which WNT pathway-related molecules contribute to cervical cancer has not been clarified and should be further verified. In this study, we identified the role of GPX2 in cervical cancer and determined how GPX2 affects the development of cervical cancer, thus providing a new target for the clinical treatment of cervical cancer.
Conclusion
In summary, we found that GPX2 was highly expressed in cervical cancer tissues and promoted the proliferation and metastasis of cervical cancer by affecting oxidative stress. Our study provides a new target for the clinical treatment of cervical cancer.
Acknowledgment
We thank the Pathology Department of First Affiliated Hospital of Dalian Medical University for their technical support in IHC and staining evaluation
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jia Y, Li H, Liu G, Song F. SMAGP a novel biomarker of cervical cancer development and progression. Onco Targets Ther. 2018;11:6925–6935. doi: 10.2147/OTT.S175808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang X, Zheng Z, Yingji S, et al. Downregulation of glutathione peroxidase 3 is associated with lymph node metastasis and prognosis in cervical cancer. Oncol Rep. 2014;31(6):2587–2592. doi: 10.3892/or.2014.3152 [DOI] [PubMed] [Google Scholar]
- 3.Bachtiary B, Boutros PC, Pintilie M, et al. Gene expression profiling in cervical cancer: an exploration of intratumor heterogeneity. Clin Cancer Res. 2006;12(19):5632–5640. doi: 10.1158/1078-0432.CCR-06-0357 [DOI] [PubMed] [Google Scholar]
- 4.Margis R, Dunand C, Teixeira FK, Margis-Pinheiro M. Glutathione peroxidase family - an evolutionary overview. Febs J. 2008;275(15):3959–3970. doi: 10.1111/j.1742-4658.2008.06542.x [DOI] [PubMed] [Google Scholar]
- 5.Toppo S, Vanin S, Bosello V, Tosatto SC. Evolutionary and structural insights into the multifaceted glutathione peroxidase (Gpx) superfamily. Antioxid Redox Signal. 2008;10(9):1501–1514. doi: 10.1089/ars.2008.2057 [DOI] [PubMed] [Google Scholar]
- 6.Kipp AP. Selenium-dependent glutathione peroxidases during tumor development. Adv Cancer Res. 2017;136:109–138. doi: 10.1016/bs.acr.2017.07.004 [DOI] [PubMed] [Google Scholar]
- 7.Nogueira V, Hay N. Molecular pathways: reactive oxygen species homeostasis in cancer cells and implications for cancer therapy. Clin Cancer Res. 2013;19(16):4309–4314. doi: 10.1158/1078-0432.CCR-12-1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez-Outschoorn UE, Balliet RM, Lin Z, et al. Hereditary ovarian cancer and two-compartment tumor metabolism: epithelial loss of BRCA1 induces hydrogen peroxide production, driving oxidative stress and NFkappaB activation in the tumor stroma. Cell Cycle. 2012;11(22):4152–4166. doi: 10.4161/cc.22226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brigelius-Flohe R, Kipp A. Glutathione peroxidases in different stages of carcinogenesis. Biochim Biophys Acta. 2009;1790(11):1555–1568. doi: 10.1016/j.bbagen.2009.03.006 [DOI] [PubMed] [Google Scholar]
- 10.De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13(2):97–110. doi: 10.1038/nrc3447 [DOI] [PubMed] [Google Scholar]
- 11.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–196. doi: 10.1038/nrm3758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J, Zhang H, Chen Y, et al. miR-598 inhibits metastasis in colorectal cancer by suppressing JAG1/Notch2 pathway stimulating EMT. Exp Cell Res. 2017;352(1):104–112. doi: 10.1016/j.yexcr.2017.01.022 [DOI] [PubMed] [Google Scholar]
- 13.Kipp A, Banning A, Brigelius-Flohe R. Activation of the glutathione peroxidase 2 (GPx2) promoter by beta-catenin. Biol Chem. 2007;388(10):1027–1033. doi: 10.1515/BC.2007.137 [DOI] [PubMed] [Google Scholar]
- 14.Gamallo C, Palacios J, Moreno G, Calvo de Mora J, Suarez A, Armas A. Beta-catenin expression pattern in stage I and II ovarian carcinomas: relationship with beta-catenin gene mutations, clinicopathological features, and clinical outcome. Am J Pathol. 1999;155(2):527–536. doi: 10.1016/s0002-9440(10)65148-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee CM, Shvartsman H, Deavers MT, et al. Beta-catenin nuclear localization is associated with grade in ovarian serous carcinoma. Gynecol Oncol. 2003;88(3):363–368. [DOI] [PubMed] [Google Scholar]
- 16.Kildal W, Risberg B, Abeler VM, et al. Beta-catenin expression, DNA ploidy and clinicopathological features in ovarian cancer: a study in 253 patients. Eur J cancer. 2005;41(8):1127–1134. doi: 10.1016/j.ejca.2005.01.022 [DOI] [PubMed] [Google Scholar]
- 17.Emmink BL, Laoukili J, Kipp AP, et al. GPx2 suppression of H2O2 stress links the formation of differentiated tumor mass to metastatic capacity in colorectal cancer. Cancer Res. 2014;74(22):6717–6730. doi: 10.1158/0008-5472.CAN-14-1645 [DOI] [PubMed] [Google Scholar]
- 18.Liu T, Kan XF, Ma C, et al. GPX2 overexpression indicates poor prognosis in patients with hepatocellular carcinoma. Tumour Biol. 2017;39(6):1010428317700410. doi: 10.1177/1010428317700410 [DOI] [PubMed] [Google Scholar]
- 19.Trachootham D, Zhang H, Zhang W, et al. Effective elimination of fludarabine-resistant CLL cells by PEITC through a redox-mediated mechanism. Blood. 2008;112(5):1912–1922. doi: 10.1182/blood-2008-04-149815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dannenmann B, Lehle S, Hildebrand DG, et al. High glutathione and glutathione peroxidase-2 levels mediate cell-type-specific DNA damage protection in human induced pluripotent stem cells. Stem Cell Reports. 2015;4(5):886–898. doi: 10.1016/j.stemcr.2015.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes DJ, Kunicka T, Schomburg L, Liska V, Swan N, Soucek P. Expression of selenoprotein genes and association with selenium status in colorectal adenoma and colorectal cancer. Nutriets. 2018;10(11):E1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finamore A, Ambra R, Nobili F, Garaguso I, Raguzzini A, Serafini M. Redox role of lactobacillus casei shirota against the cellular damage induced by 2,2ʹ-azobis (2-amidinopropane) dihydrochloride-induced oxidative and inflammatory stress in enterocytes-like epithelial cells. Front Immunol. 2018;9:1131. doi: 10.3389/fimmu.2018.01131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azzariti A, Iacobazzi RM, Di Fonte R, et al. Plasma-activated medium triggers cell death and the presentation of immune activating danger signals in melanoma and pancreatic cancer cells. Sci Rep. 2019;9(1):4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan W, Chen X. GPX2, a direct target of p63, inhibits oxidative stress-induced apoptosis in a p53-dependent manner. J Biol Chem. 2006;281(12):7856–7862. doi: 10.1074/jbc.M512655200 [DOI] [PubMed] [Google Scholar]
- 25.Nose K. Role of reactive oxygen species in the regulation of physiological functions. Biol Pharm Bull. 2000;23(8):897–903. doi: 10.1248/bpb.23.897 [DOI] [PubMed] [Google Scholar]
- 26.Chen XJ, Butow RA. The organization and inheritance of the mitochondrial genome. Nat Rev Genet. 2005;6(11):815–825. doi: 10.1038/nrg1708 [DOI] [PubMed] [Google Scholar]
- 27.Dakubo GD, Parr RL, Costello LC, Franklin RB, Thayer RE. Altered metabolism and mitochondrial genome in prostate cancer. J Clin Pathol. 2006;59(1):10–16. doi: 10.1136/jcp.2005.027664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei L, Yao Y, Zhao K, et al. Oroxylin A inhibits invasion and migration through suppressing ERK/GSK-3beta signaling in snail-expressing non-small-cell lung cancer cells. Mol Carcinog. 2016;55(12):2121–2134. doi: 10.1002/mc.22456 [DOI] [PubMed] [Google Scholar]
- 29.Nushtaeva AA, Karpushina AA, Ermakov MS, et al. Establishment of primary human breast cancer cell lines using “pulsed hypoxia” method and development of metastatic tumor model in immunodeficient mice. Cancer cell Int. 2019;19:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Touyz RM. Mitochondrial redox control of matrix metalloproteinase signaling in resistance arteries. Arterioscler Thromb Vasc Biol. 2006;26(4):685–688. doi: 10.1161/01.ATV.0000216428.90962.60 [DOI] [PubMed] [Google Scholar]
- 31.Chen X, Jiang Z, Zhou C, et al. Activation of Nrf2 by sulforaphane inhibits high glucose-induced progression of pancreatic cancer via AMPK dependent signaling. Cell Physiol Biochem. 2018;50(3):1201–1215. doi: 10.1159/000494547 [DOI] [PubMed] [Google Scholar]
- 32.van de Wetering M, Sancho E, Verweij C, et al. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111(2):241–250. doi: 10.1016/s0092-8674(02)01014-0 [DOI] [PubMed] [Google Scholar]